Abstract
Spotted fever group (SFG) rickettsioses are notifiable conditions in the United States caused by the highly pathogenic Rickettsia rickettsii and less pathogenic rickettsial species such as Rickettsia parkeri and Rickettsia sp. 364D. Surveillance data from 2008 to 2012 for SFG rickettsioses are summarized. Incidence increased from 1.7 cases per million person-years (PY) in 2000 to 14.3 cases per million PY in 2012. During 2008–2012, cases of SFG rickettsiosis were more frequently reported among males, persons of white race, and non-Hispanic ethnicity. Overall, case fatality rate (CFR) was low (0.4%), however, risk of death was significantly higher for American Indian/Alaska Natives (relative risk [RR] = 5.4) and Asian/Pacific Islanders (RR = 5.7) compared with persons of white race. Children aged < 10 years continue to experience the highest CFR (1.6%). Higher incidence of SFG rickettsioses and decreased CFR likely result from increased reporting of tick-borne disease including those caused by less pathogenic species. Recently, fewer cases have been confirmed using species-specific laboratory methods (such as cell culture and DNA detection using polymerase chain reaction [PCR] assays), causing a clouded epidemiological picture. Use of PCR and improved documentation of clinical signs, such as eschars, will better differentiate risk factors, incidence, and clinical outcomes of specific rickettsioses in the future.
Introduction
Spotted fever group (SFG) rickettsioses are caused by a group of closely related intracellular bacteria transmitted by arthropod vectors including ticks, fleas, and mites. Tick-borne SFG Rickettsia species cause a wide range of human illness from highly pathogenic to asymptomatic, but usually involve fever, headache, and later, rash. Rocky Mountain spotted fever (RMSF), caused by the bacterium Rickettsia rickettsii, is the most severe and most commonly reported SFG rickettsiosis in the United States with seven cases per million persons reported in 2007.1,2 RMSF is a rapidly progressing illness, which, when left untreated, can lead to widespread vasculitis resulting in death, even in previously healthy individuals. Untreated case fatality rates (CFRs) for RMSF may be up to 20–25%.3,4 Early treatment with doxycycline is the best way to reduce the likelihood of severe disease or fatal outcome for patients of all ages.5
Tick-borne SFG Rickettsia species in the United States known to cause human illness include R. rickettsii, Rickettsia parkeri, and Rickettsia sp. 364D. Several other SFG Rickettsia species, including Rickettsia massiliae, Rickettsia montanensis, and Rickettsia amblyommii have been isolated from ticks, and may cause some human pathogenesis, but have not yet been isolated from human specimens in the United States.6–9 Speculation has arisen as to how often low or nonpathogenic rickettsial species may be misdiagnosed as RMSF.8,10–12 The available serological assays for R. rickettsii cross react with other pathogens in the SFG Rickettsia, as well as member of the and transitional group (TRG) Rickettsia, including Rickettsia akari and Rickettsia felis.13 Because of this, passive surveillance in the United States may capture these rickettsioses, as they technically meet the case definition. The separation of TRG and SFG Rickettsia species, based on vector and phylogenetic age, is a relatively recent development (2007) and much of the past and current literature still refers to members of both groups collectively as SFG Rickettsia species.14 This report uses the term SFG rickettsiosis to refer to human infections of both SFG and TRG Rickettsia represented in passive surveillance data reported under the category “spotted fever rickettsiosis.”
In 2009, the Council for State and Territorial Epidemiologists (CSTE) changed the name of the notifiable condition of “RMSF” to the more broad “spotted fever rickettsiosis” because of the inability to distinguish a specific agent using serological laboratory methods.15 Spotted fever rickettsioses, including RMSF, are notifiable conditions in every state except Alaska and Hawaii. We summarize the passive surveillance data regarding SFG rickettsioses reported to the Centers for Disease Control and Prevention (CDC) between 2008 and 2012 to characterize the epidemiology of reported cases and analyze trends in incidence rates (IRs).
Methods
National surveillance systems.
CDC's Nationally Notifiable Diseases Surveillance System (NNDSS) provides a partnership of local, state, territorial, and federal health organizations to share information relating to the monitoring, control, and prevention of notifiable infectious diseases. As part of this partnership, CDC receives electronic reports of notifiable diseases as well as basic demographic information such as state of residence, race, age, and gender. While the majority of state and local health departments use the CSTE case definition, clinical and laboratory components suggestive of SFG rickettsiosis may differ by locality, and case classification reported in NNDSS is made based on state or local criteria. Supplementary case data including characterization of clinical course, diagnostic test utilization, and illness outcome are reported to the CDC through case report forms (CRFs) (http://www.cdc.gov/ticks/forms/2010_tbrd_crf.pdf) or via abstracted information from state-based surveillance systems. These provide more detailed information not currently reported to CDC through NNDSS. For the purposes of this report, all supplementary case data provided by the states are referred to as CRFs. Case classifications for CRFs were applied retrospectively, using the CSTE case definitions, using data provided on the CRF.15 CRFs generally represent a subset of data reported through NNDSS; therefore, NNDSS data are used for incidence-related calculations. The completeness and quality of the data submitted to both systems varies widely and are reliant on the appropriate reporting of cases to state and local health departments from health-care providers.
Case definition.
15Following the CSTE case definition, cases of SFG rickettsiosis can be categorized as either probable or confirmed and are defined using both clinical and laboratory characteristics. Clinical criteria include a febrile illness with one or more of the following symptoms: rash, eschar, headache, myalgia, anemia, thrombocytopenia, or elevated hepatic transaminases. The presence or absence of an eschar was added to the CDC CRF in 2010. Laboratory criteria provide either confirmatory or supportive documentation for each case. Confirmatory evidence includes one or more of the following: 1) demonstration of a 4-fold rise in immunoglobulin G (IgG)–specific antibodies reactive to R. rickettsii or other SFG Rickettsia species by indirect immunofluorescence assay (IFA) between paired specimens (one taken in the first week of illness and the second taken 2–4 weeks later), 2) detection of R. rickettsii or other SFG rickettsial DNA in clinical specimens via polymerase chain reaction (PCR) assay, 3) demonstration of SFG antigen in biopsied specimens by immunohistochemistry (IHC), and 4) isolation of R. rickettsii or other SFG Rickettsia from a clinical specimen using cell culture. Supportive laboratory evidence includes any serological evidence by elevated IgG or immunoglobulin M (IgM) antibodies reactive to R. rickettsii or another SFG Rickettsia by IFA, enzyme-linked immunosorbent assay (ELISA), dot-ELISA, or latex agglutination assay.
Analytic and statistical methods.
