Abstract
Human ehrlichiosis is a potentially fatal disease caused by Ehrlichia chaffeensis and Ehrlichia ewingii. Cases of ehrlichiosis are reported to Centers for Disease Control and Prevention through two national surveillance systems: Nationally Notifiable Diseases Surveillance System (NNDSS) and Case Report Forms. During 2008–2012, 4,613 cases of E. chaffeensis infections were reported through NNDSS. The incidence rate (IR) was 3.2 cases per million person-years (PYs). The hospitalization rate (HR) was 57% and the case fatality rate (CFR) was 1%. Children aged < 5 years had the highest CFR of 4%. During 2008–2012, 55 cases of E. ewingii infection were reported through NNDSS. The national IR was 0.04 cases per million PY. The HR was 77%; no deaths were reported. Immunosuppressive conditions were reported by 26% of cases. The overall rate for ehrlichiosis has increased 4-fold since 2000. Although previous literature suggests E. ewingii primarily affects those who are immunocompromised, this report shows most cases occurred among immunocompetent patients. This is the first report to show children aged < 5 years with ehrlichiosis have an increased CFR, relative to older patients. Ongoing surveillance and reporting of tick-borne diseases are critical to inform public health practice and guide disease treatment and prevention efforts.
Introduction
Human ehrlichioses are potentially fatal tick-borne infections caused by the obligate intracellular bacterium of the Ehrlichia genus, including Ehrlichia chaffeensis and Ehrlichia ewingii. The first reported case of human monocytic ehrlichiosis (HME) caused by E. chaffeensis was documented in 1991.1 Ehrlichia ewingii was first identified as the etiologic agent of canine granulocytic ehrlichiosis in 1992 and as one of the etiologic agents of human ehrlichiosis in 1999.2,3 The majority of reported human ehrlichiosis cases are caused by E. chaffeensis, which is the most frequently diagnosed tick-borne disease in the southern United States.4,5 Human ehrlichiosis caused by E. ewingii is less commonly reported and most infections have historically occurred among immunocompromised patients.2,6–9
Symptoms of ehrlichiosis are nonspecific and typically begin within 7–14 days of exposure.6 Patients are most likely to seek medical attention approximately 3 days after onset of symptoms.6,9–11 Presenting clinical features most often include fever, chills, headache, malaise, myalgia, and nausea.2,6 Fewer than 30% of adult patients and 60% of children infected with E. chaffeensis have a rash, which is maculopapular in early stages and can be petechial in late stages.4,6,9,10,12 Rash typically appears on the trunk around 7 days after onset of illness, and often spares the hands and feet.9,13 In E. ewingii cases, rash is rare.9 Leukopenia, thrombocytopenia, and elevations in serum hepatic aminotransferase levels are frequent laboratory findings.6 Severe illness may include cough, diarrhea, confusion, and lymphadenopathy in adults; and edema of the hands or feet in children.6 When left untreated or when treatment is delayed, severe complications may occur and include adult respiratory distress syndrome (ARDS), a disseminated intravascular coagulation-like syndrome (DIC), central nervous system involvement, and renal failure.6,14 Ehrlichiosis generally manifests as a moderate to severe illness, and 50–70% of patients are hospitalized.9–11 In E. chaffeensis infections, death can occur as early as the second week of illness and has been reported in 1–3% of cases.9,10 Infection by E. ewingii usually results in milder illness compared with E. chaffeensis, and no E. ewingii deaths have been reported.6,10 Patients with ehrlichiosis and who are immunocompromised, especially from human immunodeficiency virus (HIV), cancer treatments, or organ transplants, are at highest risk for severe outcome.8,15–23 As recommended by the American Academy of Pediatrics (AAP) and the Centers for Disease Control and Prevention (CDC), doxycycline, a tetracycline-class antibiotic, is the treatment of choice for children and adults of all ages with rickettsial disease, including ehrlichiosis; treatment should never be withheld pending laboratory confirmation.9,13,24 Treatment with doxycycline within the first 5 days of illness has been shown to decrease severity of disease in patients when compared with patients who were treated later in the course of illness.11,13
The primary vector for E. chaffeensis and E. ewingii in the United States is the lone star tick, Amblyomma americanum.25,26 Transmission to humans is caused almost exclusively by the bites of infected ticks and occurs in areas where the lone star tick is prevalent, most commonly in the southeastern, southcentral, and, recently, northeastern United States.4,5,10,27 Lone star ticks are aggressive, nonspecific feeders, which bite humans at all stages of their life cycle.25 The feeding cycle of the lone star tick coincides with the seasonality of ehrlichiosis, particularly the nymphal and adult stages, which transmit the bacteria, with cases peaking from May through July.4,6,10 White-tailed deer (Odocoileus virginianus), which are naturally infected with E. chaffeensis, maintain the enzootic cycle of E. chaffeensis.25,28,29 In addition, canids, domestic dogs, goats, rabbits, red foxes, raccoons, opossums, rodents, birds, and wild turkeys can serve as hosts for the lone star tick; though few mammals or birds are exempt as potential hosts.10,25,28 In addition, dogs may serve as transport hosts by carrying infected ticks in closer proximity to humans in households; this can lead to establishment of a focus of infection at or near the residence.10,25 Ehrlichia ewingii is maintained in a similar enzootic cycle, with white-tailed deer and domestic dogs serving as reservoir species.5,29 Transmission of E. chaffeensis through transplants and transfusions, such as of the liver and kidneys, has also been reported.6,10,13,18,30 Similarly, a case of E. ewingii in an immunocompromised young boy transmitted through a platelet transfusion has been reported.17
Ehrlichiosis has been a nationally notifiable disease since 1999.31 In 2008, the Council of State and Territorial Epidemiologists (CSTE) case definition changed to differentiate between ehrlichiosis and anaplasmosis, and to include the following categories for ehrlichiosis: E. chaffeensis infection (formerly HME); E. ewingii infection (formerly ehrlichiosis [unspecified, or other agent]); and ehrlichiosis/anaplasmosis, human, undetermined. Presented here is a summary of passive surveillance of E. chaffeensis and E. ewingii infections in the United States with onset dates during 2008–2012.
Materials and Methods
National surveillance systems.
Individual state and territory health departments report surveillance data to CDC through the Nationally Notifiable Diseases Surveillance System (NNDSS) and through manually completed Tick-Borne Rickettsial Disease Case Report Forms (CRFs) (http://www.cdc.gov/ticks/forms/2010_tbrd_crf.pdf). Demographic data, including county and state of residence, sex, ethnicity, and race, are reported through NNDSS. Cases are reported as probable or confirmed by the state and local health departments. Data reported through CRFs includes county and state of residence, clinical course, immunosuppressive conditions, hospitalization status, laboratory results, and patient outcome.
