Abstract
Spotted fever group (SFG) Rickettsia species are etiologic agents of a wide range of human infections from asymptomatic or mild infections to severe, life-threatening disease. In the United States, recent passive surveillance for SFG rickettsiosis shows an increased incidence and decreased severity of reported cases. The reasons for this are not well understood; however, we hypothesize that less pathogenic rickettsiae are causing more human infections, while the incidence of disease caused by more pathogenic rickettsiae, particularly Rickettsia rickettsii, is relatively stable. During the same period, the range of Amblyomma americanum has expanded. Amblyomma americanum is frequently infected with Candidatus Rickettsia amblyommii, a SFG Rickettsia of unknown pathogenicity. We tested our hypothesis by modeling incidence rates from 1993 to 2013, hospitalization rates from 1981 to 2013, and case fatality rates from 1981 to 2013 regressed against the presence of A. americanum, the decade of onset of symptoms, and the county of residence. Our results support the hypothesis, and we show that the expanding range of A. americanum is associated with changes in epidemiology reported through passive surveillance. We believe epidemiological and acarological data collected on individual cases from enhanced surveillance may further elucidate the reasons for the changing epidemiology of SFG rickettsiosis.
Introduction
Rocky Mountain spotted fever (RMSF) is a severe, life-threatening illness caused by infection with Rickettsia rickettsii, the most virulent of all known spotted fever group (SFG) Rickettsia species. Early treatment with doxycycline typically results in rapid recovery; but, after the fifth day of illness, untreated cases have high chances of severe illness, including disseminated intravascular coagulopathy and multisystem organ failure, potentially leading to permanent disabilities or death.1–6 Confirmed cases of RMSF occur sporadically across the United States with concentration in the south central and southeastern states.3,7,8
In the United States, several other SFG Rickettsia species cause human disease, but these are characteristically far milder infections compared with RMSF and are not associated with deaths. Rickettsia akari causes rickettsialpox, a febrile illness with a rash, associated with mites and house mice.9,10 Rickettsia parkeri causes a febrile illness with a characteristic eschar.11,12 The Gulf Coast tick (Amblyomma maculatum) was the first discovered vector of R. parkeri, but the lone star tick (Amblyomma americanum) is also a potential vector.13,14 In California, Rickettsia species 364D causes a febrile illness associated with an eschar, and the cases reported to date have not required hospitalization.15,16 The Pacific Coast tick (Dermacentor occidentalis) is the suspected vector of Rickettsia species 364D, and reported cases have so far been within its range.16
Serological evidence suggests other SFG Rickettsia species may cause mild or abortive infections. Rickettsia montanensis has historically been considered nonpathogenic to humans because of the absence of human cases and the high prevalence of R. montanensis in the American dog tick (Dermacentor variabilis), which is also the primary vector of R. rickettsii.17 However, a single case report associated R. montanensis with a mild, afebrile illness.18 Cases of SFG rickettsiosis reported through public health surveillance systems in North Carolina may be attributed to Candidatus Rickettsia amblyommii.19 Using polymerase chain reaction, researchers demonstrated the presence of Ca. R. amblyommii in ticks removed from humans, including a case with a rash localized to the site of tick attachment.20,21 In addition, a longitudinal study of dogs in Oklahoma demonstrated rickettsemia and seroconversion to several Rickettsia species following tick exposure, despite no clinical illness.22 These various rickettsiae are antigenically related, and serologic assays developed for the diagnosis of RMSF may react nonspecifically with antigens of less pathogenic SFG Rickettsia species.7 In this context, it is not possible to differentiate SFG rickettsioses by serology alone.
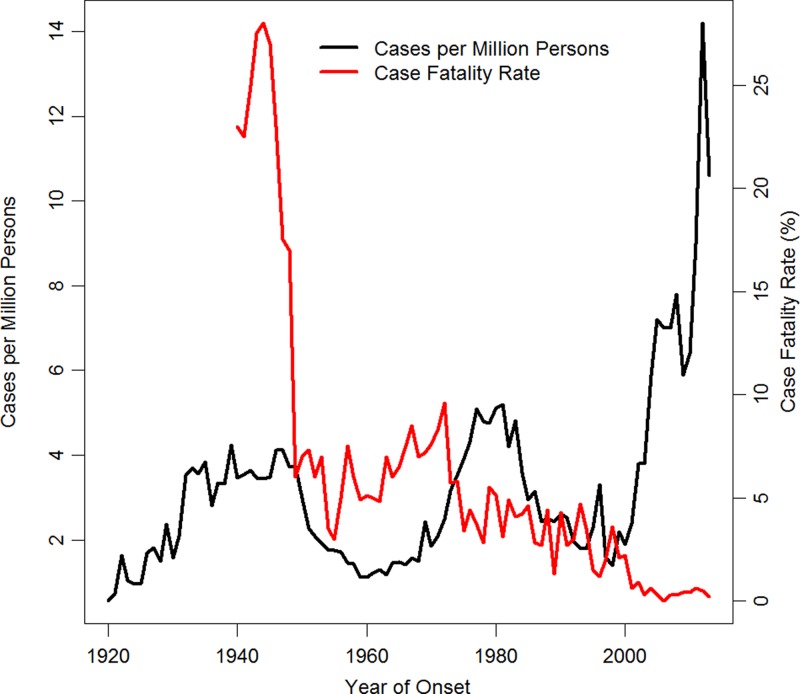
Passive surveillance for SFG rickettsiosis in the United States began in 1920.23 The case fatality rate (CFR) for RMSF, which was 1–10% in the United States during 1950–2000, dropped precipitously in 2001, and has remained under 1% for 12 consecutive years (Figure 1 ).3,8 Accompanying this trend was a sizeable increase in reported incidence, with the reported annual incidence rate (IR) topping historical highs of six cases per million persons, reaching into cases per 100,000 for the first time in recorded history (Figure 1).3,8 Possible explanations for these changes in reported epidemiology include changes in public health surveillance, clinical practice, use of enzyme-linked immunosorbent assay, and the underlying epidemiology of RMSF and other SFG rickettsiosis.3 Determining whether this trend observed in passive surveillance is a result of changes in the epidemiology of SFG rickettsiosis or an artifact of surveillance is a matter of public health importance.
Figure 1.
