Abstract
Knowledge of variables influencing serology is crucial to evaluate serology results for the diagnosis and clinical management of cystic echinococcosis (CE). We analyzed retrospectively a cohort of patients with hepatic CE followed in our clinic in 2000–2012 to evaluate the influence of several variables on the results of commercial enzyme-linked immunosorbent assay (ELISA) and indirect hemagglutination (IHA) tests. Sera from 171 patients with ≥ 1 hepatic CE cyst, and 90 patients with nonparasitic cysts were analyzed. CE cysts were staged according to the WHO-IWGE classification and grouped by activity. A significant difference in ELISA optical density (OD) values and percentage of positivity was found among CE activity groups and with controls (P < 0.001). The serological response was also influenced by age (P < 0.001) and cyst number (P = 0.003). OD values and cyst size were positively correlated in active cysts (P = 0.001). IHA test showed comparable results. When we analyzed the results of 151 patients followed over time, we found that serology results were significantly influenced by cyst activity, size, number, and treatment ≤ 12 months before serum collection. In conclusion, serological responses as assessed by commercial tests depend on CE cyst activity, size and number, and time from treatment. Clinical studies and clinicians in their practice should take this into account.
Introduction
Cystic echinococcosis (CE) is a zoonosis caused by the larval stage (metacestode) of Echinococcus granulosus, a tapeworm found in the small intestine of canids with livestock, especially sheep, as its natural intermediate host. Human CE is highly endemic in pastoral communities, particularly in regions of central Asia and China, South America, the Mediterranean, Eastern Europe, the Middle East, and East Africa, causing an estimated burden of 1–3 million disability-adjusted life years and annual loss of over US$2,000 million in livestock production.1,2
In human primary infection, CE cysts may develop in almost any organ. Up to 80% of patients have a single organ involved with a solitary cyst located most frequently in the liver.3,4 The diagnosis of hepatic (and more in general, abdominal) CE is based mainly on ultrasound (US), and the standardized World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) classification of CE cysts is used to guide the clinical management of patients.5,6 In the WHO-IWGE classification, CE cysts are grouped into active (CE1 and CE2), transitional (CE3), and inactive (CE4 and CE5). CE3 cysts have been further differentiated into CE3a (with detached endocyst or water lily sign) and CE3b (predominantly solid with daughter vesicles).7 According to a study on the metabolic profiles of these stages, CE3b cysts should be classified as active, while CE3a can be equally likely to be active or inactive.8
Serology has a complementary role to imaging in the diagnosis of CE. However, even the combined use of imaging and serology can sometimes fail to establish a definitive diagnosis. In particular, small active cysts, inactive cysts, or extrahepatic cysts with suggestive but not pathognomonic imaging features require invasive diagnosis if a definitive diagnosis is needed. The performances of immunoassays are heterogeneous and influenced by the antigen and technique used and by many clinical variables.9,10 Unfortunately, these data are often not taken into account when test performances are assessed and, in the clinical setting, when serology results are interpreted. Knowledge of serology behavior and influencing variables is crucial for clinicians to evaluate serology results in the context of each patient's condition. This work aimed to broaden the knowledge on the variables influencing serology results using two commercial serology kits marked with European Conformity. Among the variables explored, stage, number, and location of cysts, presence of complications, collection of serum after treatment, and infection with other parasitic or nonparasitic diseases have been reported to influence serology results.10 Cyst dimensions, however, have never been taken into account as a continuous variable in previous studies. In this retrospective analysis of a cohort of patients diagnosed with hepatic CE and followed in our clinic from May 2000 to June 2012, we found that not only cyst activity and time from treatment but also antigen mass influence the results of two commercial enzyme-linked immunosorbent assays (ELISA and IHA) test used in the diagnostic parasitology laboratory of our hospital.
Materials and Methods
Patients and cysts classification.
