Abstract
With the expansion of DNA sequencing technology, quantifying evolution during emerging viral outbreaks has become an important tool for scientists and public health officials. Although it is known that the degree of sequence divergence significantly affects the calculation of evolutionary metrics in viral outbreaks, the extent and duration of this effect during an actual outbreak remains unclear. We have analyzed how limited divergence time during an early viral outbreak affects the accuracy of molecular evolutionary metrics. Using sequence data from the first 25 months of the 2009 pandemic H1N1 (pH1N1) outbreak, we calculated each of three different standard evolutionary metrics—molecular clock rate (i.e., evolutionary rate), whole-gene dN/dS, and site-wise dN/dS—for hemagglutinin and neuraminidase, using increasingly longer time windows, from 1 month to 25 months. For the molecular clock rate, we found that at least 3–4 months of temporal divergence from the start of sampling was required to make precise estimates that also agreed with long-term values. For whole-gene dN/dS, we found that at least 2 months of data were required to generate precise estimates, but 6–9 months were required for estimates to approach their long term values. For site-wise dN/dS estimates, we found that at least 6 months of sampling divergence was required before the majority of sites had at least one mutation and were thus evolutionarily informative. Furthermore, 8 months of sampling divergence was required before the site-wise estimates appropriately reflected the distribution of values expected from known protein-structure-based evolutionary pressure in influenza. In summary, we found that evolutionary metrics calculated from gene sequence data in early outbreaks should be expected to deviate from their long-term estimates for at least several months after the initial emergence and sequencing of the virus.
Keywords: influenza, molecular clock rate, dN/dS, emerging infectious diseases, evolution
1 Introduction
While modern medicine has been able to control the prevalence of traditional human pathogens through vaccines and medications, emerging infectious diseases remain a major threat to public health. Many emerging infectious diseases are caused by zoonotic viruses, which are normally endemic to a reservoir animal and are transmitted to humans upon exposure to the respective reservoir. Some viruses that have successfully undergone zoonoses into humans during the last century include HIV, Zaire ebolavirus, Hantavirus, Machupo virus, Marburg virus, and Chickungya virus, as well as most major influenza virus subtypes, including pandemic 1918 H1N1, H2N2, H3N2, pH1N1, H5N1, and the recent H7N9.
Quantifying the evolutionary dynamics of emerging zoonotic epidemics has become an important tool in understanding and controlling such epidemics. Computational methods that extract evolutionary information from gene-sequence data can produce valuable hypotheses regarding viral adaptation (Bush et al. 1999b; Luksza and Lassig 2014). Two particular metrics have been widely used to analyze such evolutionary dynamics from genetic data: the molecular clock rate (also known as the clock rate, the nucleotide substitution rate, or the evolutionary rate) and the evolutionary rate ratio, dN/dS (Bush et al. 1999a; Rambaut and Holmes 2009; Bhatt et al. 2013). The molecular clock rate indicates the rate of sequence divergence, whereas the dN/dS rate ratio gives the relative rate of non-synonymous to synonymous sequence changes and is widely used to infer positive selection (when ). In viral sequences, is thought to reflect viral adaptation, typically in response to selection pressure exerted by the host immune system. Most commonly, the clock rate is measured on an entire genome or occasionally on a single gene, and dN/dS is measured either on a gene or at individual sites in a gene.
Importantly, each of these metrics is computed using models of molecular evolution which assume that observed mutations represent fixed population differences, not intra-population polymorphisms. This assumption has wide-reaching consequences for inferences. A growing body of theoretical work has demonstrated that data collected over short-time scales may yield biased estimates for both the molecular clock rate (Ho et al. 2005, 2007; Peterson and Masel 2009; Ho et al. 2011; Biek et al. 2015) and dN/dS (Rocha et al. 2006; Kryazhimskiy and Plotkin 2008; dos Reis and Yang 2013; Mugal, Wolf, and Kaj 2014). Moreover, a pattern of over-estimation in the molecular clock rate relative to long-term estimates has been shown in a small number of real viral systems (Wertheim and Kosakovsky-Pond 2011). This would be expected either because sufficient time has not passed for natural selection to purge slightly deleterious (often non-synonymous) mutations from the gene pool or because not enough mutations have accumulated to correctly compute a dN/dS ratio. Much of this work has additionally demonstrated that, after sequences have sufficiently diverged, the clock rate and dN/dS estimates do converge to a long-term steady-state value (Ho et al. 2007; Peterson and Masel 2009; Mugal, Wolf, and Kaj 2014). We therefore expect that evolutionary estimates computed with sequences from early outbreak stages will not be reliable approximations of longer term values. It is not known, however, how much time is required to obtain long-term clock rates or dN/dS estimates either per gene or in the site-wise distribution.
Here, we have investigated the extent to which limited sampling divergence time produces molecular clock rate and dN//dS estimates in an emerging virus that do not agree with long-term estimates for that virus. Specifically, we have analyzed the hemagglutinin (pH1) and neuraminidase (pN1) sequences from the 2009 pandemic H1N1 (pH1N1) to systematically examine how divergence time influences clock rate and dN/dS estimates both across the entire protein and at each site individually. Previously, Hedge, Lycett, and Rambaut (2013) conducted a similar time-series study of pH1N1. Their analysis included an estimate of R0, an analysis of the time dependence in the molecular clock rate, and the identification of the most recent common ancestor with whole-genome data. We have performed a more detailed analysis of the two genes that dominate influenza antigenicity and have included calculations of the gene-wise molecular clock rate, the whole-gene dN/dS, and the site-wise dN/dS (Bush et al. 1999a; Luksza and Lassig 2014; Meyer and Wilke 2015). Thus, the two studies are complementary for quantifying the evolution of pH1N1.
We have found that, early in the outbreak, both clock rate and dN/dS estimates are not equal to their long-term, steady-state values. In particular, when only the first month of sequence data is used to generate estimates, the clock rate is three to five times higher than the value obtained after 25 months of divergence in the sample. Similarly, we have found that whole-gene dN/dS for pH1 and pN1 are approximately 30 per cent higher for pH1 and 50 per cent lower for pN1 than their values after 25 months. Additionally, the majority of site-wise dN/dS estimates are completely uninformative until at least 6 months of mutations have accumulated. Finally, we have found that at least 8 months of accumulated sample divergence are required for site-wise dN/dS calculations to reflect structural constraints on protein evolution.