Case definition.31
To meet the confirmed case definition, a case must meet both clinical and laboratory criteria. Clinical presentation must include fever and one or more of the following symptoms: headache, myalgia, anemia, leukopenia, thrombocytopenia, or any hepatic transaminase elevation. Laboratory criteria for a confirmed case of E. chaffeensis infection includes demonstration of one of the following: a 4-fold change in immunoglobulin G (IgG)-specific antibody titer against E. chaffeensis antigen by indirect immunofluorescence antibody assay (IFA) between paired serum samples (one taken in first week of illness and a second 2–4 weeks later); detection of E. chaffeensis DNA in a clinical specimen via polymerase chain reaction (PCR) assay; demonstration of E. chaffeensis antigen in a biopsy or autopsy sample by immunohistochemical methods; or isolation of E. chaffeensis from a clinical specimen in cell culture. To meet the probable case definition for E. chaffeensis infection, the clinical criteria must be met and one of the following should be met for laboratory evidence: serological evidence of elevation of IgG or IgM antibody reactive with E. chaffeensis antigen by IFA, enzyme-linked immunosorbent assay (ELISA), dot-ELISA, or assays in other formats, or identification of morulae in the cytoplasm of monocytes or macrophages by microscopic examination. Because E. ewingii had not been cultured during 2008–2012, antigen was not available; thus, E. ewingii infections could only be diagnosed by PCR. The CSTE case definition is applied by CDC to data provided on CRFs.
Analysis.
Confirmed and probable cases of ehrlichiosis reported to CDC through both surveillance systems from January 2008 through December 2012 were included in this analysis. When date of onset of symptoms was not reported, the earliest known date associated with the case was used as a proxy. The year of symptom onset instead of reporting year was used for analyses; therefore, the number of cases presented here may differ from reports of the annual number of cases published in Morbidity and Mortality Weekly Report annual summaries. Data from NNDSS were used to calculate incidence rates (IRs) by year, state, and age. IRs were calculated as the number of ehrlichiosis cases per million person-years (PYs) using U.S. Census Bureau population estimates.32,33 Because of a large proportion of cases with missing data, IRs were not reported for race and ethnic groups. Ehrlichiosis caused by E. chaffeensis was not considered a notifiable disease during some years in Iowa (2008–2012), North Dakota (2008–2010), District of Columbia (2008–2012), Colorado (2008–2012), Idaho (2008–2012), Montana (2008–2012), Nevada (2008–2010), New Mexico (2008–2012), Alaska (2008–2012), Hawaii (2008–2012), and Washington (2008–2009). Ehrlichiosis caused by E. ewingii was not considered a notifiable disease during some years in Iowa (2008–2012), Nebraska (2008–2010), North Dakota (2008–2010), District of Columbia (2008–2012), Colorado (2008–2012), Idaho (2008–2012), Montana (2008–2012), Nevada (2008–2009), New Mexico (2008–2012), Alaska (2008–2012), Hawaii (2008–2012), and Washington (2008–2009). State populations were only considered at risk in years in which ehrlichiosis was reportable. CRFs are received by CDC on a rolling basis; therefore, only CRFs received before March 10, 2015 were included in this analysis. Confirmed and probable CRFs were used to calculate hospitalization and case fatality rates (CFRs), laboratory diagnostic test usage, seasonality, and severe complications related to ehrlichiosis. Reports missing these data were excluded from that segment of the analysis. Data analysis was performed using SAS v9.3 software (SAS Institute, Cary, NC). Because these reported cases are not generalizable to unreported cases or to other reporting time periods, we do not present confidence intervals or results from statistical hypotheses testing.
Results
Ehrlichia chaffeensis.
NNDSS data.
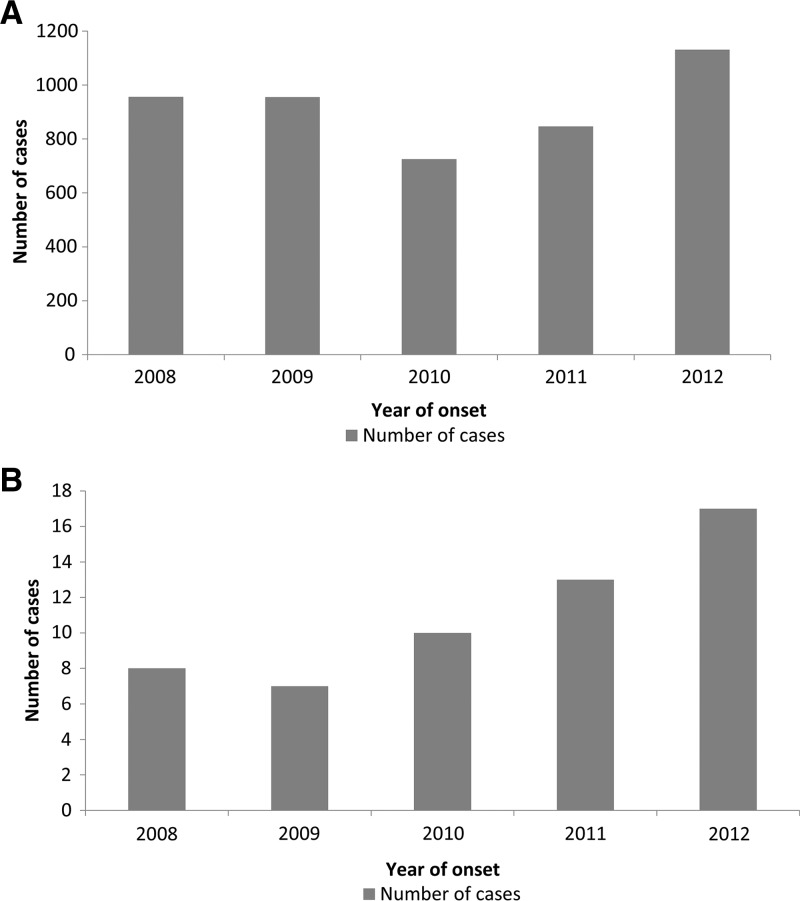
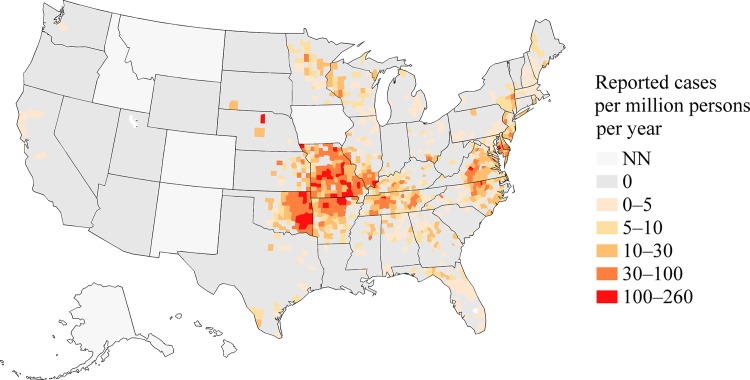
Through NNDSS, a total of 4,613 cases of E. chaffeensis were reported with an illness onset date between 2008 and 2012; 1,461 (32%) were reported as confirmed cases (Table 1). The percent of cases meeting a confirmed case definition increased during the study period from 24% in 2008 to 39% in 2012. The national reported IR was 3.2 cases per million PY (Figure 1 ). States with the highest reported IRs include: Oklahoma (30.9), Missouri (26.3), Delaware (19.8), Arkansas (19.4), Virginia (10.8), and Tennessee (9.7) (Table 2 and Figure 2 ). These six states accounted for 54% of all reported cases of human ehrlichiosis caused by E. chaffeensis in the United States.
