Abstract
Information on the infectious causes of undifferentiated acute febrile illness (AFI) in Georgia is essential for effective treatment and prevention. In May 2008, a hospital-based AFI surveillance was initiated at six hospitals in Georgia. Patients aged ≥ 4 years with fever ≥ 38°C for ≥ 48 hours were eligible for surveillance. Blood culture and serologic testing were conducted for Leptospira spp., Brucella spp., West Nile virus (WNV), Crimean–Congo hemorrhagic fever virus, Coxiella burnetii, tick-borne encephalitis virus (TBEV), hantavirus, Salmonella enterica serovar Typhi (S. Typhi), and Rickettsia typhi. Of 537 subjects enrolled, 70% were outpatients, 54% were males, and the mean age was 37 years. Patients reported having fatigue (89%), rigors (87%), sweating (83%), pain in joints (49%), and sleep disturbances (42%). Thirty-nine (7%) patients were seropositive for R. typhi, 37 (7%) for Brucella spp., 36 (7%) for TBEV, 12 (2%) for Leptospira spp., 10 (2%) for C. burnetii, and three (0.6%) for S. Typhi. None of the febrile patients tested positive for WNV antibodies. Of the patients, 73% were negative for all pathogens. Our results indicate that most of the targeted pathogens are present in Georgia, and highlight the importance of enhancing laboratory capacity for these infectious diseases.
Introduction
A wide spectrum of infectious agents causes febrile illness syndromes, and the relative burden of any particular etiology may vary by geographic region and time of year.1 An understanding of the relevant causes of fever could improve clinical decision making and inform public health programming. However, determining the infectious etiologies of febrile illnesses requires advanced laboratory facilities and trained laboratory staff because many pathogens cause similar clinical presentations. Since gaining independence in the early 1990s, the health-care and public health sectors of the former Soviet Republic of Georgia have suffered because of the socioeconomic collapse that resulted from civil war and the rapid transition to a free-market economy.2 As a consequence, only limited information has been published on the infectious etiologies of acute febrile illnesses (AFI) in the country.3–5
A retrospective review of 52 cases of fever of unknown origin presenting to a single institute in Georgia showed that the most common diagnoses included sepsis, tuberculosis (TB), pneumonia, and pyelonephritis.6 However, the authors reported neither the laboratory diagnostic methodology nor the associated etiologies. At the time of surveillance initiation, TB and brucellosis were thought to be the common causes of febrile illnesses in Georgia with an estimated rate of 98 TB cases per 100,000 population (ranked fifth highest in the European region) and 2.8 brucellosis cases per 100,000 population.7,8 Sporadic outbreaks of typhoid fever were reported in Georgia in 1999 (77 cases), 2001 (59 cases), and 2002 (12 cases), presumably due to contaminated drinking water.9 According to the National Center for Disease Control and Public Health of Georgia, the estimated incidence rate of leptospirosis was 0.3 cases per 100,000 population in 2008.10 This estimate was based on a combination of clinical manifestations and enzyme-linked immunosorbent assay (ELISA) results.10,11
Herein, we describe hospital-based sentinel surveillance for infectious etiologies of AFI cases that sought care at selected hospitals in Georgia. This surveillance is an initial step toward an accurate assessment of the background rate of occurrence of these infections. Further, this project provides substantial epidemiologic data useful for the design and implementation of future studies focused on particular pathogens or more comprehensive assessments.
Materials and Methods
Study population.
In 2008, a hospital-based surveillance project for AFI was implemented at six hospitals throughout Georgia to determine the frequency of nine infectious causative agents of febrile illness. Of the selected hospitals, three are major infectious disease referral centers in the capital city, Tbilisi; two are multi-profile hospitals located in the second largest city, Kutaisi; and one is in a rural area in Sachkhere. The study protocol was approved by institutional review boards at U.S. Naval Medical Research Unit No. 3 (NAMRU-3), U.S. Army Medical Research Institute of Infectious Diseases, Walter Reed Army Institute of Research, and the National Center for Disease Control and Public Health. Patients aged ≥ 4 years with fever ≥ 38°C for ≥ 48 hours and without a diagnosis were considered eligible for surveillance. Enrolling physicians were asked to exclude cases with focal infections (e.g., urinary tract infection, cellulites, and abscess) suggestive of common bacterial or viral causes. Human immunodeficiency virus (HIV)–positive cases as well as patients suspected for noninfectious causes of fever (e.g., rheumatic diseases, neoplasms) were excluded as well. As of 2012, the estimated adult population HIV prevalence was 0.3% in Georgia. Since HIV testing was not supported by this study, HIV status was defined based on patient reports.12,13 Each eligible patient who signed an informed consent or assent form was enrolled in the study. Epidemiologic information was collected through a standardized questionnaire and blood and serum samples were obtained for laboratory determination of infectious agents. In addition to sample collection, biphasic blood culture bottles (bioMerieux, Lyon, France) and Ellinghausen-McCullough-Johnson-Harris (EMJH) media bottles (Becton, Dickinson and Company, Franklin Lakes, NJ) were inoculated on enrollment. Biphasic blood culture bottles were incubated at 37°C for 21 days with blind plating. Inoculated EMJH bottles were incubated at 30°C for 2 months with weekly follow-up using dark field microscopy to detect Leptospira. Patients were encouraged to return to hospital sites for the 2- to 6-week voluntary follow-up visit.
Laboratory analyses.