Probable and confirmed cases with an onset of illness between January 1, 2008 and December 31, 2012 were included in this summary. When onset date were not available, date of first test or date of report were used to approximate onset date for inclusion criteria. National, state, and age-specific IRs were calculated using population estimates obtained from the U.S. Census Bureau.16,17 Rates are reported as cases per million person-years (PY). Race-specific IRs were calculated using U.S. Census-bridged race population estimates provided by the National Center for Health Statistics.18,19 All states except Hawaii and Alaska, where SFG rickettsioses are not notifiable, were included in the population at risk. State and county IRs exclude cases reported as acquired outside of the state or the United States. National IRs exclude cases that are reportedly acquired outside the United States. Geographic regions are defined using the U.S. Census Bureau categories.20 Data reported through CRFs were used to calculate CFRs and hospitalization rates (HRs), and allow for the description of diagnostic test utilization, seasonality, and severe complications relating to SFG rickettsioses. Regional seasonality analyses exclude cases reporting a history of travel. Analyses were performed using SAS® software version 9.3 (SAS Institute, Cary, NC).21 Frequencies and rates do not include missing values in the denominator unless otherwise noted.
Results
NNDSS data.
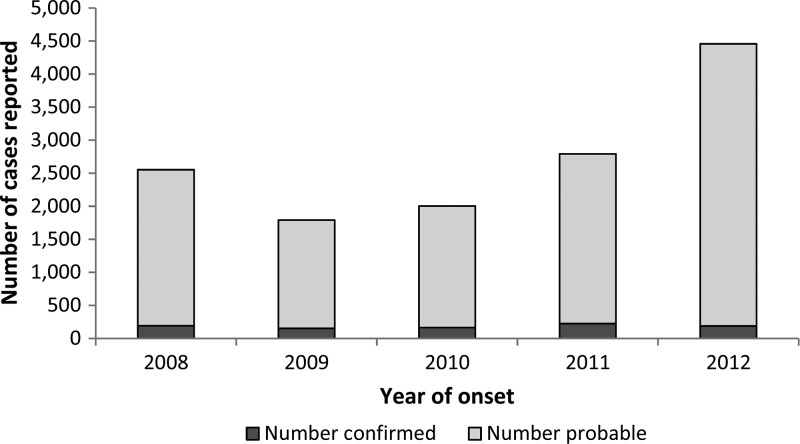
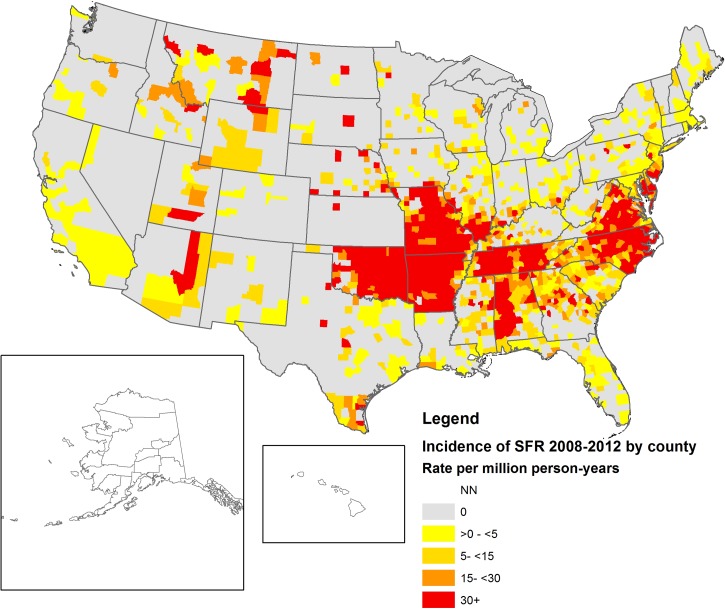
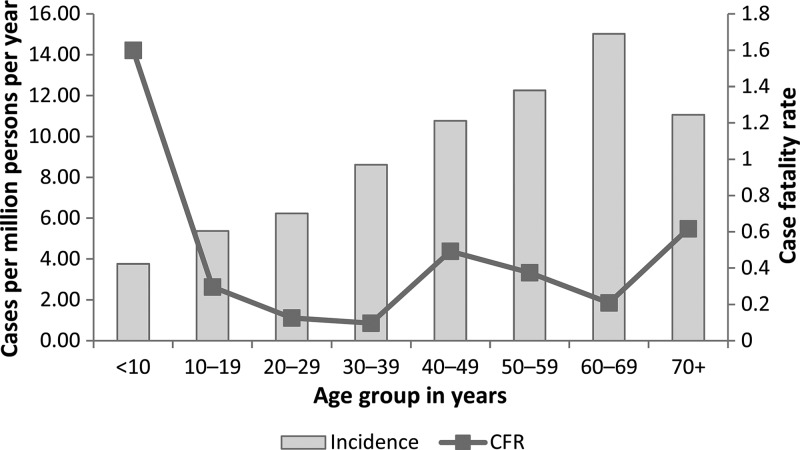
During 2008–2012, 13,599 cases of SFG rickettsiosis were reported; 93% (N = 12,678) of which were reported as probable and 7% (N = 921) as confirmed. Average national incidence was 8.9 cases per million PY. Annual incidence increased from 2009 to 2012, with the most substantial change between 2011 and 2012 (58% increase in national incidence, Figure 1 ). Cases were reported from 47 states and the District of Columbia (Table 1). State-based IRs ranged from zero in Connecticut to 128 cases per million PY in Arkansas. Of all cases, 63% (N = 8,544) were reported out of Arkansas, Missouri, North Carolina, Oklahoma, and Tennessee. Geographic distribution of cases indicated by county-level IRs can be seen in Figure 2 . Importation status of cases was largely missing (N = 8,236, 61%); however, of those reporting importation status, only 38 cases (0.7%) were reported to have been acquired outside the United States. Demographic characteristics from both NNDSS and CRFs can be seen in Table 2. More than half of cases (N = 8,338, 63%) were reported among males, 4% (N = 383) were reported in Hispanic ethnicity, and white race was the most frequently reported (N = 8,559, 87%). Race was missing in 27% (N = 3,706) of cases and ethnicity was missing in 35% of cases (N = 4,817) reported through NNDSS. Incidence was highest among American Indian/Alaska Natives (IR = 29.6), followed by whites (IR = 7.0), blacks (IR = 2.0), and Asian/Pacific Islanders (IR = 0.9). Incidence of SFG rickettsiosis increased with age, with the lowest incidence among children < 10 years (IR = 3.8) and the highest incidence (IR = 15.0) among persons aged 60–69 years (Figure 3 ).
Figure 1.
Probable and confirmed cases of spotted fever group (SFG) rickettsiosis 2008–2012, United States (Nationally Notifiable Diseases Surveillance System [NNDSS]).
Table 1.