Historical incidence rate and case fatality rate of spotted fever group rickettsiosis in the United States, 1920–2013 (2013 CDC unpublished data).3,8 The two measures are negatively correlated (Spearman's ρ = −0.37, P = 0.001).
A regional study suggests that changes in the epidemiology of SFG rickettsiosis may be unrelated to changes in the ecology of the American dog tick, historically identified as the principal vector of R. rickettsii in the United States.24 Another study mapped the approximate distribution of the American dog tick, the lone star tick, the brown dog tick (Rhipicephalus sanguineus), the Gulf Coast tick, and the area with the highest reported RMSF incidence during 2001–2005 to illustrate the overlap of reported RMSF with the ranges of the lone star tick, the brown dog tick, and the Gulf Coast tick populations, suggesting most cases reported as RMSF might be other SFG rickettsiosis.17 The range of the lone star tick is expanding into areas previously thought uninhabited by the tick, which may affect changes in the epidemiology of SFG rickettsiosis.25,26 We hypothesize that less pathogenic rickettsiae are causing more human infections, while the incidence of disease caused by R. rickettsii is relatively stable. Here, we use data from passive surveillance for human SFG rickettsiosis and data on the presence of the lone star tick to test our hypothesis by investigating the association of the widening range of the lone star tick with an increase in incidence, a decrease in CFR, and a decrease in hospitalization.27
Methods
Tick data.
We used county-level distribution data for A. americanum from 1980 to 2013 adopted from the work of Springer and others,27 maintaining the designations of established, reported, or absent as defined by the authors. Counties were designated as
-
•
established, if at least six ticks from one life stage or at least two life stages were found,
-
•
reported, if one to five ticks of a single life stage were found, or
-
•
absent otherwise.
Springer and others used the years the ticks were collected to tie reports in the literature to decades, and when absent, the year of publication was used as a proxy. We allowed each decade to inherit the previous decades' evidence for the tick's status in our data, as investigators may not have been interested in reestablishing the presence of the tick decade after decade in the literature.
Human data.
The Centers for Disease Control and Prevention (CDC) has two passive surveillance systems that collect data for SFG rickettsiosis. Since 1981, state and territorial public health departments report cases of SFG rickettsiosis to the CDC by case report forms (CRFs) (http://www.cdc.gov/ticks/forms/2010_tbrd_crf.pdf), and these CRFs include the county of residence, year of onset, and data regarding hospitalization and survival or death from disease. Since 1993, public health departments report cases of SFG rickettsiosis to the CDC through the Nationally Notifiable Disease Surveillance System (NNDSS), and these data also include the county of residence and onset date (http://wwwn.cdc.gov/NNDSS/). Incidence rates were computed using county population estimates from the U.S. Census Bureau, Population Division, for 1993–2013. Because Rickettsia species 364D, R. parkeri, and R. akari are not known to cause fatal SFG rickettsiosis, we used the CFR as a marker for the presence of RMSF in the passive surveillance data. Similarly, we used hospitalization as a softer marker for the presence of RMSF, as cases of R. parkeri rickettsiosis may be diagnosed as RMSF and subsequently hospitalized.
Statistical methods.
We used three generalized additive models to investigate the relationship between SFG rickettsiosis and the status of A. americanum populations: absent, reported, or established. Cases were assigned latitude and longitude based on the centroid of their county of residence, and this spatial component was included in our models using thin plate splines with a basis of dimension 40.28,29 The parametric part of each model included the distribution status of A. americanum, the decade of onset of symptoms, and an interaction term between the main effects. A log-binomial model was constructed for both the hospitalization rate (HR) and the CFR. Data from 1981 to 2013 were included in these analyses using the CRFs. A Poisson model was constructed for the number of reported cases from the NNDSS using population estimates from the U.S. Census Bureau, Population Division, to compute the offset for each county for each decade. Data from 1993 to 2013 were used in this analysis. Nonparametric models were regressed only on thin plate splines to generate maps. We used the Wald χ2 test for linear hypotheses from our regression models at α = 0.05, and we used the Wald interval to construct 95% confidence intervals (CIs). Because the interaction terms in our model were all significant, we did not perform hypothesis testing or construct CIs for results outside our models. Analysis was performed using SAS software (Version 9.3 of the SAS System for Windows; Copyright 2010, SAS Institute Inc., Cary, NC) and R software (Version 3.1.1; Copyright 2014, R Foundation for Statistical Computing, Vienna, Austria).