Electronic demographic and clinical records of patients with echinococcal and nonparasitic hepatic cysts seen in our clinic between May 2000 and June 2012 were searched for subject selection. Patients with extrahepatic CE were excluded from this study because of the known very poor sensitivity and negative predictive value of serology in the diagnosis of extrahepatic CE. Data extracted were gender, age at visit, cyst etiology (echinococcal or nonparasitic), number, stage and largest diameter of cysts, and date and type of treatment. Echinococcal cysts were classified based on US morphology according to the WHO-IWGE classification and grouped as follows: active (CE1, CE2, and CE3b), transitional (CE3a), and inactive (CE4, CE5). This grouping was chosen on the basis of the results of the metabolic profile of CE cysts assessed by magnetic resonance spectroscopy in a previous work by Hosch and others.8 When ≥ 2 CE cysts were present, the patient was grouped according the most active cyst, and, when more cysts of the same stage were present, the patient was grouped according to the cyst of largest diameter. Patients with nonparasitic hepatic cysts were used as controls because nonparasitic cysts represent the most common differential diagnosis of CE cysts. Patients without previous therapy obtained at the first visit in our center were included in the cross-sectional analysis. For the analysis of influence of time from therapy on serology, patients with post-intervention residual lesions due to conservative surgery or percutaneous treatment, and patients previously treated with albendazole with at least one follow-up visit after treatment were included. For this analysis, patients who had received treatment were further divided into those who received treatment ≤ 12 and > 12 months before serum collection. This period was arbitrarily chosen based on the reported kinetics of antibody titers after surgery.11
Serology.
Sera were analyzed by ELISA test in the parasitology laboratory of our hospital using the European Conformity-marked kit Ridascreen® Echinococcus IgG (R-Biopharm, Darmstadt, Germany), based on cyst fluid purified antigens, according to the manufacturer's instructions, showing a repeatability coefficient of variation (CV) = 6.3–8.6% and a reproducibility CV = 5.0–5.7% (N = 24). Cutoff for a positive test was ≥ 1.1 optical density (OD). Sera were also processed with European Conformity-marked test Cellognost® Echinococcosis IHA (Siemens Healthcare Diagnostics, Marburg, Germany) with a cutoff for a positive test of 1:64 titer, as indicated by the manufacturer.
Statistical analysis.
Quantitative variables were expressed as the mean and standard deviation if normally distributed and median and interquartile range (IQR) if not normally distributed. Qualitative variables were described as number and percentage. Differences between groups were evaluated using χ2 or Fisher exact test, as appropriate. ELISA OD results were compared between groups using Kruskal–Wallis analysis of variance, and post hoc tests were performed to correct for multiple comparisons. Correlations between two variables were assessed with Pearson's correlation coefficient. ELISA OD values and indirect hemagglutination (IHA) titers were log transformed to achieve normality and included as the dependent variable in univariable and multivariable regression models. Age, gender, cyst stage and activity group, diameter of the largest cyst, and number of cysts were considered as independent variables after testing for collinearity with Spearman correlation coefficient. The results of the regression models were expressed as the coefficient and relative 95% confidence interval (CI). Log-transformed ELISA OD values and IHA titers over time were compared between groups using a longitudinal data analysis method. Population-averaged generalized estimating equation (GEE) models with an autocorrelation of order 1 were fitted considering age, gender, treatment (received ≤ 12 or > 12 months from serum collection, or never received), cyst stage and activity group, diameter of the largest cyst, and number of cysts as independent variables. The results of the GEE models were expressed as the coefficient and relative 95% CI. The coefficient indicates the mean change of log-transformed ELISA OD per unit change of the independent variable. A P < 0.05 was considered statistically significant. All tests were two sided. Data analysis was performed with the STATA statistical package (version 13.1; Stata Corporation, College Station, TX).
Results
Patient characteristics.