ELISA serology testing was conducted for antibodies against Leptospira (Panbio, Brisbane, Australia), Brucella (the in-house ELISA of the U.S. NAMRU-3 and U.S. Naval Medical Research Center [NMRC]), West Nile virus (WNV; Focus Diagnostic, Cypress, CA), Coxiella burnetii (Panbio), tick-borne encephalitis virus (TBEV; IBL International, Hamburg, Germany), Salmonella enterica serovar Typhi (S. Typhi; NAMRU-3/NMRC in-house ELISA), and Rickettsia typhi (Fuller Laboratory, Fullerton, CA). Positive Leptospira ELISA samples were confirmed by the microscopic agglutination test (MAT) and positive C. burnetii and WNV results were confirmed by an immunofluorescence assay (IFA; Focus Diagnostics). Commercial ELISA and IFA results were interpreted in accordance with the manufacturers' inserts. For the Leptospira MAT, a single titer of ≥ 400 was defined as positive. For in-house Brucella and S. Typhi ELISAs, a single titer cutoff was set at ≥ 320.12,14,15 Cases were also tested for the presence of antibodies against Crimean–Congo hemorrhagic fever (CCHF) virus and hantavirus antibodies; those results are published separately.16,17
Statistical analyses.
Data were entered into an Epi Info database (CDC, Atlanta, GA). Double data entry was performed for quality control. Epi Info version 3.5.3 and SPSS version 19 (IBM, Chicago, IL) were used for data analysis. Odds ratios (ORs) along with 95% confidence intervals (95% CI) were calculated to estimate associations between risk factors and study outcomes by univariate logistic regression analysis. Adjusted OR (AOR) were calculated using multiple logistic regression analysis after controlling for age in years, gender, and the year of enrollment.
Results
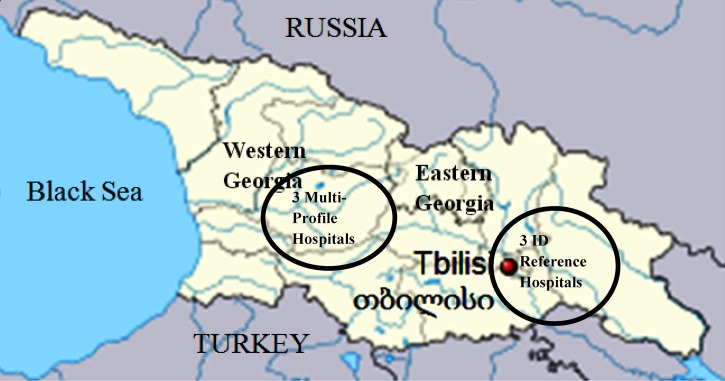
From 2008 to 2011, a total of 537 patients were enrolled. Of these, 54% were males and 89% were aged ≥ 15 years. The mean age (±standard deviation) of the participants was 37 (±18) years. Half of the participants were from Tbilisi, the capital city located in eastern Georgia, and 22% were from western Georgia (Figure 1 ). Of the patients, 89% were enrolled at either the V. Bochorishvili Sepsis Center and Infectious Diseases (64%) or AIDS Clinical Immunology Research Center (25%), both of which are in Tbilisi. The remaining 11% of patients were enrolled from the four other hospitals participating in the surveillance. Office workers, unemployed individuals, school-aged children (14%), and housewives comprised the majority of patients (Table 1).
Figure 1.
Map of Georgia.
Table 1.
Demographic characteristics of 537 febrile patients, febrile illness surveillance study, 2008–2011
| Characteristic | N (%) |
|---|---|
| Age (in years) | |
| 4–14 | 57 (11) |
| ≥ 15 | 474 (89) |
| Mean age (SD): 37 (±18) | |
| Median: 35 | |
| Gender | |
| Male | 292 (54) |
| Female | 245 (46) |
| Occupation | |
| Office workers | 117 (22) |
| Unemployed | 128 (24) |
| Pupils | 77 (14) |
| Housewife | 65 (12) |
| Pensioners | 54 (10) |
| Students | 28 (5) |
| Other | 68 (13) |
| Regional distribution | |
| Tbilisi | 266 (50) |
| Imereti | 57 (11) |
| Kvemo Kartli | 51 (9.5) |
| Shida Karti | 39 (7) |
| Kakheti | 35 (6.5) |
| Samegrelo-Zemo Svaneti | 24 (4.5) |
| Other | 65 (12) |
Denominators may vary because of missing data; the amount of missing data was within the range of ≤ 1%.
Up to 50% of febrile patients presented to the study sites > 20 days beyond disease onset, 70% were on antibiotic therapy before enrollment and 25% were on self-treatment with antibiotics. Fatigue, shaking, excessive sweating, joint pain, and muscle soreness were the most frequently reported complaints among febrile patients, whereas pallor, hepatomegaly, splenomegaly, pharyngeal injection, and rash were often observed during physical examination (Table 2).
Table 2.
