Abstract
Hypoglycemia is a life-threatening complication of several diseases in childhood. We describe the prevalence and incidence of hypoglycemia among admitted Mozambican children, establishing its associated risk factors. We retrospectively reviewed clinical data of 13 years collected through an ongoing systematic morbidity surveillance in Manhiça District Hospital in rural Mozambique. Logistic regression was used to identify risk factors for hypoglycemia and death. Minimum community-based incidence rates (MCBIRs) for hypoglycemia were calculated using data from the demographic surveillance system. Of 49,089 children < 15 years hospitalized in Manhiça District Hospital, 45,573 (92.8%) had a glycemia assessment on admission. A total of 1,478 children (3.2%) presented hypoglycemia (< 3 mmol/L), of which about two-thirds (972) were with levels < 2.5 mmol/L. Independent risk factors for hypoglycemia on admission and death among hypoglycemic children included prostration, unconsciousness, edema, malnutrition, and bacteremia. Hypoglycemic children were significantly more likely to die (odds ratio [OR] = 7.11; P < 0.001), with an associated case fatality rate (CFR) of 19.3% (245/1,267). Overall MCBIR of hypoglycemia was 1.57 episodes/1,000 child years at risk (CYAR), significantly decreasing throughout the study period. Newborns showed the highest incidences (9.47 episodes/1,000 CYAR, P < 0.001). Hypoglycemia remains a hazardous condition for African children. Symptoms and signs associated to hypoglycemia should trigger the verification of glycemia and the implementation of life-saving corrective measures.
Introduction
Critical illness seriously deranges metabolism in children and adults.1–3 Alterations in blood glucose homeostasis are the most common metabolic abnormalities found in critically ill children,4 and both hyper- and hypoglycemia are associated with poor outcomes.1,3,4 Hyperglycemia in critically ill patients is an adaptive response to stress related to hypovolemia, surgery, sepsis, or trauma.5,6 The prevalence in tropical settings has been estimated between 2.9% and 10.9%,7,8 and its presence on admission is associated with mortality.1,9,10 Insulin therapy is the treatment of choice, but is often unavailable in resource-constrained settings and can also cause iatrogenic hypoglycemia, potentially more harmful than sustained hyperglycemia.
On the other side of the spectrum, hypoglycemia is also a common and life-threatening complication of several diseases such as severe malaria, bacterial sepsis, severe malnutrition, and neonatal illness, among others.11–15 Hypoglycemia has been extensively reported to have important influence on the outcome of very ill patients, both children and adults.1,16,17 In Africa, its prevalence among pediatric admissions has been estimated to range between 1.8% and 7.3%.7,18 Use of toxic herbal preparations and delays in seeking medical assistance may all cause or further aggravate hypoglycemia in these settings. Severe and prolonged hypoglycemia can result in mental retardation, neurological deficits, and recurrent seizures.19,20 Management of hypoglycemia according to World Health organization (WHO) guidelines11 includes rapid administration of exogenous glucose, preferably through an intravenous access. In the developing world, hypoglycemia remains an insufficiently recognized killer of children, as it is seldom diagnosed and whenever detected, often poorly managed mainly because of the lack of simple equipment or trained staff.
We analyzed data collected throughout 13 consecutive years of systematic morbidity surveillance among children admitted to a rural Mozambican hospital to determine prevalence, incidence, and risk factors associated with hypoglycemia on admission and mortality in those children.
Material and Methods
Study site and population.
The study was conducted in Manhiça in southern Mozambique. The Manhiça Health Research Center (CISM) runs a demographic surveillance system (DSS) in the area and a morbidity surveillance system (MSS) at Manhiça District Hospital (MDH), which admits around 3,000 children annually. Malaria, pneumonia, diarrhea, malnutrition, and neonatal pathologies are among the main causes of admission and under-five mortality in Manhiça,21 where human immunodeficiency virus (HIV) prevalence is among the highest in the world.22 A detailed description of MDH, CISM, and the study area can be found elsewhere.23 CISM provides personnel as well as valuable resources and laboratory diagnosis to MDH.
Study design.
We present a retrospective analysis of data collected through the Manhiça MSS from children < 15 years, who were admitted to MDH during a 13-year-long study period (2001–2013).
Hospital surveillance system.
A standardized admission questionnaire, which includes demographic, clinical, laboratory, and outcome data, was filled in for all hospitalized children < 15 years of age by a clinician. On arrival, a finger-prick blood sample was collected to measure packed cell volume (PCV) and blood glucose concentration, and thick and thin blood films were prepared to quantify Plasmodium falciparum parasitemia. Blood cultures were systematically performed for all children under the age of 2 years, or in older children with clinical severity, as part of the routine microbiological surveillance in MDH.
HIV status information was not routinely collected. On discharge or death, up to four final diagnoses—based on the International Classification of Diseases 10 —were recorded on the questionnaire after review of all available results.
Laboratory methods.
Glycemia was determined using Accu-Chek® (Roche Inc., Manheim, Germany) at the bedside, with blood being usually collected by finger prick. Glycemia results were provided either in mmol/L or mg/dL units. To simplify the analysis, all mg/dL values were converted into mmol/L by multiplying them by 0.0555. PCV was measured using a microcentrifuge and a Hawksley hematocrit reader card (Hawksley and Sons Ltd., Lancing, United Kingdom). Thick and thin blood films for malaria diagnosis and blood cultures were processed as previously described.24,25
Clinical definitions.
All case definitions were based on admission data from the standardized questionnaires. Children were categorized into three groups according to blood glucose levels: 1) hypoglycemia: blood glucose levels < 3.0 mmol/L (categorized as severe if < 2.5 mmol/L), 2) hyperglycemia: glycemia > 11 mmol/L, and 3) normoglycemia: values between 3 and 11 mmol/L.
A malaria case was defined as a child admitted with a clinical diagnosis of malaria with a P. falciparum asexual parasitemia > 0 parasites/μL. Prostration was defined as the inability to sit unsupported or breast-feed in children not yet capable of sitting. Impaired consciousness was defined as a child having a Blantyre coma score less than 5. Severe anemia was defined as a PCV < 15% on admission. Hypothermia was defined as axillary temperature < 35°C. Increased respiratory rate followed age-specific WHO definitions.11 Respiratory distress included the presence of deep breathing or indrawing. Nutritional status was based on weight-for-age z scores (WAZ), calculated using the least mean square method and the WHO and Centers for Disease Control and Prevention growth charts.26 Malnutrition was defined if WAZ was < −1 and severe malnutrition as WAZ of < −3.
Case management.
Children with hypoglycemia were managed according to Mozambican national guidelines, based on the WHO guidelines,11 which recommend a rapid intravenous correction with 5 mL/kg of 10% glucose or dextrose solution, repeated if necessary. Ten percent dextrose in normal saline or Ringer's lactate for maintenance infusion was used to prevent further episodes, and feeding encouraged as soon as possible. Facilities for intensive care are not available at MDH. All clinical assistance and treatment of admitted children is free of charge. Children requiring specialized care were transferred to Maputo Central Hospital.