Results
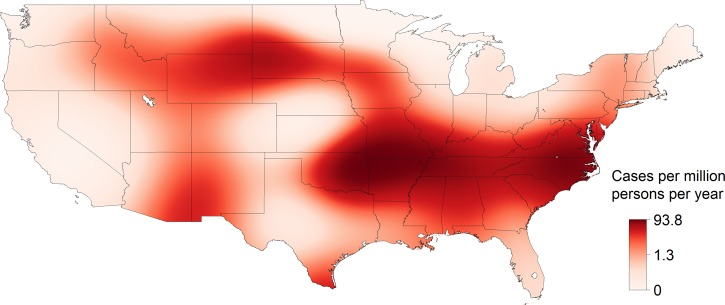
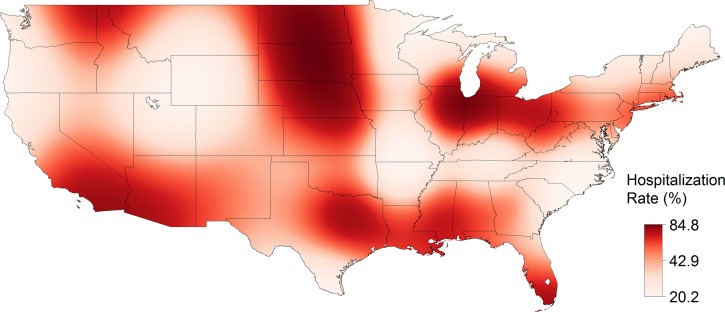
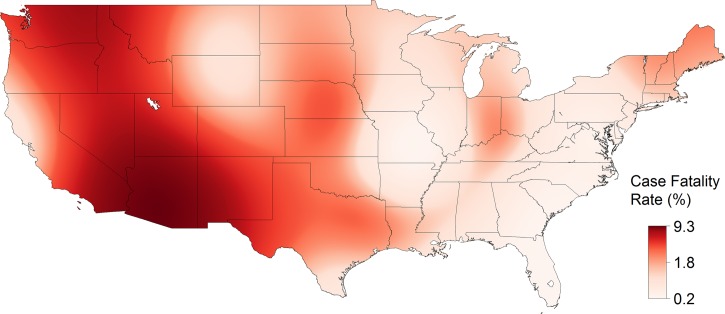
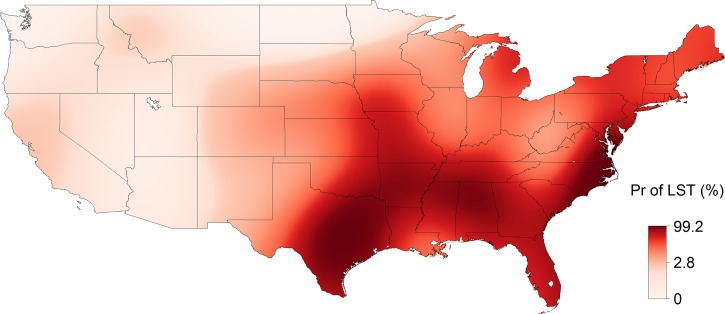
From 1993 to 2013, 31,234 cases of SFG rickettsiosis were reported through NNDSS, which included a county of residence, for an IR of 5.20 cases per million persons per year. The reported IR increased each decade and increased with the strength of evidence (absent < reported < established) for the presence of A. americanum (Table 1). The estimated IR was highest, up to 94 cases per million persons per year, in a region spanning from Arkansas and Missouri to Virginia (Figure 2 ). From 1981 to 2013, 21,483 cases of SFG rickettsiosis were reported through CRFs, which included hospitalization status and county of residence. Of these cases, 7,597 were hospitalized (HR = 35.4%). In general, the reported HR decreased with time and with the presence of A. americanum (Table 1). The estimated HR was high along the Gulf of Mexico, from 65% to 85% (Figure 3 ). From 1981 to 2013, 21,443 cases of SFG rickettsiosis were reported through CRFs with data on whether the case survived or died and county of residence, and 210 of these cases were fatal (CFR = 0.98%). In general, the reported CFR decreased with time and decreased with the presence of A. americanum (Table 1). The estimated CFR was high in a region spanning Arizona to Montana, from 2.4% to 9.3% (Figure 4 ). Amblyomma americanum was either reported or established in much of the South, parts of the Midwest, and parts of the Northeast by the 2010s (Figure 5 ).
Table 1.
The IR, HR, and CFR of reported cases of spotted fever group rickettsiosis by status of Amblyomma americanum within a county and decade of onset of symptoms
| IR* | HR (%) | CFR (%) | |
|---|---|---|---|
| Amblyomma americanum within county | |||
| Absent | 2.28 | 48.6 | 1.68 |
| Reported | 6.07 | 33.2 | 0.74 |
| Established | 10.3 | 27.8 | 0.65 |
| Decade of onset of symptoms | |||
| 1980s | – | 72.0 | 2.80 |
| 1990s | 1.68 | 51.0 | 2.03 |
| 2000s | 5.38 | 24.4 | 0.43 |
| 2010s | 10.0 | 27.3 | 0.47 |
CFR = case fatality rate; HR = hospitalization rate; IR = incidence rate.
The IRs were calculated using case counts from the Nationally Notifiable Disease Surveillance System and population estimates from the United States Census Bureau, Population Division, for 1993–2013. The HRs and CFRs were calculated using the case report form data for 1981–2013. The statistics in this table are crude statistics, not adjusted statistics from our regression models.
Cases per million persons per year.
Figure 2.
The incidence rate (IR) of spotted fever group rickettsiosis in the United States, 1993–2013. IRs were estimated from a nonparametric Poisson model using thin plate splines (P < 0.0001), with case counts from the Nationally Notifiable Disease Surveillance System and population estimates from the U.S. Census Bureau, Population Division.
Figure 3.
The hospitalization rate (HR) of spotted fever group rickettsiosis in the United States, 1981–2013. HRs were estimated from a nonparametric log-binomial model using thin plate splines (P < 0.0001). Data are from case report forms.
Figure 4.
The case fatality rate (CFR) of spotted fever group rickettsiosis in the United States, 1981–2013. CFRs were estimated from a nonparametric log-binomial model using thin plate splines (P < 0.0001). Data are from case report forms.
Figure 5.
The distribution of the lone star tick (Amblyomma americanum) in the United States, 2013. The probability of the lone star tick being reported or established (vs. absent) was estimated from a nonparametric binomial logistic regression model using thin plate splines (P < 0.0001). Data are from Springer and others.27
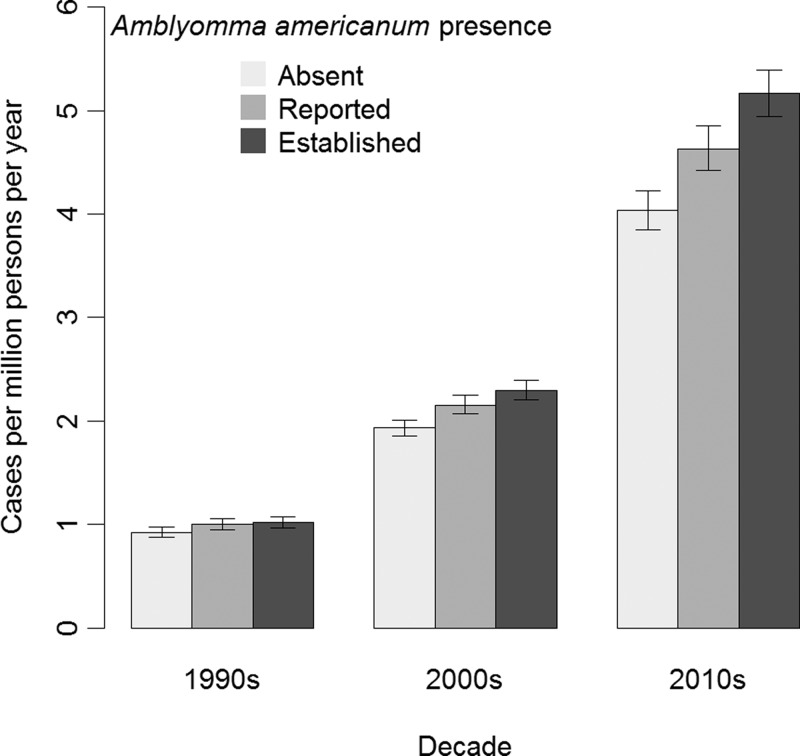
Our full model of the IR of SFG rickettsiosis was statistically significant (P < 0.0001). The effect of the presence of the lone star tick and IRs was not the same for each decade (P = 0.0007; Figure 6 ). In the 1990s, the estimated IR was greater in counties where A. americanum was reported than counties where A. americanum was absent (IR ratio [IRR] = 1.08, 95% CI = 1.01–1.16), and the estimated IR in counties where A. americanum was established was greater than counties where A. americanum was absent (IRR = 1.10, CI = 1.03–1.17). By the 2010s, counties where A. americanum was reported had a higher IR than counties where A. americanum was absent (IRR = 1.15, CI = 1.09–1.21) and counties where A. americanum was established had a higher IR than counties where A. americanum was absent (IRR = 1.28, CI = 1.22–1.34). The IR increased between decades (IRR = 2.08, CI = 2.02–2.16). The correlation between regression parameters for the decade of onset with A. americanum being reported was 0.65 and with A. americanum being established was 0.71.
