Abstract
Relapsing fever borreliae, produced by ectoparasite-borne Borrelia species, cause mild to deadly bacteremia and miscarriage. In the perspective of developing inexpensive assays for the rapid detection of relapsing fever borreliae, we produced 12 monoclonal antibodies (MAbs) against Borrelia crocidurae and characterized the two exhibiting the highest titers. P3A10 MAb reacts with the 35.6-kDa flagellin B (flaB) of B. crocidurae while P6D9 MAb recognizes a 35.1-kDa variable-like protein (Vlp) in B. crocidurae and a 35.2-kDa Vlp in Borrelia duttonii. Indirect immunofluorescence assay incorporating relapsing fever and Lyme group borreliae and 11 blood-borne organisms responsible for fever in West Africa confirmed the reactivity of these two MAbs. Combining these two MAbs in indirect immunofluorescence assays detected relapsing fever borreliae including B. crocidurae in ticks and the blood of febrile Senegalese patients. Both antibodies could be incorporated into inexpensive and stable formats suited for the rapid point-of-care diagnosis of relapsing fever. These first-ever MAbs directed against African relapsing fever borreliae are available for the scientific community to promote research in this neglected field.
Introduction
Relapsing fever borreliae are arthropod-borne pathogens causing mild to deadly spirochetemia, most commonly resulting in malaria-like symptoms.1 In Africa, cultured representatives include tick-borne Borrelia crocidurae, Borrelia duttonii, and Borrelia hispanica, transmitted by Ornithodoros soft ticks, and louse-borne Borrelia recurrentis.1 Recently, we detected, using molecular tools, a new Borrelia sp. named Candidatus Borrelia algerica in the blood of febrile patients in Algeria.2 Resource-consuming molecular methods are required to detect these pathogens in vectors and clinical specimens, which may not be routinely available in endemic regions,3 in addition to nonspecific direct microscopic examination. Microscopic examination is not able to distinguish between the various Borrelia species and molecular methods are not widely available, mainly used in a few reference centers not necessarily located in the endemic regions. However, prognosis of relapsing fever depends on the causative species, with the mortality rate being significantly higher for B. recurrentis than that for B. crocidurae.4 Indeed, part of the deadly mortality is due to Jarisch–Herxheimer reactions when treating patients infected by B. recurrentis; such a reaction has never been described with B. crocidurae.5 Development of inexpensive assays for the rapid detection of relapsing fever borreliae is therefore warranted. In this perspective, a few antibodies have been made using new world Borrelia hermsii and Borrelia afzelii (for the Lyme disease group) antigens, which may eventually cross-react with some African borreliae6,7 and five murine monoclonal antibodies (MAbs): MAbs H5332 and H5TS specific for Borrelia burgdorferi outer surface protein A (OspA),8,9 MAbs H6831 and H614 specific for OspB,10 and MAb H9724 that reacts with a protein of the periplasmic flagella of the genus Borrelia.11 However, no antibody has been developed using African relapsing fever borreliae as antigens. Borrelia crocidurae is the most common parasite of such relapsing fever borreliae in west Africa. We therefore produced and characterized MAbs against B. crocidurae with a view to incorporating them in a point-of-care laboratory test for rapid diagnosis.3
Materials and Methods
Ethics statement.
The Ornithodoros sonrai ticks studied here are not registered as endangered species. The study protocol was approved by the Steering Committee of the Institut Recherche et Développement (IRD) Special Program Evolution Climatique et Santé (Montpellier, France), reference project ATI-ECS-07-H/2002. As for the mammals, the study protocol was approved by Comité d'éthique de Marseille C2EA-14 and experimentation was performed according to the recommendations of reference project no. 60 12-11-2012. Regarding the human specimens, the study protocol was approved by the National Ethics Committee of Senegal Br 00081 04-06-12 and experimentation was performed according to the recommendations of reference projects SEN21/09 and SEN37/09.
Borrelia culture.
The B. crocidurae Achema strain, (tick strain), B. crocidurae 03-02 strain, (clinical strain), B. duttonii Ly strain, B. recurrentis A1 strain, and B. burgdorferi B31 strain were grown at 32°C in a Barbour-Stoenner-Kelly-H medium (Sigma, Saint-Quentin-Fallavier, France) supplemented with 10% heat-inactivated rabbit serum (Eurobio, Courtaboeuf, France). Dark-field microscopic observation was performed to ensure the absence of any contaminant organisms and confirm the growth of the borreliae. To prepare antigens, broth inoculated with B. crocidurae was centrifuged at 14,000 × g for 10 minutes at 4°C; the pellet was washed twice with 5% phosphate-buffered saline (PBS) and Tween-20 (Euromedex, Souffelweyersheim, France), suspended in PBS and inactivated by incubation at 70°C for 1 hour.
Production of MAbs.