Patient complaints and physical examination findings on enrollment for 537 patients, febrile illness surveillance study, 2008–2011
| Patient complaints | N (%) | Physical examination findings | N (%) |
|---|---|---|---|
| Fever | 537 (100) | Pallor | 203 (38) |
| Fatigue | 482 (89) | Hepatomegaly | 145 (27) |
| Shaking/rigors | 467 (87) | Splenomegaly | 93 (17) |
| Excessive sweating | 447 (83) | Pharyngeal injection | 68 (13) |
| Pain in joints | 262 (49) | Rash | 72 (13) |
| Headache | 253 (47) | Lymphadenopathy | 55 (10) |
| Sleep disturbances | 225 (42) | Heart murmur | 36 (7) |
| Muscle soreness | 218 (41) | Abdominal distention | 35 (7) |
| Cough | 187 (35) | Conjunctival injection | 31 (6) |
| Depressed mood | 175 (33) | Icterus | 28 (5) |
| Shortness of breath | 154 (29) | Jaundice | 19 (4) |
| Sore throat | 129 (24) | Abdominal tenderness to palpation | 22 (4) |
| Nausea/vomiting | 110 (21) | Respiratory crackles | 19 (4) |
| Rash | 72 (13) | Skin lesions | 14 (3) |
| Abdominal pain | 66 (12) | Neurological findings | 9 (2) |
| Pain behind the eyes | 52 (10) | Neck stiffness | 8 (2) |
| Diarrhea | 51 (10) | Edema | 12 (2) |
| Stiff neck | 34 (6) | Joint effusions | 12 (2) |
| Unusual bleeding | 16 (3) | Bleeding | 9 (2) |
| Mental status changes | 6 (1) |
Denominators may vary because of missing data; the amount of missing data was within the range of ≤ 1%.
The majority of febrile patients were treated as outpatients (70%), and only 14% of patients returned for the follow-up visit. No positive blood culture case was detected. On the basis of serologic analyses, 73% of febrile patients were negative for all pathogens of interest. Immunologic response was detected to Brucella spp. (7% of patients), TBEV (7%), R. typhi (7%), Leptospira spp. (2%), C. burnetii (2%), S. Typhi (0.6%), CCHF virus (0.6%), and hantavirus (0.4%) (Table 3).
Table 3.
Laboratory testing results, febrile illness surveillance study, 2008–2011
| Assay | Seropositivity, N (%) |
|---|---|
| Rickettsia typhi IgM ELISA | 39 (7.0) |
| Brucella spp. Ab ELISA | 37 (7.0) |
| TBEV IgM ELISA | 36 (7.0) |
| Leptospira spp. IgM ELISA and MAT | 12 (2.0) |
| Coxiella burnetii IgM ELISA and Phase II and I IgM/IgG IFA | 10 (2.0) |
| Salmonella enterica serovar Typhi Ab ELISA | 3 (0.6) |
| CCHFV IgM ELISA | 3 (0.6) |
| Hantavirus IgM ELISA, IgM/IgG IFA, and immunoblotting | 2 (0.4) |
| Unknown | 368 (72) |
Ab = antibody; CCHFV = Crimean–Congo hemorrhagic fever virus; ELISA = enzyme-linked immunosorbent assay; IFA = immunofluorescence assay; IgM = immunoglobulin M; MAT = microscopic agglutination test; TBEV = tick-borne encephalitis virus.
Brucellosis.
Thirty-seven patients tested positive for brucellosis antibodies. Of those, the majority (30, or 83%) were from eastern regions of Georgia. Male gender, contact with animal abortus material, engagement in agricultural activities, participation in animal slaughter, exposure to sheep or cattle, consumption of undercooked meat, and visiting forests were significantly associated with brucellosis infection, according to univariate analysis (Table 4). Our analyses revealed a negative association between brucellosis and having a water tap at home. The majority of these factors remained associated with brucellosis in the multiple logistic regression analysis with two exceptions—participating in animal slaughter and visiting forests (Table 4). Excessive sweating (87%), fatigue (84%), joint pain (60%), and hepatomegaly (38%) were the most frequently reported clinical symptoms and signs. No association was found between brucellosis seropositivity and the observation of particular signs and symptoms, with the exception of neck stiffness (OR = 3.2, 95% CI = 1.3–8.4, P = 0.016).
Table 4.
Disease determinants analysis for brucellosis seropositive patients, febrile illness surveillance study, 2008–2011
| Exposures | Univariate logistic regression OR (P value) | 95% CI | Multiple logistic regression* AOR (P value) | 95% CI |
|---|---|---|---|---|
| Contact with animal abortus materials in the month before getting sick | 7.7 (0.005) | 1.8–32.1 | 7.7 (0.007) | 1.7–32.4 |
| Agricultural activities in the month before getting sick | 3.6 (0.000) | 1.8–7.5 | 3.1 (0.003) | 1.5–6.5 |
| Involvement in animal slaughter in the month before getting sick | 3.1 (< 0.001) | 1.1–8.5 | 2.5 (0.080) | 0.9–7.2 |
| Cattle exposure | 2.6 (0.011) | 1.2–5.6 | 2.5 (0.020) | 1.2–5.3 |
| Sheep exposure | 8.5 (0.001) | 2.4–30.6 | 7.2 (0.003) | 1.9–26.5 |
| Goat exposure | 3.4 (0.274) | 0.4–31.6 | 2.9 (0.351) | 0.3–27.8 |
| Consumption of raw or unpasteurized milk products in the month before getting sick | 2.5 (0.160) | 0.7–8.9 | 2.3 (0.224) | 0.6–8.3 |
| Consumption of meat products that were red from inside in the month before getting sick | 5.2 (0.001) | 2.1–13.2 | 4.4 (0.002) | 1.7–11.6 |
| Rodents inside or around the household in the month before getting sick | 2.0 (0.085) | 0.9–4.2 | 2.0 (0.086) | 0.9–4.5 |
| Collection of berries or mushrooms or cutting wood in the forest in the month before getting sick | 3.2 (0.026) | 1.2–9.1 | 2.8 (0.061) | 1.0–8.1 |
| Insect bites in the month before getting sick | 1.6 (0.339) | 0.6–3.9 | 1.6 (0.356) | 0.6–4.2 |
| Contact with water from ponds and rivers in the month before getting sick | 2.0 (0.173) | 0.7–5.5 | 2.4 (0.120) | 0.8–7.0 |
| Having water tap at home | 0.4 (0.000) | 0.2–07 | 0.4 (0.003) | 0.2–0.7 |
| Gender† | 2.9 (< 0.001) | 1.3–6.0 | 2.9 (0.007) | 1.3–6.4 |
AOR = adjusted odds ratio; CI = confidence interval.