Figure 6.
A plot of the incidence rate (IR) of spotted fever group rickettsiosis vs. decade of report by Amblyomma americanum presence, 1993–2013. The estimates are from our Poisson model of the IR using case counts from the Nationally Notifiable Disease Surveillance System and population estimates from the U.S. Census Bureau, Population Division. The error bars represent Wald 95% confidence intervals.
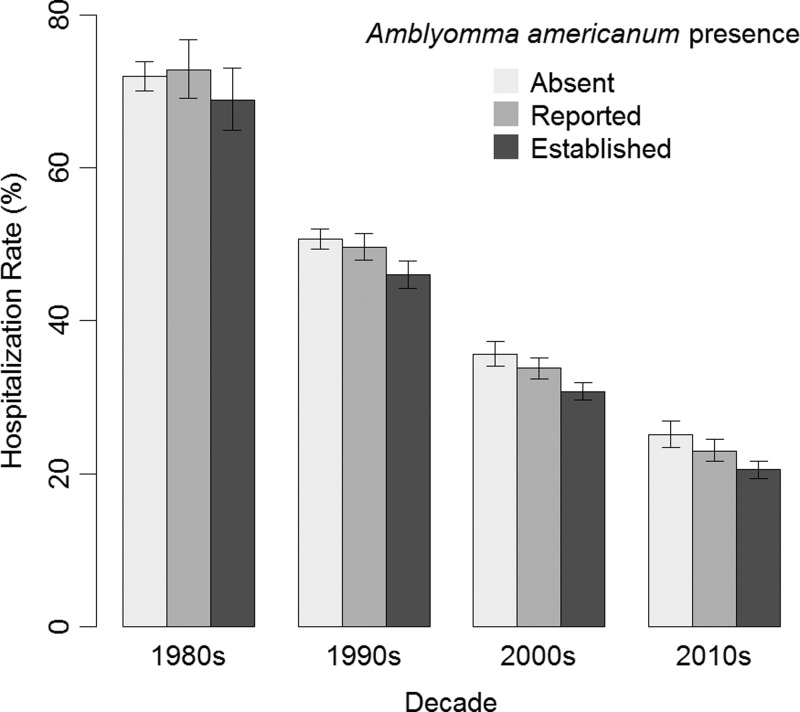
Our model of the HR was statistically significant (P < 0.0001). The effect of A. americanum populations and HR was not the same for each decade (P = 0.004, Figure 7 ). In the 1980s, whether A. americanum was absent, reported, or established within a county was not associated with HR (P = 0.31). By the 2010s, the HR in counties where A. americanum was reported was similar to counties where A. americanum was absent (risk ratio [RR] = 0.92, CI = 0.83–1.01), and the HR in counties where A. americanum was established was lower than counties where A. americanum was absent (RR = 0.82, CI = 0.74–0.90). The HR decreased each decade (RR = 0.70, CI = 0.68–0.73). The correlation between the regression parameters for the decade of onset with A. americanum being reported was 0.16 and with A. americanum being established was 0.16.
Figure 7.
A plot of the hospitalization rate of spotted fever group rickettsiosis vs. decade of report by Amblyomma americanum presence, 1981–2013. The estimates are from our log-binomial model of the hospitalization rate using data from case report forms. The error bars represent Wald 95% confidence intervals.
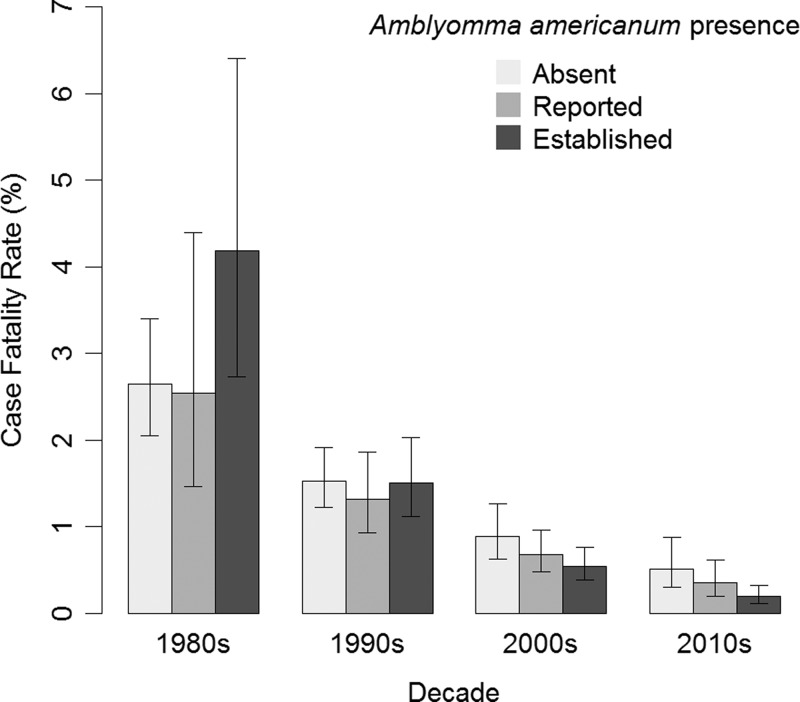
Our model of the CFR was statistically significant (P < 0.0001). The effect of the presence of A. americanum on CFR was not the same for each decade (P = 0.03; Figure 8 ). In the 1980s, whether A. americanum was absent, reported, or established was not associated with CFR (P = 0.15). By the 2010s, the CFR among counties where A. americanum was reported was similar to that among counties where A. americanum was absent (RR = 0.68, CI = 0.46–1.01), and the CFR among counties where A. americanum was established was lower than counties where A. americanum was absent (RR = 0.38, CI = 0.26–0.56). The CFR decreased each decade (RR = 0.58, CI = 0.47–0.72). The correlation between the regression parameters for the decade of onset with A. americanum being reported was 0.20 and with A. americanum being established was 0.24.