Taken together, our results indicate that some of the most commonly used metrics in molecular evolution can be very different in early outbreaks relative to long-term estimates. In addition, considering the relatively rapid evolution of influenza, it is likely that most other emerging viruses will require substantially more divergence time than reported here for influenza. Therefore, investigators should expect that early estimates of the clock rate, the whole-gene dN/dS, and the site-wise dN/dS will likely not agree with their long-term values. Specifically, the early molecular clock rate will probably be elevated, the early whole-gene dN/dS may be unpredictably increased or decreased, and the early site-wise dN/dS will likely be uninformative in emerging outbreaks.
2 Materials and methods
2.1 Data collection and processing
All data analyzed were taken from the Influenza Research Database (IRD) (Squires et al. 2012). We specifically selected pH1N1 sequences for the genes hemagglutinin (pH1) and neuraminidase (pN1) collected from humans in North America, beginning in April 2009. We used the built-in IRD filter to include only pH1N1 sequences. Further, we used the built-in IRD filters to select specifically the pandemic strains. We downloaded sequences sampled from April 2009 to April 2011. We chose to exclude data dated after April 2011 because the number of available sequences began dropping quickly as pH1N1 became a seasonal strain, peaking in the winter and dropping to almost undetectable levels in the summer. We selected only sequences that represented the entire coding regions of the pH1 and pN1 genes. All laboratory strains, duplicate strains, and sequences which contained ambiguous nucleotides were omitted.
2.2 Molecular clock rate estimation
For molecular-clock rate estimation, we grouped sequences for each gene into time-aggregated datasets, as follows. We established a dataset for April 2009, a dataset for April–May 2009, a dataset for April–June 2009, and so on, until all months were included. Because datasets containing more than 2 months of data were too large for the estimation procedure to be computationally tractable, we down-sampled the datasets, as follows: For estimates in the third month, we aggregated twenty-five sequences from the first month, twenty-five sequences from the second month, and twenty-five sequences from the third month. Thus, estimates from June 2009 contained seventy-five sequences sampled at random from each month. For any month that did not have twenty-five sequences, we simply added all of the sequences from that month. For each subsequent time window, we added an additional twenty-five sequences from the next month (or all), such that the 25-month time window contained 470 sequences. For all subsampling, we used completely random sampling within each month’s sequences.
After compiling all datasets for all time windows, we converted each set of nucleotide sequences to amino acids and aligned them with MAFFT (Katoh and Standley 2013), specifying the ‘–auto’ flag. We then backtranslated each amino-acid alignment to the original codons.
For each alignment, we used BEAST (Drummond and Rambaut 2007) to infer the molecular clock rate. Here, a temporally dated phylogeny was estimated using BEAST with a logistic growth coalescent demographic model, an HKY nucleotide substitution model, and a strict molecular clock. The molecular clock rate was given a non-informative CTMC reference prior (Ferreira and Suchard 2008). Inclusion of gamma rate heterogeneity was avoided as preliminary analysis showed little effect on molecular clock rate with this data. Using a strict clock reduced the risk of model over-parameterization, as relaxed clock models add an additional parameter for every branch in the phylogeny, and strict clock models have been commonly used when studying influenza molecular evolution (Bedford et al. 2014). We ran Markov chain Monte Carlo (MCMC) for 500 million steps for most datasets and confirmed Bayesian convergence using Tracer. For the 25-month set, we ran MCMC until the runs had clearly converge via Tracer. All runs had an effective sample size in the 165–1,383 range.
To be sure that codon site heterogeneity was not affecting the molecular clock rate calculations, we repeated the MCMC calculation adding the gamma site heterogeneity model with four rate categories. The results were statistically identical to those we found previously (Supplementary Fig. S5). In addition, to ensure that sample divergence was increasing during the sampling period, we plotted the root height of the tree against the sampling time. We found a progressively taller phylogenetic tree as the sample time increased (Supplementary Fig. S6).
2.3 Estimating dN/dS for the whole-gene and individual sites
Estimation of dN/dS is commonly carried out by maximum likelihood, which scales to much larger dataset sizes than the Bayesian approach implemented in BEAST. Therefore, down-sampling of the data was not necessary for dN/dS estimates. Thus, for all dN/dS analyses, we established time-aggregated datasets as described in the previous subsection but did not down-sample. Again, we aligned each dataset by amino acids with MAFFT and then backtranslated to codons for subsequent dN/dS inference. We estimated a phylogeny for each alignment using FastTree v2.1.7 (Price, Dehal, and Arkin 2009), specifying the flags ‘-nt -gtr –nosupport’ to use the generalized time- reversible model.
Next, we used HyPhy (Kosakovsky Pond, Frost, and Muse 2005) to compute both whole-gene and site-wise dN/dS estimates for all alignments. We specifically used HyPhy’s MG94xHKY85 model (Kosakovsky Pond and Frost 2005; Kosakovsky Pond and Muse 2005), which is less biased than the more frequently used GY94 model (Spielman and Wilke 2015). In our estimates, we specified a single parameter ω to represent the dN/dS ratio, rather than using separate parameters for dN and dS as is often done in MG94 models (Kosakovsky Pond and Muse 2005). Site-specific dN/dS values were inferred using the fixed-effects likelihood method (Yang and Swanson 2002; Kosakovsky Pond and Frost 2005). As influenza proteins undergo substantial post-translational modification, we considered dN/dS values only for those sites which could be aligned to a known crystal structure, that is, sites which appeared in the mature protein. We obtained protein structures from the Protein Data Bank (Berman et al. 2000) with PDBID: 1RD8 for hemagglutinin and PDBID: 3TI3 for neuraminidase.