Multiple binary regression analysis was conducted after controlling for age, gender, and year of enrollment.
Multiple binary regression analysis was conducted after controlling for age and year of enrollment.
Tick-borne encephalitis virus.
Thirty-six patients tested positive for TBEV antibodies; the majority of them (29 [81%]) were from eastern regions of Georgia. Tick bite was the only risk factor positively associated with TBEV antibody seroprevalence (Table 5). Change in mental status was reported in one case. It was the only neurologic manifestation observed. Fatigue (92%), excessive sweating (86%), joint pain (58%), and headache (56%) were the frequently reported signs among those testing positive for TBEV antibodies. No statistically significant associations were found between TBE seropositivity and the occurrence of particular clinical signs and symptoms.
Table 5.
Univariate and multiple logistic regression analysis for TBE, rickettsiosis, Q-fever, leptospirosis, and typhoid fever seropositive patients, febrile illness surveillance study, 2008–2011
| Risk factors and clinical symptoms | Univariate logistic regression OR (P value) | 95% CI | Multiple logistic regression* AOR (P value) | 95% CI |
|---|---|---|---|---|
| TBE | ||||
| Contact with animals abortus material | 4.1 (0.085) | 0.8–20.5 | 4.1 (0.129) | 0.7–24.8 |
| Tick bite | 5.8 (0.039) | 1.1–31.2 | 6.6 (0.052) | 1.0–44.1 |
| Rickettsiosis | ||||
| Consumption of unpasteurized milk products | 6.3 (0.000) | 2.3–17.3 | 9.5 (0.000) | 3.0–29.4 |
| Consumption of undercooked meat | 2.9 (< 0.001) | 1.0–8.0 | 3.5 (< 0.001) | 1.2–2.4 |
| Sore throat | 2.1 (0.033) | 1.1–4.1 | 2.1 (0.032) | 1.1–4.4 |
| Dyspnea | 2.0 (0.037) | 1.0–3.9 | 2.2 (0.026) | 1.1–4.4 |
| Q-fever | ||||
| Nausea | 4.0 (0.030) | 1.1–14.1 | 3.6 (0.055) | 1.0–13.6 |
| Stiff neck | 3.8 (0.097) | 0.8–18.9 | 3.8 (0.109) | 0.7–19.3 |
| Neurologic findings | 18.6 (0.001) | 3.3–103.7 | 14.7 (0.004) | 2.4–89.9 |
| Jaundice | 7.5 (0.015) | 1.5–38.0 | 8.1 (0.018) | 1.4–45.5 |
| Leptospirosis | ||||
| Agricultural works | 2.9 (0.084) | 0.9–10.0 | 3.1 (0.091) | 0.8–11.8 |
| Visiting forests | 3.8 (0.094) | 0.8–18.4 | 3.2 (0.181) | 0.6–17.0 |
| Consumption of raw or unpasteurized milk products in the month before getting sick | 5.6 (0.033) | 1.1–27.5 | 3.3 (0.177) | 0.6–18.2 |
| Typhoid fever | ||||
| Consumption of undercooked meat | 9.0 (0.077) | 0.8–101.7 | 5.0 (0.225) | 0.4–66.6 |
| Rodents near home | 10.2 (0.059) | 1.0–113.6 | 5.5 (0.178) | 0.5–66.8 |
| Abdominal pain | 14.7 (0.029) | 1.3–164.3 | 10.4 (0.065) | 0.9–125.2 |
| Nausea | 7.9 (0.093) | 0.7–87.8 | 5.9 (0.168) | 0.5–73.0 |
| Splenomegaly | 9.7 (0.065) | 0.9–108.3 | 7.9 (0.104) | 0.7–94.4 |
| Heart murmur | 29.4 (0.006) | 2.6–332.6 | 21.0 (0.016) | 1.8–248.1 |
AOR = adjusted odds ratio; CI = confidence interval; TBE = tick-borne encephalitis.
Multiple binary regression analysis was conducted after controlling for age, gender, and year of enrollment.
Rickettsiosis.
Thirty-nine patients tested positive for R. typhi antibodies; the majority of them (31 [80%]) were from eastern regions of Georgia. According to univariate logistic analysis, consumption of unpasteurized milk products and undercooked meat were significantly associated with rickettsiosis and remained so after multiple regression analysis. Excessive sweating, shaking, fatigue, joint pain, and headache were the main symptoms and signs among rickettsiosis cases. Seropositive cases had increased odds of having sore throat and dyspnea on enrollment (Table 5).
Coxiella burnetii.
Only 10 patients had anti-C. burnetii antibodies; all of these patients were residents of Tbilisi. No statistically significant associations were found between C. burnetii seropositivity and particular risk factors. The majority of seropositive cases had a nonspecific clinical manifestation. According to a univariate analysis, seropositive cases were at increased odds of having nausea, neurologic findings, and jaundice. After multiple regression analysis, only neurologic findings and jaundice remained significantly associated with this outcome (Table 5).
Leptospirosis.