Of the patients, 171 with 205 hepatic CE cysts (median = 1, range = 1–3 cysts/patient) and 90 controls with 90 nonparasitic cysts were included in the cross-sectional analysis. A total of 151 patients with 194 hepatic CE cysts (median = 1, range = 1–3 cysts/patient) examined over 674 visits (median = 4, IQR = 2–6 visits/patient; median of follow-up 40 months, IQR = 14–80) were included in the longitudinal analysis. Of these patients, 89 (68.9%) were receiving therapy at the first time point of the analysis. Demographic and clinical characteristics of included subjects are summarized in Table 1.
Table 1.
Patient characteristics
| Patient groups | Cross-sectional analysis | Longitudinal analysis* | |
|---|---|---|---|
| Variable | CE patients (N = 171) | Controls (N = 90) | CE patients (N = 151) |
| Age (years) [mean (SD)] | 46.89 (16.92) | 56.27 (14.18) | 44.55 (16.64) |
| Male gender [N (%)] | 84 (49.12) | 20 (22.22) | 74 (49.01) |
| Distribution of number of cysts [patients N (%)] | |||
| One nonparasitic cysts | 0 (0) | 90 (100) | 0 (0) |
| One parasitic cyst | 142 (83.04) | 0 (0) | 110 (72.85) |
| Two parasitic cysts | 24 (14.04) | 0 (0) | 39 (25.83) |
| Three parasitic cysts | 5 (2.92) | 0 (0) | 2 (1.32) |
| Cyst diameter (mm) [mean (SD)]† | 65.63 (32.79) | 64.28 (44.38) | 65.63 (32.79) |
| Distribution of cysts stages [cysts N (%)]† | |||
| Nonparasitic cyst | 0 (0) | 90 (100) | 0 (0) |
| CE1 | 7 (4.09) | 0 (0) | 6 (3.97) |
| CE2 | 16 (9.36) | 0 (0) | 14 (9.27) |
| CE3a | 22 (12.87) | 0 (0) | 27 (17.88) |
| CE3b | 46 (26.9) | 0 (0) | 46 (30.46) |
| CE4 | 43 (25.15) | 0 (0) | 33 (21.85) |
| CE5 | 37 (21.64) | 0 (0) | 25 (16.56) |
CE = cystic echinococcosis.
Data refer to the first visit.
Data refer to the most active cyst, and, when more cysts of the same stage were present, according to the cyst of largest diameter.
Cross-sectional analysis.
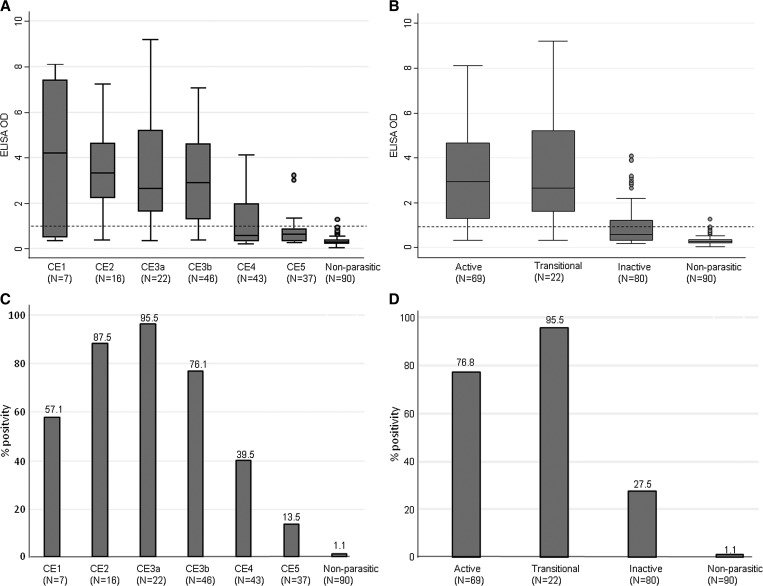
Cyst size (mean largest diameter) did not differ significantly between cyst stage groups (P = 0.780). As expected, a statistically significant difference in ELISA OD values was found between CE cysts (of all stages and activity groups) and nonparasitic cysts (P < 0.0001 for all comparisons, with adjusted P = 0.001 and 0.004, respectively) (Figure 1 ). Furthermore, ELISA OD values differed significantly between activity groups of cysts (P < 0.0001 for all comparisons, with adjusted P = 0.004) with the exception of transitional versus active cysts, which had comparable serology results (Figure 1). When the same analysis was carried out by cyst stage, these results were confirmed (Figure 1).