To produce MAbs, 6-week-old female BALB/c mice were immunized by intraperitoneal inoculation of 10 μg of purified B. crocidurae Achema strain mixed with 100 μL of Imject Alum adjuvant (Thermoscientific, Courtaboeuf, France) (aluminum hydroxide and magnesium hydroxide mixture to stimulate the immune response for antibody production when incubated with immunogens v/v for 30 minutes). The mice were boosted three times at 2-week intervals, and then 5 days after the last injection, the spleens were sampled. Spleen cells were fused with myeloma cells (X63 Ag 8.653), then cultured in a supplemented Roswell Park Memorial Institute medium (Invitrogen, Cergy Pontoise, France) as previously described.12 Hybridoma supernatants were screened by an immunofluorimetric assay. The purified B. crocidurae Achema isolate was spotted on glass slides and then fixed with methanol for 10 minutes. The hybridoma supernatants (200 μL) were deposited on spot and then incubated for 30 minutes at 37°C. The slides were washed twice with PBS containing 0.1% Tween for 5 minutes and with distilled water for 5 minutes. After drying, antibodies were tagged using a goat anti-mouse IgG conjugated to fluorescein isothiocyanate (FITC) (Immunotech, Marseille, France) at 1:400 dilution in PBS. After washing, slides were dried, mounted with Fluoprep (bioMérieux, Marcy l'Etoile, France), then observed under ultraviolet light using an epifluorescent Leica DM 2500 microscope (Leica, Saint-Jorioz, France) at a magnification of ×400. Sera from immunized mice were used as positive controls and those from healthy unexposed mice were used as negative controls. Cells of the wells secreted antibodies (determined by immunofluorescence assay with B. crocidurae) were cloned and subcloned two times. The isotypes of the MAbs were determined with an IsoStrip Mouse Monoclonal Isotyping Kit (Roche Diagnostic, Meylan, France) containing sensitized strips against IgG1, IgG2a, IgG2b, IgG3, IgA, and IgM. Then specificities of the MAbs were assessed by western immunoblotting.
Reactivity of MAbs.
The MAbs produced were tested against B. duttonii, B. burgdorferi, B. recurrentis, and 11 blood-borne organisms responsible for fever in West Africa (Salmonella paratyphi, Streptococcus agalactiae, Streptococcus pneumoniae, Rickettsia felis, Plasmodium falciparum, Escherichia coli, Coxiella burnetii, Bartonella quintana, Pseudomonas aeruginosa, Haemophilus influenzae, and Acinetobacter baumannii)3,13 by indirect immunofluorescence as described in the above section Production of MAbs. The sources and strains of the Borrelia species and other organisms used to screen hybridomas and test the reactivity of MAbs are presented in Table 1. To confirm the reactivity of these MAbs against B. crocidurae, we also tested a clinical strain B. crocidurae 03-02 isolated from a patient with relapsing fever in Senegal, and its genome sequenced in our laboratory.14
Table 1.
Strains and sources of Borrelia spp. and other organisms used to screen hybridomas and to test the specificity of mouse MAbs against Borrelia crocidurae
| Species | Strain | Source |
|---|---|---|
| B. crocidurae | Achema | Ornithodoros sonrai, Mauritania |
| B. crocidurae | 03-02 | Clinical, Senegal |
| Borrelia duttonii | Ly | Clinical, Tanzania |
| Borrelia recurrentis | A1 | Clinical, Ethiopia |
| Borrelia burgdorferi | B31 | Ixodes scapularis, ATCC 35210 |
| Salmonella paratyphi | DK336 | Clinical, Senegal |
| Streptococcus agalactiae | DK003 | Clinical, Senegal |
| Streptococcus pneumoniae | 8CV | Clinical, France |
| Rickettsia felis | URRWXCal(2)(T) | Flea, France |
| Plasmodium falciparum | Clinical | Clinical, Senegal |
| Escherichia coli | DK031 | Clinical, Senegal |
| Coxiella burnetii | RSA 493 | Clinical, France |
| Bartonella quintana | URBQMTF14 | Clinical, France |
| Pseudomonas aeruginosa | DK 069 | Clinical, Senegal |
| Haemophilus influenzae | DK 102 | Clinical, Senegal |
| Acinetobacter baumannii | DK 007 | Clinical, Senegal |
Gel electrophoresis and western blotting.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western-blot analysis of Borrelia sp. were performed as previously described.15 Antigens were treated with proteinase K, and heat denaturation was performed at 100°C for 10 minutes. Borrelia crocidurae, B. burgdorferi, and B. duttonii whole-cell proteins resolved by 10% SDS-PAGE were visualized by silver staining. For one-dimensional western blotting, the gels were transferred to nitrocellulose and incubated with supernatants of MAbs P6D9 and P3A10, diluted at 1:100 and 1:500, respectively.