2.4 Calculating proximity to the receptor-binding site as a constraint on site-wise dN/dS
It has been shown that protein structure is a major determinant of hemagglutinin and neurminidase evolution (Meyer and Wilke 2013; Meyer, Dawson, and Wilke 2013; Sikosek and Chan 2014); in particular in hemagglutinin, distance to the sialic acid-binding site is a major constraint on hemagglutinin evolution (Meyer and Wilke 2015). For each time point, we evaluated the degree to which the distance from the sialic acid-binding site can predict the site-wise dN/dS of hemagglutinin.
To start, we first calculated the distances from every alpha–carbon to every other alpha–carbon. For hemagglutinin, this produced a square, symmetric matrix of 490 × 490 sites in the protein structure where the diagonal is all zeros (the diagonal represents the distance from a site to itself). To be clear, for hemagglutinin, the first column in the matrix (a 1 × 490 column) represents the distances from the first amino acid to all amino acids in the protein; the second column represents to distances from the second amino acid to all amino acids in the protein, etc. Then, for each column in the matrix, we calculated the correlation of site-wise dN/dS to the inverse distances for each site in the structure; again, for hemagglutinin, there were 490 dN/dS values (one for every site in the structure) and a set of 490 distances in each column (i.e., for each reference ). We then plotted the correlation onto the structure at each amino acid site, using PyMOL (Schrödinger, LLC 2010). The same procedure was used to calculate distances using the neuraminidase structure and site-wise dN/dS values. As with hemagglutinin, we also used the inverse distance to compute correlations for neuraminidase.
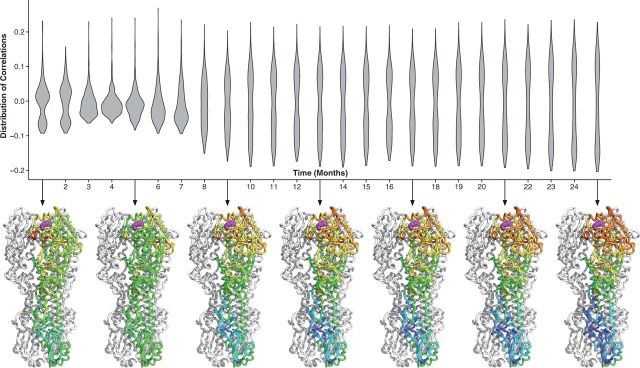
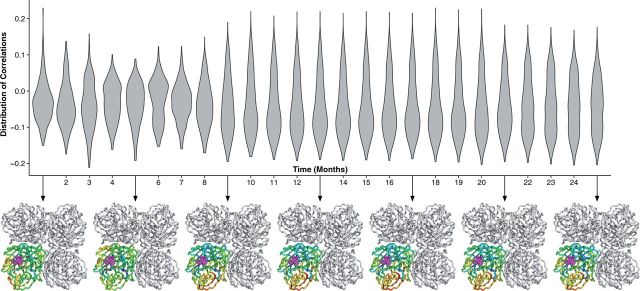
We repeated this process for each month, where the site-wise dN/dS estimates were calculated with the aggregate of data for all previous months. Thus, at each time point, we were left with a distribution of correlations between distance and rates calculated with data up to that time point. The distribution of correlations was plotted as a violin plot for each time point (Figs 5 and 6); the violin plot should be seen as a horizontal, symmetric histogram. Thus, wider regions represent higher counts. For select time points (every 5 months), we plotted the distribution of correlations directly onto the protein structure below the violin plot. The figure shows that as more divergence was aggregated, the ability of this distance constraint to predict evolution in hemagglutinin and neuraminidase improved dramatically. This accompanies a characteristic flattening of the distribution of correlations; the flattening implies that as data are added, all of the sites in the protein appear to encode the same directional evolutionary pressure.
Figure 5.
Temporal development of geometric evolutionary constraints in hemagglutinin. The violin plots show the distribution of dN/dS–proximity correlations using each possible site in the hemagglutinin protein as the reference point. The violin plot should be viewed as a horizontal histogram; thus, the wider the violin plot, the higher the number of reference sites with that correlation. Underneath the violin plots, we map these correlations onto the protein structure at 4-month time intervals. The hemagglutinin protein (PDB ID 1RD8) is shown in its native trimer structure, but the correlations are plotted onto just one of the monomers. The correlation pattern stabilizes after approximately 8 months of divergence
Figure 6.
Temporal development of geometric evolutionary constraints in neuraminidase. The violin plots show the distribution of dN/dS–proximity correlations using each possible site in the neuraminidase protein as the reference point. The violin plot should be viewed as a horizontal histogram; thus, the wider the violin plot, the higher the number of reference sites with that correlation. Underneath the violin plots, we map these correlations onto the protein structure at 4-month time intervals. The neuraminidase protein (PDB ID 3TI3) is shown in its native tetramer structure, but the correlations are plotted onto just one of the monomers. The correlation pattern stabilizes after approximately 8 months of divergence
2.5 Statistical analysis and plotting
We used the R statistical programming language and the ggplot2 R package for all statistical analyses and plots, respectively (Ihaka and Gentleman 1996; Wickham 2009).
2.6 Availability
All code and data used are freely available from the github repository, https://github.com/wilkelab/influenza_pH1N1_timecourse.
3 Results
3.1 Brief history of pH1N1
The 2009 pandemic influenza (pH1N1) emerged in Mexico during March and April of 2009 and spread across the globe within weeks (Neumann, Noda, and Kawaoka 2009). Although the most common seasonal influenza strain in 2009 was H3N2, a descendant of the 1968 Hong Kong strain (Neumann, Noda, and Kawaoka 2009), pH1N1 accounted for as much as 99 per cent of the sub-typed, type A influenza infections after only a single season. The pH1N1 virus itself is thought to be a descendant of the 1998 triple re-assortment H3N2 strain after mixing with existing swine influenza strains (Neumann, Noda, and Kawaoka 2009; Smith et al. 2009; Vijaykrishna et al. 2011). Moreover, the two genes studied here, hemagglutinin and neuraminidase, descended from two separate influenza lineages (Smith et al. 2009). The pH1 hemagglutinin gene originated in the classical swine strain that diverged early last century from the human-infecting H1N1 virus, and the pN1 neuraminidase gene originated in the avian H1N1 lineage. Thus, pH1N1 represents a valuable study system for examining the evolution of two viral genes that had not adapted to humans for nearly 100 years, and this influenza strain is therefore an effective and representative model for a rapidly spreading infectious disease.