Figure 1.
Enzyme-linked immunosorbent assay optical density (ELISA OD) values and positivity rates in the investigated groups. Median, interquartile range (IQR), and minimum and maximum values of ELISA OD values in patients groups according to cyst stage (A) and activity (B) and control patients with nonparasitic cysts. Dashed lines represent the OD cutoff for positivity. (A) CE2 vs. CE4, CE2 vs. CE5, CE3a vs. CE4, CE3a vs. CE5, CE3b vs. CE4, and CE3b vs. CE5 P < 0.001. Any cyst stage vs. nonparasitic P < 0.001. All other comparisons are nonsignificant. Adjusted P value for significance = 0.001. (B) Active vs. transitional P = 0.324; all other comparisons P < 0.001. Adjusted P value for significance = 0.004. Percentage ELISA positivity rate in patients groups according to cyst stage (C) and activity (D). (C) CE2 vs. CE4 and CE3b vs. CE4 P = 0.001; CE2 vs. CE5, CE3a vs. CE4, CE3a vs. CE5, and CE3b vs. CE5 P < 0.001. All other comparisons are nonsignificant. (D) Active vs. transitional P = 0.062; all other comparisons P < 0.001.
When ELISA positivity rates were compared between cyst activity groups and cyst stages, results mirrored those obtained by the analysis of ELISA OD values. Positivity rates differed significantly between CE cysts (of all stages and activity groups) and nonparasitic cysts (P < 0.001 for all comparisons) (Figure 1). Moreover, positivity differed significantly between cyst activity groups (P ≤ 0.001 for all comparisons) with the exception of transitional versus active cysts that had comparable serology results (Figure 1).
To evaluate the factors associated with serology results at first visit in the absence of any previous treatment, univariable and multivariable analyses were carried out. The results are summarized in Table 2. Cyst activity group, size, and number of cysts were positively correlated, whereas age at visit was negatively correlated, with ELISA OD values. This result may be due to the significant difference of mean age between the groups (P < 0.001). On the contrary, gender of patients was not significantly correlated with ELISA OD values. All variables that were significantly correlated with serology results in the univariable analysis were also found to be independent factors influencing ELISA OD results when multivariable analysis was carried out.
Table 2.
Association between demographic and clinical variables and ELISA OD values* at first visit (cross-sectional analysis)
| Variable | Univariable analysis | Multivariable analysis‡ | ||||
|---|---|---|---|---|---|---|
| Coefficient† | 95% CI | P | Coefficient† | 95% CI | P | |
| Age (years) | ||||||
| ELISA | −0.02 | −0.03 to −0.12 | < 0.001 | −0.01 | −0.02 to −0.01 | < 0.001 |
| IHA | 0.08 | 0.05–0.12 | < 0.001 | 0.06 | 0.03–0.09 | < 0.001 |
| Male gender | ||||||
| ELISA | 0.15 | −0.16 to 0.47 | 0.339 | – | – | – |
| IHA | −0.70 | −1.93 to −0.53 | 0.264 | – | – | – |
| Cysts size (mm)§ | ||||||
| ELISA | 0.01 | 0.01–0.02 | < 0.001 | 0.004 | 0.00–0.01 | 0.056 |
| IHA | −0.04 | −0.06 to −0.03 | < 0.001 | −0.03 | −0.04 to −0.01 | 0.001 |
| Number of cysts | ||||||
| ELISA | 0.48 | 0.15–0.81 | 0.005 | 0.39 | 0.13–0.64 | 0.003 |
| IHA | −1.54 | −2.84 to −0.23 | 0.021 | −1.25 | −2.34 to −0.15 | 0.026 |
| Type of cyst§ | ||||||
| Active vs. inactive | ||||||
| ELISA | 1.23 | 0.95–1.50 | < 0.001 | 1.02 | 0.74–1.30 | < 0.001 |
| IHA | −4.32 | −6.03 to −2.61 | < 0.001 | −2.91 | −4.63 to −1.19 | 0.001 |
| Transitional vs. inactive | ||||||
| ELISA | 1.40 | 1.0–1.80 | < 0.001 | 1.05 | 0.65–1.45 | < 0.001 |
| IHA | −3.79 | −4.96 to −2.62 | < 0.001 | −2.61 | −3.83 to −0.01 | < 0.001 |
CI = confidence interval; ELISA = enzyme-linked immunosorbent assay; IHA = indirect hemagglutination; OD = optical density.