Table 1.
Demographic profiles and case classification for Ehrlichia chaffeensis and Ehrlichia ewingii as reported to the Nationally Notifiable Diseases Surveillance System (NNDSS) and Case Report Forms (CRFs), United States, 2008–2012
| NNDSS | CRFs | |||
|---|---|---|---|---|
| E. chaffeensis | E. ewingii | E. chaffeensis | E. ewingii | |
| N = 4,613, no. (%) | N = 55, no. (%) | N = 3,593, no. (%) | N = 54, no. (%) | |
| Case classification | ||||
| Confirmed | 1,461 (31.7) | 55 (100) | 993 (28.1) | 54 (100) |
| Probable | 3,147 (68.2) | 2,546 (71.9) | ||
| Unknown* | 5 (0.1) | |||
| Sex | ||||
| Male | 2,606 (56.5) | 28 (50.9) | 2,070 (58.5) | 28 (51.9) |
| Female | 1,872 (40.6) | 24 (43.6) | 1,430 (40.4) | 26 (48.1) |
| Unknown | 135 (3.0) | 3 (5.5) | 39 (1.1) | 0 (0) |
| Race | ||||
| White | 2,959 (64.1) | 35 (63.6) | 2,554 (72.1) | 42 (77.8) |
| Black | 134 (2.9) | 2 (3.6) | 87 (2.4) | 1 (1.9) |
| American Indian† | 132 (2.9) | 0 (0) | 129 (3.6) | 0 (0) |
| Asian‡ | 18 (0.4) | 0 (0) | 14 (0.4) | 0 (0) |
| Unknown | 1,370 (29.7) | 18 (32.7) | 755 (21.0) | 11 (20.4) |
| Ethnicity | ||||
| Hispanic | 90 (2.0) | 0 (0) | 57 (1.6) | 0 (0) |
| Non-Hispanic | 2,723 (59.7) | 37 (67.3) | 2,269 (63.2) | 37 (68.5) |
| Unknown | 1,750 (38.4) | 18 (32.7) | 1,209 (33.6) | 17 (31.5) |
| Age (years) | ||||
| < 5 | 82 (1.8) | 0 (0) | 69 (1.9) | 0 (0) |
| 5–9 | 132 (2.9) | 1 (1.8) | 100 (2.9) | 1 (1.9) |
| 10–14 | 123 (2.7) | 0 (0) | 102 (2.8) | 0 (0) |
| 15–19 | 165 (3.6) | 0 (0) | 122 (3.4) | 0 (0) |
| 20–24 | 138 (3.0) | 1 (1.8) | 107 (3.0) | 1 (1.9) |
| 25–29 | 114 (2.5) | 3 (5.5) | 86 (2.4) | 3 (5.6) |
| 30–34 | 196 (4.3) | 0 (0) | 145 (4.0) | 0 (0) |
| 35–39 | 230 (5.0) | 4 (7.3) | 157 (4.4) | 4 (7.4) |
| 40–44 | 269 (5.8) | 4 (7.3) | 192 (5.3) | 4 (7.4) |
| 45–49 | 391 (8.5) | 5 (9.1) | 279 (7.8) | 4 (7.4) |
| 50–54 | 408 (8.8) | 4 (7.3) | 312 (8.7) | 4 (7.4) |
| 55–59 | 510 (11.1) | 8 (14.6) | 382 (10.6) | 9 (16.7) |
| 60–64 | 502 (11.0) | 6 (10.9) | 401 (11.2) | 4 (7.4) |
| 65–69 | 467 (10.1) | 6 (10.9) | 361 (10.0) | 8 (14.8) |
| 70+ | 853 (18.5) | 13 (23.6) | 637 (17.7) | 11 (20.4) |
| Unknown | 31 (0.7) | 0 (0) | 87 (2.4) | 1 (1.9) |
California.
Or Alaskan native.
Or Pacific Islander.
Figure 1.
Number of annual cases vs. the year of onset of symptoms, 2008–2012, for (A) Ehrlichia chaffeensis infections and (B) Ehrlichia ewingii infections in the United States. The number of incident cases is from the Nationally Notifiable Diseases Surveillance System.
Table 2.
The number of reported cases and the reported incidence rate (IR) per million person-years by state for Ehrlichia chaffeensis and Ehrlichia ewingii, 2008–2012
| State | E. chaffeensis | E. ewingii |
|---|---|---|
| No. of cases (rate*) | No. of cases (rate*) | |
| Alabama | 45 (1.9) | 0 (0) |
| Alaska | NN | NN |
| Arizona | 0 (0) | 0 (0) |
| Arkansas | 283 (19.4) | 0 (0) |
| California | 5 (0.03) | 0 (0) |
| Colorado | NN | NN |
| Connecticut | 2 (0.1) | 0 (0) |
| Delaware | 89 (19.8) | 5 (1.1) |
| District of Columbia | NN | NN |
| Florida | 69 (0.7) | 0 (0) |
| Georgia | 104 (2.1) | 1 (0.02) |
| Hawaii | NN | NN |
| Idaho | NN | NN |
| Illinois | 138 (2.2) | 1 (0.02) |
| Indiana | 4 (0.1) | 0 (0) |
| Iowa | NN | NN |
| Kansas | 71 (5) | 1 (0.1) |
| Kentucky | 87 (4) | 0 (0) |
| Louisiana | 2 (0.1) | 1 (0.04) |
| Maine | 10 (1.5) | 0 (0) |
| Maryland | 183 (6.3) | 2 (0.1) |
| Massachusetts | 55 (1.7) | 0 (0) |
| Michigan | 18 (0.4) | 0 (0) |
| Minnesota | 50 (1.9) | 2 (0.1) |
| Mississippi | 14 (0.9) | 0 (0) |
| Missouri | 788 (26.3) | 33 (1.1) |
| Montana | NN | NN |
| Nebraska | 8 (0.9) | 0 (0) |
| Nevada | 0 (0) | 0 (0) |
| New Hampshire | 18 (2.7) | 0 (0) |
| New Jersey | 326 (7.4) | 0 (0) |
| New Mexico | NN | NN |
| New York City | 35 (0.9) | 0 (0) |
| New York State | 250 (4.5) | 0 (0) |
| North Carolina | 374 (7.8) | 0 (0) |
| North Dakota | 0 (0) | 0 (0) |
| Ohio | 40 (0.7) | 1 (0.02) |
| Oklahoma | 580 (30.9) | 0 (0) |
| Oregon | 0 (0) | 0 (0) |
| Pennsylvania | 27 (0.4) | 0 (0) |
| Rhode Island | 43 (8.2) | 0 (0) |
| South Carolina | 12 (0.5) | 1 (0.04) |
| South Dakota | 1 (0.2) | 0 (0) |
| Tennessee | 309 (9.7) | 5 (0.2) |
| Texas | 43 (0.3) | 0 (0) |
| Utah | 0 (0) | 0 (0) |
| Vermont | 1 (0.3) | 0 (0) |
| Virginia | 432 (10.8) | 2 (0.05) |
| Washington | 1 (0.05) | 0 (0) |
| West Virginia | 5 (0.5) | 0 (0) |
| Wisconsin | 91 (3.2) | 0 (0) |
| Wyoming | 0 (0) | 0 (0) |
The number of incident cases is from the Nationally Notifiable Diseases Surveillance System and the number of person-years at risk is from the U.S. Census Bureau, 2008–2012. States where the disease was not notifiable for the duration of 2008–2012 are designated as “NN.”