3.2 Over-estimation of molecular clock rate
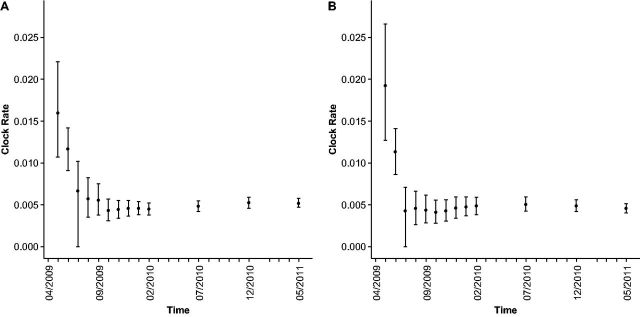
We calculated the molecular clock rate for pH1 and pN1 for each month in the outbreak from April 2009 to 2011, aggregating all available data up to each respective month (Fig. 1). We found that analyzing only the first month of data produced dramatically inflated molecular clock rates; the rate was overestimated roughly threefold for pH1 and fourfold for pN1. Upon adding the second month of data, the clock rate for pH1 declined substantially, and the pH1 clock rate converged to its long-term rate only after 5–6 months of data were incorporated. Similarly, for pN1, adding just 2–3 additional months of data to the first month produced molecular clock rate estimates that were essentially identical to the long term estimates. Thus, for influenza, molecular clock estimates could be computed with reasonable accuracy after approximately 4–6 months of sequence sample divergence. Note that for the remainder of this study, we will use the term ‘accuracy’ to refer to the extent to which any of the monthly estimates equal the long-term estimate. Similarly, we will use the terms ‘reliability’ or ‘precision’ to refer to the extent to which any of the estimates have low error of estimation.
Figure 1.
Molecular clock rate computed by BEAST for pH1 hemagglutinin and pN1 neuraminidase from the pH1N1 outbreak. In panel A, we show the molecular clock rate over time for pH1, and in panel B, we show the molecular clock rate over time for pN1. The error bars represent the HPD 95 per cent of the mean, as reported by BEAST. The plot shows a fourfold decline in the substitution rate estimates from single month of data to 25 months of aggregated data. Further, the molecular clock HPD 95 per cent for the first 2 months of data, for both pH1 and pN1, does not overlap the final clock rate, indicating that these early estimates are in no way representative of the long-term estimates
Importantly, the 95 per cent highest probability density (HPD) intervals estimated 1–2 months after the start of the pandemic, although wide, did not include the long-term average (Fig. 1). Thus, the inflated clock rates measured early in the outbreak seem to represent a systematic bias in the estimates, and not simply high variance (i.e., low precision) resulting from a small dataset.
3.3 Variability in the evolutionary rate ratio dN/dS
Although the molecular clock rate tells us the extent to which a virus is accumulating changes in its DNA, it does not imply anything about the rate of adaptation. Instead, adaptive evolution is frequently inferred from the dN/dS rate ratio. Traditionally, a value of indicates positive selection, under the assumption that an excess of non-synonymous changes is driven by adaptation. (However, on the whole-gene level, one is unlikely to observe even under positive selection, because purifying selection across the entire gene tends to dominate the overall estimate to produce .) At short timescales, there are relatively few observed sequence differences, and selection has not yet had sufficient time to purge slightly deleterious mutations from the population. As a result, sequence data from early in emerging outbreaks can either bias the dN/dS rate ratio (Rocha et al. 2006; Kryazhimskiy and Plotkin 2008; Mugal, Wolf, and Kaj 2014) or make the dN/dS estimate unreliable.
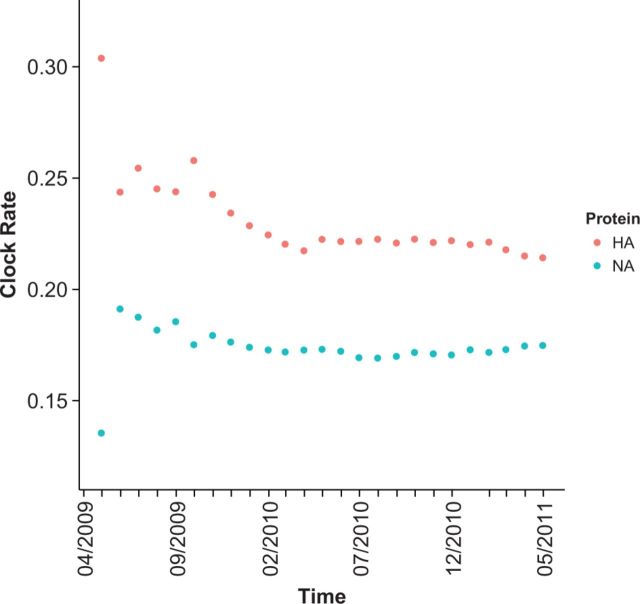
We inferred whole-gene dN/dS values for each monthly aggregate of data (Fig. 2). For both pH1 and pN1, our estimates that included only the first month of data were highly inaccurate, where pH1 was substantially overestimated and pN1 was substantially underestimated. After this first month, variability in monthly estimates leveled out, but, as had been the case for the molecular clock, estimated dN/dS values continued to decrease over time, until stabilizing after approximately 12 months. This trend suggests that early estimates are inaccurate and biased upwards with respect to long-term averages. In general, even though short divergence times do tend to produce elevated dN/dS values, exceedingly short time frames of 1 month may yield largely imprecise estimates, due to lack of accumulated variation (see also next subsection).
Figure 2.