Data management and statistical methods.
All admission questionnaires were double entered using a program written in FoxPro version 5.0 (Microsoft Corp., Seattle, WA). Statistical analyses were done with Stata 13.1 (Stata Corp., College Station, TX).
Minimum community-based incidence rates (MCBIRs) for hypoglycemia were calculated referring cases to population denominators establishing time at risk (child years at risk [CYAR]) inferred from the DSS. Children did not contribute to the numerator or denominator for a period of 28 days after each episode of hypoglycemia or when they were outside the study area. The analysis of MCBIRs only takes into account children with a permanent identification number issued by the demography department allowing the linkage of their demographic data with the morbidity surveillance. Children not living within the study area were excluded for incidence calculations. Negative binomial regression models with random effects using likelihood ratio test were used to assess differences in incidence rates between calendar years and age groups.
Case fatality rates (CFRs) were calculated for different glycemia levels as the number of patients who died with a specific glycemia level divided by the total number of patients with known outcome admitted in the study period. These CFRs represent in-hospital mortality and do not include patients absconding or being transferred.
Qualitative variables were compared using a χ2 test or Fisher's exact test. Means of normally distributed variables were compared using the Student t test or analysis of variance.
Multivariate logistic regression was used to investigate 1) adjusted associations for potential risk factors for hypoglycemia on admission and 2) adjusted associations for potential risk factors for hypoglycemia-related deaths. In the second analysis, given that the dependent variable was the final outcome (dead/alive), only children with a known outcome were included in the analysis (those absconding or being transferred were excluded). P values from analyses performed in large samples may be confounded because of their dependence on sample size and may reach the significance level even when the association is negligible. Thus, significant associations will be interpreted in accordance with the effect size (odds ratio [OR]) and will be classified as small (< 2), medium (2–3), or large (> 3).27
Ethics.
This study retrospectively assessed data collected in the context of routine clinical practice. The morbidity surveillance in place at MDH has been approved by the Mozambican Ethics Committee. The analytical plan of this specific analysis was assessed and approved by Manhiça's Internal Scientific Committee.
Results
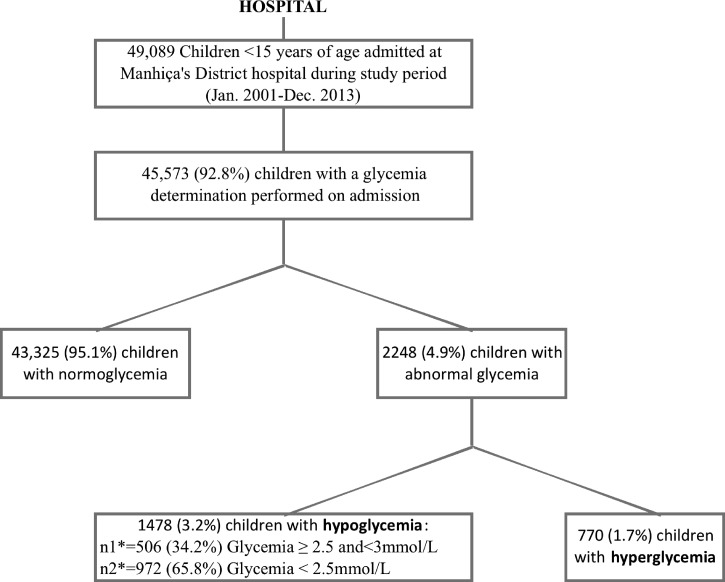
During the 13-year study period (January 1, 2001 to December 31, 2013), 49,089 children < 15 years of age were admitted to MDH, including 17,115 infants and 2,774 newborns. Median age on admission was 18 months (interquartile range [IQR] = 8–35). Glycemia results were available for 45,573 (92.8%) children (Figure 1 ) and in-hospital outcome information was missing in 4,013 (8.2%) cases. Children without glycemia data were excluded from the analysis.
Figure 1.
Study profile.
Prevalence of dysglycemia.
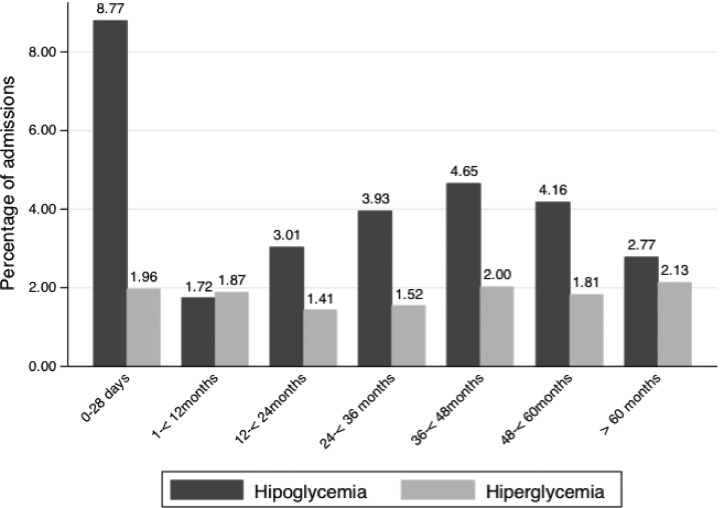
On admission, 1,478/45,573 (3.2%) children had hypoglycemia, two-thirds of these episodes (972; 2.1%) being severe. Hyperglycemia was detected in 770/45,573 (1.7%) patients. By age group, hypoglycemia prevalence was the highest among newborns (8.8%), but present in all ages. Hyperglycemia was low and present in all age groups (Figure 2 ).
Figure 2.
Distribution of normo-, hypo-, and hyperglycemia according to age group.
Clinical presentation and CFR of children with hypoglycemia admitted to the hospital.