Figure 8.
A plot of the case fatality rate (CFR) of spotted fever group rickettsiosis vs. decade of report by Amblyomma americanum presence, 1981–2013. The estimates are from our log-binomial model of the CFR using data from case report forms. The error bars represent Wald 95% confidence intervals.
Discussion
Our results support the hypothesis that the expanding range of the lone star tick contributes to the increasing incidence and decreasing severity, as measured by HR and CFR, of reported SFG rickettsiosis because of a decreasing proportion of reported cases being RMSF. However, other factors may also play an important role, because the effect of time in our models was always stronger than the effects of the presence of the lone star tick. From our model of incidence, the IR was highest in counties with an established presence of the lone star tick each decade. But, counties with no reports of the lone star tick in the literature, largely in the western United States, also saw large increases in incidence over time (Figure 6). Similarly, the HR was lowest in counties with an established presence of the lone star tick each decade (Figure 7). The trend was not as simple for the CFR. The presence of the lone star tick was not associated with CFR in the 1980s, but by the 2010s, the CFR was lower in counties with stronger evidence of the lone star tick (Figure 8). Yet, the primary factor explaining the decrease in CFR in our model is again time, as the differences in the presence of the lone star tick within a decade are much less than the differences between decades (Figure 8). Generally, the dose–response relationship of the strength of evidence for the presence of the lone star tick with both the incidence and the severity of SFG rickettsiosis supports the hypothesis that the expanding range of the lone star tick is responsible for some of the changes in the epidemiology of SFG rickettsiosis.
Doxycycline has been a recommended treatment of RMSF and other SFG rickettsiosis for decades, and the association between treatment with chloramphenicol and an increased risk of fatal outcome led to doxycycline becoming the single recommended treatment.2 This discovery alone may be sufficient to explain the decreased CFR of SFG rickettsiosis between the 1990s and the 2000s (Table 1). However, oral chloramphenicol was no longer manufactured in the United States by 1991, so only the intravenous formulations—exclusive to the hospital setting—were available for treating SFG rickettsiosis.30 Removal of chloramphenicol as a treatment for SFG rickettsiosis could not have decreased the HR between the 1990s and the 2000s. Either preventing severe illness by appropriate, early treatment or a change in the distribution of the etiologic agents causing SFG rickettsiosis could explain the reported changes.
In most regions of the United States, several distinct etiologic agents contribute to the epidemiology of SFG rickettsiosis. An exception is Arizona, where peridomestic RMSF is associated with pet dogs and the brown dog tick.31 No other tick vectors or SFG Rickettsia have been associated conclusively with human disease in this state. Here, the IR, HR, and CFR are all higher than the national average (Figures 2–4). These trends in Arizona are consistent with the historical epidemiology of RMSF. Our maps also suggest that a similar phenomenon continues in Idaho and Montana, where the first reports originated, giving RMSF its namesake.32,33
While the prevalence of R. rickettsii in D. variabilis is typically under 1%, the prevalence of Ca. R. amblyommii in A. americanum is often over 50%.17,20,34–44 Because of these large differences in prevalence, exposure to R. rickettsii may be rare relative to exposure to Ca. R. amblyommii. A prospective case series in North Carolina demonstrated that most reported cases of RMSF were another SFG rickettsiosis, as these illnesses were milder than classical RMSF and serological titers were generally higher for Ca. R. amblyommii than for R. rickettsii.19 A similar study at Fort Chaffee, Arkansas, produced similar results: Guardsmen who seroconverted to R. rickettsii often did not have an illness compatible with a tickborne disease.45 The association of the presence of the lone star tick with both decreased severity and increased incidence of SFG rickettsiosis supports these findings, and most cases of SFG rickettsiosis reported through passive surveillance are unlikely to be RMSF. Besides the lone star tick and Ca. R. amblyommii, other vectors and SFG Rickettsia species may contribute to the changes in epidemiology of SFG rickettsiosis, as the temporal trend toward less severe disease and higher incidence is only partly explained by changes in the range of the lone star tick.
The ecology of R. parkeri, which is thought to be vectored primarily by the Gulf Coast tick, may also play an important role in the changes of the epidemiology of SFG rickettsiosis.12 The Gulf Coast tick is an aggressive biter of humans, and the prevalence of R. parkeri in the Gulf Coast tick is often > 20%.46 Despite its name, the range of the Gulf Coast tick has expanded beyond the coast of the Gulf of Mexico.47 Our results suggest a contribution of the Gulf Coast tick to the national statistics for SFG rickettsiosis. In the United States, the HR is high along the Gulf Coast tick (between the Brazos River and the Chattahoochee River) relative to the areas with highest incidence, while the CFR is not (Figures 3 and 4). This finding is more consistent with R. parkeri rickettsiosis than with either RMSF or infections with less pathogenic SFG Rickettsia species, suggesting the importance of R. parkeri and its vector may be underappreciated.46 In Kansas and Oklahoma, the Gulf Coast tick may not carry R. parkeri because of high prevalence of Candidatus Rickettsia andeanae, an agent of unknown human pathogenicity.48 In our results, the HR in Kansas and Oklahoma was lower than other parts of the Gulf Coast tick's range, suggesting that the Gulf Coast tick may not be a vector associated with severe SFG rickettsiosis in these states.