IR per million persons per year.
Figure 2.
A map of incidence rates determined from reported Ehrlichia chaffeensis infections in the counties in the United States, 2008–2012. States where the disease was not notifiable for the duration of 2008–2012 are shaded with the “NN” category. The number of incidence cases is from the Nationally Notifiable Diseases Surveillance System, and the number of person-years at risk is from the U.S. Census Bureau.
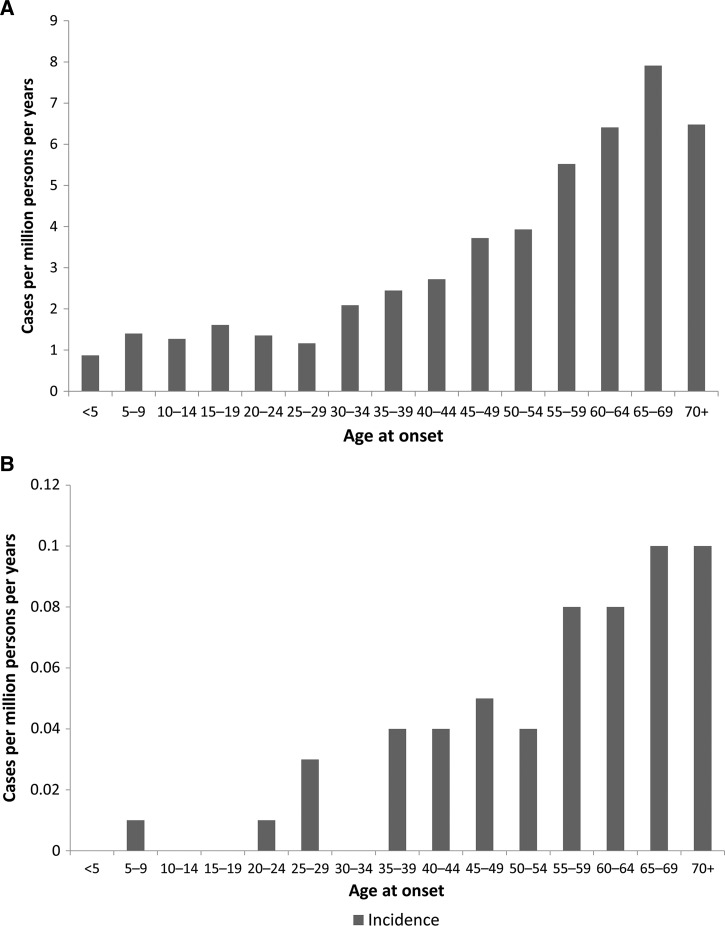
More males (57%) than females were reported with E. chaffeensis infection through NNDSS (Table 1). Of those reporting race, cases were primarily among whites (64%), followed by blacks (3%), and American Indians (3%). Hispanic ethnicity was reported for 2% of cases. The overall mean and median age of onset was 51 and 55 years of age, respectively. The reported IR increased with age, and the rate among persons 65–69 years of age (7.9) was the highest when compared with the entire population (Figure 3 ).
Figure 3.
Incidence rates (IRs) by age group of (A) Ehrlichia chaffeensis and (B) Ehrlichia ewingii based on cases reported through the National Notifiable Diseases Surveillance System, United States, 2008–2012. IRs are cases per million person-years.
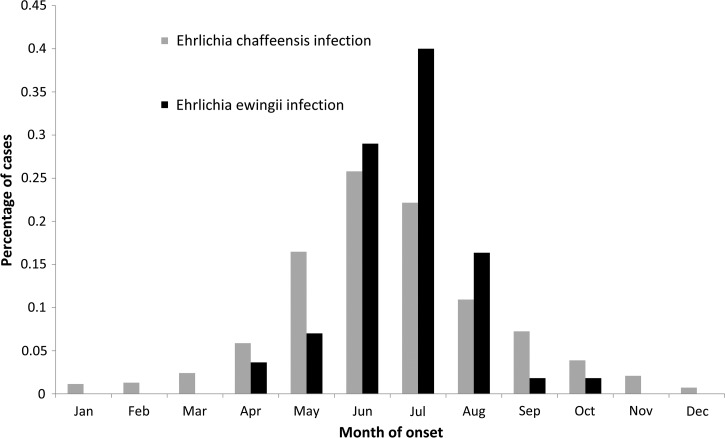
Reported symptom onset dates peaked in the summer months from May through July (N = 2,972, 64%); the fewest cases were reported during the winter months of December, January, and February (N = 146, 3%) (Figure 4 ).
Figure 4.
Percentage of cases by month of onset for Ehrlichia chaffeensis (N = 4,563) and Ehrlichia ewingii (N = 53) infections based on cases reported through the Nationally Notifiable Diseases Surveillance System, United States, 2008–2012.
CRF data.
During the same study period, 3,593 cases of E. chaffeensis were reported to CDC via CRFs. Cases reported through CRFs were similar to NNDSS cases in terms of confirmed cases (28%), gender (59% male), age (mean age 51 years and median age 55 years), and race distribution (72% white, 4% American Indian, and 3% black) (Table 1). The percent of confirmed cases reported through CRFs also increased during the study period from 20% in 2008 to 37% in 2012.
Among cases reporting hospitalization status, 1,584 (57%) were hospitalized during the course of illness. There was a higher hospitalization rate among confirmed cases (77%) than probable cases (48%). More males (60%) were hospitalized than females (54%). A higher proportion of cases was hospitalized among persons over 60 than among other age groups. For those reporting race and hospitalization status, blacks were most frequently hospitalized (75%), followed by Asian/Pacific Islanders (60%) and whites (59%). Of those reporting the presence of life-threatening complication, 399 cases were (11%) reported suffering from a life-threatening complication. These complications included renal failure (8%), meningitis (5%), ARDS (3%), DIC (2%), pneumonia (1%), and sepsis (1%).