Whole-gene dN/dS estimates for pH1 hemagglutinin and pN1 neuraminidase. Each point represents the average dN/dS at the specified time point for the pH1 (red points) and pN1 (blue points) genes. All of the dN/dS values were calculated by maximum likelihood using HyPhy (Kosakovsky Pond, Frost, and Muse 2005). For each gene, the first month yielded unpredictable, inaccurate dN/dS estimates, but estimates from the data were systematically elevated between months 2–8 for pH1 and 2–11 for pN1. After approximately 11 months for pH1 and 8 months for pN1, the mean dN/dS value largely converged to the long term estimate obtained after 25 months
3.4 Distribution of site-wise dN/dS
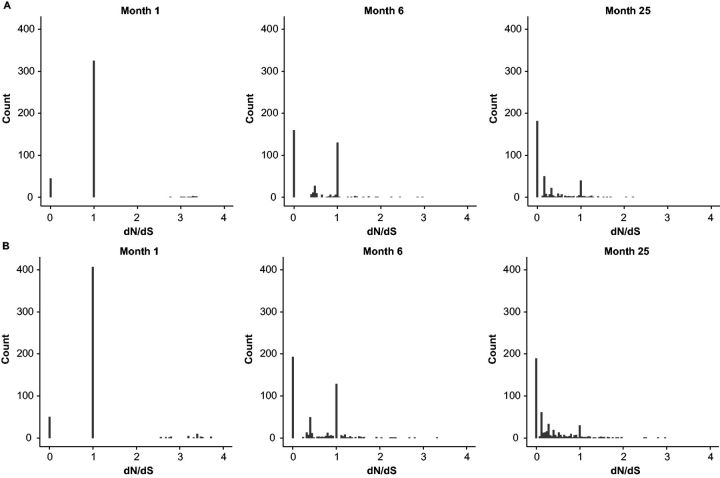
We next analyzed how sample divergence time influenced site-specific dN/dS estimates for pH1 and pN1. In Fig. 3, we show how the sample divergence time influenced the overall distribution of site-wise dN/dS estimates for pH1 (Fig. 3A) and pN1 (Fig. 3B). Both genes displayed the same general trend. With only a single month of data, the dN/dS distribution was unimodal, centered around . As we considered increasingly longer time windows, the mode around decayed, while a second mode near arose and took up an increasingly larger percentage of the data. At 25 months, the mode around had almost completely disappeared and the distribution consisted almost entirely of the mode near . By comparison, the distribution of site-wise dN/dS for all human H3 hemagglutinin sequences from 1991 to 2004 showed a completely unimodal distribution centered near (Supplementary Fig. S1). Thus, the site-wise dN/dS distribution had after 25 months largely, but not completely, reached its final shape.
Figure 3.
Distribution of site-wise dN/dS for pH1 hemagglutinin and pN1 neuraminidase. dN/dS distributions containing aggregated data from the 1st, 6th, and 25th month are shown for pH1 in panel A and for pN1 in panel B. The first month of data featured a majority of sites with . Most of these sites were uninformative sites that had not experienced any mutations. The maximum likelihood inference approach sets dN/dS to the arbitrary value of 1 for these sites. After 6 months, roughly half of the sites were informative, although half still showed . Finally, after 25 months of divergence, the majority of sites had informative dN/dS values. Distributions for all months are shown in Supplementary Figures S3 and S4
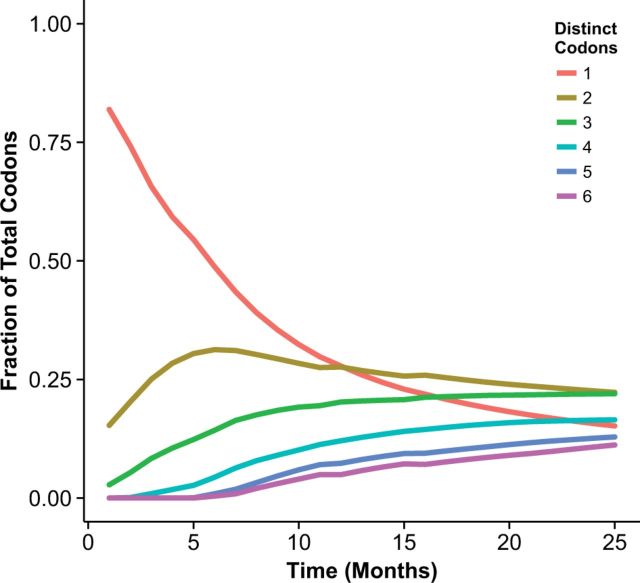
We investigated the origin of the mode at and found that it could generally be traced to completely conserved codon sites in the alignment. If a site has experienced neither a synonymous nor a non-synonymous mutation, both dN and dS are undefined. In this case, the maximum-likelihood inference algorithm outputs the arbitrary starting value for dN/dS of 1. More generally, for site-wise dN/dS estimates to be reliable, the sequence alignment needs to be sufficiently diverged at each codon site. We estimated overall divergence by counting the fraction of codon sites in each alignment that had 1, 2, 3, etc. distinct codons, and plotted these fractions over time (Fig. 4 and Supplementary Fig. S2). The fraction of sites with one distinct codon corresponds to the sites that have not experienced any mutations, while the fractions of sites with two, three, etc. distinct codons correspond to the sites that have undergone at least one, two, etc. mutations. We found that the mode at dN/dS declined in proportion to the number of completely conserved sites. At 5–6 months, approximately 50 per cent of all sites were still completely conserved in both proteins (Fig. 4 and Supplementary Fig. S2), and even after 25 months, 16 out of 503 sites in hemagglutinin and 14 out of 387 sites in neuraminidase still did not show any evidence of evolutionary divergence.
Figure 4.
Fraction of alignment columns with distinct numbers of codons, plotted over time for hemagglutinin. Alignment columns with 1 distinct codon are completely conserved, while columns with two, three, etc. distinct codons have experienced at least one, two, etc. mutations. At 6 months, approximately half of all sites had not yet experienced a mutation, and even after 25 months, 16 out of 503 sites in pH1 remained completely conserved
3.5 Effect of limited divergence on inferring important regions of hemagglutinin and neuraminidase
We have previously shown that proximity to the sialic acid-binding site in H3 hemagglutinin is one of the two best predictors (the other being relative solvent accessibility [RSA]) of site-wise dN/dS (Meyer and Wilke 2015). More specifically, if we calculate correlations between site-wise dN/dS and the inverse distance to a reference site, then reference sites near the sialic-acid binding region yield significant positive correlations while reference sites away from the sialic-acid binding region yield either no correlation or negative correlations. Thus, sites closer to the sialic-acid binding region tend to have higher dN/dS. By mapping these correlations onto the protein structure, we can visualize this result as a heat map that shows the sialic acid-binding region as the hottest region, and sites progressively farther away from this reference are proportionately colder (Meyer and Wilke 2015).
