Abstract
In November 2013, a Plasmodium falciparum malaria outbreak of 11 cases occurred in Cusco, southern Peru, where falciparum malaria had not been reported since 1946. Although initial microscopic diagnosis reported only Plasmodium vivax infection in each of the specimens, subsequent examination by the national reference laboratory confirmed P. falciparum infection in all samples. Molecular typing of four available isolates revealed identity as the B-variant (BV1) strain that was responsible for a malaria outbreak in Tumbes, northern Peru, between 2010 and 2012. The P. falciparum BV1 strain is multidrug resistant, can escape detection by PfHRP2-based rapid diagnostic tests, and has contributed to two malaria outbreaks in Peru. This investigation highlights the importance of accurate species diagnosis given the potential for P. falciparum to be reintroduced to regions where it may have been absent. Similar molecular epidemiological investigations can track the probable source(s) of outbreak parasite strains for malaria surveillance and control purposes.
Peru reported 43,468 malaria cases in 2013, 84% of which were attributed to Plasmodium vivax infection while 16% (7,371) were due to Plasmodium falciparum.1 As Peru and other South American countries progress toward the malaria pre-elimination phase, the utilization of appropriate parasite detection tools will facilitate effective disease surveillance and case management. Plasmodium falciparum transmission in Peru is mainly found in the Amazonic region; three departments (Loreto, San Martin, and Ucayali) reported P. falciparum malaria cases between January and the first week of December 2013.2 Between 2010 and 2012, a P. falciparum malaria outbreak of 210 cases occurred in the north coastal department of Tumbes, where falciparum transmission had been interrupted for 5 years.3 Using molecular tools, it was determined that a multidrug resistant, Pfhrp2 gene–deleted lineage (BV1) originating from the Peruvian Amazon caused this outbreak.3
Between November 1 and 20, 2013, 11 malaria cases occurred in two communities—Rosalina and Ivanqui (Palma Real)—in Echarate District, La Convención Province, Cusco Department, which is located in southern Peru.2 Information about the 11 malaria-infected patients, eight males and three females, is presented in Table 1. The community of Rosalina, where six malaria cases were detected, consisted of 70 families residing in 54 households whereas the community of Ivanqui, where five of the malaria-infected patients resided, had 92 families in 32 homes at the time of the malaria outbreak.2 All 11 patients were initially found to be infected with P. vivax based on microscopic analysis and received a 7-day chloroquine (CQ) and primaquine (PQ) treatment regimen.
Table 1.
Characteristics of the 11 patients that presented with malaria during the 2013 outbreak in Palma Real, La Convención Province, Cusco Department, Peru
| Sample ID | Age | Sex | Parasitemia | Health center | Diagnosis date | Residence |
|---|---|---|---|---|---|---|
| 854* | 26 | Male | F§ | Palma real | November 14, 2013 | Rosalina |
| 1885* | 32 | Male | F∥ | Palma real | November 14, 2013 | Rosalina |
| 1886* | 30 | Male | F∥ | Hospital Quillabamba | November 14, 2013 | Rosalina |
| 1930* | 33 | Male | F∥ | Palma real | November 15, 2013 | Rosalina |
| 1913 | 17 | Male | F§ | Palma real | November 15, 2013 | Ivanqui |
| 1952 | 25 | Female | F§ | Palma real | November 16, 2013 | Ivanqui |
| 2033 | 32 | Male | F§ | Palma real | November 17, 2013 | Ivanqui |
| 2109 | 52 | Male | F§ | Palma real | November 18, 2013 | Rosalina |
| 2139 | 2 | Female | F§ | Palma real | November 18, 2013 | Ivanqui |
| 2303 | 24 | Male | F‡ | Palma real | November 22, 2013 | Rosalina |
| 2443 | 28 | Female | No slide available† | Palma real | November 25, 2013 | Ivanqui |
F = Plasmodium falciparum.
Patient specimens available for molecular analysis.
Plasmodium falciparum malaria was positively diagnosed (but undocumented) locally at the Palma Real Health Center. Blood film was not available to the Cusco Department of Health for confirmatory diagnosis.
One parasite per field in 100 fields.