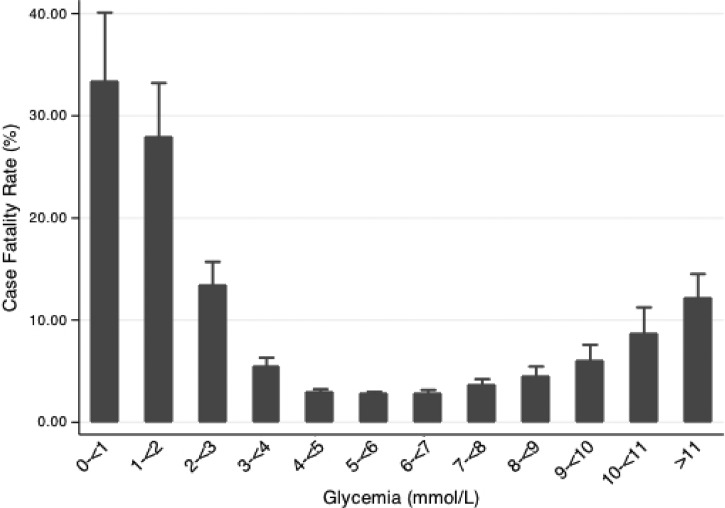
Table 1 compares some key characteristics in children with hypoglycemia and normoglycemia. A significantly higher proportion of the patients with hypoglycemia were newborns, but median age was not significantly different between the two glycemia groups. Children with hypoglycemia were significantly more prone to refer feeding difficulties, have hypothermia, or neurological impairment than their normoglycemic peers. They were also significantly more malnourished, with lower mean weight and WAZ scores, and, more frequently, severely anemic or bacteremic. Vomiting and diarrhea were not associated with having more hypoglycemia on admission. The odds of dying in the hospital were higher among hypoglycemic children (OR = 7.11, 95% confidence interval [CI] = 6.11–8.27; associated CFR = 19.3% versus 3.3%, P < 0.001). Figure 3 summarizes CFRs by categorized glycemia. CFRs increased with decreasing glycemia, peaking at 33.3% in patients with values < 1 mmol/L. Importantly, CFRs also rose significantly for patients with hyperglycemia (associated CFR = 12.1%, P < 0.001, when compared with CFR of normoglycemia).
Table 1.
Univariate analysis of clinical variables and diagnosis according to glycemia group
| Variables | Normoglycemia (N = 43,325) | Hypoglycemia (N = 1,478) | OR and 95% CI | P value |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Age in months (median, IQR) | 17 (8–34) | 21 (8–37) | 1.00 | 0.068* |
| Newborn, n (%) | 2,304 (5.3) | 226 (15.3) | 3.21 (2.77–3.73) | < 0.001 |
| Male, n (%) | 23,249 (53.7) | 841 (57.0) | 1.14 (1.03–1.27) | 0.013 |
| Symptoms before admission | ||||
| Fever, n (%) | 39,655 (91.5) | 1,207 (81.7) | 0.41 (0.36–0.47) | < 0.001 |
| Cough, n (%) | 28,429 (65.6) | 825 (55.8) | 0.66 (0.60–0.73) | < 0.001 |
| Vomiting, n (%) | 10,134 (23.4) | 362 (24.5) | 1.06 (0.94–1.20) | 0.32 |
| Diarrhea, n (%) | 9,326 (21.5) | 308 (20.8) | 0.96 (0.84–1.09) | 0.52 |
| Difficulties to breast-feed/anorexia, n (%) | 5,291 (13.5) | 406 (30.1) | 2.76 (2.45–3.12) | < 0.001 |
| Seizures, n (%) | 3,486 (8.1) | 211 (14.3) | 1.90 (1.64–2.21) | < 0.001 |
| Anthropometrics | ||||
| Weight in kilogram (mean ± SD) | 10.3 (5.9) | 9.6 (5.9) | 0.98 (0.97–0.99) | < 0.001 |
| Malnutrition (WAZ < −1), n (%) | 24,111 (55.7) | 908 (61.4) | 1.27 (1.14–1.41) | < 0.001 |
| Severe malnutrition (WAZ < −3), n (%) | 5,884 (14.6) | 252 (19.4) | 1.41 (1.22–1.62) | < 0.001 |
| WAZ score (mean ± SD) | −1.41 (1.5) | −1.76 (1.4) | 1.17 (1.13–1.22) | < 0.001 |
| Symptoms and signs on admission | ||||
| Axillary temperature (°C) (mean ± SD) | 37.9 (1.3) | 37.6 (1.5) | 0.86 (0.83–0.89) | < 0.001 |
| Hypothermia (axillary temperature < 35°C), n (%) | 99 (0.2) | 26 (1.8) | 7.82 (5.06–12.09) | < 0.001 |
| Respiratory rate (mean ± SD) | 45.5 (15.2) | 47.0 (18.5) | 1.01 (1.00–1.01) | < 0.001 |
| Increased respiratory rate, n (%) | 24,211 (55.9) | 834 (56.5) | 1.02 (0.92–1.14) | 0.67 |
| Respiratory distress, n (%) | 9,373 (21.7) | 427 (29.0) | 1.47 (1.31–1.65) | < 0.001 |
| Dehydration, n (%) | 6,935 (16.0) | 292 (19.8) | 1.29 (1.14–1.47) | < 0.001 |
| Pallor, n (%) | 7,789 (18.0) | 316 (21.4) | 1.24 (1.09–1.41) | < 0.001 |
| Jaundice, n (%) | 613 (1.4) | 55 (3.7) | 2.69 (2.03–3.57) | < 0.001 |
| Edema, n (%) | 2,678 (6.2) | 134 (9.1) | 1.51 (1.26–1.81) | < 0.001 |
| Stiff neck, n (%) | 399 (0.9) | 20 (1.4) | 1.48 (0.94–2.33) | 0.09 |
| Prostration, n (%) | 6,613 (15.3) | 489 (33.1) | 2.75 (2.45–3.07) | < 0.001 |
| BCS on admission (mean ± SD) | 4.9 (0.5) | 4.5 (1.2) | 4.10 (3.72–4.52) | < 0.001 |
| Unconsciousness (BCS < 5), n (%) | 2,270 (5.3) | 289 (19.7) | 4.42 (3.85–5.06) | < 0.001 |
| Deep coma (BCS ≤ 2), n (%) | 693 (1.6) | 146 (9.9) | 6.77 (5.61–8.17) | < 0.001 |
| Investigation | ||||
| Plasmodium falciparum malaria, n (%) | 22,186 (54.4) | 716 (56.2) | 1.07 (0.96–1.20) | 0.21 |
| HIV, n (%) | 341 (22.1) | 12 (27.9) | 0.73 (0.37–1.45) | 0.37 |
| Severe anemia, n (%) | 1,653 (4.2) | 77 (6.5) | 1.59 (1.26–2.02) | < 0.001 |
| Positive blood culture, n (%) | 2,803 (7.9) | 177 (14.8) | 2.02 (1.72–2.38) | < 0.001 |
| Clinical severe pneumonia (WHO criteria), n (%) | 9,934 (23.0) | 370 (25.1) | 1.12 (1.00–1.27) | 0.053 |
| Outcome | ||||
| Length of admission (mean ± SD) | 4.8 (5.8) | 5.0 (6.1) | 1.00 (0.99–1.01) | 0.21 |
| Died, n (%) | 1,307/40,063 (3.3) | 245/1,267 (19.3) | 7.11 (6.11–8.27) | < 0.001 |
BCS = Blantyre coma score; CI = confidence interval; HIV = human immunodeficiency virus; IQR = interquartile range; OR = odds ratio; SD = standard deviation; WHO = World Health Organization; WAZ = weight-for-age z score.