Assuming sufficient divergence for accurate site-wise estimates of dN/dS, we expected to find a similar trend in both pH1 hemagglutinin and pN1 neuraminidase. In contrast to the receptor binding protein hemagglutinin, however, neuraminidase is an enzyme with a functionally constrained chemical active site. Therefore, we expected neuraminidase to display the opposite trend from that of hemagglutinin: sites closer to the active site of neuraminidase should appear colder, that is, more evolutionarily conserved.
We found for both proteins that these expected trends emerged with sufficient evolutionary divergence. For pH1, just as for H3, sites closer to the sialic acid-binding site appeared hotter on our maps (Fig. 5). Similarly, for pN1, sites nearer the catalytic core were colder than the exposed surface (Fig. 6). Moreover, we found that these trends stabilized after approximately 8–10 months for both proteins. The temporal development of these geometric evolutionary constraints is also shown in Supplementary Videos S1 and S2.
4 Discussion
Molecular evolutionary metrics, namely the molecular clock rate (nucleotide substitution rate) and the dN/dS evolutionary rate ratio, are widely used to infer the evolutionary dynamics of viruses. With the uptake of rapid sequencing technologies, such evolutionary estimates have found widespread use in tracking emerging epidemics. However, the underlying models used to infer these metrics make a key assumption that genetic variation arises solely from fixed differences among sequences, and the influence of segregating mutations is ignored. Theoretical work has demonstrated that this assumption produces biased or inaccurate clock rate and dN/dS estimates at small divergence times (Ho et al. 2005; Rocha et al. 2006; Ho et al. 2007; Kryazhimskiy and Plotkin 2008; Peterson and Masel 2009; Ho et al. 2011; Mugal, Wolf, and Kaj 2014). Importantly, during the initial stages of an emerging infectious disease, viral sequences are separated almost exclusively by transient polymorphisms rather than fixed differences, making evolutionary estimates unreliable. It is not known, however, how much divergence time is required to obtain accurate and reliable clock rate and dN/dS estimates.
We found that reasonably accurate measurements for each metric could be obtained with 4–6 months of data for clock rate and 8 months of data for dN/dS. Moreover, we found that site-specific dN/dS values were largely uninformative during the first 6 months of the outbreak but started to become reliable for longer time windows. Our results provide empirical evidence of the theoretical predictions that short-time scales produce biased and inaccurate estimates for both the molecular clock and dN/dS. Since we obtained these results for influenza virus, which is one of the most rapidly evolving viruses, it is possible that more slowly evolving viruses will require substantially more time before estimates converge to their long-term values.
The pH1N1 influenza that swept the globe in 2009 is among the most studied pandemics in history. Indeed, one of the most widely cited early epidemic studies was performed on pH1N1 (Smith et al. 2009); in addition, there are several studies that are narrower in scope (Rambaut and Holmes 2009; Furuse et al. 2010) but have comparable results to our own. For example, one earlier study which included pH1N1 data from March 2009 to May 2009 also used the molecular clock rate and whole-gene dN/dS (Smith et al. 2009). In pH1, they found a similarly elevated whole-gene in the early epidemic and in non-outbreak swine clades. By comparison, we found nearly identical values for whole-gene dN/dS with the 1st month and 25th-fifth month of data, respectively. For neuraminidase, previous work found during the initial outbreak and for the non-outbreak swine data. In contrast, we found for the first month and for the 25th month. Thus, with the exception of the first month of data for neuraminidase, our results are in excellent agreement with prior work. It is unclear what caused the discrepancy in the first month; however, estimates are highly unreliable for these very short time scales, and minor differences in the set of selected sequences may result in very different dN/dS estimates.
In addition to the good agreement between the non-outbreak and long-divergence-time pH1N1 dN/dS estimates, we also found that our estimates of the molecular clock rate were similar to earlier work (Rambaut and Holmes 2009; Smith et al. 2009; Hedge, Lycett, and Rambaut 2013). In particular, we used essentially the same approach and data as did Hedge, Lycett, and Rambaut (2013). In that study, the authors used whole-genome data to calculate, among other things, the molecular clock rate of pH1N1. As with our analysis, the rate calculated in the first month was very different from that of the rest of the outbreak. After the second month of sampling, they found a mean clock rate of subst./site/year for the entire pH1N1 genome. Likewise, Hedge, Lycett, and Rambaut (2013) found a slight decrease in clock rate and a narrowing of the HPD interval as more data was added to the estimates. For comparison, with 2 years of data, we found a molecular clock rate of subst./site/year for hemagglutinin and subst./site/year for neuraminidase. Thus, both hemagglutinin and neuraminidase have higher mean molecular clock rates than the average of the pH1N1 genome; this is not surprising considering the pressure these immune-exposed proteins should experience. In addition, our estimates were similar to those found earlier for the immediately pre-outbreak reference from swine of subst./site/year for hemagglutinin and subst./site/year for neuraminidase. With 25 months of data, our estimates for both hemagglutinin and neuraminidase were virtually identical to the whole-genome estimate of subst./site/year with early pH1N1 data (Rambaut and Holmes 2009). Therefore, 25 months of data were sufficient to capture the long-term evolutionary trends.
There are at least two distinct mechanisms that contribute to inaccurate estimates of clock rates and dN/dS early in an outbreak. First, segregating polymorphisms lead to inflated estimates for both quantities. This effect has been discussed extensively in the literature (Ho et al. 2005; Rocha et al. 2006; Ho et al. 2007; Kryazhimskiy and Plotkin 2008; Peterson and Masel 2009; Ho et al. 2011; Mugal, Wolf, and Kaj 2014). Second, limited sampling divergence results in estimates with low precision. When the total number of accumulated mutations is low, it is simply not possible to obtain precise estimates of clock rates or dN/dS, regardless of whether the observed mutations are fixed substitutions or transient polymorphisms. This effect will be more pronounced for per-site estimates than for whole-gene estimates, but it can be present in either case. For the data we analyzed here, limited sampling divergence was likely a major contributing factor for the observed whole-gene estimates for the first month of data. For the per-site estimates, on the other hand, we needed 8–10 months of data to obtain reasonably precise estimates for a majority of sites.