Between 2 and 20 parasites per field in 100 fields.
Between 21 and 200 parasites per field in 100 fields.
However, symptoms persisted in four of the patients, who then received CQ and PQ for 14 more days. The four patients continued to experience symptoms for over a month posttreatment until one of them visited a private practitioner on December 12, 2013. Microscopic examination of the patient's thick blood smear revealed P. falciparum infection.2 Between December 16 and 17, 2013, blood samples were obtained from the remaining three patients in whom symptoms had persisted after the initial P. vivax-targeted treatment. Giemsa-stained blood films were read by microscopy at which time the patients were confirmed to have P. falciparum malaria and treated with artesunate plus mefloquine.2
Since 1946, only P. vivax malaria transmission had been reported in Cusco Department; all 608 malaria cases reported in 2013 were attributed to P. vivax.2 For this reason, the P. falciparum diagnoses prompted active surveillance for febrile cases in Rosalina and Ivanqui. In addition, the 11 smears confirmed to be positive for P. vivax were reexamined and by December 2013, the Cusco Regional Directorate of Health confirmed that the malaria cases from Palma Real corresponded to a P. falciparum outbreak.2
Blood samples from the four patients with persistent symptoms were sent to the Centers for Disease Control and Prevention malaria laboratory for further characterization. We determined the molecular profile (by genotyping microsatellites and drug-resistance-associated genes) of these four isolates and evaluated the possible origin(s) and/or relatedness to other P. falciparum parasite lineages previously reported in Peru.4 Information about the four patients from whom the samples were collected is shown in Table 1.
Neutral microsatellite markers were analyzed to delineate the genetic background of the parasites. These seven markers (TA1 [chromosome 6]; Poly-α [chromosome 4]; PfPK2 [chromosome 12]; TA109 [chromosome 6], 2490 [chromosome 10]; C2M34 [chromosome 2]; and C3M69 [chromosome 3]) have been previously used to characterize P. falciparum clonal lineages in Peruvian isolates.5,6 Moreover, they were used to determine the source of a P. falciparum malaria outbreak in Tumbes.3
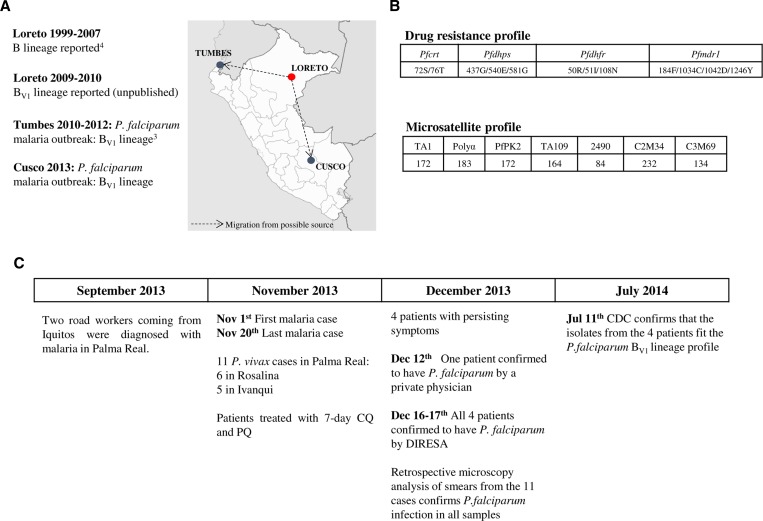
Our analyses revealed that all four parasite isolates from Cusco had an identical microsatellite background consistent with the BV1 lineage previously reported in at least two other departments of Peru: Tumbes (malaria outbreak of 20123) and Loreto (specimens collected in Yurimaguas and Requena in 2009–2010; D. Gamboa, J. Bendezu, K. Torres, and others, unpublished data) (Figure 1 ). To further verify the extent of similarity of the Cusco parasites to the BV1 parasite lineage, four drug resistance–associated genes; Pfcrt (CQ resistance), Pfmdr1 (multidrug resistance), Pfdhfr (pyrimethamine resistance), and Pfdhps (sulfadoxine resistance) were polymerase chain reaction amplified and sequenced as described previously.3 Finally, we genotyped histidine-rich protein 2 (Pfhrp2) and Pfhrp3 as reported by Akinyi and others.6
Figure 1.