Significance: P-value of the statistical test comparing normoglycemic and hypoglycemic children on admission.
Mann–Whitney test for the difference of two medians.
Figure 3.
Case fatality rates (CFRs) according to glycemia level.
The multivariate analysis showed nine risk factors independently associated with the presence of hypoglycemia on admission (Table 2). History of seizures, unconsciousness, refusing to feed, malnutrition, edema, jaundice, prostration, P. falciparum infection, and having a positive blood culture were all associated with hypoglycemia, while a history of cough was protective against it. All identified factors showed small effect size (OR = < 2 for all risk factors and OR = > 0.5 for history of cough), except unconsciousness, whose effect size was medium (OR = 2.13, 95% CI = 1.66, 2.72).
Table 2.
Multivariate analysis of independent risk factors associated to hypoglycemia on admission
| Risk factors | Hypoglycemia (N = 1,478), n (%) | Adjusted OR | 95% CI | P value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Newborn | 226 (15.3) | 1.05 | 0.56 | 1.95 | 0.879 |
| Male | 841 (57.0) | 1.12 | 0.96 | 1.30 | 0.143 |
| Malnutrition (per unit WAZ decrease) | 908 (61.4)* | 1.12 | 1.07 | 1.18 | < 0.001 |
| History of fever | 1,207 (81.7) | 0.72 | 0.50 | 1.02 | 0.062 |
| History of cough | 825 (55.8) | 0.82 | 0.69 | 0.97 | 0.020 |
| Hypothermia on admission | 26 (1.8) | 3.21 | 0.79 | 12.94 | 0.101 |
| History of seizures | 211 (14.3) | 1.47 | 1.18 | 1.84 | 0.001 |
| Anorexia/refusing to feed | 406 (30.1) | 1.67 | 1.38 | 2.02 | < 0.001 |
| Unconsciousness (BCS < 5) | 289 (19.7) | 2.13 | 1.66 | 2.72 | < 0.001 |
| Edema | 134 (9.1) | 1.56 | 1.17 | 2.08 | 0.002 |
| Oral candidiasis | 66 (4.5) | 1.14 | 0.74 | 1.74 | 0.550 |
| Jaundice | 55 (3.7) | 1.95 | 1.25 | 3.03 | 0.003 |
| Respiratory distress | 427 (29.0) | 1.22 | 0.97 | 1.54 | 0.092 |
| Pallor | 316 (21.4) | 1.20 | 0.99 | 1.45 | 0.066 |
| Dehydration | 292 (19.8) | 0.94 | 0.76 | 1.15 | 0.536 |
| Neck stiffness | 20 (1.4) | 1.00 | 0.57 | 1.77 | 0.994 |
| Positive blood culture | 177 (14.8) | 1.66 | 1.32 | 2.09 | < 0.001 |
| Plasmodium falciparum malaria | 716 (56.2) | 1.29 | 1.09 | 1.52 | 0.003 |
| Prostration | 489 (33.1) | 1.64 | 1.36 | 1.98 | < 0.001 |
BCS = Blantyre coma score; CI = confidence interval; OR = odds ratio; WAZ = weight-for-age z score.
Significance: P-value of the multivariate analysis of independent risk factors associated to hypoglycemia.
Number and proportion of children with hypoglycemia who had severe malnutrition (i.e., WAZ < −3).
Risk factors for death in children admitted with hypoglycemia.
Of the 1,267 children with hypoglycemia on admission and outcome results, 245 died, yielding an overall CFR of 19.3%. Independent risk factors for death in children admitted with hypoglycemia included anorexia and malnutrition with a small effect size (OR = < 2), prostration and edema with medium effect size (OR = 2–3), and unconsciousness, oral candidiasis-positive blood culture, and respiratory distress with large effect size (OR = > 3) (Table 3).
Table 3.
Multivariate analysis of independent risk factors associated to mortality in children with hypoglycemia on admission
| Risk factors for adverse outcome | Hypoglycemia deaths (N = 245), n/N (%) | Adjusted OR | 95% CI | P value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Newborn | 46/245 (18.8) | 0.99 | 0.39 | 2.49 | 0.977 |
| History of fever | 182/245 (74.3) | 0.52 | 0.23 | 1.15 | 0.106 |
| History of seizures | 53/245 (21.6) | 1.08 | 0.59 | 1.98 | 0.798 |
| Anorexia/refusing to feed | 117/224 (52.2) | 1.74 | 1.02 | 2.97 | 0.042 |
| Hypothermia on admission | 11/245 (4.5) | 0.58 | 0.04 | 9.03 | 0.695 |
| Unconsciousness (BCS < 5) | 106/245 (43.3) | 3.10 | 1.75 | 5.49 | < 0.001 |
| Prostration | 160/245 (65.3) | 2.34 | 1.30 | 4.23 | 0.005 |
| Oral candidiasis | 31/244 (12.7) | 4.80 | 1.80 | 12.8 | 0.001 |
| Edema | 45/245 (18.4) | 2.67 | 1.25 | 5.71 | 0.011 |
| Pallor | 72/245 (29.4) | 1.40 | 0.81 | 2.42 | 0.225 |
| Dehydration | 67/245 (27.4) | 1.06 | 0.59 | 1.89 | 0.846 |
| Jaundice | 17/245 (6.9) | 2.43 | 0.84 | 7.06 | 0.102 |
| Positive blood culture | 75/211 (35.6) | 3.24 | 1.85 | 5.66 | < 0.001 |
| Plasmodium falciparum malaria | 77/206 (837.4) | 0.62 | 0.37 | 1.04 | 0.069 |
| Malnutrition (per unit WAZ decrease) | 143/245 (58.4)* | 1.21 | 1.03 | 1.42 | 0.019 |
| Respiratory distress | 139/245 (56.7) | 2.03 | 1.25 | 3.30 | 0.004 |
BCS = Blantyre coma score; CI = confidence interval; OR = odds ratio; WAZ = weight-for-age Z score.
Significance: P-value of the multivariate analysis of independent risk factors associated to mortality in children with hypoglycemia.
Number and proportion of children who died with hypoglycemia and severe malnutrition (i.e., WAZ < −3).
Minimum community-based incidence rates.