Log transformed.
The coefficient indicates the mean change of log-transformed ELISA OD per unit change of the independent variable.
Only independent variable significantly associated with the dependent variable in the univariable analysis were included in the multivariable analysis.
Data refer to the most active, and, when more cysts of the same stage were present, according to the cyst of largest diameter.
To assess whether our results were test- or antigen-specific, we carried out univariable and multivariable analysis on the results of the IHA test. As shown in Table 2, results obtained with the IHA test were found comparable with the ELISA test, supporting the hypothesis that results have a biological basis independent from the test and antigen used.
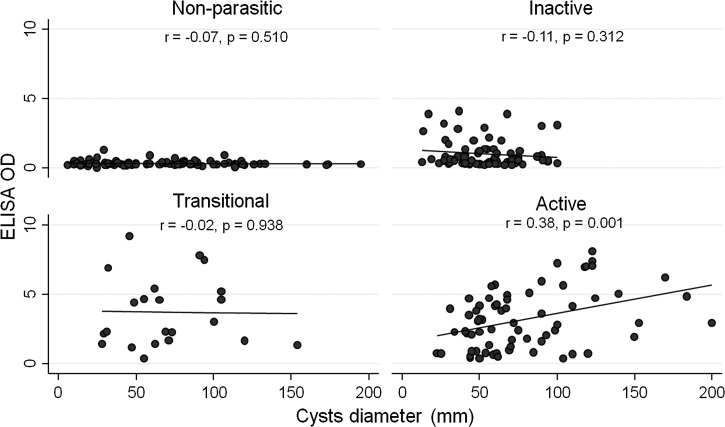
To our knowledge, size of cysts as a continuous variable has not been taken into account in previous studies investigating the factors influencing serology in CE. Therefore, we investigated whether this factor was correlated with serology within all cyst activity groups. As shown in Figure 2 , we found that ELISA OD values and cyst diameter positively correlated only within the active cysts group (r = 0.038; P = 0.001), while no significant correlation was found within the other investigated groups. Comparable results (r = −0.28; P = 0.018 within the active cyst group) were found when analyzing the IHA titers (data not shown).
Figure 2.
Correlation between cyst size and enzyme-linked immunosorbent assay optical density (ELISA OD) results in patients groups according to cyst activity.
Longitudinal analysis.
Using a GEE population-averaged model, we evaluated the factors influencing serology results over time, including time from therapy. Similarly to what was found in the cross-sectional analysis, the results from our cohort showed that cyst activity, size, and number were independently associated with ELISA OD values. Moreover, having been treated ≤ 12 months before serum collection was also independently associated with ELISA results, while age at visit and gender were not. The results of the longitudinal analysis are detailed in Table 3.
Table 3.