Incidence (per million PY) of SFG rickettsiosis by state, 2008–2012, United States (NNDSS)
| State | n | Incidence (per million PY) |
|---|---|---|
| Alabama | 472 | 19.77 |
| Alaska | NN | NN |
| Arizona | 181 | 5.65 |
| Arkansas | 1,870 | 128.25 |
| California | 34 | 0.18 |
| Colorado | 9 | 0.36 |
| Connecticut | 0 | 0 |
| Delaware | 124 | 27.55 |
| District of Columbia | 14 | 4.62 |
| Florida | 62 | 0.66 |
| Georgia | 366 | 7.54 |
| Hawaii | NN | NN |
| Idaho | 13 | 1.66 |
| Illinois | 379 | 5.91 |
| Indiana | 93 | 2.87 |
| Iowa | 27 | 1.77 |
| Kansas | 1 | 0.07 |
| Kentucky | 76 | 3.50 |
| Louisiana | 29 | 1.28 |
| Maine | 12 | 1.81 |
| Maryland | 215 | 7.43 |
| Massachusetts | 17 | 0.52 |
| Michigan | 17 | 0.34 |
| Minnesota | 33 | 1.24 |
| Mississippi | 95 | 6.40 |
| Missouri | 1,521 | 50.84 |
| Montana | 21 | 4.24 |
| Nebraska | 54 | 5.91 |
| Nevada | 3 | 0.22 |
| New Hampshire | 7 | 1.06 |
| New Jersey | 473 | 10.76 |
| New Mexico | 10 | 0.97 |
| New York | 169 | 1.74 |
| New York City | 49 | 1.20 |
| North Carolina | 1,893 | 39.67 |
| North Dakota | 4 | 1.18 |
| Ohio | 93 | 1.61 |
| Oklahoma | 1,582 | 84.38 |
| Oregon | 6 | 0.31 |
| Pennsylvania | 103 | 1.62 |
| Rhode Island | 20 | 3.80 |
| South Carolina | 188 | 8.12 |
| South Dakota | 5 | 1.23 |
| Tennessee | 1,678 | 52.83 |
| Texas | 242 | 1.92 |
| Utah | 22 | 1.59 |
| Vermont | 1 | 0.32 |
| Virginia | 1,040 | 25.95 |
| Washington | 3 | 0.09 |
| West Virginia | 17 | 1.84 |
| Wisconsin | 41 | 1.44 |
| Wyoming | 23 | 8.17 |
| Average national incidence per million PY*† | 13,561 | 8.81 |
NNDSS = Nationally Notifiable Diseases Surveillance System; PY = person-years; SFG = spotted fever group; NN = not notifiable.
Because SFG rickettsioses are not a reportable conditions in Alaska and Hawaii, these states are not included in the national incidence calculation.
National incidence includes cases acquired outside of state of residence, but within the United States.
Figure 2.
Incidence of spotted fever group (SFG) rickettsiosis by county, United States 2008–2012 (Nationally Notifiable Diseases Surveillance System [NNDSS]).
Table 2.
Demographic profiles and outcomes among SFG rickettsiosis cases, 2008–2012, United States (NNDSS and CRF)
| NNDSS (N = 13,599) | CRF (N = 10,356) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Case classification | ||||
| Confirmed | 921 | 6.8 | 139 | 1.3 |
| Probable | 12,678 | 93.2 | 10,217 | 98.7 |
| Unknown | 0 | – | 0 | – |
| Sex | ||||
| Male | 8,338 | 61.3 | 6,558 | 63.3 |
| Female | 4,996 | 36.7 | 3,621 | 35.0 |
| Unknown | 268 | 2.0 | 177 | 1.7 |
| Race | ||||
| White | 8,559 | 62.9 | 7,245 | 70.0 |
| Black | 416 | 3.1 | 293 | 2.8 |
| American Indian/Alaska Native | 607 | 4.5 | 526 | 5.1 |
| Asian/Pacific Islander | 71 | 0.5 | 54 | 0.5 |
| Not specified/unknown | 3,706 | 27.3 | 2,232 | 21.6 |
| Other | 240 | 1.8 | 6 | 0.1 |
| Ethnicity | ||||
| Hispanic | 383 | 2.8 | 332 | 3.2 |
| Non-Hispanic | 8,399 | 61.8 | 6,878 | 66.4 |
| Unknown | 4,817 | 35.4 | 3,146 | 30.4 |
| Age group | ||||
| < 10 | 755 | 5.6 | 597 | 5.8 |
| 10–19 | 1,137 | 8.4 | 819 | 7.9 |
| 20–29 | 1,329 | 9.8 | 944 | 9.1 |
| 30–39 | 1,718 | 12.6 | 1,225 | 11.8 |
| 40–49 | 2,327 | 17.1 | 1,689 | 16.3 |
| 50–59 | 2,556 | 18.8 | 1,914 | 18.5 |
| 60–69 | 2,197 | 16.2 | 1,682 | 16.2 |
| 70+ | 1,542 | 11.3 | 1,151 | 11.1 |
| Unknown | 38 | 0.3 | 335 | 3.2 |
| Imported | ||||
| Acquired in state | 5,122 | 37.7 | – | – |
| Acquired outside the United States | 38 | 0.3 | – | – |
| Acquired in the United States outside state | 203 | 1.5 | – | – |
| Unknown | 8,236 | 60.6 | – | – |
| Eschar | ||||
| Present | – | – | 33 | 0.3 |
| Not present | – | – | 1,108 | 10.7 |
| Not reported | – | – | 9,215 | 89.0 |
| Immunosuppressive condition | ||||
| Yes | – | – | 458 | 4.4 |
| No | – | – | 5,099 | 49.2 |
| Unknown | – | – | 4,799 | 46.3 |
| Hospitalization status | ||||
| Hospitalized | – | – | 2,216 | 21.4 |
| Not hospitalized | – | – | 6,229 | 60.1 |
| Unknown | – | – | 1,911 | 18.5 |
| Outcome | ||||
| Died | – | – | 36 | 0.3 |
| Did not die | – | – | 8,618 | 83.2 |
| Unknown | – | – | 1,702 | 16.4 |
CRF = case report forms; NNDSS = Nationally Notifiable Diseases Surveillance System.