Because of insufficient sample volume, MAT was carried out with only 31% of study samples that tested positive or equivocal for leptospirosis by immunoglobulin M (IgM) ELISA. Only 12 patients tested positive for leptospirosis by MAT and half of them were from western Georgia. The following serogroups were found to be positive by MAT: Autumnalis (Leptospira interrogans serovar Autumnalis), Australis (L. interrogans serovar Bratislava), Bataviae (L. interrogans serovar Bataviae), Icterohemorrhagiae (L. interrogans serovar Mankarso), Hebdomadis (L. interrogans serovar Hebdomadis), Sejroe (L. interrogans serovar Wolfii), Sejroe (L. interrogans serovar Hardjo), and Pyrogenes (L. santarosai serovar Alexi).
Consumption of raw milk products was significantly associated with leptospirosis seropositivity, but only by univariate analysis (Table 5). Nonspecific symptoms and hepatomegaly (33%) were the most commonly reported symptoms and signs among those testing positive for leptospirosis. Statistical associations with leptospirosis were not found for any of the clinical variables.
Typhoid fever.
Only three cases of S. Typhi were found in this febrile population. Two of these cases did not have a centralized water supply at home. No statistically significant risk factors for typhoid fever were found as a result of univariate analysis; however, seropositive cases had greater odds of experiencing abdominal pain and a heart murmur (Table 5). Heart murmur still was associated with this outcome infection in multiple logistic analysis.
West Nile virus.
No WNV-seropositive cases were found.
Discussion
In this hospital-based surveillance, the majority of patients (up to 60%) were urban dwellers and were enrolled from eastern Georgia. Most of the patients were enrolled from the major tertiary care centers for infectious diseases in the capital city. In our study, western Georgian regions were represented to a lesser extent, but the urban-to-rural population ratio resembled the 2008 national population statistics.18 Nevertheless, the study design precluded estimation of the population prevalence of the studied pathogens. A variety of occupations were reported, but occupations linked to the agricultural works were reported in only a few cases (three cases [0.6%]). Engagement in animal husbandry or other agricultural works is perceived as informal employment in the country. Compared with national employment data, the employment rate in the agricultural sector was very low, which can be explained by the underreporting of informal jobs in Georgia.19,20
Nonspecific symptoms predominated in this study because of the inclusion and exclusion criteria. On the basis of the average time reported between disease onset and enrollment, one might speculate that either enrolled febrile patients were experiencing moderate-to-severe forms of the disease, requiring tertiary care, or the primary health-care providers were not able to manage these patients adequately. Since most of our patients were recruited from referral centers, the majority of them had already been treated with antibiotics before admission to the hospital sites. The reported rate of self-treatment with antibiotics is explained by unregulated antibiotic use in Georgia during the study period. Negative blood culture results can be attributed to the high rate of antibiotic use before sample collection. Because few patients showed up for the voluntary follow-up visit, data regarding final health status and diagnosis were not collected for most cases. Hence, results reported here represent cases that were seropositive at enrollment, but do not indicate whether the patients were diagnosed with the particular disease within the health-care system.
Brucellosis is an endemic zoonosis in Georgia; thus, the seropositivity rate we observed for brucellosis, in contrast to the rates for TBE and rickettsiosis, was not an unexpected finding.21 As expected, brucellosis seropositive cases were predominantly from the eastern regions of Georgia; however, we did find a few cases from western Georgia as well.21,22 Our analyses identified several risk factors for brucellosis, including well-known exposures that have been previously reported in Georgia (e.g., exposure to sheep and cattle, contact with animal abortus material).8 We also found that male patients were more likely to be seropositive for brucellosis, which has been found previously.21,22 In developed countries, brucellosis is considered an occupational disease affecting mostly males. In underdeveloped settings, such as in rural Georgia, women and children commonly engage in animal care and animal product handling, putting them at risk for infection, as well.22,23 To determine this association in greater depth, a study focusing specifically on brucellosis risk factors should be carried out. Interestingly, in our study, consumption of unpasteurized dairy products was not associated with brucellosis. Moreover, we found a negative association with having a centralized water supply and positive associations with engagement in agricultural activities and forest exposures. The latter three exposures are indicative of rural residence and, thus, could be considered a surrogate factor for brucellosis. Since usually meat does not contain large concentrations of bacteria, undercooked meat is a rare source of brucellosis. However, consumption of undercooked offal puts people at greater risk of acquiring this infection. Traditionally dishes from offal are common in Georgian cuisine; therefore, this factor needs further exploration to determine its impact on brucellosis transmission.
Another pathogen with a relatively high seropositivity rate in this study was TBEV, a virus in the family Flaviviridae. Despite a recent publication in Georgia suggesting that this virus may be an etiologic agent for central nervous system infections, no published data are available on the burden of TBEV in Georgia.24 The presence of the tick vector, however, is documented in the southern Caucasus.25 Accordingly, our risk factor analysis identified tick bites as a risk factor for TBEV. Tick bites and the consumption of raw dairy products are major transmission routes for TBEV. Because of diagnostic challenges, further laboratory testing is required to confirm the IgM ELISA findings.25,26 TBEV has a biphasic clinical manifestation: febrile and neurologic. Out of the seropositive cases in our surveillance study, only one experienced mental changes and the remaining patients had febrile, nonspecific clinical symptoms and signs. No antibodies to WNV, another flavivirus, were detected in this study.