What do our results imply for the practice of analyzing disease outbreaks as they occur? Although there is little doubt that the molecular clock rate and whole-gene dN/dS are important for understanding and quantifying emerging outbreaks, both metrics by themselves provide relatively limited information about a particular outbreak. In a given outbreak scenario, after estimating these quantities, one has to assess what these estimates mean, and in particular, whether any deviations from estimates obtained for past outbreaks reflect an actual biological difference in the current outbreak or simply a biased or imprecise estimate due to limited sampling divergence and transient polymorphisms. One possible approach towards answering this question is to proceed as we have done here, by subdividing the data into successively longer temporal intervals and assessing whether estimates seem to converge to the long-term values. However, this approach requires a large amount of sequence data collected over a substantial time window.
Alternatively, one could attempt to internally verify that a sufficient level of accumulated variation exists by investigating whether or not the data fit an expected pattern. For example, there are a number of structure-based constraints that guide protein evolution. Various studies have shown that RSA, weighted contact number, energy of mutation, and local packing density of sites can account for some portion of variation in site-wise dN/dS for diverse proteins (Meyer and Wilke 2013; Meyer, Dawson, and Wilke 2013; Huang et al. 2014; Shahmoradi et al. 2014; Sikosek and Chan 2014; Yeh et al. 2014). Moreover, in influenza specifically, it is known that proximity to the sialic acid-binding site is a strong constraint on hemagglutinin evolution (Bush et al. 1999b; Hensley et al. 2009; Koel et al. 2013; Meyer and Wilke 2015); in fact, a combined model with RSA and proximity to the sialic acid-binding site performs similarly to a cross-species comparison of site-wise dN/dS estimates (Meyer, Dawson, and Wilke 2013; Meyer and Wilke 2015).
We showed here that, after 25 months of divergence, the number of uninformative sites dropped to a sufficiently low number, and the resultant site-wise estimates were sufficiently accurate, to display the same proximity-based pattern as seen in H3 hemagglutinin (Supplementary Fig. S1). For pH1, that pattern started to emerge clearly in November of 2009 with 8 months of accumulated mutations. Moreover, this date could be inferred from the distribution of site-wise dN/dS estimates; it is the first month when substantially more sites are informative than are uninformative (Supplementary Fig. S3). Likewise, for pN1, we showed that a similar proximity metric can be used for the enzyme active site. However, in the case of neuraminidase, the active site was more constrained rather than less constrained, as was the case in hemagglutinin. This finding makes sense intuitively, since enzymes must retain a functional active site at all costs. Further, we found that the expected pattern began to emerge after the same 8-month interval, and again, this interval could have been inferred based on the site-wise dN/dS distribution (Supplementary Fig. S4). Therefore, we have found that by inspecting the change in the distribution of site-wise dN/dS estimates over time, we could identify the time point at which site-wise estimates became reliable.
Supplementary data
Supplementary data is available at VEVOLU Journal online.
Acknowledgements
This work was supported by NIH grant R01 GM088344, DTRA grant HDTRA1-12-C-0007, and NSF Cooperative Agreement No. DBI-0939454 (BEACON Center) to C.O.W. Computational resources were provided by the Center for Computational Biology and Bioinformatics (CCBB) at UT Austin.
Conflict of interest: None declared.
References
- Bedford T., et al. (2014) ‘Integrating Influenza Antigenic Dynamics with Molecular Evolution,’ eLife 3: e01914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H. M., et al. (2000) ‘The Protein Data Bank,’ Nucleic Acids Research, 28: 235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S., et al. (2013) ‘The Evolutionary Dynamics and Influenza A Virus Adaptation to Mammalian Host,’ Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 368: 20120382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biek R., et al. (2015) ‘Measurably Evolving Pathogens in the Genomic Era,’ Trends in Ecology and Evolution, 30: 306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush R. M., et al. (1999a) ‘Predicting the Evolution of Human Influenza A,’ Science, 286: 1921–5. [DOI] [PubMed] [Google Scholar]
- Bush R. M., et al. (1999b) ‘Positive Selection on the H3 Hemagglutinin Gene of Human Influenza Virus A,’ Molecular Biology and Evolution, 16: 1457–65. [DOI] [PubMed] [Google Scholar]
- dos Reis M., Yang Z. (2013) ‘Why Do More Divergent Sequences Produce Smaller Nonsynonymous/Synonymous Rate Ratios in Pairwise Sequence Comparisons?,’ Genetics, 1: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. J., Rambaut A. (2007) ‘BEAST: Bayesian Evolutionary Analysis by Sampling Trees,’ BMC Evolutionary Biology, 7: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M. A. R., Suchard M. A. (2008) ‘Bayesian Analysis of Elapsed Times in Continuous-Time Markov Chains,’ Canadian Journal of Statistics, 36: 355–68. [Google Scholar]
- Furuse Y., et al. (2010) ‘Comparison of Selection Pressures on the ha Gene of Pandemic (2009) and Seasonal Human and Swine Influenza A H1 Subtype Viruses,’ Virology, 405: 314–21. [DOI] [PubMed] [Google Scholar]
- Hedge J., Lycett S. J., Rambaut A. (2013) ‘Real-Time Characterization of the Molecular Epidemiology of an Influenza Pandemic,’ Biology Letters, 9: 20130331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley S. E., et al. (2009) ‘Hemagglutinin Receptor Binding Avidity Drives Influenza A Virus Antigenic Drift,’ Science, 326: 734–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. Y. W., et al. (2005) ‘Time Dependency of Molecular Rate Estimates and Systematic Overestimation of Recent Divergence Times,’ Molecular Biology and Evolution, 22: 1561–8. [DOI] [PubMed] [Google Scholar]