The lone star tick is also the vector of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME).25 The CFR of HME is low (< 2%), but the HR of HME is 50%.49,50 The maps in Figure 2 show an elevated IR from Missouri and Arkansas to Virginia. However, the HR in these areas is well below the reported HR of HME (Figure 3). Although the difference is less striking, the CFR in these areas is also below the reported CFR of HME (Figure 4). While the serological assays for R. rickettsii do cross-react with E. chaffeensis, the reported epidemiology of SFG rickettsiosis in this region is not severe enough to be consistent with reported epidemiology of HME.7
Our results are subject to limitations. Importantly, our methods are limited by the ecological fallacy: the associations we report at the county level do not imply that the lone star tick is responsible for any human disease. This limitation is particularly problematic in studies involving data generated through passive surveillance, as identifying the tick vector responsible for a representative sample of cases of SFG rickettsiosis is not feasible. Without epidemiological and acarological data on individual case-patients, inference is limited to the county level. A challenge to interpreting the results from our model is whether the observed associations with the changing epidemiology of SFG rickettsiosis and the expanding range of the lone star tick are collinear. When modeling hospitalization and fatality, the presence of the lone star tick is not correlated with the decade of onset. When modeling incidence, the two are highly correlated; although, our methods should be sufficiently robust to a correlation of 0.71 and 0.66 between regression parameters. This implies that as the range of the tick vector expands with time and the IR of SFG rickettsiosis increases with time, teasing out the effects of the expanding tick vector on IR is subject to more uncertainty. We observed no other issues with multicollinearity (data not shown). The human data presented here are based on passive surveillance, which is subject to a variety of artifacts. Similarly, the tick data are based on the interests of individual investigators. Especially, investigators may have found the lone star tick incidentally during extensive investigations into the ecology of Ixodes scapularis. Altogether, our data are less than ideal, with systematic gaps in coverage and completeness. Further, the degree of completeness at any particular location may change over time. Given the length of the periods we investigated, we expect these limitations to contribute to bias toward the null. The lone star tick data do not exactly match periods of the human data. For the analysis of IR, we included data on the lone star tick from 1980 to 2013 and human data from 1981 to 2013. For the analysis of HR and CFR, we included data on the lone star tick from 1990 to 2013 and human data from 1993 to 2013. However, we felt this discrepancy was preferable to using only data from 2000 to 2013 in the analysis of IR and using only data from 1990 to 2013 for the analyses of HR and CFR.
Despite the limitations of our data and methods, we have demonstrated that the expansion of the lone star tick is associated with increasing incidence and decreasing severity of reported SFG rickettsiosis in the United States. As a variety of SFG Rickettsia species, vectors, and reservoirs drive the epidemiology of SFG rickettsiosis in the United States, we are not surprised that our models were able to explain only a fraction of the trends observed in passive surveillance. Including the status of other tick vectors, such as the Gulf Coast tick, as well as the prevalence of SFG Rickettsia in these vectors in our models may also expand our understanding; however, such an analysis will still be inferior to a study at the individual case-patient level. Enhanced surveillance for tickborne rickettsial diseases at endemic sites may provide the ecological, clinical, epidemiological, and laboratory data needed to further elucidate the complex, dynamic natural history of SFG rickettsiosis in the United States.
ACKNOWLEDGMENTS
We thank Naomi Drexler, Kristen Nichols Heitman, Eric Mandel, John Krebs, Lindsey Pool, Alison Mondul, and Lois Vernon for their work in building the human data, and the countless entomologists, laboratorians, health-care providers, and epidemiologists who contributed to these data.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Financial support: This study was supported by the Centers for Disease Control and Prevention.
Authors' addresses: F. Scott Dahlgren, Christopher D. Paddock, and Casey Barton Behravesh, Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: iot0@cdc.gov, cdp9@cdc.gov, and dlx9@cdc.gov. Yuri P. Springer and Rebecca J. Eisen, Bacterial Diseases Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Fort Collins, CO, E-mails: yurispringer@gmail.com and dyn2@cdc.gov.
References
- 1.Dalton MJ, Clarke MJ, Holman RC, Krebs JW, Fishbein DB, Olson JG, Childs JE. National surveillance for Rocky Mountain spotted fever, 1981–1992: epidemiologic summary and evaluation of risk factors for fatal outcome. Am J Trop Med Hyg. 1995;52:405–413. doi: 10.4269/ajtmh.1995.52.405. [DOI] [PubMed] [Google Scholar]
- 2.Holman RC, Paddock CD, Curns AT, Krebs JW, McQuiston JH, Childs JE. Analysis of risk factors for fatal Rocky Mountain spotted fever: evidence for superiority of tetracyclines for therapy. J Infect Dis. 2001;184:1437–1444. doi: 10.1086/324372. [DOI] [PubMed] [Google Scholar]
- 3.Openshaw JJ, Swerdlow DL, Krebs JW, Holman RC, Mandel E, Harvey A, Haberling D, Massung RF, McQuiston JH. Rocky Mountain spotted fever in the United States, 2000–2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg. 2010;83:174–182. doi: 10.4269/ajtmh.2010.09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Consequences of delayed diagnosis of Rocky Mountain spotted fever in children—West Virginia, Michigan, Tennessee, and Oklahoma, May–July 2000. MMWR. 2000;49:885–888. [PubMed] [Google Scholar]