To prepare crude extracts for two-dimensional (2D) gel electrophoresis, purified bacteria were lysed by sonication in a rehydration solution (7 M urea, 2 M thiourea, 4% w/v 3-[(3-chloramidopropyl) diméthylammonio]-1-propanesulfonate hydrate) and centrifuged (10,000 × g, 20 minutes, 4°C) to remove cell debris and unbroken cells. The whole-cell protein extract was precipitated using the PlusOne 2-D Clean-Up Kit (GE Healthcare, Chalfont St. Giles, United Kingdom). The final pellet was resuspended again in rehydration solution, and the protein concentration was determined using the modified Bradford method.16 All IEF (Immobiline DryStrip gels [13 cm, pH 3–10]; GE Healthcare; rehydrated with 30 μg of solubilized proteins) and 2D electrophoresis steps were carried out as previously described.17 The proteins were resolved by electrophoresis through a 10% SDS-polyacrylamide gel (EttanTM DALT; GE Healthcare) at 5 watts (W)/gel for 30 minutes, followed by 17 W/gel for 4–5 hours. After electrophoresis, the gels were either silver stained or transferred onto a nitrocellulose membrane. Digital images were generated using transmission scanning (Image Scanner; GE Healthcare).
For 2D western blotting, Borrelia sp. proteins resolved by 2D gel electrophoresis (13 cm, pH 3-10) were transferred onto nitrocellulose membranes (Trans-blot Transfer Medium, pure nitrocellulose membrane, 0.45 μm; Bio-Rad, Ville-d'Avray, France). Membranes were then blocked in PBS supplemented with 0.2% Tween 20 and 5% non-fat dry milk (PBS-Tween-milk) for 1.5 hours at room temperature before incubation with MAbs P3A10 and P6D9 (dilution 1:100 or 1:500 for SDS-PAGE and western blot in the blocking buffer and a dilution of 1:1,000 for 2D western blotting). After 1 hour of incubation, membranes were washed three times with PBS-Tween and probed with horseradish peroxidase–conjugated goat antihuman IgG (1:5,000; GE Healthcare). Membranes were subsequently incubated with the secondary antibody (biotin-conjugated antibody, 20 μg/mL) for 1 hour before three successive washes as described above. Immunostained spots were visualized using a commercially available chemiluminescence kit (ECL™ western blotting Analysis System; GE Healthcare). Then, the membranes were exposed to Hyperfilm™ ECL and subsequently developed using an automated film processor (Hyperprocessor™; GE Healthcare).
Digestion peptides and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis.
The protein spots were excised from silver-stained gels either manually or by using Bio-Rad spot picker and identified using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) on a Bruker Ultraflex II spectrometer (Bruker Daltonics, Wissembourg, France) as previously described.18 After destaining, in-gel digestion with trypsin (sequencing-grade modified porcine trypsin; Promega, Madison, WI) was done as previously described.19 The peptides obtained from protein digestion were dissolved in 10–20 μL of 0.1% trifluoroacetic acid (TFA). The peptide mixture was then analyzed using MALDI-TOF-MS. The 0.3 μL peptide mixture was cocrystallized in the presence of 0.5% TFA onto the MALDI-TOF target with an equal amount of matrix solution (3 mg/mL of α-cyano-4-hydroxycinnamic acid in 50% acetonitrile). Alternatively, the peptide mixtures derived from protein digestion were desalted and concentrated using zip tips (Millipore, Bedford, MA) and deposited onto the MALDI-TOF target by elution with the matrix solution. Mass spectra were internally calibrated using autolytic peptides from trypsin. The peptide mass fingerprints were used to identify the proteins, using Mascot software for comprehensive sequence databases.20 Searches were performed against all available sequences in public databases, including those for eukaryotes.21
Immunofluorescence detection of B. crocidurae.
Twelve O. sonrai were collected in Senegal, and the presence of B. crocidurae was confirmed in ticks as previously described.22 The ticks were tested for the presence of B. crocidurae by glpQ gene real-time polymerase chain reaction (PCR) using a Ct ≤ 35 cutoff.23 The blind immunofluorescence assay incorporating both antibodies P3A10 and P6D9 was done using hemolymph of ticks as described.24 The distal portion of a leg of tick was amputated, and the hemolymph that appeared at the leg extremity was smeared onto a microscope slide, stained by Giemsa staining or immuno-detection methods, and examined for the presence of bacteria.25 The hemolymph drops were directly deposited onto glass slides, air-dried, and fixed with methanol for 5 minutes. The slides were stored at 4°C before use. For the immunofluorescence assay, slides were saturated by incubation with PBS-5% bovine serum albumine (BSA) at 37°C for 30 minutes and then washed twice with PBS-0.1% Tween for 10 minutes and once with sterile distilled water for 5 minutes. Samples were incubated with mouse MAbs P3A10 or P6D9, with serum from healthy unexposed mice as negative controls and serum of immunized mice as positive controls at a respective 1:100 or 1:400 dilution in PBS-3% BSA-0.1% Tween for 30 minutes at 37°C. After a washing step as described above, bound antibodies were revealed with FITC-conjugated IgG goat anti-mouse antibody (Immunotech, Marseille, France) diluted at 1:400 in PBS Evans blue (bioMérieux) and one spot of secondary antibody FITC with a sample for secondary controls. For image scanning, slides were mounted with Fluoprep (bioMérieux) after subsequent washing procedures and examined under an Olympus BX-51 epifluorescence microscope (Olympus, Rungis, France).