Available limited published data on “rat rickettsiosis” in Georgia date back to the middle of twentieth century.27 Recent tick studies showed the presence of the spotted fever group rickettsiae (Rickettsia raoultii, Rickettsia slovaca, and Rickettsia aeschlimannii) in the country.28,29 Rickettsia typhi antibodies were tested in our study. This particular agent causes endemic typhus; rats and domestic animals, such as cats and dogs, serve as reservoirs and their fleas as vectors. We found that consumption of undercooked meat and unpasteurized milk products were associated with endemic typhus. Nevertheless, these risk factors can be indicative of rural residence and lower socioeconomic status contributing to disease occurrence. As to clinical symptoms and signs, both sore throat and shortness of breath due to pneumonitis or pleural effusion were previously described in murine typhus cases.30,31
Limited epidemiologic data exist on Q-fever in Georgia, but outbreaks of this infection were reported in the middle of twentieth century in the country and region (Aleksenyan, 1962).32 This infection has a myriad of clinical manifestations ranging from self-limited febrile illness to pneumonia, hepatitis, and endocarditis. Neurologic manifestations belong to the more rare forms of this disease.33 Inhalation of air contaminated with excreta from infected animals and other routes including tick bites are the modes of transmission. Compared with non-cases, Q-fever seropositive cases had greater odds of having nausea, jaundice, and neurologic findings. The latter two symptoms remained significant after multiple regression analysis; this may be suggestive of Q-fever hepatitis and neurologic syndrome.
Leptospirosis, a disease that is reported annually in Georgia, may manifest as flu-like, hepatitis-like, or neurologic syndromes.10,34 Exposure to open water reservoirs has been implicated as a possible infection source in most settings. Leptospirosis can also be acquired either from direct or indirect exposure to infected animals (including domestic ones) and their excreta. Our seroprevalence and risk factor estimates for this infection are not accurate because insufficient specimen volumes affected the scale of the MAT confirmation. Several patients responded to more than one serovars, which can be explained by cross-reactivity between the serovars characteristic for leptospirosis serology.11 Despite the limitations, we found that leptospirosis seropositivity was associated with consumption of unpasteurized milk products, a behavior observed in rural parts of Georgia.
We found typhoid fever antibodies in only a few cases (three cases [0.6%]). The disease is transmitted through contaminated food or water.35 Long-term carriage of the bacterium is possible as well. In general, the disease demonstrates a nonspecific febrile manifestation. Seropositive cases in this study had greater odds of having consumed undercooked meat and having a heart murmur at enrollment. Because the causative agent of typhoid fever can, in rare cases, cause infective endocarditis, the presence of uncommon etiologies should be considered for febrile cases with symptoms suggestive of endocarditis.36
This study provides a snapshot of communicable disease frequency at the selected hospitals from 2008 to 2011 in Georgia. On the basis of these results, one could speculate that, with the exception of WNV, the targeted pathogens are prevalent among febrile patients in the country (to varying degrees). We suggest that greater clinical suspicion and improved laboratory capacity are needed to improve case detection and to further confirmation as part of routine public health surveillance. In addition, identified associations should be confirmed using disease specific case–control studies.
ACKNOWLEDGMENTS
We thank all of the volunteers for participating in this study. This study was made possible by the hard work and dedication of the physicians, epidemiologists, and laboratory technicians engaged in this surveillance study. We acknowledge University Research Program grant (no. S-GE800-13-GR-122), U.S. Embassy in Georgia for its contribution to the article development.
Disclaimer: The views expressed herein are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, the U.S. Government, or any organization listed. Some authors are employees of the U.S. government. This work was prepared as part of their official duties and, as such, there is no copyright to be transferred.
Footnotes
Financial support: This study was funded by the Global Emerging Infections Surveillance Program (GEIS).
Authors' addresses: Tinatin Kuchuloria, Javakhishvili Tbilisi State University, Tbilisi, Georgia, E-mail: drkuchuloriasyahoo.com. Paata Imnadze, Javakhishvili Tbilisi State University, Tbilisi, Georgia, and National Center for Disease Control and Public Health, Tbilisi, Georgia, E-mail: pimnadze@ncdc.ge. Nana Mamuchishvili and Maiko Chokheli, National Center for Disease Control and Public Health, Tbilisi, Georgia, E-mails: nanamamuchishvili@yahoo.com and chokhelimaiko@yahoo.com. Tengiz Tsertsvadze, Javakhishvili Tbilisi State University, Tbilisi, Georgia, and Infectious Diseases, AIDS and Clinical Immunology Research Center, Tbilisi, Georgia, E-mail: aids@gol.ge. Marina Endeladze, Ketevan Mshvidobadze, and Lana Gatserelia, Infectious Diseases, AIDS and Clinical Immunology Research Center, Tbilisi, Georgia, E-mails: marinaendeladze@ymail.com, katemshvidobadze@yahoo.com, and lgatserelia@yahoo.com. Manana Makhviladze, Marine Kanashvili, and Teona Mikautadze, V. Bochorishvili Sepsis Center, Tbilisi, Georgia, E-mails: makhviladze_manana@yahoo.com, mkanashvili@mail.ru, and mikautadze.teona@mail.ru. Alexander Nanuashvili and Khatuni Kiknavelidze, Sachkhere Hospital, Sachkhere, Georgia, E-mails: tamari.davitashvili@gmail.com and xatuna-kiknavelidze@rambler.ru. Nora Kokaia and Manana Makharadze, S. Virsaladze Research Institute of Medical Parasitology and Tropical Medicine, Tbilisi, Georgia, E-mails: irma_kokaia@yahoo.com and mananamaxaradze@yahoo.com. Danielle V. Clark and Christian T. Bautista, Walter Reed Army Institute of Research, Silver Spring, MD, E-mails: dvclark@gmail.com and marcos.c.bautista.ctr@mail.mil. Margaret Farrell, Moustafa Abdel Fadeel, Mohamed Abdel Maksoud, Guillermo Pimentel, and Brent House, Global Disease Detection and Response Program, U.S. Naval Medical Research Unit No. 3, Cairo, Egypt, E-mails: marleefar@gmail.com, moustafa.abdelfadeel.eg@med.navy.mil, mohamed.abdelmaksoud.eg@med.navy.mil, gpiment@gmail.com, and brent.l.house2.mil@mail.mil. Matthew J. Hepburn and Robert G. Rivard, U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD, E-mails: matthewhepburn@yahoo.com and robert.g.rivard.mil@mail.mil.