- 5.Traeger MS, Regan JJ, Humpherys D, Mahoney DL, Martinez M, Emerson GL, Tack DM, Geissler A, Yasmin S, Lawson R, Hamilton C, Williams V, Levy C, Komatsu K, McQuiston JH, Yost DA. Rocky Mountain spotted fever characterization and comparison to similar illnesses in a highly endemic area—Arizona, 2002–2011. Clin Infect Dis. 2015;60:1650–1658. doi: 10.1093/cid/civ115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regan JJ, Traeger MS, Humpherys D, Mahoney DL, Martinez M, Emerson GL, Tack DM, Geissler A, Yasmin S, Lawson R, Williams V, Hamilton C, Levy C, Komatsu K, Yost DA, McQuiston JH. Risk factors for fatal outcome from Rocky Mountain spotted fever in a highly endemic area—Arizona, 2002–2011. Clin Infect Dis. 2015;60:1659–1666. doi: 10.1093/cid/civ116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichiosis, and anaplasmosis—United States. MMWR Recomm Rep. 2006;55:1–27. [PubMed] [Google Scholar]
- 8.Drexler N, Dahlgren FS, Nichols Heitman K, Massung RF, Paddock CD, Barton Behravesh C. National surveillance of spotted fever group rickettsioses in the United States, 2008–2012. Am J Trop Med Hyg. 2016;94:26–34. doi: 10.4269/ajtmh.15-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huebner RJ, Jellison WL, Armstrong C. Rickettsial pox: a newly recognized rickettsial disease: V. Recovery of Rickettsia akari from a house mouse (Mus musculus) Public Health Rep. 1947;62:777–780. [PubMed] [Google Scholar]
- 10.Greenberg M, Pellitteri O, Klein IF, Huebner RJ. Rickettsialpox—a newly recognized rickettsial disease: II. Clinical observations. J Am Med Assoc. 1947;133:901–906. doi: 10.1001/jama.1947.02880130001001. [DOI] [PubMed] [Google Scholar]
- 11.Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SL, Tamminga CL, Ohl CA. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- 12.Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, McLellan SL, Goddard J, Holman RC, Openshaw JJ, Sumner JW, Zaki SR, Eremeeva ME. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis. 2008;47:1188–1196. doi: 10.1086/592254. [DOI] [PubMed] [Google Scholar]
- 13.Parker RR. Observations on an infectious agent from Amblyomma maculatum. Public Health Rep. 1939;54:1482–1484. [Google Scholar]
- 14.Cohen SB, Yabsley MJ, Garrison LE, Freye JD, Dunlap BG, Dunn JR, Mead DG, Jones TF, Moncayo AC. Rickettsia parkeri in Amblyomma americanum ticks, Tennessee and Georgia, USA. Emerg Infect Dis. 2009;15:1471–1473. doi: 10.3201/eid1509.090330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston SH, Glaser CA, Padgett K, Wadford DA, Espinosa A, Espinosa N, Eremeeva ME, Tait K, Hobson B, Shtivelman S, Hsieh C, Messenger SL. Rickettsia spp. 364D causing a cluster of eschar-associated illness, California. Pediatr Infect Dis J. 2013;32:1036–1039. doi: 10.1097/INF.0b013e318296b24b. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro MR, Fritz CL, Tait K, Paddock CD, Nicholson WL, Abramowicz KF, Karpathy SE, Dasch GA, Sumner JW, Adem PV, Scott JJ, Padgett KA, Zaki SR, Eremeeva ME. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis. 2010;50:541–548. doi: 10.1086/649926. [DOI] [PubMed] [Google Scholar]
- 17.Stromdahl EY, Jiang J, Vince M, Richards AL. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group rickettsiae in the United States. Vector Borne Zoonotic Dis. 2011;11:969–977. doi: 10.1089/vbz.2010.0099. [DOI] [PubMed] [Google Scholar]
- 18.McQuiston JH, Zemtsova G, Perniciaro J, Hutson M, Singleton J, Nicholson WL, Levin ML. Afebrile spotted fever group Rickettsia infection after a bite from a Dermacentor variabilis tick infected with Rickettsia montanensis. Vector Borne Zoonotic Dis. 2012;12:1059–1061. doi: 10.1089/vbz.2012.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW. Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. doi: 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- 20.Jiang J, Yarina T, Miller MK, Stromdahl EY, Richards AL. Molecular detection of Rickettsia amblyommii in Amblyomma americanum parasitizing humans. Vector Borne Zoonotic Dis. 2010;10:329–340. doi: 10.1089/vbz.2009.0061. [DOI] [PubMed] [Google Scholar]
- 21.Billeter SA, Blanton HL, Little SE, Levy MG, Breitschwerdt EB. Detection of Rickettsia amblyommii in association with a tick bite rash. Vector Borne Zoonotic Dis. 2007;7:607–610. doi: 10.1089/vbz.2007.0121. [DOI] [PubMed] [Google Scholar]
- 22.Barrett A, Little SE, Shaw E. “Rickettsia amblyommii” and R. montanensis infection in dogs following natural exposure to ticks. Vector Borne Zoonotic Dis. 2014;14:20–25. doi: 10.1089/vbz.2013.1325. [DOI] [PubMed] [Google Scholar]
- 23.National Office of Vital Statistics Reported incidence of selected notifiable diseases: United States, each division and state, 1920–1950. Vital Statistics. Special Reports. 1953;37:179–243. [Google Scholar]
- 24.Atkinson SF, Sarkar S, Avina A, Schuermann JA, Williamson P. Modelling spatial concordance between Rocky Mountain spotted fever disease incidence and habitat probability of its vector Dermacentor variabilis (American dog tick) Geospat Health. 2012;7:91–100. doi: 10.4081/gh.2012.108. [DOI] [PubMed] [Google Scholar]
- 25.Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- 26.Blanton LS, Mendell NL, Walker DH, Bouyer DH. “Rickettsia amblyommii” induces cross protection against lethal Rocky Mountain spotted fever in a guinea pig model. Vector Borne Zoonotic Dis. 2014;14:557–562. doi: 10.1089/vbz.2014.1575. [DOI] [PubMed] [Google Scholar]
- 27.Springer YP, Eisen L, Beati L, James AM, Eisen RJ. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae) J Med Entomol. 2014;51:342–351. doi: 10.1603/me13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood SN. Generalized Additive Models: An Introduction with R. Boca Raton, FL: Chapman and Hall/CRC; 2006. [Google Scholar]
- 29.Bockman SF. Generalizing the formula for areas of polygons to moments. Am Math Mon. 1989;96:131–132. [Google Scholar]