Association between demographic and clinical variables and ELISA OD values* (longitudinal analysis)
| Variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| Coefficient† | 95% CI | P | Coefficient† | 95% CI | P | |
| Age (years) | −0.0014 | −0.0046 to 0.0017 | 0.375 | 0.0003 | −0.0026 to 0.0032 | 0.842 |
| Male gender | 0.0828 | −0.0357 to 0.2012 | 0.171 | 0.0608 | −0.0458 to 0.1674 | 0.264 |
| Cyst diameter (mm)‡ | 0.0033 | 0.002–0.0046 | < 0.001 | 0.0025 | 0.0012–0.0038 | < 0.001 |
| Number of cysts | 0.0919 | 0.0241–0.1597 | 0.008 | 0.0784 | 0.0141–0.1427 | 0.017 |
| Type of cyst‡ | ||||||
| Active vs. inactive | 0.1313 | 0.0623–0.2004 | < 0.001 | 0.0982 | 0.029–0.1675 | 0.005 |
| Transitional vs. inactive | 0.2302 | 0.1467–0.3137 | < 0.001 | 0.1879 | 0.1036–0.2722 | < 0.001 |
| Time between treatment and serum collection | ||||||
| ≤ 12 months | 0.075 | 0.0113–0.1388 | 0.021 | 0.0857 | 0.0247–0.1468 | 0.006 |
| > 12 months | −0.0144 | −0.0773–0.0484 | 0.652 | 0.0113 | −0.0484 to 0.0709 | 0.711 |
CI = confidence interval; ELISA = enzyme-linked immunosorbent assay; OD = optical density.
Log transformed.
The coefficient indicates the mean change of log-transformed ELISA OD per unit change of the independent variable.
Data refer to the most active, and, when more cysts of the same stage were present, according to the cyst of largest diameter.
Discussion
The diagnosis and follow-up of CE are based on imaging, while serology has a complementary role supporting imaging diagnosis when imaging features are not pathognomonic. However, the performances of immunoassays are heterogeneous and influenced by many clinical and technique-related variables, which need to be taken into account by the clinician when interpreting serology results of individual patients.9,10 Unfortunately, the majority of studies evaluating serology test performance have used the presence or absence of infection only and have not taken into account other clinical variables. The stage of the cyst is the variable most often investigated in correlation with serology results, with a consensus finding that patients with CE1 and CE4-CE5 (hepatic) cysts are seronegative in a high percentage of cases (30–58% and 50–87%, respectively), while rates of negativity are lower in the presence of CE2 and CE3 cysts (5–20%).10,12–14 When the variables associated with serology results were investigated in more detail, serodiagnosis performance correlated with stage, number (single versus multiple), location (liver versus lungs), and size (< or > than 15 cm) of cysts; presence of complications; and collection of serum after treatment.10,11,13,15
Although in the majority of cases of hepatic CE the combined use of imaging and serology allows for a reliable diagnosis, in some instances they are inconclusive, and differential diagnosis with other lesions may be challenging. This is particularly true in the case of small CE1 and old inactive CE4-CE5 cysts that may lack clear pathognomonic signs of echinococcal etiology on US and are serology negative in a high proportion of cases. Currently, no reliable marker of infection or infection activity is available. Specific antibodies may be detectable for years in the presence of stably inactive cysts and even after radical surgery and do not imply the presence of active infection.16–19 As a result, clinicians with little experience with this disease infer that positive serology always means presence of active infection, a wrong assumption often resulting in unnecessary treatment, with attendant side effects, cost, and patient (and physician) anxiety. A good understanding of the behavior of serology and its influencing variables, beyond stage of cysts, is crucial for the diagnosis and clinical management of CE, and allows clinicians to evaluate serology results in the context of each patient's condition.
In our study, we investigated the correlation between serology results and activity, size and number of hepatic cysts, and collection of serum after treatment. Besides confirming previous results,10 we clarified that the behavior of serology before and after 12 months from treatment differ. Furthermore, when we investigated the size of the cyst as a continuous variable, we found that diameter of cyst and serology results positively correlated only within the active cyst group, while no significant correlation was found within the other investigated groups.
From a biological point of view, the increase in the antigenic mass exposed to the host's immune system because of the loss of integrity of the cyst wall, either spontaneously or as the result of therapy, and the increase in the antigenic mass when multiple and large cysts are present, may at least partly explain our results. However, further studies should address the biological mechanisms at the basis of the variability of the immune response to CE.