Five 7-week-old female guinea pigs were inoculated intraperitoneally with 107 B. crocidurae or buffer (one negative control). Blood obtained by cardiac puncture 5 days postinoculation was tested by immunofluorescence assay incorporating P6D9 or P3A10 as described above.15
Six blood smears from patients diagnosed by real-time PCR with B. crocidurae relapsing fever and from two patients negative for B. crocidurae in Senegal26 were analyzed by immunofluorescence assay incorporating P6D9 or P3A10.
Results
Reactivity.
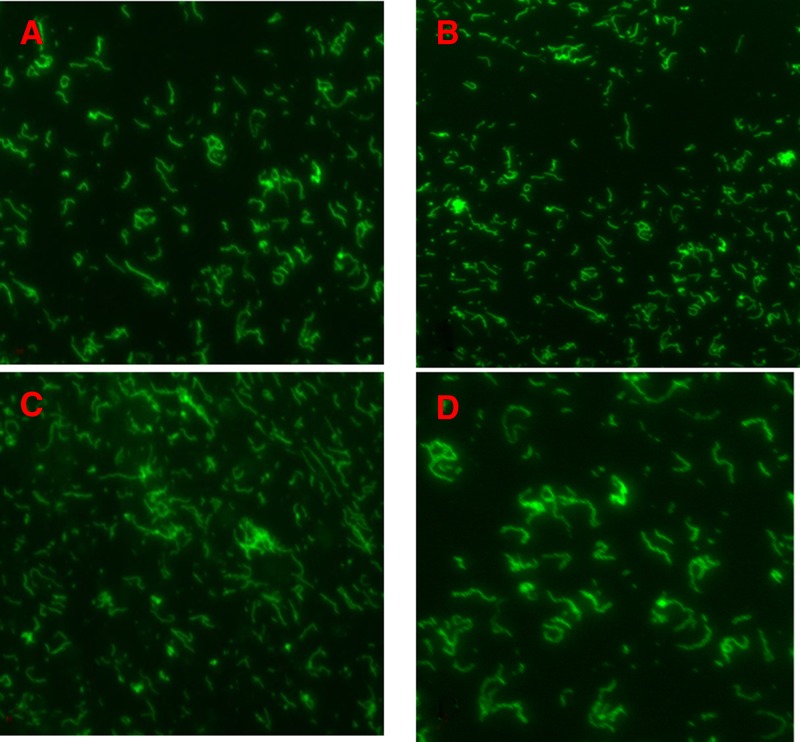
A total of 12 MAbs were produced and isotyped as IgG1k, IgG2ak, IgG2bk, and IgM k (Table 2). Indirect immunofluorescence assay incorporating relapsing fever borreliae and 11 blood-borne organisms responsible for fever in West Africa showed that 11 MAbs reacted for relapsing fever borreliae, with MAb P5A7 also reacting with a Bartonella quintana clinical strain at 1:40 titer. Antibodies P3A10 and P6D9 exhibited the highest titers against the B. crocidurae Achema strain (Tables 1 and 2) and further reacted against the B. crocidurae 03-02 clinical strain in immunofluorescence assay (Figure 1 ). In silico, multispacer sequence typing (MST) of B. crocidurae 03-02 strain found sequence type 6 (ST6), which differed in the five spacer sequences used from the reference strain Achema (ST13). In particular, P3A10 recognized the B. crocidurae Achema strain at a 1:500 dilution and the B. crocidurae 03-02 clinical strain at a 1:1,000 dilution.
Table 2.