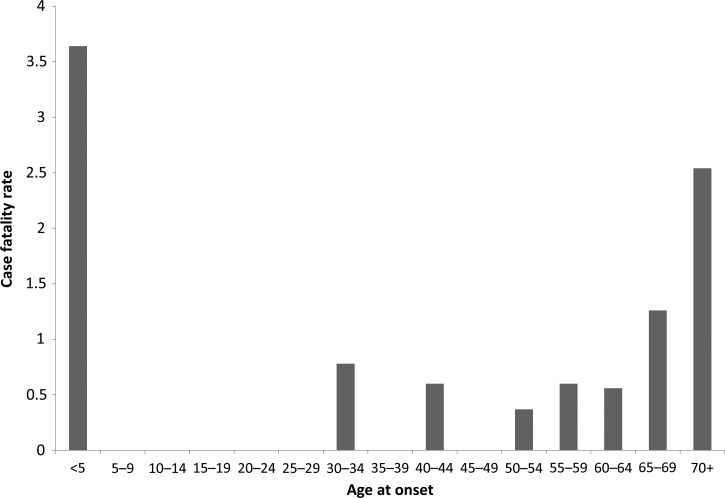
Of those reporting clinical outcome, there were 29 (1%) fatal cases. Confirmed cases had a higher percentage of patients with fatal outcome (2%) compared with probable cases (0.6%). The annual CFR remained consistent throughout the study period (range = 0.7–1%). Of those reporting race and fatal outcome, American Indians, blacks, and whites experienced the highest reported CFR (1%). CFRs were highest among children < 5 years of age (4%) and persons ≥ 70 years of age (3%) than among other age groups (Figure 5 ). Among confirmed cases, the CFR among children < 5 years of age increases to 14% and the CFR among persons ≥ 70 increases to 53%.
Figure 5.
Case fatality rate by age group of Ehrlichia chaffeensis infection based on cases reported through Case Report Forms, United States, 2008–2012.
Among those reporting immune status (N = 2,319), 327 cases (14%) reported immunosuppressive conditions. These conditions included diabetes (3%), cancer (2%), history of organ transplant (1%), asplenia (0.5%), hepatitis C (0.5%), and HIV (0.4%). The median age of those with an immunosuppressive condition was 60 years, and the median age among immunocompetent persons was 55 years. When compared with immunocompetent case outcomes, risk for severe outcome for immunosuppressed cases was higher for hospitalization (relative risk [RR] = 1.4), presence of life-threatening complication (RR = 2.4), and death (RR = 2.3).
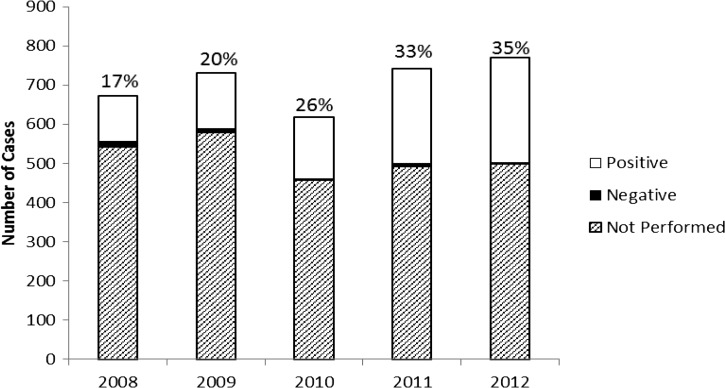
Most cases of infection with E. chaffeensis were diagnosed using IFA specific for IgG (N = 2,240, 63%). However, only a small number of these serologic diagnoses (N = 112, 3%) demonstrated seroconversion and were reported as confirmed cases. A single positive, acute-phase serologic result was the only supporting laboratory evidence for 2,126 cases (60%). Among probable cases reporting positive IgG or IgM titers, onset date, and serology collection date, the median number of days from onset date to date of first serology collection date was 7 days (interquartile range [IQR] = 2–14). A total of 935 cases (26%) were confirmed by PCR. The proportion of cases confirmed by PCR increased from less than 20% during 2008–2009 to 35% in 2012 (Figure 6 ). Of all confirmed cases (N = 1,047, 30%), most (89%) were confirmed by PCR. Among confirmed cases reporting positive PCR or culture results, onset date, and serology collection date, the median number of days from onset date to date of first serology collection date was 5.5 days (IQR = 3–9).
Figure 6.
Summary of the number of annual cases of Ehrlichia chaffeensis (N = 3,593), reported through Case Report Forms, United States, 2008–2012. The annual number of cases is subdivided into polymerase chain reaction (PCR)-positive cases, PCR-negative cases, and cases for which PCR was not performed. The annotation above each vertical bar indicates the annual percent of reported cases diagnosed by PCR.
Ehrlichia ewingii.
NNDSS data.
Through NNDSS, 55 cases of E. ewingii infection were reported with an onset date between 2008 and 2012. All cases were confirmed by PCR (N = 55), as required to meet the case definition. The national reported IR was 0.04 cases per million PY. During the study period, reported IR increased from 0.03 cases per million PY in 2008 to 0.06 cases per million PY in 2012 (Figure 1). States with the highest reported IRs were Delaware (1.1) and Missouri (1.1) (Table 2). These two states accounted for 69% of all E. ewingii cases reported in the United States.
Slightly more males (51%) than females were reported with E. ewingii infection through NNDSS (Table 1). Among those reporting race, cases were primarily among persons of white race (64%). The overall mean and median ages were 56 and 58 years, respectively. Reported IR increased with age, and rates among persons over 65 years of age (0.1) were the highest when compared with the national average (Figure 3).
Reported symptom onset dates peaked in the summer months from June through August (N = 47, 85%), with fewer cases reported during the fall months of September and October (N = 2, 4%) (Figure 4). No cases were reported in January, February, March, November, or December.
CRFs data.
During the same study period, 54 cases of E. ewingii were reported to CDC via CRFs. The cases reported through CRFs were similar to NNDSS cases in terms of gender (52%), age (mean age: 55.6 years and median age: 58 years), and race distribution (78% white and 2% black) (Table 1). As written in the case definition, all cases were confirmed by PCR (N = 54).
Among cases reporting hospitalization status (N = 52), 40 (77%) were hospitalized during the course of illness. More females (79%) were hospitalized than males (75%). There was one case (2%) for which a specific life-threatening complication (renal failure) was reported. Of the cases reporting clinical outcome (N = 52), there were no fatalities. Among those reporting immune status (N = 38), 10 cases (26%) reported immunosuppressive conditions, including five cases (13%) with history of organ transplantation and three cases (8%) with history of cancer. There were no cases reporting HIV infection. The median age of those with an immunosuppressive condition was 65.5 years and the median age among immunocompetent persons was 58.5 years. Cases reported for patients having an immunosuppressive condition were more likely to be hospitalized (RR = 1.6) than for those who were immunocompetent.
Discussion
The IR for ehrlichiosis has continued to increase since becoming a nationally notifiable disease in 1999. In our previous report, the incidence of ehrlichiosis caused by E. chaffeensis was 1.4 cases per million PY during 2000–2007.14 In this report, the incidence of ehrlichiosis caused by E. chaffeensis more than doubled to 3.2 cases per million PY during 2008–2012. This is the first report to show children aged < 5 years with ehrlichiosis may have an increased CFR relative to older patients. Additionally, this is the first report to summarize the reported epidemiology of E. ewingii infections in the United States.