(A) Proposed origin and observed migration pattern of Plasmodium falciparum BV1 lineage parasites in Peru. The dotted arrows in the map indicate human migration from Loreto Department, which is proposed to be the potential origin of the P. falciparum BV1 clonal lineage in Peru, to Tumbes3 and Cusco departments, resulting in two separate malaria outbreaks. (B) Molecular profile of P. falciparum isolates from the malaria outbreak in Cusco. In the top panel, the numbers indicate the amino acid codons while the letter symbols represent the amino acid changes at those particular codons within each respective gene. In the bottom panel, the top row lists the names of the microsatellite loci while the number listed below each locus corresponds to the size (in bases) of the polymerase chain reaction (PCR)–amplified fragment. The antimalarial drug resistance marker and microsatellite allele profiles of the isolates studied were identical to those of the BV1 lineage parasites identified in the Tumbes outbreak3 and in Loreto Department. Pfdhfr = dihydrofolate reductase; Pfcrt = chloroquine resistance transporter; Pfdhps = dihydropteroate synthase; Pfmdr1 = multidrug-resistant gene, where pf denotes P. falciparum. (C) Timeline of the malaria outbreak in Cusco. The two road construction workers from Loreto who presented with malaria in Cusco in September 2013 are the likely index cases to the subsequent outbreak that occurred in November 2013. CQ = chloroquine; DIRESA = Direccion Regional de Salud (Regional Health Directorate); PQ = primaquine.
All four isolates had drug resistance profiles identical to the BV1 lineage, with an S72V73M74N75T76 haplotype in the Pfcrt gene, N86D144F184C1034D1042Y1246 in Pfmdr1, R50I51C59N108I164 in Pfdhfr, and S436G437E540G581A613 in Pfdhps (bold font represents the mutant amino acid at each respective position). Also, similar to BV1, all four isolates had deleted Pfhrp2 and Pfhrp3. Overall, the genetic background of these isolates was identical to that of the BV1 lineage that caused the P. falciparum outbreak in Tumbes in 2012 (Figure 1). This finding suggests that the BV1 lineage is the most likely source of the falciparum malaria outbreak in Cusco. This is the second malaria outbreak in Peru that involved the same P. falciparum lineage.
It is important to point out that the BV1 lineage derived from the original “B clonal lineage” found in Peru since 1999.5 The highly clonal dynamic observed in this P. falciparum population is the result of decreased outcrossing and genetic drift primarily resulting from inbreeding and asexual replication.7 In general, the low P. falciparum genetic diversity found in South America relates to the low transmission intensity in this region. With fewer parasite types, there are limited opportunities for outcrossing during meiosis in the mosquito, which results in highly related or even clonal parasites.8,9 Moreover, since the P. falciparum population underwent strong drug selection, expansions of few drug-resistant parasite lineages is expected.10
Epidemiological data suggest that the Cusco P. falciparum outbreak was the result of human migration from Loreto Department when a road construction company set up a camp in Rosalina to build a road to Kiteni, Echarate District.2 In September 2013, prior to the outbreak, two road workers who were originally from Iquitos were diagnosed with severe malaria and, because of their critical condition, were subsequently transferred to another city for medical examination. It is suspected that these may have been the index cases to the ensuing outbreak.2 Unfortunately, specimens from these suspected index cases were not available for molecular analysis to confirm the proposed link. Nevertheless, even with the limited number of samples analyzed, this molecular investigation corroborated the epidemiological data suggesting that the P. falciparum BV1 lineage from Loreto is the most likely source of the Cusco outbreak.