Isotyping, titration, and specificity of 12 MAbs by immunofluorescence assay using Borrelia spp. and 11 blood-borne organisms
| Hybridoma | P4F7 | P6G9 | P10H6 | P2E1 | P3A10 | P6D9 | P2E9 | P9B1 | P6A1 | P5A7 | P7D7 | P1D4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isotype | IgG1k | IgG1k | IgG2ak | IgG1k | IgG2bk | IgG1k | IgG1k | IgG1k | IgG2bk | IgG1k | IgM k | IgG2bk |
| Titer | 21:320 | 1:320 | 1:80 | 1:40 | 1:640 | 1:1,280 | 1:320 | 1:160 | 1:80 | 1:40 | 1:320 | 1:160 |
| Borrelia crocidurae | pos | pos | pos | pos | pos | pos | pos | pos | pos | pos | pos | pos |
| Borrelia duttonii | pos | pos | pos | pos | neg | pos | pos | pos | pos | pos | pos | pos |
| Borrelia recurrentis | pos | neg | pos | neg | neg | neg | neg | pos | pos | pos | pos | pos |
| Borrelia burgdorferi | pos | pos | neg | neg | neg | neg | neg | pos | pos | neg | pos | pos |
| Salmonella paratyphi | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg |
| Streptococcus agalactiae | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg |
| Streptococcus pneumoniae | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg |
| Rickettsia felis | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg |
| Plasmodium falciparum | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg |
| Escherichia coli | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg |
| Coxiella burnetii | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg |
| Bartonella quintana | neg | neg | neg | neg | neg | neg | neg | neg | neg | pos | neg | neg |
| Pseudomonas aeruginosa | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg |
| Haemophilus influenzae | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg |
| Acinetobacter baumanii | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg | neg |
MAbs = monoclonal antibodies; neg = negative; pos = positive.
Samples were considered positive when the fluorescence intensity of bacteria was comparable to that of polyclonal sera from immunized mice.
Figure 1.
Indirect immunofluorescence assay detection of Borrelia crocidurae Achema strain (A and B) and clinical isolate B. crocidurae 03-02 (C and D) by purified mouse monoclonal antibodies (MAbs). P3A10 (panel A and C at 1:500 and 1:1,000 dilution, respectively) and P6D9 (panel B and D at 1:500 and 1:1,000 dilution, respectively). MAbs were tagged using a goat anti-mouse IgG conjugated to fluorescein isothiocyanate at 1:400 dilution. Magnification: ×1,000.
SDS-PAGE and western blotting of B. crocidurae.
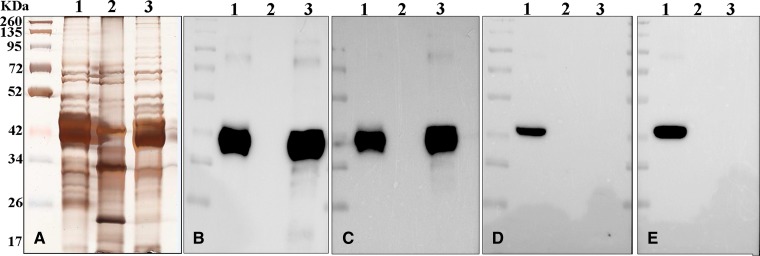
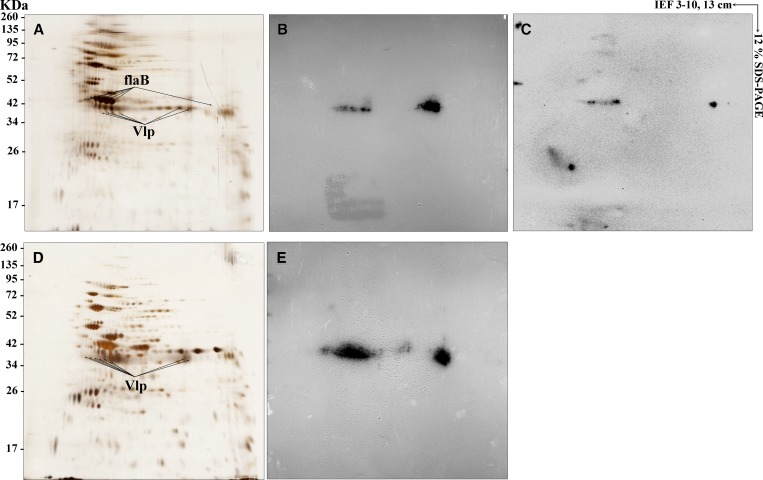
To identify the epitopes recognized by the MAbs, B. crocidurae Achema and B. duttonii Ly extracts were subjected to 2D electrophoresis and subsequent western blotting analysis. SDS-PAGE and western-immunobloting using P3A10 and P6D9 found no reactivity against B. burgdorferi while P6D9 cross-reacted with B. crocidurae and B. duttonii and P3A10 reacted with B. crocidurae (Figure 2 ). Protein profiles of silver-stained 2D gels (13 cm, pI 3–10) were very similar for both B. crocidurae and B. duttonii. Protein spots were distributed within molecular weight ranging from 17 to 135 kDa. The majority of spots are clustered around a narrow range of pH 3.5–7. The immunoreactivity patterns revealed by 2D immunoblot profiles obtained for both B. crocidurae and B. duttonii were very similar and homogeneous with MAb P6D9. In-gel trypsin digestion of reacting spots excised from silver-stained 2D gel followed by MALDI-TOF-MS identification indicated that P3A10 recognized flagellin Borrelia flaB (molecular weight 35.6 kDa) and P6D9 recognized a variable-like protein (Vlp) (molecular weight 35.1 kDa in B. crocidurae and 35.2 kDa in B. duttonii) (Figure 3 ). P3A10 and P6D9 have been deposited in the Deutsche Sammlung von Mikroorganismen (DSM) collection (DSM ACC3255 and DSM ACC3256, respectively) as required by the Budapest Treaty on the international recognition of the deposit of microorganisms for the purposes of patent procedure.