In conclusion, our results expand current knowledge on the behavior of CE serology in respect to a number of variables present in the clinical history of patients with this infection, providing useful information to clinicians for the interpretation of serology results in the context of each patient's condition.
ACKNOWLEDGMENTS
We are grateful to Sam Goblirsch, who edited the article for grammar and style.
Footnotes
Financial support: The research leading to these results has been partly funded from the European Union Seventh Framework Programme (FP7/2007-2013) under the project HERACLES (grant agreement no. 602051) to Enrico Brunetti.
Authors' addresses: Raffaella Lissandrin and Enrico Brunetti, Department of Infectious Diseases, IRCCS San Matteo Hospital Foundation, Pavia, Italy, WHO Collaborating Centre for Clinical Management of Cystic Echinococcosis, Pavia, Italy, and Department of Clinical, Surgical, Diagnostic and Pediatric Sciences, University of Pavia, Pavia, Italy, E-mails: raffaella.lissandrin@unipv.it and enrico.brunetti@unipv.it. Francesca Tamarozzi, WHO Collaborating Centre for Clinical Management of Cystic Echinococcosis, Pavia, Italy, and Department of Clinical, Surgical, Diagnostic and Pediatric Sciences, University of Pavia, Pavia, Italy, E-mail: f_tamarozzi@yahoo.com. Luca Piccoli, Department of Infectious Diseases, IRCCS san Matteo Hospital Foundation, Pavia, Italy, Current address: Institute for Research in Biomedicine, Bellinzona, Switzerland, E-mail: luca.piccoli@irb.usi.ch. Carmine Tinelli and Annalisa De Silvestri, Clinical Epidemiology and Biometry Unit, IRCCS San Matteo Hospital Foundation, Pavia, Italy, E-mails: ctinelli@smatteo.pv.it and a.desilvestri@smatteo.pv.it. Mara Mariconti, Department of Infectious Diseases, IRCCS San Matteo Hospital Foundation, Pavia, Italy, and WHO Collaborating Centre for Clinical Management of Cystic Echinococcosis, Pavia, Italy, E-mail: mara.mariconti@guest.unimi.it. Francesca Genco, Department of Microbiology and Virology, IRCCS San Matteo Hospital Foundation, Pavia, Italy, E-mail: francesca.genco@unipv.it. Valeria Meroni, Department of Microbiology and Virology, IRCCS San matteo Hospital Foundation, Department of Internal Medicine and Clinical Therapy, University of Pavia, Italy, E-mail: v.meroni@smatteo.pv.it.
References
- 1.Budke CM. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis. 2006;12:296–303. doi: 10.3201/eid1202.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig PS, Budke CM, Schantz P, Li T, Qiu J, Yang JY, Zeyhle E, Rogan MT, Ito A. Human echinococcosis: a neglected disease? Trop Med Health. 2007;35:283–292. [Google Scholar]
- 3.Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid disease from head to toe. Radiographics. 2003;23:475–494. doi: 10.1148/rg.232025704. quiz 536–537. [DOI] [PubMed] [Google Scholar]
- 4.Pedrosa I, Saiz A, Arrazola J, Ferreiros J, Pedrosa CS. Hydatid disease: radiologic and pathologic features and complications. Radiographics. 2000;20:795–817. doi: 10.1148/radiographics.20.3.g00ma06795. [DOI] [PubMed] [Google Scholar]
- 5.Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 6.WHO Informal Working Group International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003;85:253–261. doi: 10.1016/s0001-706x(02)00223-1. [DOI] [PubMed] [Google Scholar]
- 7.Junghanss T, da Silva AM, Horton J, Chiodini PL, Brunetti E. Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am J Trop Med Hyg. 2008;79:301–311. [PubMed] [Google Scholar]