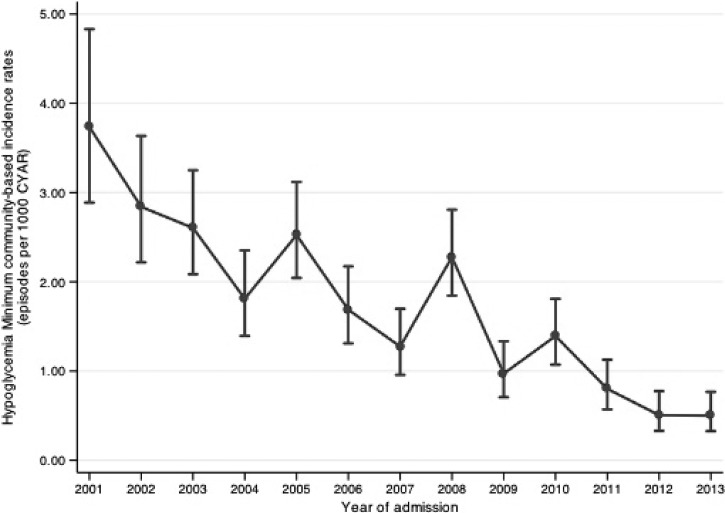
Overall MCBIR throughout the study period was 1.57 episodes/1,000 CYAR. MCBIR trends for hypoglycemia in all pediatric age groups during the 13-year-long study period are shown in Figure 4 . MCBIR peaked at 3.73/1,000 CYAR in 2001 (first year of the study) and significantly decreased subsequently, reaching the nadir in 2013 (0.50/1,000 CYAR; P < 0.001). MCBIR were significantly higher in newborns (9.47 episodes/1,000 CYAR) than in any other age group (P < 0.001; Table 4).
Figure 4.
Minimum community-based incidence rates (MCBIRs) of hypoglycemia according to year (vertical bars indicate 95% confidence intervals [CI]).
Table 4.
MCBIRs of hypoglycemia among children admitted to Manhiça District Hospital, according to age group
| Age groups | Subjects | Episodes | Time at risk (CYAR) | Rate estimations | Model estimations | |||
|---|---|---|---|---|---|---|---|---|
| Incidence rate (episodes per 1,000 CYAR) | 95% CI | IRR | 95% CI | P value | ||||
| Newborns | 38,442 | 28 | 2,957.53 | 9.47 | (6.54, 13.71) | 1 | – | < 0.0001 |
| 28 days to < 1year | 42,682 | 117 | 33,651.90 | 3.48 | (2.90, 4.17) | 0.37 | (0.24, 0.55) | |
| 1 to < 2 years | 41,429 | 170 | 34,865.01 | 4.88 | (4.20, 5.67) | 0.51 | (0.34, 0.76) | |
| 2 to < 3 years | 39,774 | 139 | 33,825.08 | 4.11 | (3.48, 4.85) | 0.43 | (0.29, 0.65) | |
| 3 to < 4 years | 38,418 | 113 | 33,157.82 | 3.41 | (2.83, 4.10) | 0.35 | (0.23, 0.53) | |
| 4 to < 5 years | 37,461 | 55 | 32,422.22 | 1.70 | (1.30, 2.21) | 0.17 | (0.11, 0.27) | |
| 5 to < 15 years | 63,103 | 82 | 276,806.07 | 0.30 | (0.24, 0.37) | 0.03 | (0.02, 0.05) | |
| Total | 90,472 | 704 | 447,685.62 | 1.57 | (1.46, 1.69) | – | – | – |
CYAR = child years at risk; CI = confidence interval; IRR = incidence rate ratio; MCBIRs = minimum community-based incidence rates.
P value from negative binomial regression model with random effects using likelihood ratio test.
Discussion
The MSS ongoing at MDH, in rural Mozambique, has allowed us to retrospectively review the prevalence of hypoglycemia in nearly 50,000 pediatric admissions, spanning across a 13-year-long period. This is perhaps the largest series examined in the developing world for the occurrence of this common (3.2% of children in this series) albeit insufficiently highlighted life-threatening complication. A study performed in an urban referral center in Mozambique two decades ago28 showed a higher prevalence (7.1%), similar to the studies conducted in a rural Kenyan hospital7 and in a Nigerian pediatric emergency ward.29 Other more recent studies in a high-malaria-endemic area in Mali30 or among febrile children in Tanzania12 have shown hypoglycemia prevalence similar to this study. More important than its frequency, its associated mortality risk needs to be overemphasized. Indeed, in this series, a fifth of all hypoglycemia cases on admission ended up in death. Although it remains to be seen how much did hypoglycemia contribute to each individual outcome, it is clear, and has robustly been shown, that hypoglycemia carries an excessive and unacceptable risk of death7,12,24,28,29 even if detected only as a single episode.31,32 The CFR associated with hypoglycemia was lower in this cohort (19.3%) than in other studies.12,29 This possibly reflects variations in case management and access to health care, and also relates to the fact that no clear consensus has yet been reached regarding the glycemia cutoff level at which to define hypoglycemia. Indeed, different authors have proposed different hypoglycemia thresholds, regardless of the presence or absence of malaria in the area.7,8,12,29 To allow comparisons, simplify guidelines, and homogenize management, the scientific community should align in defining a unique value. Irrespective of the threshold used, mortality dramatically increases with glycemia lower than 3 mmol/L, with a linear and steep inverse relationship between mortality and glycemia levels below this value. In our series, however, borderline values still carried a worse prognosis, with risk of death doubling in children with admission glycemia between 3 and 4 mmol/L when compared with those with higher levels.
Of all hypoglycemia episodes, 15.9% affected newborns, and 8.8% of all admitted newborns had hypoglycemia, highlighting the importance of hypoglycemia in this age group. However, hypoglycemia also commonly affected—and killed—children of all ages, which justifies universal screening for hypoglycemia among all admitted children in the developing world.
Most studies describing the incidence of hypoglycemia and associated outcomes are based on the determination of glycemia at a fixed point, usually admission. Our results are also limited by this lack of follow-up. We probably missed children who were normoglycemic on admission, but developed hypoglycemia subsequently. So we may be underestimating the incidence of hypoglycemia and the OR of death associated with hypoglycemia occurring during hospitalization. Similarly, we were unable to determine how many recurrent hypoglycemia episodes occurred in our series. Few studies in developing countries have monitored glycemia throughout the whole hospitalization. This is now possible through the innovative use of continuous glucose monitoring (CGM). CGM can be performed through a subcutaneous sensor that measures the interstitial glucose level—closely related to the blood glucose level—every 5 minutes, uninterruptedly 24 hours a day, and for as long as a week. CGM is slowly being introduced in pediatric intensive care units of developed countries,33,34 but has not reached yet in the developing world, possibly due to its high cost.