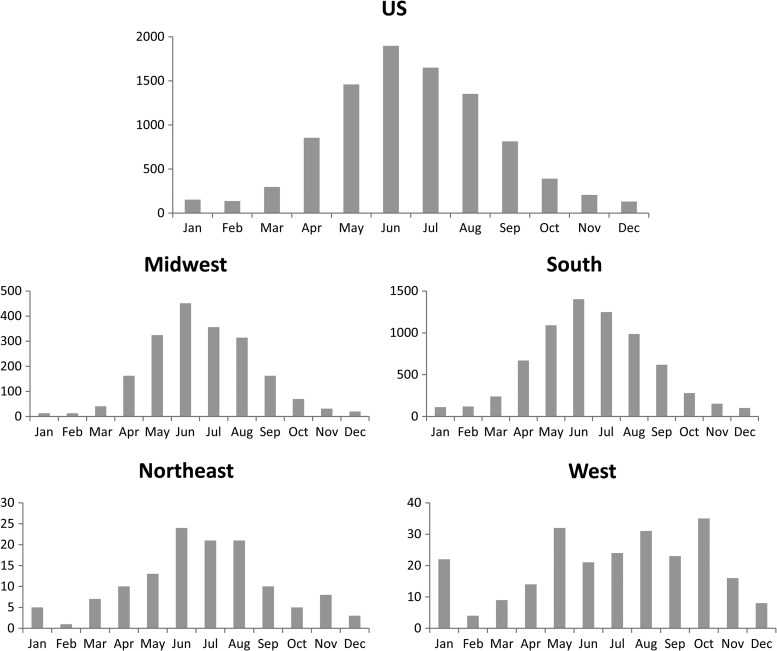
Figure 3.
Month of onset of spotted fever group (SFG) rickettsiosis cases in the United States and by region, 2008–2012 (case report forms [CRFs]).
CRF data.
The CDC received 10,356 CRFs, or equivalent data, meeting the SFG rickettsiosis case definition for the 2008–2012 reporting period, of which 99% (N = 10,217) were probable cases and only 1% (N = 139) were confirmed cases. Sex, ethnicity, and race distributions among reported cases were similar to those reported by NNDSS (see Table 2). Of those cases reporting month of onset, most (N = 7,052, 68%) occurred between May and August, with the highest number of cases (N = 2,094, 20%) in June (Figure 4 ). Nationally, “off-season” (November–February) cases are documented infrequently (7%); however, regional differences are evident. Seasonal trends were less pronounced, and off-season cases were reported more frequently in the western (21%) and northeast (13%) regions. Presence or absence of an eschar was largely missing (N = 9,215, 89%), however, where eschar status was reported, 3% (N = 33) of persons reported an eschar associated with their illness. Comparison of demographics and outcome between confirmed and probable cases are shown in Table 3. Differences were evident for variables relating to disease severity and outcome between confirmed and probable cases.
Figure 4.
Incidence per million person-years (PY) and case fatality rate (CFR) of spotted fever group (SFG) rickettsiosis by 10-year age group, United States 2008–2012 (incidence from Nationally Notifiable Diseases Surveillance System [NNDSS], CFR from case report forms [CRFs]).
Table 3.
Demographics by confirmed and probable case definitions, 2008–2012, (CRFs)
| Confirmed, N = 139 | Probable, N = 10,217 | |||
|---|---|---|---|---|
| n | % | n | % | |
| Gender | ||||
| Male | 77 | 55 | 6,481 | 63 |
| Female | 46 | 33 | 3,575 | 35 |
| Unknown | 16 | 12 | 161 | 2 |
| Age group (years) | ||||
| < 10 | 20 | 14 | 577 | 6 |
| 10–19 | 14 | 10 | 805 | 8 |
| 20–29 | 3 | 2 | 941 | 9 |
| 30–39 | 13 | 9 | 1,212 | 12 |
| 40–49 | 21 | 15 | 1,668 | 16 |
| 50–59 | 26 | 19 | 1,888 | 18 |
| 60–69 | 23 | 17 | 1,659 | 16 |
| 70+ | 9 | 6 | 1,142 | 11 |
| Unknown | 10 | 7 | 325 | 3 |
| Race | ||||
| White | 89 | 64 | 7,156 | 70 |
| Black | 1 | 1 | 292 | 3 |
| American Indian/Alaska Native | 16 | 12 | 510 | 5 |
| Asian/Pacific Islander | 0 | 0 | 37 | 0 |
| Other | 0 | 0 | 17 | 0 |
| Unknown | 33 | 24 | 2,205 | 22 |
| Ethnicity | ||||
| Hispanic | 4 | 3 | 328 | 3 |
| Non-Hispanic | 81 | 58 | 6,797 | 67 |
| Unknown | 54 | 39 | 3,092 | 30 |
| Immune status | ||||
| Immunocompromised | 7 | 5 | 451 | 4 |
| Not immunocompromised | 82 | 59 | 5,017 | 49 |
| Unknown | 50 | 36 | 4,749 | 46 |
| Life-threatening complications | ||||
| One or more | 14 | 10 | 315 | 3 |
| None | 122 | 88 | 9,675 | 95 |
| Unknown | 3 | 2 | 227 | 2 |
| Hospitalization status | ||||
| Hospitalized | 51 | 37 | 2,165 | 21 |
| Not hospitalized | 61 | 44 | 6,168 | 60 |
| Unknown | 27 | 19 | 1,884 | 18 |
| Final outcome | ||||
| Died | 12 | 9 | 24 | 0.2 |
| Did not die | 100 | 72 | 8,518 | 83 |
| Unknown | 27 | 19 | 1,675 | 16 |
CRFs = case report forms.
Among those reporting hospitalization status, just over one quarter (HR = 26%, N = 2,216) reported hospitalization during their illness, and rates were highest in the oldest (≥ 70 years, HR = 40%) and youngest (< 10 years, HR = 29%) age groups (Table 4). HR was higher for persons of black race (HR = 44%, RR = 1.7) and Asian/Pacific Islanders (HR = 41%, RR = 1.5) compared with persons of white race and was slightly higher among persons of Hispanic ethnicity (HR = 34%, RR = 1.4).
Table 4.
Demographics of SFG rickettsiosis cases by hospitalization status and fatal outcome, 2008–2012 (CRFs)
| Hospitalized cases, N = 2,216 | Fatal cases, N = 36 | |||
|---|---|---|---|---|
| n (HR, %) | RR | n (CFR, %) | RR | |
| Gender | ||||
| Male | 1,474 (28) | – | 17 (0.3) | – |
| Female | 711 (24) | 1.2 | 19 (0.6) | 2.0 |
| Age group (years) | ||||
| < 10 | 131 (29) | 1.0 | 8 (1.6) | 7.7 |
| 10–19 | 140 (21) | 0.8 | 2 (0.3) | 1.4 |
| 20–29 | 174 (23) | 0.8 | 1 (0.1) | 0.6 |
| 30–39 | 229 (23) | 0.8 | 1 (0.1) | 0.5 |
| 40–49 | 302 (22) | 0.8 | 7 (0.5) | 2.4 |
| 50–59 | 376 (24) | 0.9 | 6 (0.4) | 1.8 |
| 60–69 | 401 (28) | – | 3 (0.2) | – |
| 70+ | 399 (40) | 1.4 | 6 (0.6) | 3.0 |
| Race | ||||
| White | 1,674 (27) | – | 23 (0.4) | – |
| Black | 118 (44) | 1.7 | 1 (0.4) | 1.1 |
| American Indian/Alaska Native | 67 (34) | 1.3 | 7 (2.0) | 5.4 |
| Asian/Pacific Islander | 19 (41) | 1.5 | 1 (2.0) | 5.7 |
| Ethnicity | ||||
| Hispanic | 104 (34) | 1.4 | 4 (1.3) | 3.2 |
| Non-Hispanic | 1,570 (26) | – | 25 (0.4) | – |
| Immune status | ||||
| Immunocompromised | 205 (50) | 2.0 | 7 (1.7) | 4.4 |
| Not immunocompromised | 1,066 (25) | – | 17 (0.4) | – |
| Life-threatening complications | ||||
| One or more | 241 (79) | 11.7 | 17 (6.0) | 27.9 |
| None | 1,949 (24) | – | 19 (0.2) | – |
CFR = case fatality rate; CRF = case report forms; HR = hospitalization rate; RR = relative risk; SFG = spotted fever group.