References
- 1.Lo Re V, 3rd, Gluckman SJ. Fever in the returned traveler. Am Fam Physician. 2003;68:1343–1350. [PubMed] [Google Scholar]
- 2.Gamkrelidze A, Atun R, Gotsadze G, MacLehose L. Healthcare Systems in Transition: Georgia. Vol. 4. Copenhagen, Denmark: European Observatory on Health Care Systems; 2002. pp. 1–72. [Google Scholar]
- 3.Sakvarelidze LA, Nersesov VA, Maskharashvili PA. Complex method for epizootiological and epidemiological surveillance. Zh Mikrobiol Epidemiol Immunobiol. 1985;10:51–54. [PubMed] [Google Scholar]
- 4.Ivanidze EA, Sakvarelidze LA, Tkachenko EA, Rezapkin GV, Dzagurova TK. Detection of natural foci of the hemorrhagic fever with renal syndrome in the Georgian SSR. Vopr Virusol. 1987;32:607–610. [PubMed] [Google Scholar]
- 5.Chitadze N, Kuchuloria T, Clark D, Tsertsvadze E, Chokheli M, Tsertsvadze N, Trapaidze N, Lane A, Bakanidze L, Tsanava S. Water-borne outbreak of oropharyngeal and glandular tularemia in Georgia: investigation and follow-up. Infection. 2009;37:514–521. doi: 10.1007/s15010-009-8193-5. [DOI] [PubMed] [Google Scholar]
- 6.Bakashvili LZ, Makhviladze MA, Pagava EK, Pagava KI. Fever of unknown origin in children and adolescents in Georgia: a review of 52 patients. Georgian Med News. 2006;135:66–69. [PubMed] [Google Scholar]
- 7.World Health Organization Global Tuberculosis Control. 2010. http://apps.who.int/iris/bitstream/10665/44425/1/9789241564069_eng.pdf?ua=1 Available at. Accessed May 22, 2015.
- 8.Havas KA, Ramishvili M, Navdarashvili A, Hill AE, Tsanava S, Imnadze P, Salman MD. Risk factors associated with human brucellosis in the country of Georgia: a case-control study. Epidemiol Infect. 2013;141:45–53. doi: 10.1017/S0950268812000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Organization for Economic Cooperation and Development (OECD) Financing Strategy for the Urban Water Supply and Sanitation Sector in Georgia. 2007. http://www.oecd.org/countries/georgia/36472918.pdf Available at. Accessed May 22, 2015.
- 10.National Center for Disease Control and Public Health of Georgia Epidemiology Bulletin. 2014. http://www.ncdc.ge/AttachedFiles/GEO972.pdf Available at. Accessed May 22, 2015.
- 11.World Health Organization Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control. 2003. http://www.who.int/csr/don/en/WHO_CDS_CSR_EPH_2002.23.pdf Available at. Accessed May 22, 2015.
- 12.Afifi S, Earhart K, Azab MA, Youssef FG, Sakka HE, Wasfy M, Mansour H, Oun SE, Rakha M, Mahoney F. Hospital-based surveillance for acute febrile illness in Egypt: a focus on community-acquired bloodstream infections. Am J Trop Med Hyg. 2005;73:392–399. [PubMed] [Google Scholar]
- 13.Chkhartishvili N, Sharvadze L, Chokoshvili O, Bolokadze N, Rukhadze N, Kempker RR, Makrelidze A, DeHovitz J, Del Rio C, Tsertsvadze T. Mortality and causes of death among HIV-infected individuals in the country of Georgia: 1989–2012. AIDS Res Hum Retroviruses. 2014;30:560–566. doi: 10.1089/aid.2013.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadeel MA, Wasfy MO, Pimentel G, Klena JD, Mahoney FJ, Hajjeh RA. Rapid enzyme-linked immunosorbent assay for the diagnosis of human brucellosis in surveillance and clinical settings in Egypt. Saudi Med J. 2006;27:975–981. [PubMed] [Google Scholar]
- 15.Fadeel MA, House B, Wasfy M, Klena K, Emil E, Said MM, Maksoud MA, Raham B, Pimentel G. Evaluating of a newly developed ELISA against Widal, Typhi dot and TUBEX-TF for typhoid fever surveillance in an endemic area. J Infect Dev Ctries. 2011;5:169–175. doi: 10.3855/jidc.1339. [DOI] [PubMed] [Google Scholar]
- 16.Kuchuloria T, Clark DV, Hepburn MJ, Tsertsvadze T, Pimentel G, Imnadze P. Hantavirus infection in the Republic of Georgia. Emerg Infect Dis. 2009;15:1489–1491. doi: 10.3201/eid1509.090617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuchuloria T, Imnadze P, Chokheli M, Tsertsvadze T, Endeladze M, Mshvidobadze K, Clark DV, Bautista CT, Fadeel MA, Pimentel G, House B, Hepburn MJ, Wölfel S, Wölfel R, Rivard RG. Viral hemorrhagic fever cases in the country of Georgia: acute febrile illness surveillance study results. Am J Trop Med Hyg. 2014;91:246–248. doi: 10.4269/ajtmh.13-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Statistics Office of Georgia Main Statistics: Population. 2008. http://www.geostat.ge/index.php?action=page&p_id=152&lang=eng Available at. Accessed February 16, 2015.