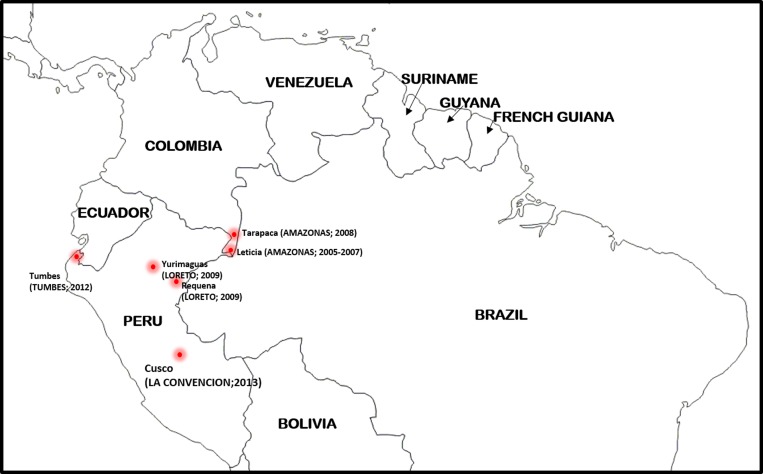
In addition to Peru, BV1 lineage parasites have been detected in isolates collected in Amazonas Department, Colombia, from as far back as 200511 (Figure 2 ). Anopheles darlingi and Anopheles oswaldoi are the main malaria species in Amazonas Department.12 The discovery of BV1 lineage parasites in other parts of the Amazon region of South America presents a major challenge for regional malaria control and elimination due to the persistence and wide distribution of this P. falciparum lineage, which seems highly adaptive to different transmission settings and can escape detection by PfHRP2-based rapid diagnostic tests because of Pfhrp2/Pfhrp3 deletions.
Figure 2.
Sites in South America where BV1 lineage parasites (based on neutral microsatellite allele information) have been identified to date. The departments (or states) of each respective country are indicated in uppercase letters. The year(s) of collection of the specimens containing BV1 lineage parasites is shown in parentheses.
Finally, this study highlights the need for local health posts to perform high-quality malaria diagnosis to detect P. falciparum introductions, particularly in areas where P. vivax transmission is known or thought to occur exclusively, because P. falciparum lineages could possibly establish themselves in the long term. In addition, although there have been some reports on the use and interpretation of Plasmodium molecular genetics tools for outbreak investigations,3,7 this methodology should be standardized for routine use to identify not only the Plasmodium species but the source(s) of a malaria outbreak and the prevalence of long-lasting drug-resistant P. falciparum strains.
ACKNOWLEDGMENTS
We are grateful to the personnel at the Ministry of Health in Cusco and Lima as well as the Reference Laboratory in La Convención, Cusco, Peru, who were involved in the response to the outbreak, assisted with patient sample collection, and provided specimens to the Malaria Laboratory at the CDC.
Footnotes
Financial support: Sheila Akinyi Okoth was supported by the Atlanta Research and Education Foundation. Stella M. Chenet was supported by the American Society of Microbiology/CDC Postdoctoral Fellowship Program. We acknowledge the support of the CDC Antimicrobial Resistance Working Group and the Atlanta Research and Education Foundation for the investigation.
Authors' addresses: Sheila Akinyi Okoth and Stella M. Chenet, Division of Parasitic Diseases and Malaria, CDC, Atlanta, GA, E-mails: jyo3@cdc.gov and ynw0@cdc.gov. Nancy Arrospide, Sonia Gutierrez, and Cesar Cabezas, Instituto Nacional de Salud del Peru, Centro Nacional de Salud Publica, Lima, Peru, E-mails: narrospide@hotmail.com, scgg68@hotmail.com, and salljaruna@yahoo.com. Jose Antonio Matta, Laboratorio de Referencia de La Convención, Cusco, Peru, E-mail: antonio_matta@hotmail.com. Venkatachalam Udhayakumar, Division of Parasitic Diseases and Malaria, Atlanta, GA, E-mail: vxu0@cdc.gov.