Of all cases, 54% (N = 5,557) reported presence or absence of immunosuppressive conditions; of those, 8% (N = 458) reported having an immunosuppressive condition. Most commonly noted immunosuppressive conditions included diabetes (N = 102), cancer (N = 74), autoimmune disorders (N = 71), hepatitis C (N = 20), and human immunodeficiency virus infection/acquired immune deficiency syndrome (N = 18). The reported HR among immunosuppressed cases was 50% (N = 205), which is twice (RR = 2.0) that of persons reporting no immunosuppression (HR = 25%, N = 1,066). The majority of cases (56%, N = 5,844) reported whether a life-threatening complication resulted from their acute infection. Of those, 329 (6%) reported one or more life-threatening complication associated with their illness. Meningitis/encephalitis was the most commonly reported complication (N = 100), followed by renal failure (N = 87) and acute respiratory distress syndrome (N = 43). Persons reporting severe or life-threatening complications were considerably more likely to be hospitalized (RR = 11.7), and result in a fatal outcome (RR = 27.9).
Thirty-six fatal cases (CFR = 0.4%) of SFG rickettsiosis were reported during 2008–2012. Among those reporting date of death, the median time from onset to death was 7 days (range = 0–111 days). Although incidence of SFG rickettsiosis was generally low for children aged < 10 years, this age group suffered the highest CFR (1.6%, N = 8), which is more than five times that of all other age groups combined (RR = 5.2). Fatal outcomes were reported more frequently among females (RR = 2.0), the immunosuppressed (RR = 4.4), and persons of Hispanic ethnicity (RR = 3.2). Fatalities were significantly higher for American Indian/Alaska Natives (RR = 5.4) and Asian/Pacific Islanders (RR = 5.7) compared with persons of white race. Of note, the highest number of fatal cases were reported out of Arizona (N = 7, CFR = 10%), making fatal outcome 30 times more likely in Arizona than all other states (RR = 30.3). High numbers of fatal outcomes were also reported out of Georgia (N = 3, CFR = 0.9%), Tennessee (N = 5, CFR = 0.5%), Arkansas (N = 5, CFR = 0.3%), Missouri (N = 4, CFR = 0.3%), and Oklahoma (N = 3, CFR = 0.3%); however, fatality rates were likely low due to the high case burdens in these localities.
Only 139 cases reported through CRFs met the confirmed case definition. Confirmatory laboratory criteria were, most commonly, a 4-fold rise in IgG antibody titers using IFA (N = 105, 76%), followed by PCR (N = 23, 17%), IHC (N = 11, 8%), and culture (N = 4, 3%). Single IgG titers by IFA were the most common laboratory criteria (N = 7,335) during the study period, with 72% of total case reports noting it as a used criteria. Among these cases, titer values ranged from 1:16 to ≥ 1:4,096 (geometric mean IgG = 115). In contrast, cases confirmed by 4-fold rise in titer had high titer values ranging from 1:128 to ≥ 1:16,384 (geometric mean IgG = 456). Laboratory methods contributing to supportive laboratory criteria for probable SFG rickettsioses can be seen in Table 5. Agglutination techniques were reported infrequently in this reporting period (N = 9, 0.1% of probable cases). Although less common than IFA, ELISA techniques (including enzyme immunoassay and PanBio; PanBio Pty Ltd., Brisbane, Australia) were still used relatively frequently (N = 1,903, 19% of probable cases) as supportive laboratory criteria. Although IgM positive results were reported for 1,590 cases (16% of probable cases), elevated IgM by IFA served as the sole supportive laboratory criteria in 231 cases (2% of total cases). The median time between onset and blood collection was 4 days (interquartile range [IQR] = 1–10 days) for the 10,356 cases with known dates. The median time was 3 days (IQR = 1–5 days) for confirmed cases.
Table 5.
Frequency of laboratory methods used in the diagnosis of probable SFG rickettsiosis in the United States, 2008–2012 (CRFs)
| Methodology | Number of probable cases | Frequency among probable cases (N = 10,217) |
|---|---|---|
| Fourfold IgG by IFA* | 265 | 2.6% |
| Less than 4-fold rise in IgG among paired samples by IFA | 790 | 7.7% |
| Single elevated IgG by IFA | 7,335 | 71.8% |
| IFA IgM | 1,590 | 15.6% |
| ELISA tests | 1,903 | 18.6% |
| Agglutination | 9 | 0.1% |
CRFs = case report forms; ELISA = enzyme-linked immunosorbent assay; IFA = indirect immunofluorescence assay; IgG = immunoglobulin G; IgM = immunoglobulin M; SFG = spotted fever group.
Cases may meet one or more laboratory criteria and does not include cases where test type was not specified, therefore, representative of minimum values.
Does not meet timing criteria for confirmatory laboratory evidence.
Discussion
Incidence of SFG rickettsiosis between 2008 and 2012 increased from 8.5 cases per million PY in 2008 to 14.3 cases per million PY in 2012, with an average national incidence of 8.9 cases per million PY. The annual incidence in 2012 represents the highest incidence of SFG rickettsiosis to date, nearly a 7-fold higher annual incidence from the 1.7 cases per million PY in 2000.2 This demonstrates an overall upward trend in national incidence of SFG rickettsiosis reported since the mid-1990s.2,22 Although the burden of SFG rickettsiosis has increased considerably, the overall geographic distribution of cases of SFG rickettsiosis has not changed (Figure 2), and the majority of cases (63%) continue to be reported by five states (Arkansas, Missouri, North Carolina, Oklahoma, and Tennessee).