Figure 2.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western-blot analysis of Borrelia sp. Borrelia crocidurae, Borrelia burgdorferi, and Borrelia duttonii whole-cell proteins resolved by 10% SDS-PAGE (lane 1, 2, and 3, respectively) were visualized by silver staining (A) or transferred to nitrocellulose before probing with the purified mouse monoclonal antibodies P6D9 (panel B and C at 1:100 or 1:500 dilution, respectively) and P3A10 (panel D and E at 1:500 or 1:100 dilution, respectively). Molecular weight markers for all aligned panels are indicated on the left in kilodaltons (kDa).
Figure 3.
Immunoreactive proteins with mouse monoclonal antibodies (MAbs) P6D9 and P3A10. Borrelia crocidurae and Borrelia duttonii proteins were resolved in the first dimension over a isoelectric point gradient of 3–10 followed by 12% linear sodium dodecyl sulfate polyacrylamide gel electrophoresis for the second dimension. Proteins were then detected by silver staining (A and D for B. crocidurae and B. duttonii, respectively) or transferred to a nitrocellulose membrane and probed with purified mouse MAbs P6D9 (B and E) and P3A10 (C) at 1:1,000 dilution. Standard molecular weight markers are indicated on the left in kilodaltons (kDa). Identified immunoreactive proteins, achieved by matrix-assisted laser desorption/ionization time-of-flight analysis, are indicated by protein name.
Immunofluorescence detection of B. crocidurae.
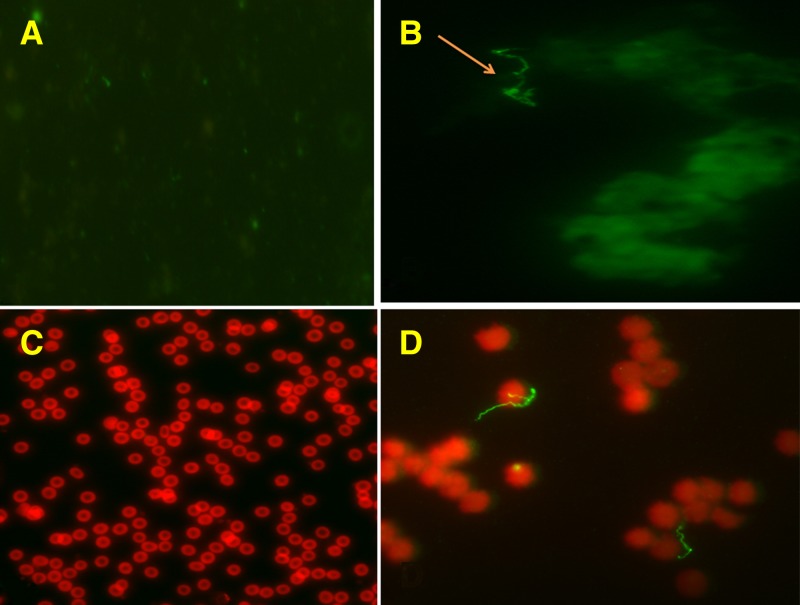
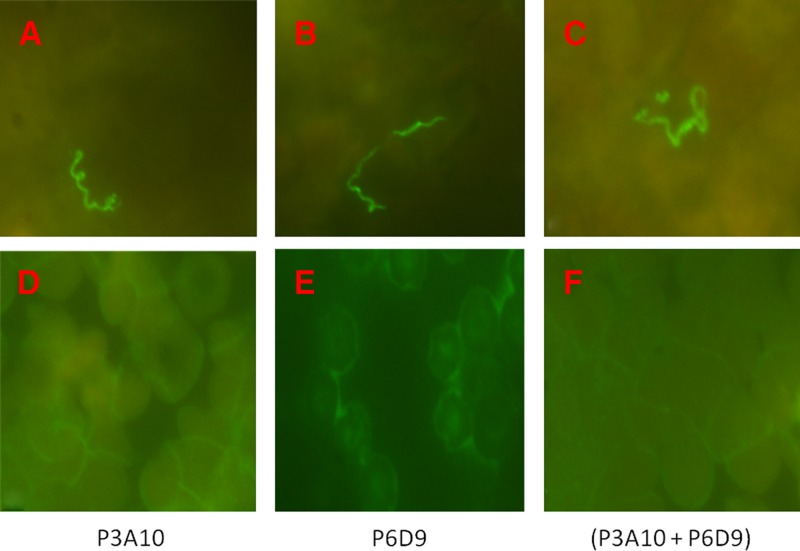
Among the 12 O. sonrai collected in Senegal, four were positive for B. crocidurae DNA. Blind immunofluorescence assay incorporating P3A10 and P6D9 on the hemolymph, using the hemolymph test technique for detection of rickettsiae in ticks by immunofluorescence,24 found no Borrelia in noninfected ticks and Borrelia-like organisms in 2/4 infected ticks (Figure 4 ). No Borrelia was detected in the negative control animal whereas P3A10 and P6D9 detected Borrelia-like organisms in 4/5 challenged female guinea pigs inoculated intraperitoneally with B. crocidurae (Figure 4). Finally, immunofluorescence assay incorporating P6D9 or P3A10 detected Borrelia-like organisms in 6/6 blood smears from patients diagnosed by real-time PCR with B. crocidurae relapsing fever in Senegal and no organisms in two negative controls (Figure 5 ).