- 19.Bernabè S. Informal Employment in Countries in Transition: A Conceptual Framework. 2002. http://sticerd.lse.ac.uk/dps/case/cp/CASEpaper56.pdf Available at. Accessed February 16, 2015.
- 20.Economic Policy Research Center Employment and Unemployment Trends in Georgia. 2011. http://www.osgf.ge/files/publications/2011/EPRC_Georgian_Economic_Outlook_II,_Nov_2011_ENG.pdf Available at. Accessed February 16, 2015.
- 21.Akhvlediani T, Clark D, Chubabria G, Zenaishvili O, Hepburn M. The changing pattern of human brucellosis: clinical manifestations, epidemiology, and treatment outcomes over three decades in Georgia. BMC Infect Dis. 2010;10:346. doi: 10.1186/1471-2334-10-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanodze L, Bautista CT, Garuchava N, Chubinidze S, Tsertsvadze E, Broladze M, Chitadze N, Sidamonidze K, Tsanava S, Akhvlediani T, Rivard RG, Mody R, Hepburn MJ, Elzer PH, Nikolich MP, Trapaidze N. Expansion of brucellosis detection in the country of Georgia by screening household members of cases and neighboring community members. BMC Public Health. 2015;15:459. doi: 10.1186/s12889-015-1761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO, OIE Brucellosis in Humans and Animals. 2006. http://www.who.int/csr/resources/publications/Brucellosis.pdf Available at. Accessed May 22, 2015.
- 24.Akhvlediani T, Bautista CT, Shakarishvili R, Tsertsvadze T, Imnadze P, Tatishvili N, Davitashvili T, Samkharadze T, Chlikadze R, Dvali N, Dzigua L, Karchava M, Gatserelia L, Macharashvili N, Kvirkvelia N, Habashy EE, Farrell M, Rowlinson E, Sejvar J, Hepburn M, Pimentel G, Dueger E, House B, Rivard R. Etiologic agents of central nervous system infections among febrile hospitalized patients in the country of Georgia. PLoS One. 2014;9:e111393. doi: 10.1371/journal.pone.0111393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amicizia D, Domnich A, Panatto D, Lai PL, Cristina ML, Avio U, Gasparini R. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum Vaccin Immunother. 2013;9:1163–1171. doi: 10.4161/hv.23802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Centre for Disease Prevention and Control Epidemiological Situation of Tick-Borne Encephalitis in the European Union and European Free Trade Association Countries. 2012. http://ecdc.europa.eu/en/publications/Publications/TBE-in-EU-EFTA.pdf Available at. Accessed May 22, 2015.
- 27.Babalova EG. On the eradication of rat rickettsiosis in some areas of the Georgia SSR [in Russian] Zh Mikrobiol Epidemiol Immunobiol. 1964;41:70–75. [PubMed] [Google Scholar]
- 28.Jiang J, You BJ, Liu E, Apte A, Yarina TR, Myers TE, Lee JS, Francesconi SC, O'Guinn ML, Tsertsvadze N, Vephkhvadze N, Babuadze G, Sidamonidze K, Kokhreidze M, Donduashvili M, Onashvili T, Ismayilov A, Agayev N, Aliyev M, Muttalibov N, Richards AL. Development of three quantitative real-time PCR assays for the detection of Rickettsia raoultii, Rickettsia slovaca, and Rickettsia aeschlimannii and their validation with ticks from the country of Georgia and the Republic of Azerbaijan. Ticks Tick Borne Dis. 2012;3:327–331. doi: 10.1016/j.ttbdis.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Zghenti E, Sukhiashvili R, Khmaladze E, Tsertsvadze N, Pisarcik S, Imnadze P. Rickettsia and Borrelia prevalence study among ticks in Georgia. Online J Public Health Inform. 2014;6:e63. [Google Scholar]
- 30.Peniche Lara G, Dzul-Rosado KR, Zavala Velázquez JE, Zavala-Castro J. Murine typhus: clinical and epidemiological aspects. Colomb Med. 2012;43:175–180. [PMC free article] [PubMed] [Google Scholar]
- 31.Dzul-Rosado K, González-Martínez P, Peniche-Lara G, Zavala-Velázquez J, Zavala-Castro J. Murine typhus in humans, Yucatan, Mexico. Emerg Infect Dis. 2013;19:1021–1022. doi: 10.3201/eid1906.121400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babalova EG. Q-fever in some areas of the Georgian SSR. Zh Mikrobiol Epidemiol Immunobiol. 1964;41:117–122. [PubMed] [Google Scholar]
- 33.Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, Kersh GJ, Limonard G, Marrie TJ, Massung RF, McQuiston JH, Nicholson WL, Paddock CD, Sexton DJ. Diagnosis and Management of Q Fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62:1–30. [PubMed] [Google Scholar]
- 34.Mamuchishvili N, Kuchuloria T, Mchedlishvili I, Imnadze P. Leptospirosis in Georgia. Georgian Med News. 2014;228:63–66. [PubMed] [Google Scholar]
- 35.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 36.Khan GQ, Kadri SM, Hassan G, Shahid IT, Gazanfar A, Kak M, Showkat H. Salmonella typhi endocarditis: a case report. J Clin Pathol. 2003;56:801–802. doi: 10.1136/jcp.56.10.801. [DOI] [PMC free article] [PubMed] [Google Scholar]