National seasonality trends of SFG rickettsiosis reflect periods of peak feeding activity by the ticks that transmit rickettsial agents, with the majority of cases reported during early summer months.23,24 This trend varied widely by region with some areas, such as the west and northeast, showing no distinct seasonality. Year-round moderate temperatures and longer periods of tick-feeding activity, such as those observed in Arizona and California, may account for less characteristic seasonal distribution in warmer areas.25,26 Off-season cases of SFG rickettsiosis in the northeast may be influenced by cases of rickettsialpox caused by infection with R. akari. Rickettsialpox cases often occur during winter months when contact with mice and their mites are more common. Cases of rickettsialpox have been reported out of urban centers of the northeast in recent years.27,28 Antigens for R. akari and SFG Rickettsia species cross-react in serological and IHC assays, and these reports cannot be excluded from the SFG rickettsiosis surveillance.
Burden of disease by sex, race, and ethnicity are similar to those reported in 2000–2007, with cases more frequently reported among males, persons of white race, and non-Hispanic ethnicity. As in the previous decade, incidence increases incrementally with age, with persons aged 60–69 years reporting the highest age-specific incidence.2,22 Although children aged < 10 years experienced the lowest incidence of SFG rickettsiosis for 2008–2012, they also reported the highest CFR (1.6%). It has been theorized that higher CFRs among young children may be due to the reticence of physicians to prescribe doxycycline for this age group relating to concerns about dental staining and enamel hypoplasia from long courses of tetracycline products.2,5,29,30 No evidence to date has documented dental staining to permanent teeth with use of short courses of doxycycline, such as those used to treat suspected rickettsial infections.31–33 Doxycycline is the treatment of choice recommended by the CDC and American Academy of Pediatrics for suspected RMSF and other rickettsioses in both adult and pediatric patients.23,34 Non-doxycycline antibiotic therapy in the treatment of RMSF has been associated with a higher likelihood of fatal outcome.23,34
Incidence of SFG rickettsiosis among American Indians and Alaska Natives was more than four times the incidence in whites (IRR = 4.2). Because of the large number of missing fields on race, these rates likely represent minimum values of race-specific incidences. Of concern, American Indian/Alaska Natives and Asian/Pacific Islanders experienced a 5-fold increased risk of fatal outcome compared with white individuals. Focal and ongoing epidemics of RMSF have been reported from American Indian reservations in eastern Arizona contributing to the high case counts and fatalities in this region.25,35–37 Continued high incidence and CFRs among American Indians compared with other race groups suggests this health disparity remains a prevalent issue that needs to be addressed with increased prevention and control programs on tribal lands.37
Rickettsial infections generally produce similar and nonspecific symptomologies making them difficult to distinguish from one another at a point-of-care level. Although species specificity is not necessary for clinical management (as all human infections with Rickettsia species can be effectively treated with doxycycline), aggregated data can be dangerously misleading. Historical evidence has shown RMSF to have a CFR of 20–25% among untreated cases in the pre-antibiotic era and CFR among treated cases to be roughly 5%.4,38 Surveillance data over the past decade have shown a decreasing CFR for SFG rickettsioses with fatalities reported in less than 1% of cases.2 Although part of this trend may be attributed to increased awareness of the disease and availability of effective antibiotic therapy, we believe this rate to be heavily confounded by the frequency of other SFG rickettsioses. Non-RMSF SFG rickettsioses, including infections with R. parkeri, R. akari, and Rickettsia sp. 364D, are known to be less severe than RMSF, and no human deaths have been attributed to infections by any of these species in the United States. By contrast, areas in which RMSF is the solely identified SFG rickettsiosis, such as Arizona, as many as 10% of cases have resulted in death.
During 2000–2007, more than 5% of cases reported through CRFs were classified as confirmed; whereas from 2008 to 2012 only 1% of cases met the confirmed case definition. Serological antibody tests continue to serve as the most common laboratory methodology.2,13 The majority of SFG rickettsiosis cases with illness onset between 2008 and 2012 were supported by single elevated IgG titers by IFA, many of which reported titers just above the ≥ 1:64 cutoff. Low, single titers may represent previous infection and without documentation of an active rise or fall of serum antibodies in a convalescent specimen, recent infection cannot be distinguished from historic infection. The median time from date of onset to date of specimen collection was only 4 days. As it may take 7–10 days to generate a measurable antibody response after rickettsial infection, it is likely that many of the low-level titers drawn early in illness represent antibody persistence rather than recent infection. Cross-reactivity among members of the SFG and TRG Rickettsia species limits the ability to draw antigen-specific conclusions, as such, molecular detection of rickettsial DNA by PCR and culture are the only methods capable of providing species-specific results. However, molecular methods are not widely available and culture is rarely used for diagnostic purposes because of the labor-intensive methods requiring living host cells.38–40 Furthermore, PCR of whole blood is not considered a sensitive test for RMSF until late in disease progression when rickettsiae begin circulating in the blood resulting from vasculitic injury. Low utilization of PCR and culture to diagnose SFG rickettsioses means few cases reported to national surveillance systems are able to be attributed to a specific rickettsial species.
It has been suggested that up to one-third of cases diagnosed and reported as RMSF are actually caused by R. parkeri.10 SFG rickettsioses of unknown pathogenicity, including R. amblyommii, have been shown to result in quantifiable antibody responses and may also be captured within this reporting category.8 Contributions of non-RMSF rickettsioses will likely vary widely by region with R. akari frequently reported out of the northeast, R. parkeri and R. amblyommii out of the south, and Rickettsia sp. 364D out of the west, corresponding with the ranges of their respective vectors.8,11,12,41 Clues, such as presence of an eschar, seasonality, and geographic distribution may suggest a causative agent; however, without the use of species-specific diagnostic tests, it remains a challenge to differentiate the epidemiology of the individual SFG rickettsioses in the United States. Biopsies of rash and eschars, when present, serve as good sample sources for acute infection when taken before administration of doxycycline.13 Swabs of eschar exudate have been shown to be sensitive diagnostic specimens for detection of R. akari, R. parkeri, and Rickettsia sp. 364D and provide a less invasive collection method than skin punch biopsies.41–43 Because the window of opportunity for detection of rickettsial DNA in acute clinical samples may be small, it is important to additionally collect acute and convalescent serum samples to look for significant changes in serum antibody levels indicative of recent infection. Utilization of better laboratory methods and documentation of specific clinical findings will improve our knowledge of national trends, and help clarify species-specific risk factors for SFG rickettsioses.