Previous case reports suggested E. ewingii primarily affects those who are immunocompromised, which may be because immunocompromised persons are more likely to develop serious infections, be hospitalized, and have extensive laboratory diagnostic tests.2,7,8,15,17,34 In addition, because E. ewingii is a milder illness, it is possible that immunocompetent patients may have less severe symptoms and not seek medical attention, which may have led to overrepresentation of immunocompromised cases in previous reports. In our report, the prevalence of reporting an immunosuppressive condition was 26% among E. ewingii cases; cases with E. ewingii infections reporting an immunocompromised condition were more likely to be hospitalized. Of the 985 PCR-positive reports of Ehrlichia species infections reported through CRFs, 54 were positive for E. ewingii; increased availability and utilization of species-specific PCR assays may further elucidate the epidemiology of E. ewingii infections in the United States.
The diagnostic laboratory gold standard for confirming E. chaffeensis infection is seroconversion: a 4-fold titer increase when comparing an acute and convalescent serum using an IFA specific for IgG.9,14 Here, only 112 (3%) cases were reported as confirmed with paired sera. During the first week, IFA is often negative.10 Since most patients develop diagnostic IFA titers by 4 weeks after illness onset, it is important to obtain a convalescent-phase serum sample, as this may be the only laboratory evidence to support the diagnosis.9,14 However, convalescent samples are often not obtained or provided to the diagnostic laboratory. Another caveat of serologic testing is that because Ehrlichia spp. and Anaplasma phagocytophilum are antigenically related, serologic assays may be positive for more than one agent and results must be interpreted with consideration of epidemiological and clinical findings.2,5,6,10 PCR on whole blood collected during acute illness may provide early laboratory evidence of infection with an Ehrlichia species, and, unlike serologic methods, PCR can differentiate between Ehrlichia species and can provide confirmation from a single acute specimen.6,8 Therefore, PCR on acute specimens is an effective and efficient method for providing laboratory evidence of ehrlichiosis. During 2000–2007, 11% of cases were confirmed by PCR, whereas 26% of cases were confirmed by PCR during 2008–2012.14 Without PCR confirmation, it is likely that cases of ehrlichiosis will be missed, and E. chaffeensis, E. ewingii, and Anaplasma infections will be misclassified based on serologic test results. When ehrlichiosis is suspected, PCR testing should be requested in addition to paired-IFA IgG serology for completeness.
The results presented here are subject to several limitations. Persons with poor access to care and diagnostic services may be underrepresented in these data because of the requirement for laboratory evidence for reported cases. Unfortunately, many persons infected with E. ewingii and other Ehrlichia and Anaplasma agents (e.g., Ehrlichia muris-like agent [EMLA], A. phagocytophilum) also produce antibodies reactive with E. chaffeensis.35–37 Recently, researchers tested 75,077 samples from patients in the United States and found 69 samples were PCR positive for EMLA; samples from patients from Minnesota and Wisconsin made up 93% of the positive samples, which may account for some of the probable reported cases of E. chaffeensis infection from Minnesota and Wisconsin.35 Thus, probable cases of ehrlichiosis may not have been caused by E. chaffeensis; without PCR testing, the species cannot be determined for acute probable infections. Studies have shown age is a predictor of prevalence of IFA antibodies to Ehrlichia among healthy children and adults; a titer meeting the cutoff for a probable case may be found in persons with previous Ehrlichia infections.37–39 The increasing rates of incidence and hospitalization with age may reflect background seroprevalence and/or there is an age-dependent severity of illness leading to hospitalization and an accurate diagnosis. Due to limitations of our data, we are unable to estimate the number of reported cases because of seroprevalence from previous infections. This question warrants further research; enhanced surveillance in highly endemic areas may provide further insight. In addition, cases may be underestimated because of asymptomatic infections or mild illnesses, especially for E. ewingii infections, not prompting medical consultation or treatment.11,37,40 Cases reported here probably represent those of more serious illness and those from highly endemic areas. Clinical suspicion for ehrlichiosis may be lower in nonendemic areas, which will also lead to underreporting. Data for race and ethnicity were largely missing in both NNDSS and CRFs datasets, making it difficult to draw conclusions on the potential role of race and ethnicity related to infections. When measuring the rates of life-threatening complications (e.g., DIC, ARDS, etc.), we are limited to data provided on CRFs and do not receive patient symptoms or laboratory data related to these complications. Finally, results from passive surveillance are not generalizable to the whole population and likely underestimate the true burden of disease.
Ehrlichiosis and Rocky Mountain spotted fever (RMSF) have similar geographic distributions and early clinical presentations.41 Both the AAP and the CDC recommend treating suspected ehrlichiosis and RMSF with doxycycline, a tetracycline-class antibiotic. Studies from the 1960s indicated that tetracycline causes a characteristic staining of children's developing teeth.42,43 Doxycycline is a newer tetracycline-class antibiotic, which binds less readily to calcium, and no reports have linked doxycycline to staining of a child's developing permanent teeth, despite several investigations looking for this association.24,44,45 The current doxycycline label indicates unless there are no other effective antibiotics, this drug should be avoided in children under 8 years old to avoid staining of permanent teeth.46 The label does not address that there are no equally effective alternatives for the treatment of rickettsial disease, or that short courses of doxycycline have not been shown to have this effect.44,46 Recent surveys suggest health-care providers may be less willing to prescribe doxycycline for suspected cases of RMSF in children less than 8 years old when compared with older children and adults.46,47 It is possible that some health-care providers are hesitant to prescribe doxycycline to children with suspected rickettsial disease over concerns about possible dental staining. A gap between the recommendations for treating suspected ehrlichiosis and practice may explain part of the increased CFR among children under 5 years old. It is critical to increase awareness among health-care providers that the empiric treatment of patients with doxycycline is essential as soon as a tick-borne rickettsial disease is suspected.
While the timely and appropriate treatment of patients with doxycycline can save lives, the best prevention approach for human ehrlichiosis is avoiding tick bites. Tick checks should be performed regularly on people and pets, especially after returning from possible tick-infested areas. Other prevention techniques for humans primarily involve restricting exposure to ticks by wearing personal protection, such as full-coverage clothing treated with permethrin and use of repellants containing n,n-diethyl-m-toluamide (DEET). There is no vaccine and currently no evidence suggesting post-tick bite prophylaxis is effective for prevention of ehrlichiosis.9,13,48 Reducing rates of human infection often hinges on effective control of vectors or reservoir populations.25 Since dogs can be transport hosts for some ticks that carry Ehrlichia species and dogs are also considered a reservoir for E. ewingii, pet owners should use veterinary ectoparasite controls, such as acaracide-impregnated collars or topical treatments, to prevent ticks from attaching to and feeding on pets.
The increasing incidence and geographic distribution of human ehrlichiosis suggests that health-care providers in previously unaffected areas may begin to see patients present with ehrlichiosis; and, state and local public health departments may begin receiving the first positive laboratory results for ehrlichiosis—both E. chaffeensis and E. ewingii—among residents with no history of travel. As ehrlichiosis continues to spread geographically, more states might consider making ehrlichiosis a notifiable condition. Ongoing surveillance and reporting of tick-borne diseases are critical for understanding the changing epidemiology of ehrlichiosis, for developing effective prevention strategies and public health education programs, and to inform public health practice and guide disease prevention efforts.