Figure 4.
Immunofluorescence assay detection of Borrelia crocidurae in the Ornithodoros sonrai tick (arrow) (A for negative control and B for assay) and bloodstream of guinea pigs (C for negative control and D for assay) with P3A10 and P6D9 monoclonal antibodies (MAbs). Purified mouse MAbs P3A10 and P6D9 at 1:500 dilution were tagged using a goat anti-mouse IgG conjugated to fluorescein isothiocyanate at 1:400 dilution. Magnification: ×500 for guinea pig negative control and ×1,000 for other pictures.
Figure 5.
Immunofluorescence assay detection of Borrelia crocidurae in blood smears from patients diagnosed with B. crocidurae relapsing fever in Senegal using P3A10 and P6D9 purified monoclonal antibodies. A, B, and C are blood smears of three patients; D, E, and F are negative controls. P3A10 and P6D9 at 1:500 dilution were tagged using a goat anti-mouse IgG conjugated to fluorescein isothiocyanate at 1:400 dilution. Magnification: ×1,000.
Discussion
The direct detection of relapsing fever borreliae in clinical and vector specimens currently requires use of technically demanding, time- and resource-consuming molecular methods.27 Although the serological confirmation is based on either an immunofluorescence assay or enzyme-linked immunosorbent assay, this technique presents drawbacks because the antigenic variability of relapsing fever borreliae outer surface protein Vlp (proteins that, as a result of antigenic variation, allow relapsing fever borreliae to escape the host immune response)28 and antigens shared with the Lyme disease spirochete (B. burgdorferi)29 may cause both false-negative and false-positive results, while serological tests based on the glpQ antigen can discriminate between relapsing fever and Lyme borreliosis.30 In this research based on the fact that B. crocidurae is the most common recognized Borrelia causing relapsing fever in West Africa, we produced 12 MAbs against B. crocidurae. Eleven of these MAbs reacted with borreliae and did not react with 11 blood-borne organisms responsible for fever in West Africa.3,13 The identification and characterization of the immunoreactivity of the two MAbs that exhibited the highest titers (P3A10 and P6D9) indicated that P6D9 cross-reacted with only B. crocidurae and B. duttonii Vlp. As Vlp is an antigen that varies in the surface protein of Borrelia, they may not be the stable and suitable targets for diagnostic MAbs.29 However, MAb P3A10 binds to flaB epitope 35.6 kDa, unique to B. crocidurae and may be a good candidate to be incorporated into a rapid diagnosis test for relapsing fever borreliae. Indeed, MAb P3A10 was shown to react equally with two cultured B. crocidurae isolates representative of two MST types. Furthermore, it detected B. crocidurae in six human blood smears in Senegal, two ticks and four animals, although these animals were experimentally infected and thus may not truly represent the sensitivity found during natural infection. We showed previously that several B. crocidurae genotypes were circulating in Senegal by MST,31 and these data suggest that MAb P3A10 recognized several genotypes in B. crocidurae. The identity of a Borrelia isolate can be classified according to protein profiles and immunoreactivities with specific MAbs. Although the diversity of major Osps and flagellar epitopes had been demonstrated in Lyme disease isolates from various geographical areas,9,11 for relapsing fever borreliae, P3A10 MAb recognized the flaB epitope of B. crocidurae. The most recent epidemiological data indicate that 43.92 million people living in rural Africa in endemic countries and 19.17 million travelers are at risk of relapsing fever in West and North African countries.32 Extrapolating on the 11% incidence of tick-borne relapsing fever measured in rural Senegal,33 this represents about 4.82 million cases of relapsing fever a year. Indeed, both antibodies could be incorporated into a format well suited for the rapid point-of-care diagnosis of relapsing fever in both endemic countries3 and in countries with travelers.34 In Africa, relapsing fever is often confused with malaria,1 therefore requires specific treatment and a specific prophylaxis.
Disclaimer: Antibodies here reported have been patented under no. FR 2015/1461399 by Aurélien Fotso Fotso, Didier Raoult, and Michel Drancourt.