Passive surveillance reporting systems, such as NNDSS and CRFs, are subject to several limitations. To be reported, cases must be accompanied by laboratory evidence; persons with poor access to care and diagnostic services may be underrepresented in these data. Hospitalized and fatal cases may be tested and reported more frequently than less severe cases and may therefore be highly represented among confirmed cases. Missing or incomplete data, such as those reported on race and ethnicity, may severely bias the results and interpretation of trends. Variations in annual incidence may be attributed to a range of factors such as human interaction with tick habitats, vector and host dynamics, climatic or ecological changes, increased awareness and testing of tick-borne disease, or changes in surveillance practices. Statistics and conclusions summarized in this report represent descriptions of the cases captured in this reporting period and are not generalizable to all SFG rickettsioses. The quality and precision of national surveillance data are dependent on clinician awareness of testing and reporting practices for tick-borne rickettsial diseases and active collaboration with practitioners and their local health departments. Improved utilization of appropriate diagnostic tests, documentation of epidemiological factors, and timely reporting to public health officials will guide prevention messaging and shape public health policy.
ACKNOWLEDGMENTS
We would like to thank the clinicians, laboratorians, and public health partners for their continued support in the diagnosis, management, and surveillance of rickettsial diseases. We also thank Eric Mandel, John Krebs, and Jennifer McQuiston for their work in designing and building these data.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Financial support: This study was supported by the Centers for Disease Control and Prevention (CDC) and in part supported by an appointment to the Research Participation Program at the CDC administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC.
Authors' addresses: Naomi A. Drexler, F. Scott Dahlgren, Kristen Nichols Heitman, Robert F. Massung, Christopher D. Paddock, and Casey Barton Behravesh, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: isj3@cdc.gov, iot0@cdc.gov, wwd7@cdc.gov, rfm2@cdc.gov, cdp9@cdc.gov, and dlx9@cdc.gov.
References
- 1.Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Openshaw JJ, Swerdlow DL, Krebs JW, Holman RC, Mandel E, Harvey A, Haberling D, Massung RF, McQuiston JH. Rocky Mountain spotted fever in the United States, 2000–2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg. 2010;83:174–182. doi: 10.4269/ajtmh.2010.09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smadel JE. Status of the rickettsioses in the United States. Ann Intern Med. 1959;51:421–435. doi: 10.7326/0003-4819-51-3-421. [DOI] [PubMed] [Google Scholar]
- 4.Childs JE, Paddock CD. Passive surveillance as an instrument to identify risk factors for fatal Rocky Mountain spotted fever: is there more to learn? Am J Trop Med Hyg. 2002;66:450–457. doi: 10.4269/ajtmh.2002.66.450. [DOI] [PubMed] [Google Scholar]
- 5.Holman RC, Paddock CD, Curns AT, Krebs JW, McQuiston JH, Childs JE. Analysis of risk factors for fatal Rocky Mountain spotted fever: evidence for superiority of tetracyclines for therapy. J Infect Dis. 2001;184:1437–1444. doi: 10.1086/324372. [DOI] [PubMed] [Google Scholar]
- 6.Eremeeva ME, Bosserman EA, Demma LJ, Zambrano ML, Blau DM, Dasch GA. Isolation and identification of Rickettsia massiliae from Rhipicephalus sanguineus ticks collected in Arizona. Appl Environ Microbiol. 2006;72:5569–5577. doi: 10.1128/AEM.00122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beeler E, Abramowicz KF, Zambrano ML, Sturgeon MM, Khalaf N, Hu R, Dasch GA, Eremeeva ME. A focus of dogs and Rickettsia massiliae-infected Rhipicephalus sanguineus in California. Am J Trop Med Hyg. 2011;84:244–249. doi: 10.4269/ajtmh.2011.10-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW. Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. doi: 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- 9.McQuiston JH, Zemtsova G, Perniciaro J, Hutson M, Singleton J, Nicholson WL, Levin ML. Afebrile spotted fever group Rickettsia infection after a bite from a Dermacentor variabilis tick infected with Rickettsia montanensis. Vector Borne Zoonotic Dis. 2012;12:1059–1061. doi: 10.1089/vbz.2012.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raoult D, Parola P. Rocky Mountain spotted fever in the USA: a benign disease or a common diagnostic error? Lancet Infect Dis. 2008;8:587–589. doi: 10.1016/S1473-3099(08)70210-X. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro MR, Fritz CL, Tait K, Paddock CD, Nicholson WL, Abramowicz KF, Karpathy SE, Dasch GA, Sumner JW, Adem PV, Scott JJ, Padgett KA, Zaki SR, Eremeeva ME. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis. 2010;50:541–548. doi: 10.1086/649926. [DOI] [PubMed] [Google Scholar]
- 12.Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SLF, Tamminga CL, Ohl CA. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- 13.La Scola B, Raoult D. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol. 1997;35:2715. doi: 10.1128/jcm.35.11.2715-2727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillespie JJ, Beier MS, Rahman MS, Ammerman NC, Shallom JM, Purkayastha A, Sobral BS, Azad AF. Plasmids and rickettsial evolution: insight from Rickettsia felis. PLoS One. 2007;2:e266. doi: 10.1371/journal.pone.0000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Council of State and Territorial Epidemiologists Public Health Reporting and National Notification for Spotted Fever Rickettsioses (Including Rocky Mountain Spotted Fever) Position Statement; Council of State and Territorial Epidemiologists Annual Conference; Buffalo, NY. 2009. June 7–11. [Google Scholar]
- 16.U.S. Census Bureau . Intercensal Estimates of the Resident Population for Counties and States: April 1, 2000 to July 1, 2010. U.S. Census Bureau; 2011. [Google Scholar]
- 17.U.S. Census Bureau . Annual Resident Population Estimates, Estimated Components of Resident Population Change, and Rates of the Components of Resident Population Change for States and Counties: April 1, 2010 to July 1, 2013. U.S. Census Bureau; 2014. [Google Scholar]
- 18.National Center for Health Statistics . In: Vintage 2013 Postcensal Estimates of the Resident Population of the United States (April 1, 2010, July 1, 2010–July 1, 2013), by Year, County, Single-Year of Age (0, 1, 2, 85 Years and Over), Bridged Race, Hispanic Origin, and Sex. National Center for Health Statistics under cooperative agreement with U.S. Census Bureau, editor. 2014. http://www.cdc.gov/NCHS/nvss/bridged_race.htm Available at. [Google Scholar]
- 19.National Center for Health Statistics . In: Bridged-Race Intercensal Estimates of the Resident Population of the United States for July 1, 2000–July 1, 2009, by Year, County, Single-Year of Age (0, 1, 2, 85 Years and Over), Bridged Race, Hispanic Origin, and Sex. National Center for Health Statistics under cooperative agreement with U.S. Census Bureau, editor. http://www.cdc.gov/NCHS/nvss/bridged_race.htm Available at. [Google Scholar]
- 20.U.S. Census Bureau Census Regions and Divisions of the United States. 2010. http://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf Available at. Accessed February 12, 2015.