Our large sample size gave us sufficient power to assess the association of different clinical and laboratorial characteristics with the presence of hypoglycemia on admission or with hypoglycemia-related mortality. Although many of such factors have been previously described in the literature, three major groups of factors stand out as significantly associated in our series with the risk of having hypoglycemia: 1) not being able to feed35 (as directly reported by the mother or as a consequence of an altered clinical condition decreasing the capacity to feed [altered consciousness or coma,25,36–38 prostration, a history of seizures]); 2) malnutrition (including edema as a common sign typically associated with this condition); and 3) concomitant infections such as invasive bacterial disease or P. falciparum malaria. In addition, jaundice was also identified as an important risk factor for hypoglycemia, a finding also previously reported in Tanzania.12
Fasting is a recognized risk factor causing hypoglycemia in children.35 During fasting, plasma glucose levels are maintained within narrow limits by a delicate balance between endogenous glucose production deriving from glycogenolysis and gluconeogenesis.39 Studies performed in children with malaria have demonstrated a relationship between fasting, hypoglycemia, severity of disease, and mortality.7,40 Conditions that decrease consciousness may also result in a prolonged fasting state, explaining the strong association found between consciousness level and the risk of hypoglycemia. However, as hypoglycemia per se is a cause of decreased consciousness, interpreting the direction of the association is not straightforward. Hypoglycemia should, however, always be investigated in the presence of a child unable to feed or unconscious.
The association between malnutrition and hypoglycemia has also been firmly established in the past.11,13,41 In malnourished patients, glucose homeostasis41 can be compromised in several ways, including a lack of exogenous nutritional intake, decreased absorption of disaccharides because of intestinal villous atrophy, increased oxidative stress, or glucose uptake compromised by intestinal bacteria. In our series, 62% of all hypoglycemic children were malnourished, with 19.4% being severely malnourished. Kenyan7 and Tanzanian12 studies had already shown a strong association between severe malnutrition (WAZ < −3) and hypoglycemia, but not with WAZ < −1.
The association of hypoglycemia with bacterial sepsis14,18,25,26,38 or malaria7,14,26 is also well documented. Our series also support these associations, as risk of hypoglycemia was increased by 68% among bacteremic patients and by 30% in malaria-infected patients. In bacterial disease, hypoglycemia has been attributed to a series of factors, including high circulating levels of cytokines such as tumor necrosis factor and interleukin-6, both powerful stimulators of insulin secretion, which can then cause among other things inhibition of the gluconeogenic pathways.36,42 Decreased levels of glycemia secondary to the consumption of glucose by the Plasmodium parasite, hyperinsulinism caused by quinine, impaired gluconeogenesis, and lack of adequate supplementation/oral intake are possible explanations in malaria.37,38,40,43
Independent risk factors associated with hypoglycemia mortality are similar to those found associated with the risk of hypoglycemia. Again, factors related to feeding difficulties or the presence of a clinical condition hindering feeding (unconsciousness, prostration, respiratory distress), malnutrition, and concomitant severe infections (in this case only bacteremia) were all significantly and independently associated with a higher odds of death among patients with hypoglycemia. In these patients, the multivariate analysis also identified edema and oral candidiasis as important prognostic factors. The association between mortality and these factors has not been properly described in the literature, but it is likely that edema is associated with kwashiorkor (protein-deficient malnutrition) and oral candidiasis is a proxy of HIV coinfection, highly endemic in the Manhiça area22 and highly prevalent among malnourished patients.13
Regarding hyperglycemia, we found a prevalence of 1.7%, and a significantly higher associated CFR (12.1%, P < 0.001) when compared with normoglycemia. Other studies in similar settings have shown similar7 or higher8,44 prevalence rates. Hyperglycemia is an insufficiently well-known risk factor for death10 in the developing world, and efforts for its early detection and correction should parallel those devoted to hypoglycemia. Glucose variability has recently emerged as a new concept and is considered to have important influence on the outcome of critically ill patients45,46 and possibly cause more harm than sustained hyperglycemia. More studies are required, using CGM, to specifically address this issue.
We report for the first time MCBIRs for hypoglycemia in sub-Saharan Africa. Overall MCBIR throughout the study period were 1.57 episodes/1,000 CYAR, peaking at 3.73 episodes/1,000 CYAR in 2001, underscoring the high burden of this particularly dangerous complication. Reasons for the decreasing trends observed throughout the study period still need to be clarified, although we could hypothesize a better and earlier access to medical care or lower incidence of malaria and/or malnutrition in the last years in the study area. Newborns showed the highest incidence rates of hypoglycemia, underscoring the need to carefully follow this complication in this particularly vulnerable age group.
Hypoglycemia is a silent and under-recognized killer of African children and needs to be properly exposed because the correction of hypoglycemia is simple and has rapid effects on the health of the child. However, in resource-constrained settings where dextrose infusion is not readily available or is operationally challenging, other alternatives to intravenous administration should be investigated and promoted to correct hypoglycemia in children unable to feed. In this respect, sublingual sugar or preprepared dextrose gel appear as promising treatments for the prevention and correction of hypoglycemia in children with hypoglycemia.30
Our study had several limitations. There are concerns with the accuracy of commercial finger-prick blood glucose assays. Advances in glucose meter technology have resulted in significant improvement of accuracy and precision of meters. However, those meters are not available in developing countries due to higher cost. Another limitation is the use of the glucose meters instead of a laboratory serum or plasma glucose concentration to measure glycemia. It is known there are physical differences between the glucose concentration in serum or plasma and the glucose from capillary blood, but formal laboratory determination was not available in our settings. Unfortunately, study subject only had one determination of glycemia measured on admission, and we were unable to know if any recurrent hypoglycemia episodes occurred in our study. Because there is no universally applicable definition of hypoglycemia, we used the threshold established by national guidelines. Different glycemia cutoff levels could modify the results.
Conclusion
Hypoglycemia is a common complication of many conditions causing hospitalization in Mozambican children and is associated with unacceptable adverse outcomes. In settings similar to Manhiça, all admitted children should be screened for hypoglycemia and aggressively managed when found to be hypoglycemic. A single determination on admission is not enough, and glycemia should be recurrently screened during hospitalization. Better, cheaper, and more innovative diagnostic and therapeutic alternatives need to be urgently investigated to better address the consequences of hypoglycemia in developing countries.
ACKNOWLEDGMENTS
We are indebted to the children and mothers who participated in the study. The work of clinical officers, field workers, data managers, laboratory workers, and laboratory coordinator was important for the successful completion of the study. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Financial support: The CISM receives financial support from the Spanish Agency for International Cooperation (AECI). Quique Bassat has a fellowship from the program Miguel Servet of the ISCIII (Plan Nacional de I+D+I 2008-2011, grant no.: CP11/00269). Lola Madrid has a fellowship from the program Rio Hortega of the ISCIII (grant no.: CP13/00260).
Authors' addresses: Lola Madrid, Sozinho Acacio, Tacilta Nhampossa, Antonio Sitoe, Sónia Amós Maculuve, Helio Mucavele, and Betuel Sigaúque, Centro de Investigação em Saúde de Manhiça (CISM), Maputo, Mozambique, E-mails: lola.madrid@isglobal.org, sozinho.acacio@manhica.net, tacilta.nhampossa@manhica.net, antonio.sitoe@manhica.net, sonia.maculuve@manhica.net, helio.mucavele@manhica.net, and betuel.sigauque@manhica.net. Miguel Lanaspa, Llorenç Quintó, and Quique Bassat, Barcelona Institute for Global Health, Barcelona, Spain, E-mails: miguel.lanaspa@isglobal.org, lquinto@clinic.ub.es, and quique.bassat@isglobal.org.