ACKNOWLEDGMENTS
We kindly thank the health-care providers, laboratorians, and public health partners whose dedication and work is indispensable to rickettsial disease surveillance. We also thank Eric Mandel, John Krebs, and Jennifer McQuiston for their work in designing and building these data.
Disclaimer: The author was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. All authors report no potential conflicts of interest.
Footnotes
Authors' addresses: Kristen Nichols Heitman, F. Scott Dahlgren, Naomi A. Drexler, Robert F. Massung, and Casey Barton Behravesh, Rickettsial Zoonoses Branch, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: wwd7@cdc.gov, iot0@cdc.gov, isj3@cdc.gov, rfm2@cdc.gov, and dlx9@cdc.gov.
References
- 1.Dawson JE, Anderson BE, Fishbein DB, Sanchez JL, Goldsmith CS, Wilson KH, Duntley CW. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikhisa Y, Unver A, Gaudreault-Keener M, Manian FA, Liddell AM, Schmulewitz N, Storch GA. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 3.Anderson BE, Greene CE, Jones DC, Dawson JE. Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis. Int J Syst Bacteriol. 1992;42:299–302. doi: 10.1099/00207713-42-2-299. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Ehrlichiosis. 2010. http://www.cdc.gov/ehrlichiosis/ Available at. Accessed December 2, 2014.
- 5.Beall M, Alleman AR, Breitschwerdt E, Cohn L, Couto CG, Dryden M, Guptill L, Iazbik C, Kania S, Lathan P, Little S, Roy A, Sayler K, Stillman B, Welles E, Wolfson W, Yabsley M. Seroprevalence of Ehrlichia canis, Ehrlichia chaffeensis and Ehrlichia ewingii in dogs in North America. Parasit Vectors. 2012;5:29. doi: 10.1186/1756-3305-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumler JS, Walker DH. Ehrlichia chaffeensis (human monocytotropic ehrlichiosis), Anaplasma phagocytophilum (human granulocytic anaplasmosis), and other Anaplasmataceae. In: Dumler JS, Walker DH, editors. Mandell, Douglas, and Bennett's Principles and Practice of Diseases. Philadelphia, PA: Elsevier; 2014. pp. 2227–2233. [Google Scholar]
- 7.Allen MB, Pritt BS, Sloan LM, Paddock CD, Musham CK, Ramos JM, Cetin N, Rosenbaum ER. First reported case of Ehrlichia ewingii involving human bone marrow. J Clin Microbiol. 2014;58:4102–4104. doi: 10.1128/JCM.01670-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas LD, Hongo I, Bloch KC, Tang YW, Dummer S. Human ehrlichiosis in transplant recipients. Am J Transplant. 2007;7:1641–1647. doi: 10.1111/j.1600-6143.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 9.Chapman AS, Bakken JS, Folk SM, Paddock CD, Bloch KC, Krusell A, Sexton DJ, Buckingham SC, Marshall GS, Storch GA, Dasch GA, McQuiston JH, Swerdlow DL, Dumler SJ, Nicholson WL, Walker DH, Eremeeva ME, Ohl CA. MMWR Recomm Rep. 2006;55:1–27. [PubMed] [Google Scholar]
- 10.Paddock CD, Childs JE. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin Microbiol Rev. 2003;16:37–64. doi: 10.1128/CMR.16.1.37-64.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fishbein DB, Dawson JE, Robinson LE. Human ehrlichiosis in the United States, 1985 to 1990. Ann Intern Med. 1994;120:736–743. doi: 10.7326/0003-4819-120-9-199405010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Harkess JR, Ewing SA, Brumit T, Mettry CR. Ehrlichiosis in children. Pediatrics. 1991;87:199–203. [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics . Ehrlichia, Anaplasma, and related infections (human ehrlichiosis, anaplasmosis, and related infections) In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Red Book: 2015 Report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics; 2015. pp. 329–333. [Google Scholar]
- 14.Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am J Trop Med Hyg. 2011;85:124–131. doi: 10.4269/ajtmh.2011.10-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paddock CD, Folk SM, Shore GM, Machado LJ, Huycke MM, Slater LN, Liddell AM, Buller RS, Storch GA, Monson TP, Rimland D, Sumner JW, Singleton J, Bloch KC, Tang YW, Standaert SM, Childs JE. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin Infect Dis. 2001;33:1586–1594. doi: 10.1086/323981. [DOI] [PubMed] [Google Scholar]
- 16.Barenfanger J, Patel P, Dumler JS, Walker DH. Clinical pathology rounds: identifying human ehrlichiosis. Lab Med. 1996;27:372–374. [Google Scholar]
- 17.Regan J, Matthias J, Green-Murphy A, Stanek D, Bertholf M, Pritt BS, Sloan LM, Kelly AJ, Singleton J, McQuiston JH, Hocevar SN, Whittle JP. A confirmed Ehrlichia ewingii infection likely acquired through platelet transfusion. Clin Infect Dis. 2013;56:E105–E107. doi: 10.1093/cid/cit177. [DOI] [PubMed] [Google Scholar]
- 18.Antony SJ, Dummer JS, Hunter E. Human ehrlichiosis in a liver transplant recipient. Transplantation. 1995;60:879–881. [PubMed] [Google Scholar]
- 19.Sadikot R, Shaver MJ, Reeves WB. Ehrlichia chaffeensis in a renal transplant recipient. Am J Nephrol. 1999;19:674–676. doi: 10.1159/000013540. [DOI] [PubMed] [Google Scholar]
- 20.Tan HP, Dumler JS, Maley WR, Klein AS, Burdick JF, Fred Poordad F, Thuluvath PJ, Markowitz JS. Human monocytic ehrlichiosis: an emerging pathogen in transplantation. Transplantation. 2001;71:1678–1680. doi: 10.1097/00007890-200106150-00030. [DOI] [PubMed] [Google Scholar]
- 21.Cotant C, Okulicz JF, Brezina B, Riley DJ, Conger NG. Human monocytic ehrlichiosis in a renal transplant patient. Scand J Infect Dis. 2006;38:699–702. doi: 10.1080/00365540500444694. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence KL, Morrell MR, Storch GA, Hachem RR, Trulock EP. Clinical outcomes of solid organ transplant recipients with ehrlichiosis. Transpl Infect Dis. 2009;11:203–210. doi: 10.1111/j.1399-3062.2009.00373.x. [DOI] [PubMed] [Google Scholar]
- 23.Safdar N, Love RB, Maki DG. Severe Ehrlichia chaffeensis infection in a lung transplant recipient: a review of ehrlichiosis in the immunocompromised patient. Emerg Infect Dis. 2002;8:320–323. doi: 10.3201/eid0803.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todd SR, Dahlgren FS, Traeger MS, Beltrán-Aguilar ED, Marianos DW, Hamilton C, McQuiston JH, Regan JJ. No visible dental staining in children treated with doxycycline for suspected Rocky Mountain spotted fever. J Pediatr. 2015;166:1246–1251. doi: 10.1016/j.jpeds.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- 26.Killmaster LF, Loftis AD, Zemtsova GE, Levin ML. Detection of bacterial agents in Amblyomma americanum (Acari: Ixodidae) from Georgia, USA, and the use of a multiplex assay to differentiate Ehrlichia chaffeensis and Ehrlichia ewingii. J Med Entomol. 2014;51:868–872. doi: 10.1603/me13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Ticks. 2014. http://www.cdc.gov/ticks/ Available at. Accessed January 14, 2015.