- 8.Hosch W, Junghanss T, Stojkovic M, Brunetti E, Heye T, Kauffmann GW, Hull WE. Metabolic viability assessment of cystic echinococcosis using high-field 1H MRS of cyst contents. NMR Biomed. 2008;21:734–754. doi: 10.1002/nbm.1252. [DOI] [PubMed] [Google Scholar]
- 9.Carmena D, Benito A, Eraso E. Antigens for the immunodiagnosis of Echinococcus granulosus infection: an update. Acta Trop. 2006;98:74–86. doi: 10.1016/j.actatropica.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Gonzalez A, Santivanez S, Garcia HH, Rodriguez S, Munoz S, Ramos G, Orduna A, Siles-Lucas M. Improved serodiagnosis of cystic echinococcosis using the new recombinant 2B2t antigen. PLoS Negl Trop Dis. 2012;6:e1714. doi: 10.1371/journal.pntd.0001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben Nouir N, Nunez S, Gianinazzi C, Gorcii M, Muller N, Nouri A, Babba H, Gottstein B. Assessment of Echinococcus granulosus somatic protoscolex antigens for serological follow-up of young patients surgically treated for cystic echinococcosis. J Clin Microbiol. 2008;46:1631–1640. doi: 10.1128/JCM.01689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li T, Ito A, Chen X, Sako Y, Qiu J, Xiao N, Qiu D, Nakao M, Yanagida T, Craig PS. Specific IgG responses to recombinant antigen B and em18 in cystic and alveolar echinococcosis in china. Clin Vaccine Immunol. 2010;17:470–475. doi: 10.1128/CVI.00466-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li T, Ito A, Pengcuo R, Sako Y, Chen X, Qiu D, Xiao N, Craig PS. Post-treatment follow-up study of abdominal cystic echinococcosis in Tibetan communities of northwest Sichuan Province, China. PLoS Negl Trop Dis. 2011;5:e1364. doi: 10.1371/journal.pntd.0001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang YR, Craig PS, Ito A, Vuitton DA, Giraudoux P, Sun T, Williams GM, Huang Z, Li Z, Wang Y, Teng J, Li Y, Huang L, Wen H, Jones MK, McManus DP. A correlative study of ultrasound with serology in an area in China co-endemic for human alveolar and cystic echinococcosis. Trop Med Int Health. 2007;12:637–646. doi: 10.1111/j.1365-3156.2007.01834.x. [DOI] [PubMed] [Google Scholar]
- 15.Santivanez SJ, Arias P, Portocarrero M, Rodriguez S, Gonzalez AE, Gilman RH, Gavidia CM, Garcia HH. Serological diagnosis of lung cystic hydatid disease using the synthetic p176 peptide. Clin Vaccine Immunol. 2012;19:944–947. doi: 10.1128/CVI.05540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez-Gonzalez A, Muro A, Barrera I, Ramos G, Orduna A, Siles-Lucas M. Usefulness of four different Echinococcus granulosus recombinant antigens for serodiagnosis of unilocular hydatid disease (UHD) and postsurgical follow-up of patients treated for UHD. Clin Vaccine Immunol. 2008;15:147–153. doi: 10.1128/CVI.00363-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galitza Z, Bazarsky E, Sneier R, Peiser J, El-On J. Repeated treatment of cystic echinococcosis in patients with a long-term immunological response after successful surgical cyst removal. Trans R Soc Trop Med Hyg. 2006;100:126–133. doi: 10.1016/j.trstmh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Rigano R, Ioppolo S, Ortona E, Margutti P, Profumo E, Ali MD, Di Vico B, Teggi A, Siracusano A. Long-term serological evaluation of patients with cystic echinococcosis treated with benzimidazole carbamates. Clin Exp Immunol. 2002;129:485–492. doi: 10.1046/j.1365-2249.2002.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccoli L, Tamarozzi F, Cattaneo F, Mariconti M, Filice C, Bruno A, Brunetti E. Long-term sonographic and serological follow-up of inactive echinococcal cysts of the liver: hints for a “watch-and-wait” approach. PLoS Negl Trop Dis. 2014;8:e3057. doi: 10.1371/journal.pntd.0003057. [DOI] [PMC free article] [PubMed] [Google Scholar]