References
- 1.Hirshberg E, Larsen G, Van Duker H. Alterations in glucose homeostasis in the pediatric intensive care unit: hyperglycemia and glucose variability are associated with increased mortality and morbidity. Pediatr Crit Care Med. 2008;9:361–366. doi: 10.1097/PCC.0b013e318172d401. [DOI] [PubMed] [Google Scholar]
- 2.Faustino EV, Hirshberg EL, Bogue CW. Hypoglycemia in critically ill children. J Diabetes Sci Technol. 2012;6:48–57. doi: 10.1177/193229681200600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ognibene KL, Vawdrey DK, Biagas KV. The association of age, illness severity, and glycemic status in a pediatric intensive care unit. Pediatr Crit Care Med. 2011;12:e386–e390. doi: 10.1097/PCC.0b013e3182192c53. [DOI] [PubMed] [Google Scholar]
- 4.Zijlmans WC, van Kempen AA, Serlie MJ, Kager PA, Sauerwein HP. Adaptation of glucose metabolism to fasting in young children with infectious diseases: a perspective. J Pediatr Endocrinol Metab. 2013;27:5–13. doi: 10.1515/jpem-2013-0165. [DOI] [PubMed] [Google Scholar]
- 5.Kraft R, Herndon DN, Mlcak RP, Finnerty CC, Cox RA, Williams FN, Jeschke MG. Bacterial respiratory tract infections are promoted by systemic hyperglycemia after severe burn injury in pediatric patients. Burns. 2014;40:428–435. doi: 10.1016/j.burns.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirata Y, Tomioka H, Sekiya R, Yamashita S, Kaneda T, Kida Y, Nishio C, Kaneko M, Fujii H, Nakamura T. Association of hyperglycemia on admission and during hospitalization with mortality in diabetic patients admitted for pneumonia. Intern Med. 2013;52:2431–2438. doi: 10.2169/internalmedicine.52.9594. [DOI] [PubMed] [Google Scholar]
- 7.Osier FH, Berkley JA, Ross A, Sanderson F, Mohammed S, Newton CR. Abnormal blood glucose concentrations on admission to a rural Kenyan district hospital: prevalence and outcome. Arch Dis Child. 2003;88:621–625. doi: 10.1136/adc.88.7.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sambany E, Pussard E, Rajaonarivo C, Raobijaona H, Barennes H. Childhood dysglycemia: prevalence and outcome in a referral hospital. PLoS One. 2013;8:e65193. doi: 10.1371/journal.pone.0065193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr. 2005;146:30–34. doi: 10.1016/j.jpeds.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 10.Bhutia TD, Lodha R, Kabra SK. Abnormalities in glucose homeostasis in critically ill children. Pediatr Crit Care Med. 2013;14:e16–e25. doi: 10.1097/PCC.0b013e3182604998. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Pocket Book for Hospital Care of Children: Guidelines for the Management of Common Illness with Limited Resources. 2nd edition. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 12.Nadjm B, Mtove G, Amos B, Hildenwall H, Najjuka A, Mtei F, Todd J, Reyburn H. Blood glucose as a predictor of mortality in children admitted to the hospital with febrile illness in Tanzania. Am J Trop Med Hyg. 2013;89:232–237. doi: 10.4269/ajtmh.13-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nhampossa T, Sigauque B, Machevo S, Macete E, Alonso P, Bassat Q, Menendez C, Fumado V. Severe malnutrition among children under the age of 5 years admitted to a rural district hospital in southern Mozambique. Public Health Nutr. 2013;16:1565–1574. doi: 10.1017/S1368980013001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houin S, Rozance PJ. 50 years ago in the Journal of Pediatrics: the incidence of neonatal hypoglycemia in a nursery for premature infants. J Pediatr. 2014;164:1485. doi: 10.1016/j.jpeds.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris DL, Weston PJ, Signal M, Chase JG, Harding JE. Dextrose gel for neonatal hypoglycaemia (the Sugar Babies Study): a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382:2077–2083. doi: 10.1016/S0140-6736(13)61645-1. [DOI] [PubMed] [Google Scholar]
- 16.Pisarchik AN, Pochepen ON, Pisarchyk LA. Increasing blood glucose variability is a precursor of sepsis and mortality in burned patients. PLoS One. 2012;7:e46582. doi: 10.1371/journal.pone.0046582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badawi O, Waite MD, Fuhrman SA, Zuckerman IH. Association between intensive care unit-acquired dysglycemia and in-hospital mortality. Crit Care Med. 2012;40:3180–3188. doi: 10.1097/CCM.0b013e3182656ae5. [DOI] [PubMed] [Google Scholar]
- 18.Sigauque B, Roca A, Mandomando I, Morais L, Quinto L, Sacarlal J, Macete E, Nhamposa T, Machevo S, Aide P, Bassat Q, Bardaji A, Nhalungo D, Soriano-Gabarro M, Flannery B, Menendez C, Levine MM, Alonso PL. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J. 2009;28:108–113. doi: 10.1097/INF.0b013e318187a87d. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda T, Takahashi T, Sato A, Tanaka H, Igarashi S, Fujita N, Kuwabara T, Kanazawa M, Nishizawa M, Shimohata T. Predictors of outcome in hypoglycemic encephalopathy. Diabetes Res Clin Pract. 2013;101:159–163. doi: 10.1016/j.diabres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Fong CY, Harvey AS. Variable outcome for epilepsy after neonatal hypoglycaemia. Dev Med Child Neurol. 2014;56:1093–1099. doi: 10.1111/dmcn.12496. [DOI] [PubMed] [Google Scholar]
- 21.Sacarlal J, Nhacolo AQ, Sigauque B, Nhalungo DA, Abacassamo F, Sacoor CN, Aide P, Machevo S, Nhampossa T, Macete EV, Bassat Q, David C, Bardaji A, Letang E, Saute F, Aponte JJ, Thompson R, Alonso PL. A 10 year study of the cause of death in children under 15 years in Manhica, Mozambique. BMC Public Health. 2009;9:67. doi: 10.1186/1471-2458-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez R, Munguambe K, Aponte J, Bavo C, Nhalungo D, Macete E, Alonso P, Menendez C, Naniche D. High HIV prevalence in a southern semi-rural area of Mozambique: a community-based survey. HIV Med. 2012;13:581–588. doi: 10.1111/j.1468-1293.2012.01018.x. [DOI] [PubMed] [Google Scholar]