- 28.Paddock CD, Yabsley MJ. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Curr Top Microbiol Immunol. 2007;315:289–324. doi: 10.1007/978-3-540-70962-6_12. [DOI] [PubMed] [Google Scholar]
- 29.Yabsley MJ, Varela AS, Tate CM, Dugan VG, Stallknecht DE, Little SE, Davidson WR. Ehrlichia ewingii infection in white-tailed deer (Odocoileus virginianus) Emerg Infect Dis. 2002;8:668–671. doi: 10.3201/eid0807.020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sachdev SH, Joshi V, Cox ER, Amoroso A, Palekar S. Severe life-threatening Ehrlichia chaffeensis infections transmitted through solid organ transplantation. Transpl Infect Dis. 2014;16:119–124. doi: 10.1111/tid.12172. [DOI] [PubMed] [Google Scholar]
- 31.Council of State and Territorial Epidemiologists Position Statement 07-ID-03: Revision of the National Surveillance Case Definition for Ehrlichiosis (Ehrlichiosis/Anaplasmosis) 2009. http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/07-ID-03.pdf Available at. Accessed January 13, 2015.
- 32.U.S. Census Bureau, Population Division Intercensal Estimates of the Resident Population for Counties and States: April 1, 2000 to July 1, 2010. 2011. http://www.census.gov/popest/data/intercensal/county/county2010.html Available at. Accessed July 23, 2014.
- 33.U.S. Census Bureau, Population Division . Annual Resident Population Estimates, Estimated Components of Resident Population Change, and Rates of the Components of Resident Population Change for States and Counties: April 1, 2010 to July 1, 2013. 2014. https://www.census.gov/popest/data/counties/totals/2013/CO-EST2013-alldata.html Available at. Accessed July 23, 2014. [Google Scholar]
- 34.Masters EJ, Storch GA, Sumner JW. Ehrlichia ewingii in an immunocompetent adult. Mo Med. 2009;106:301–303. [PubMed] [Google Scholar]
- 35.Diep KHJ, Elizabeth S, Jeffrey PD, David N, Lynne MS, William LN, Thomas RF, Christopher RS, Julie AR, Tracy KM, Michelle AF, Timothy SU, Joni JF, Amy LL, Alecia KD, Elitza ST, Bobbi SP. Human infection with Ehrlichia muris-like pathogen, United States, 2007–2013. Emerg Infect Dis. 2015;21:1794–1799. doi: 10.3201/eid2110.150143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Comer JA, Nicholson WL, Olson JG, Childs JE. Serologic testing for human granulocytic ehrlichiosis at a national referral center. J Clin Microbiol. 1999;37:558–564. doi: 10.1128/jcm.37.3.558-564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yevich SJ, Sanchez JL, DeFraites RF, Rives CC, Dawson JE, Uhaa IJ, Johnson BJ, Fishbein DB. Seroepidemiology of infections due to spotted fever group rickettsiae and Ehrlichia species in military personnel exposed in areas of the United States where such infections are endemic. J Infect Dis. 1995;171:1266–1273. doi: 10.1093/infdis/171.5.1266. [DOI] [PubMed] [Google Scholar]
- 38.Standaert SM, Dawson JE, Schaffner W, Childs JE, Biggie KL, Singleton J, Jr., Gerhardt RR, Knight ML, Hutcheson RH. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420–425. doi: 10.1056/NEJM199508173330704. [DOI] [PubMed] [Google Scholar]
- 39.Marshall GS, Jacobs RF, Schutze GE, Paxton H, Buckingham SC, DeVincenzo JP, Jackson MA, San Joaquin VH, Standaert SM, Woods CR. Ehrlichia chaffeensis seroprevalence among children in the southeast and south-central regions of the United States. Arch Pediatr Adolesc Med. 2002;156:166–170. doi: 10.1001/archpedi.156.2.166. [DOI] [PubMed] [Google Scholar]
- 40.Eng TR, Harkess JR, Fishbein DB, Dawson JE, Greene CN, Redus MA, Satalowich FT. Epidemiologic, clinical, and laboratory findings of human ehrlichiosis in the United States, 1988. JAMA. 1990;264:2251–2258. [PubMed] [Google Scholar]
- 41.Raoult DA. Dumler JS, Walker DH. Mandell, Douglas, and Bennett's Principles and Practice of Diseases. Philadelphia, PA: Elsevier; 2014. Introduction to rickettsioses, ehrlichioses, and anplasmosis; pp. 2195–2197. [Google Scholar]
- 42.Wallman IS, Hilton HB. Teeth pigmented by tetracycline. Lancet. 1962;1:827–829. doi: 10.1016/s0140-6736(62)91840-8. [DOI] [PubMed] [Google Scholar]
- 43.Weyman J. The clinical appearances of tetracycline staining of the teeth. Br Dent J. 1965;118:289–291. [PubMed] [Google Scholar]
- 44.Volovitz B, Shkap R, Amir J, Calderon S, Varsano I, Nussinovitch M. Absence of tooth staining with doxycycline treatment in young children. Clin Pediatr (Phila) 2007;46:121–126. doi: 10.1177/0009922806290026. [DOI] [PubMed] [Google Scholar]
- 45.Lochary ME, Lockhart PB, Williams WT., Jr. Doxycycline and staining of permanent teeth. Pediatr Infect Dis J. 1998;17:429–431. doi: 10.1097/00006454-199805000-00019. [DOI] [PubMed] [Google Scholar]
- 46.Zientek J, Dahlgren FS, McQuiston JH, Regan J. Self-reported treatment practices by healthcare providers could lead to death from Rocky Mountain spotted fever. J Pediatr. 2014;164:416–418. doi: 10.1016/j.jpeds.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mosites E, Carpenter LR, McElroy K, Lancaster MJ, Ngo TH, McQuiston J, Wiedeman C, Dunn JR. Knowledge, attitudes, and practices regarding Rocky Mountain spotted fever among healthcare providers, Tennessee, 2009. Am J Trop Med Hyg. 2013;88:162–166. doi: 10.4269/ajtmh.2012.12-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brett ME, Hinckley AF, Zielinski-Gutierrez EC, Mead PS. U.S. healthcare providers' experience with Lyme and other tick-borne diseases. Ticks Tick Borne Dis. 2014;5:404–408. doi: 10.1016/j.ttbdis.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]