- 30.Salvatore M, Meyers BR. Tetracyclines and chloramphenicol. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th edition. Vol. 1. Philadelphia, PA: Elsevier Churchill Livingstone; 2010. pp. 385–402. [Google Scholar]
- 31.Drexler N, Miller M, Gerding J, Todd S, Adams L, Dahlgren FS, Bryant N, Weis E, Herrick K, Francies J, Komatsu K, Piontkowski S, Velascosoltero J, Shelhamer T, Hamilton B, Eribes C, Brock A, Sneezy P, Goseyun C, Bendle H, Hovet R, Williams V, Massung R, McQuiston JH. Community-based control of the brown dog tick in a region with high rates of Rocky Mountain spotted fever, 2012–2013. PLoS One. 2014;9:e112368. doi: 10.1371/journal.pone.0112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood MW. 1896. Spotted fever as reported from Idaho. Report to the Surgeon General of the Army; pp. 60–65. [Google Scholar]
- 33.Cobb JO. The so-called “spotted fever” of the Rocky Mountains—a new disease in Bitter Root Valley, Mont. Public Health Rep. 1902;17:1868–1870. [Google Scholar]
- 34.Williamson PC, Billingsley PM, Teltow GJ, Seals JP, Turnbough MA, Atkinson SF. Borrelia, Ehrlichia, and Rickettsia spp. in ticks removed from persons, Texas, USA. Emerg Infect Dis. 2010;16:441–446. doi: 10.3201/eid1603.091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fornadel CM, Zhang X, Smith JD, Paddock CD, Arias JR, Norris DE. High rates of Rickettsia parkeri infection in Gulf Coast ticks (Amblyomma maculatum) and identification of “Candidatus Rickettsia andeanae” from Fairfax County, Virginia. Vector Borne Zoonotic Dis. 2011;11:1535–1539. doi: 10.1089/vbz.2011.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fritzen CM, Huang J, Westby K, Freye JD, Dunlap B, Yabsley MJ, Schardein M, Dunn JR, Jones TF, Moncayo AC. Infection prevalences of common tick-borne pathogens in adult lone star ticks (Amblyomma americanum) and American dog ticks (Dermacentor variabilis) in Kentucky. Am J Trop Med Hyg. 2011;85:718–723. doi: 10.4269/ajtmh.2011.10-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moncayo AC, Cohen SB, Fritzen CM, Huang E, Yabsley MJ, Freye JD, Dunlap BG, Huang J, Mead DG, Jones TF, Dunn JR. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010;83:653–657. doi: 10.4269/ajtmh.2010.09-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagac BB, Miller MK, Mazzei MC, Nielsen DH, Jiang J, Richards AL. Rickettsia parkeri and Rickettsia montanensis, Kentucky and Tennessee, USA. Emerg Infect Dis. 2014;20:1750–1752. doi: 10.3201/eid2010.140175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mixson TR, Campbell SR, Gill JS, Ginsberg HS, Reichard MV, Schulze TL, Dasch GA. Prevalence of Ehrlichia, Borrelia, and Rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J Med Entomol. 2006;43:1261–1268. doi: 10.1603/0022-2585(2006)43[1261:poebar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Castellaw AH, Showers J, Goddard J, Chenney EF, Varela-Stokes AS. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J Med Entomol. 2010;47:473–476. doi: 10.1603/me09263. [DOI] [PubMed] [Google Scholar]
- 41.Smith MP, Ponnusamy L, Jiang J, Ayyash LA, Richards AL, Apperson CS. Bacterial pathogens in ixodid ticks from a Piedmont County in North Carolina: prevalence of rickettsial organisms. Vector Borne Zoonotic Dis. 2010;10:939–952. doi: 10.1089/vbz.2009.0178. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Ren X, Norris DE, Rasgon JL. Distribution and infection frequency of ‘Candidatus Rickettsia amblyommii’ in Maryland populations of the lone star tick (Amblyomma americanum) and culture in an Anopheles gambiae mosquito cell line. Ticks Tick Borne Dis. 2012;3:38–42. doi: 10.1016/j.ttbdis.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaines DN, Operario DJ, Stroup S, Stromdahl E, Wright C, Gaff H, Broyhill J, Smith J, Norris DE, Henning T, Lucas A, Houpt E. Ehrlichia and spotted fever group Rickettsiae surveillance in Amblyomma americanum in Virginia through use of a novel six-plex real-time PCR assay. Vector Borne Zoonotic Dis. 2014;14:307–316. doi: 10.1089/vbz.2013.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Killmaster LF, Loftis AD, Zemtsova GE, Levin ML. Detection of bacterial agents in Amblyomma americanum (Acari: Ixodidae) from Georgia, USA, and the use of a multiplex assay to differentiate Ehrlichia chaffeensis and Ehrlichia ewingii. J Med Entomol. 2014;51:868–872. doi: 10.1603/me13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCall CL, Curns AT, Rotz LD, Singleton JA, Jr, Treadwell TA, Comer JA, Nicholson WL, Olson JG, Childs JE. Fort Chaffee revisited: the epidemiology of tick-borne rickettsial and ehrlichial diseases at a natural focus. Vector Borne Zoonotic Dis. 2001;1:119–127. doi: 10.1089/153036601316977723. [DOI] [PubMed] [Google Scholar]
- 46.Paddock CD, Goddard J. The evolving medical and veterinary importance of the Gulf Coast tick (Acari: Ixodidae) J Med Entomol. 2015;52:230–252. doi: 10.1093/jme/tju022. [DOI] [PubMed] [Google Scholar]
- 47.Estrada-Pena A, Venzal JM, Mangold AJ, Cafrune MM, Guglielmone AA. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae: Amblyomminae) tick group: diagnostic characters, description of the larva of A. parvitarsum Neumann, 1901, 16S rDNA sequences, distribution and hosts. Syst Parasitol. 2005;60:99–112. doi: 10.1007/s11230-004-1382-9. [DOI] [PubMed] [Google Scholar]
- 48.Paddock CD, Denison AM, Dryden MW, Noden BH, Lash RR, Abdelghani SS, Evans AE, Kelly AR, Hecht JA, Karpathy SE, Ganta RR, Little SE. High prevalence of “Candidatus Rickettsia andeanae” and apparent exclusion of Rickettsia parkeri in adult Amblyomma maculatum (Acari: Ixodidae) from Kansas and Oklahoma. Ticks Tick Borne Dis. 2015;6:297–302. doi: 10.1016/j.ttbdis.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am J Trop Med Hyg. 2011;85:124–131. doi: 10.4269/ajtmh.2011.10-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nichols Heitman K, Dahlgren FS, Drexler N, Massung RF, Barton Behravesh C. Increasing incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med Hyg. 2016;94:52–60. doi: 10.4269/ajtmh.15-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]