- Ho S. Y. W., et al. (2007) ‘Evidence for Time Dependency of Molecular Rate Estimates,’ Systematic Biology, 56: 515–22. [DOI] [PubMed] [Google Scholar]
- Ho S. Y. W., et al. (2011) ‘Time-Dependent Rates of Molecular Evolution,’ Molecular Ecology, 20: 3087–101. [DOI] [PubMed] [Google Scholar]
- Huang T. T., et al. (2014) ‘A Mechanistic Stress Model of Protein Evolution Accounts for Site-Specific Evolutionary Rates and Their Relationship with Packing Density and Flexibility,’ BMC Evolutionary Biology, 14: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R., Gentleman R. (1996) ‘R: A Language for Data Analysis and Graphics,’ Journal of Computational and Graphical Statistics, 5: 299–314. [Google Scholar]
- Katoh K., Standley D. M. (2013) ‘MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability,’ Molecular Biology and Evolution, 30: 772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koel B. F., et al. (2013) ‘Substitutions Near the Receptor Binding Site Determine Major Antigenic Change During Influenza Virus Evolution,’ Science, 342: 976–9. [DOI] [PubMed] [Google Scholar]
- Kosakovsky Pond S. L., Frost S. D. W. (2005) ‘Not So Different After All: A Comparison of Methods for Detecting Amino Acid Sites under Selection,’ Molecular Biology and Evolution, 22: 1208–22. [DOI] [PubMed] [Google Scholar]
- Kosakovsky Pond S. L., Muse S. V. (2005) ‘Site-to-Site Variation of Synonymous Substitution Rates,’ Molecular Biology and Evolution, 22: 2375–85. [DOI] [PubMed] [Google Scholar]
- Kosakovsky Pond S. L., Frost S. D. W., Muse S. V. (2005) ‘HyPhy: Hypothesis Testing Using Phylogenies,’ Bioinformatics, 21: 676–9. [DOI] [PubMed] [Google Scholar]
- Kryazhimskiy S., Plotkin J. B. (2008) ‘The Population Genetics of dN/dS,’ PLoS Genetics, 4: e1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luksza M., Lassig M. (2014) ‘A Predictive Fitness Model for Influenza Evolution,’ Nature, 507: 57–61. [DOI] [PubMed] [Google Scholar]
- Meyer A. G., Wilke C. O. (2013) ‘Integrating Sequence Variation and Protein Structure to Identify Sites under Selection,’ Molecular Biology and Evolution, 30: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. G., Wilke C. O. (2015) ‘Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin,’ PLOS Pathogens, 11: e1004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. G., Dawson E. T., Wilke C. O. (2013) ‘Cross-Species Comparison of Site-Specific Evolutionary-Rate Variation in Influenza Hemagglutinin,’ Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 368: 20120334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugal C. F., Wolf J. B. W., Kaj I. (2014) ‘Why Time Matters: Codon Evolution and the Temporal Dynamics of dN/dS,’ Molecular Biology and Evolution, 31: 212–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann G., Noda T., Kawaoka Y. (2009) ‘Emergence and Pandemic Potential of Swine-Origin H1N1 Influenza Virus,’ Nature, 459: 931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. I, Masel J. (2009) ‘Quantitative Prediction of Molecular Clock and Ka/Ks at Short Timescales,’ Molecular Biology and Evolution, 26: 2595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M. N., Dehal P. S., Arkin A. P. (2009) ‘FastTree2—Approximately Maximum-Likelihood Trees for Large Alignments,’ PLoS One, 5: e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A, Holmes E. (2009) ‘The Early Molecular Epidemiology of the Swine-Origin A/H1N1 Human Influenza Pandemic,’ PLOS Currents, 1: RRN1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha E. P. C., et al. (2006) ‘Comparisons of dN/dS are Time Dependent for Closely Related Bacterial Genomes,’ Journal of Theoretical Biology, 239: 226–35. [DOI] [PubMed] [Google Scholar]
- Schrödinger LLC. (2010) The PyMOL Molecular Graphics System, Version 1.3r1. [Google Scholar]
- Shahmoradi A., et al. (2014) ‘Predicting Evolutionary Site Variability from Structure in Viral Proteins: Buriedness, Packing, Flexibility, and Design,’ Journal of Molecular Evolution, 79: 132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikosek T., Chan H. S. (2014) ‘Biophysics of Protein Evolution and Evolutionary Protein Biophysics,’ Journal of the Royal Society, Interface, 11: 20140419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. J., et al. (2009) ‘Origins and Evolutionary Genomics of the 2009 Swine-Origin H1N1 influenza A Epidemic,’ Nature, 459: 1122–5. [DOI] [PubMed] [Google Scholar]
- Spielman S. J., Wilke C. O. (2015) ‘The Relationship Between dN/dS and Scaled Selection Coefficients,’ Molecular Biology and Evolution, 32: 1097–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires R., et al. (2012) ‘Influenza Research Database: An Integrated Bioinformatics Resource for Influenza Research and Surveillance,’ Influenza and Other Respiratory Viruses, 6: 404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaykrishna D., et al. (2011) ‘Long-Term Evolution and Transmission Dynamics of Swine Influenza A Virus,’ Nature, 1473: 519–22. [DOI] [PubMed] [Google Scholar]
- Wertheim J. O., Kosakovsky-Pond S. L. (2011) ‘Purifying Selection can Obscure the Ancient Age of Viral Lineages,’ Molecular Biology and Evolution, 28: 3355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. (2009) ggplot2: Elegant Graphics for Data Analysis. New York: Springer. [Google Scholar]
- Yang Z., Swanson W. J. (2002) ‘Codon-Substitution Models to Detect Adaptive Evolution that Account for Heterogeneous Selective Pressures among Site Classes,’ Molecular Biology and Evolution, 19: 49–57. [DOI] [PubMed] [Google Scholar]
- Yeh S. W., et al. (2014) ‘Site-Specific Structural Constraints on Protein Sequence Evolutionary Divergence: Local Packing Density versus Solvent Exposure,’ Molecular Biology and Evolution, 31: 135–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.