References
- 1.World Health Organization . World Malaria Report, 2014—Peru. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 2.Casos confirmados de malaria por Plasmodium falciparum en el distrito de Echarate, provincia de la Convencion y departamento del Cusco, ano 2013. 2013 http://www.dge.gob.pe/portal/docs/vigilancia/boletines/2013/52.pdf Ministerio de Salud del Peru, Direccion General de Epidemiologia. Available at. [Google Scholar]
- 3.Baldeviano GC, Okoth SA, Arrospide N, Gonzalez RV, Sanchez JF, Macedo S, Conde S, Tapia LL, Salas C, Gamboa D, Herrera Y, Edgel KA, Udhayakumar V, Lescano AG. Molecular investigation of Plasmodium falciparum re-emergence in the Peruvian North Coast maps the parasite source population to an Amazon village. Emerg Infect Dis. 2015;21:797–803. doi: 10.3201/eid2105.141427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffing SM, Viana GM, Mixson-Hayden T, Sridaran S, Alam MT, de Oliveira AM, Barnwell JW, Escalante AA, Povoa MM, Udhayakumar V. Historical shifts in Brazilian P. falciparum population structure and drug resistance alleles. PLoS One. 2013;8:e58984. doi: 10.1371/journal.pone.0058984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffing SM, Mixson-Hayden T, Sridaran S, Alam MT, McCollum AM, Cabezas C, Marquino Quezada W, Barnwell JW, De Oliveira AM, Lucas C, Arrospide N, Escalante AA, Bacon DJ, Udhayakumar V. South American Plasmodium falciparum after the malaria eradication era: clonal population expansion and survival of the fittest hybrids. PLoS One. 2011;6:e23486. doi: 10.1371/journal.pone.0023486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akinyi S, Hayden T, Gamboa D, Torres K, Bendezu J, Abdallah JF, Griffing SM, Quezada WM, Arrospide N, De Oliveira AM, Lucas C, Magill AJ, Bacon DJ, Barnwell JW, Udhayakumar V. Multiple genetic origins of histidine-rich protein 2 gene deletion in Plasmodium falciparum parasites from Peru. Sci Rep. 2013;3:2797. doi: 10.1038/srep02797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obaldia N, 3rd, Baro NK, Calzada JE, Santamaria AM, Daniels R, Wong W, Chang HH, Hamilton EJ, Arevalo-Herrera M, Herrera S, Wirth DF, Hartl DL, Marti M, Volkman SK. Clonal outbreak of Plasmodium falciparum infection in eastern Panama. J Infect Dis. 2015;211:1087–1096. doi: 10.1093/infdis/jiu575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels R, Chang HH, Sene PD, Park DC, Neafsey DE, Schaffner SF, Hamilton EJ, Lukens AK, Van Tyne D, Mboup S, Sabeti PC, Ndiaye D, Wirth DF, Hartl DL, Volkman SK. Genetic surveillance detects both clonal and epidemic transmission of malaria following enhanced intervention in Senegal. PLoS One. 2013;8:e60780. doi: 10.1371/journal.pone.0060780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniels RF, Schaffner SF, Wenger EA, Proctor JL, Chang HH, Wong W, Baro N, Ndiaye D, Fall FB, Ndiop M, Ba M, Milner DA, Jr, Taylor TE, Neafsey DE, Volkman SK, Eckhoff PA, Hartl DL, Wirth DF. Modeling malaria genomics reveals transmission decline and rebound in Senegal. Proc Natl Acad Sci USA. 2015;112:7067–7072. doi: 10.1073/pnas.1505691112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chenet SM, Schneider KA, Villegas L, Escalante AA. Local population structure of Plasmodium: impact on malaria control and elimination. Malar J. 2012;11:412. doi: 10.1186/1475-2875-11-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murillo Solano C, Akinyi Okoth S, Abdallah J, Pava Z, Dorado E, Incardona S, Huber CS, Macedo de Oliveira A, Bell D, Udhayakumar V, Barnwell JW. Deletion of Plasmodium falciparum histidine-rich protein 2 (pfhrp2) and histidine-rich protein 3 (pfhrp3) genes in Colombian parasites. PLoS One. 2015;10:e0131576. doi: 10.1371/journal.pone.0131576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez M, Perez L, Caicedo JC, Prieto G, Arroyo JA, Kaur H, Suarez-Mutis M, de La Hoz F, Lines J, Alexander N. Composition and biting activity of Anopheles (Diptera: Culicidae) in the Amazon region of Colombia. J Med Entomol. 2009;46:307–315. doi: 10.1603/033.046.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]