- 21.SAS Institute Inc. SAS/STAT Statistical Software. Cary, NC: SAS Institute Inc.; 2011. [Google Scholar]
- 22.Chapman AS, Murphy SM, Demma LJ, Holman RC, Curns AT, McQuiston JH, Krebs JW, Swerdlow DL. Rocky Mountain spotted fever in the United States, 1997–2002. Vector Borne Zoonotic Dis. 2006;6:170–178. doi: 10.1089/vbz.2006.6.170. [DOI] [PubMed] [Google Scholar]
- 23.Bakken JS, Folk SM, Paddock CD, Bloch KC, Krusell A, Sexton DJ, Buckingham SC, Marshall GS, Storch GA, Dasch GA, McQuiston JH, Swerdlow DL, Dumler JS, Nicholson WL, Walker DH, Eremeeva ME, Ohl CA, Tickborne Rickettsial Diseases Working Group. CDC Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2006;55:1. [PubMed] [Google Scholar]
- 24.Burg JG. Seasonal activity and spatial distribution of host-seeking adults of the tick Dermacentor variabilis. Med Vet Entomol. 2001;15:413–421. doi: 10.1046/j.0269-283x.2001.00329.x. [DOI] [PubMed] [Google Scholar]
- 25.Traeger MS, Regan JJ, Humpherys D, Mahoney D, Martinez M, Emerson GL, Tack D, Geissler A, Yasmin S, Lawson R, Williams V, Hamilton C, Levy C, Komatsu K, McQuiston JH, Yost D. Rocky Mountain spotted fever characterization and comparison to similar illnesses in a highly endemic area—Arizona, 2002–2011. Clin Infect Dis. 2015;60:1650–1658. doi: 10.1093/cid/civ115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane R. Seasonal activity of two human-biting ticks. Calif Agric. 1990;44:23–25. [Google Scholar]
- 27.Paddock CD, Zaki SR, Koss T, Singleton J, Sumner JW, Comer JA, Eremeeva ME, Dasch GA, Cherry B, Childs JE. Rickettsialpox in New York City: a persistent urban zoonosis. Ann N Y Acad Sci. 2003;990:36–44. doi: 10.1111/j.1749-6632.2003.tb07334.x. [DOI] [PubMed] [Google Scholar]
- 28.Finn LE. Identification of Rickettsialpox and Rocky Mountain Spotted Fever in Philadelphia, 2011–2013; 2014 CSTE Annual Conference; June 22–26, 2014; Nashville, Tennessee. 2014. [Google Scholar]
- 29.Shwachman H, Fekete E, Kulczycki LL, Foley GE. The effect of long-term antibiotic therapy in patients with cystic fibrosis of the pancreas. Antibiot Annu. 1958;6:692–699. [PubMed] [Google Scholar]
- 30.Wallman IS, Hilton HB. Teeth pigmented by tetracycline. Lancet. 1962;279:827–829. doi: 10.1016/s0140-6736(62)91840-8. [DOI] [PubMed] [Google Scholar]
- 31.Lochary ME, Lockhart PB, Williams WT., Jr Doxycycline and staining of permanent teeth. Pediatr Infect Dis J. 1998;17:429–431. doi: 10.1097/00006454-199805000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Volovitz B, Shkap R, Amir J, Calderon S, Varsano I, Nussinovitch M. Absence of tooth staining with doxycycline treatment in young children. Clin Pediatr. 2007;46:121–126. doi: 10.1177/0009922806290026. [DOI] [PubMed] [Google Scholar]
- 33.Todd SR, Dahlgren FS, Traeger MS, Beltran-Aguilar ED, Marianos DW, Hamilton C, McQuiston JH, Regan JJ. No visible dental staining in children treated with doxycycline for suspected Rocky Mountain spotted fever. J. Pediatr. 2015;166:1246–1251. doi: 10.1016/j.jpeds.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 34.AAP Committee on Infectious Diseases . Rickettsial diseases. In: Pickering L, Baker C, Kimberlin D, Long S, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics Committee of Infectious Diseases; 2012. pp. 620–625. [Google Scholar]
- 35.Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, Dasch GA, Levin ML, Singleton J, Zaki SR, Cheek JE, Swerdlow DL, McQuiston JH. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353:587–594. doi: 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]
- 36.Regan JJ, Traeger MS, Humpherys D, Mahoney D, Martinez M, Emerson GL, Tack D, Geissler A, Yasmin S, Lawson R, Williams V, Hamilton C, Levy C, Komatsu K, Yost D, McQuiston JH. Risk factors for fatal outcome from Rocky Mountain spotted fever in a highly endemic area: Arizona, 2002–2011. Clin Infect Dis. 2015;60:7. doi: 10.1093/cid/civ116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drexler NA, Miller M, Gerding J, Todd SR, Adams L, Dahlgren FS, Bryant N, Weis E, Herrick K, Francies J, Komatsu K, Piontkowski S, Velascosoltero J, Shelhamer T, Hamilton B, Eribes C, Brock A, Sneezy P, Goseyun C, Bendle H, Hovet R, Williams V, Massung RF, McQuiston JH. Community-based control of the brown dog tick in a region with high rates of Rocky Mountain spotted fever, 2012–2013. PLoS One. 2014;9:e112368. doi: 10.1371/journal.pone.0112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hattwick M. Rocky Mountain spotted fever in the United States, 1920–1970. J Infect Dis. 1971;124:112–114. [Google Scholar]
- 39.Weinberg EH, Stakebake JR, Gerone PJ. Plaque assay for Rickettsia rickettsii. J Bacteriol. 1969;98:398–402. doi: 10.1128/jb.98.2.398-402.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kordova N. Plaque assay of rickettsiae. Acta Virol. 1966;10:278. [PubMed] [Google Scholar]
- 41.Cox HR. Use of yolk sac of developing chick embryo as medium for growing Rickettsiae of Rocky Mountain spotted fever and typhus groups. Publ Health Rep (1896–1970) 1938;53:2241–2247. [Google Scholar]
- 42.Johnston SH, Glaser CA, Padgett K, Wadford DA, Espinosa A, Espinosa N, Eremeeva ME, Tait K, Hobson B, Shtivelman S. Rickettsia spp. 364D causing a cluster of eschar-associated illness, California. Pediatr Infect Dis J. 2013;32:1036–1039. doi: 10.1097/INF.0b013e318296b24b. [DOI] [PubMed] [Google Scholar]
- 43.Bechah Y, Socolovschi C, Raoult D. Identification of rickettsial infections by using cutaneous swab specimens and PCR. Emerg Infect Dis. 2011;17:83–86. doi: 10.3201/eid1701.100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myers T, Lalani T, Dent M, Jiang J, Daly PL, Maguire JD, Richards AL. Detecting Rickettsia parkeri infection from eschar swab specimens. Emerg Infect Dis. 2013;19:778. doi: 10.3201/eid1905.120622. [DOI] [PMC free article] [PubMed] [Google Scholar]