- 23.Sacoor C, Nhacolo A, Nhalungo D, Aponte JJ, Bassat Q, Augusto O, Mandomando I, Sacarlal J, Lauchande N, Sigauque B, Alonso P, Macete E, Munguambe K, Guinovart C, Aide P, Menendez C, Acacio S, Quelhas D, Sevene E, Nhampossa T. Profile: Manhica Health Research Centre (Manhica HDSS) Int J Epidemiol. 2013;42:1309–1318. doi: 10.1093/ije/dyt148. [DOI] [PubMed] [Google Scholar]
- 24.Bassat Q, Guinovart C, Sigauque B, Aide P, Sacarlal J, Nhampossa T, Bardaji A, Nhacolo A, Macete E, Mandomando I, Aponte JJ, Menendez C, Alonso PL. Malaria in rural Mozambique. Part II: children admitted to hospital. Malar J. 2008;7:37. doi: 10.1186/1475-2875-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassat Q, Guinovart C, Sigauque B, Mandomando I, Aide P, Sacarlal J, Nhampossa T, Bardaji A, Morais L, Machevo S, Letang E, Macete E, Aponte JJ, Roca A, Menendez C, Alonso PL. Severe malaria and concomitant bacteraemia in children admitted to a rural Mozambican hospital. Trop Med Int Health. 2009;14:1011–1019. doi: 10.1111/j.1365-3156.2009.02326.x. [DOI] [PubMed] [Google Scholar]
- 26.Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. MMWR Recomm Rep. 2010;59:1–15. [PubMed] [Google Scholar]
- 27.Sullivan GM, Feinn R. Using effect size—or why the P value is not enough. J Grad Med Educ. 2012;4:279–282. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon T, Felix JM, Samuel M, Dengo GA, Saldanha RA, Schapira A, Phillips RE. Hypoglycaemia in paediatric admissions in Mozambique. Lancet. 1994;343:149–150. doi: 10.1016/s0140-6736(94)90937-7. [DOI] [PubMed] [Google Scholar]
- 29.Elusiyan JB, Adejuyigbe EA, Adeodu OO. Hypoglycaemia in a Nigerian paediatric emergency ward. J Trop Pediatr. 2006;52:96–102. doi: 10.1093/tropej/fmi068. [DOI] [PubMed] [Google Scholar]
- 30.Graz B, Dicko M, Willcox ML, Lambert B, Falquet J, Forster M, Giani S, Diakite C, Dembele EM, Diallo D, Barennes H. Sublingual sugar for hypoglycaemia in children with severe malaria: a pilot clinical study. Malar J. 2008;7:242. doi: 10.1186/1475-2875-7-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krinsley JS. Glycemic control in the critically ill—3 domains and diabetic status means one size does not fit all! Crit Care. 2013;17:131. doi: 10.1186/cc12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rattarasarn C. Hypoglycemia in sepsis: risk factors and clinical characteristics. J Med Assoc Thai. 1997;80:760–766. [PubMed] [Google Scholar]
- 33.Branco RG, Chavan A, Tasker RC. Pilot evaluation of continuous subcutaneous glucose monitoring in children with multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2009;11:415–419. doi: 10.1097/PCC.0b013e3181c59144. [DOI] [PubMed] [Google Scholar]
- 34.Bridges BC, Preissig CM, Maher KO, Rigby MR. Continuous glucose monitors prove highly accurate in critically ill children. Crit Care. 2010;14:R176. doi: 10.1186/cc9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thien HV, Kager PA, Sauerwein HP. Hypoglycemia in falciparum malaria: is fasting an unrecognized and insufficiently emphasized risk factor? Trends Parasitol. 2006;22:410–415. doi: 10.1016/j.pt.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Yelich MR, Filkins JP. Mechanism of hyperinsulinemia in endotoxicosis. Am J Physiol. 1980;239:E156–E161. doi: 10.1152/ajpendo.1980.239.2.E156. [DOI] [PubMed] [Google Scholar]
- 37.White NJ, Miller KD, Marsh K, Berry CD, Turner RC, Williamson DH, Brown J. Hypoglycaemia in African children with severe malaria. Lancet. 1987;1:708–711. doi: 10.1016/s0140-6736(87)90354-0. [DOI] [PubMed] [Google Scholar]
- 38.White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, Williamson DH, Turner RC. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309:61–66. doi: 10.1056/NEJM198307143090201. [DOI] [PubMed] [Google Scholar]
- 39.Robinson PJ, Rapoport SI. Glucose transport and metabolism in the brain. Am J Physiol. 1986;250:R127–R136. doi: 10.1152/ajpregu.1986.250.1.R127. [DOI] [PubMed] [Google Scholar]
- 40.Kawo NG, Msengi AE, Swai AB, Chuwa LM, Alberti KG, McLarty DG, Orskov H. Hypoglycaemia and cerebral malaria. Lancet. 1990;336:1128–1129. doi: 10.1016/0140-6736(90)92602-e. [DOI] [PubMed] [Google Scholar]
- 41.Bandsma RH, Mendel M, Spoelstra MN, Reijngoud DJ, Boer T, Stellaard F, Brabin B, Schellekens R, Senga E, Heikens GT. Mechanisms behind decreased endogenous glucose production in malnourished children. Pediatr Res. 2010;68:423–428. doi: 10.1203/PDR.0b013e3181f2b959. [DOI] [PubMed] [Google Scholar]
- 42.Romijn JA, Godfried MH, Wortel C, Sauerwein HP. Hypoglycemia, hormones and cytokines in fatal meningococcal septicemia. J Endocrinol Invest. 1990;13:743–747. doi: 10.1007/BF03349613. [DOI] [PubMed] [Google Scholar]
- 43.Dekker E, Hellerstein MK, Romijn JA, Neese RA, Peshu N, Endert E, Marsh K, Sauerwein HP. Glucose homeostasis in children with falciparum malaria: precursor supply limits gluconeogenesis and glucose production. J Clin Endocrinol Metab. 1997;82:2514–2521. doi: 10.1210/jcem.82.8.4131. [DOI] [PubMed] [Google Scholar]
- 44.Willcox ML, Forster M, Dicko MI, Graz B, Mayon-White R, Barennes H. Blood glucose and prognosis in children with presumed severe malaria: is there a threshold for ‘hypoglycaemia’? Trop Med Int Health. 2009;15:232–240. doi: 10.1111/j.1365-3156.2009.02444.x. [DOI] [PubMed] [Google Scholar]
- 45.Rake AJ, Srinivasan V, Nadkarni V, Kaptan R, Newth CJ. Glucose variability and survival in critically ill children: allostasis or harm? Pediatr Crit Care Med. 2010;11:707–712. doi: 10.1097/PCC.0b013e3181e88b1f. [DOI] [PubMed] [Google Scholar]
- 46.Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118:173–179. doi: 10.1542/peds.2005-1819. [DOI] [PubMed] [Google Scholar]