Footnotes
Authors' addresses: Aurélien Fotso Fotso, Claude Nappez, Saïd Azza, and Didier Raoult, URMITE, UMR 6236, CNRS 7278, IRD 198, INSERM 1095, IFR 48, Méditerranée Infection, Faculté de Médecine, Aix-Marseille Univesité, Marseille, France, E-mails: aurelien74000618@yahoo.fr, claude.nappez@univ-amu.fr, said.azza@univ-amu.fr, and didier.raoult@gmail.com. Oleg Mediannikov, URMITE, UMR 6236, CNRS 7278, IRD 198, INSERM 1095, IFR 48, Méditerranée Infection, Faculté de Médecine, Aix Marseille Université, Marseille, France, and IRD, URMITE UMR198, Campus Communs IRD/UCAD Hann Maristes, Dakar, Senegal, E-mail: olegusss1@gmail.com. Michel Drancourt, Méditerrannée Infection, Aix Marseille Université, Marseille, France, and Unité de Recherche sur les Maladies Infectieuses et Tropicale Émergentes, Marseille, France, E-mail: michel.drancourt@univ-amu.fr.
References
- 1.Haitham E, Raoult D, Drancourt M. Relapsing fever borreliae in Africa. Am J Trop Med Hyg. 2013;89:288–292. doi: 10.4269/ajtmh.12-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fotso Fotso A, Angelakis E, Mouffok N, Drancourt M, Raoult D. Blood-borne Candidatus Borrelia algerica in a patient with prolonged fever in Oran, Algeria. Am J Trop Med Hyg. 2015;93:1070–1073. doi: 10.4269/ajtmh.15-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sokhna C, Mediannikov O, Fenollar F, Bassene H, Diatta G, Tall A, Trape JF, Drancourt M, Raoult D. Point-of-care laboratory of pathogen diagnosis in rural Senegal. PLoS Negl Trop Dis. 2013;7:e1999. doi: 10.1371/journal.pntd.0001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutler S, Abdissa A, Trape JF. New concepts for the old challenge of African relapsing fever borreliosis. Clin Microbiol Infect. 2009;15:400–406. doi: 10.1111/j.1469-0691.2009.02819.x. [DOI] [PubMed] [Google Scholar]
- 5.Fekade D, Knox K, Hussein K, Melka A, Lalloo DG, Coxon RE, Warrell DA. Prevention of Jarisch-Herxheimer reactions by treatment with antibodies against tumor necrosis factor alpha. N Engl J Med. 1996;335:311–315. doi: 10.1056/NEJM199608013350503. [DOI] [PubMed] [Google Scholar]
- 6.Schwan T, Gage K, Karstens R, Schrumpf M, Hayes S, Barbour AG. Identification of the tick-borne relapsing fever spirochete Borrelia hermsii by using a species-specific monoclonal antibody. J Clin Microbiol. 1992;30:790–795. doi: 10.1128/jcm.30.4.790-795.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canica M, Nato F, Du Merle L, Mazie JC, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- 8.Barbour AG, Tessier SL, Todd WJ. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983;41:795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbour AG, Heiland RA, Howe TR. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985;152:478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- 10.Barbour AG, Tessier SL, Hayes SF. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984;45:94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbour AG, Hayes SF, Heiland RA, Schrumpf ME, Tessier SL. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986;52:549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 13.D'Acremont V, Kilowoko M, Kyungu E, Philipina S, Sangu W, Kahama-Maro J, Lengeler C, Cherpillod P, Kaiser L, Genton B. Beyond malaria-causes of fever in outpatient Tanzanian children. N Engl J Med. 2014;370:809–817. doi: 10.1056/NEJMoa1214482. [DOI] [PubMed] [Google Scholar]
- 14.Fotso Fotso A, Mediannikov O, Padmanabhan R, Robert C, Fournier PE, Raoult D, Drancourt M. Genome sequence of Borrelia crocidurae strain 03-02, a clinical isolate from Senegal. Genome Announc. 2014;2:e01150–e14. doi: 10.1128/genomeA.01150-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Y, Kowalczewska M, Decloquement P, Nappez C, La Scola B. Production of monoclonal antibodies to Tropheryma whipplei and identification of recognized epitopes by two-dimensional electrophoresis and mass spectrometry. J Clin Microbiol. 2006;44:4179–4185. doi: 10.1128/JCM.01714-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kowalczewska M, Fenollar F, Villard C, Azza S, Roux M, Raoult D. An immunoproteomic approach for identification of clinical biomarkers of Whipple's disease. Proteomics Clin Appl. 2008;2:504–516. doi: 10.1002/prca.200780078. [DOI] [PubMed] [Google Scholar]
- 17.Renesto P, Samson L, Ogata H, Azza S, Fourquet P, Gorvel JP, Heinzen RA, Raoult D. Identification of two putative rickettsial adhesins by proteomic analysis. Res Microbiol. 2006;157:605–612. doi: 10.1016/j.resmic.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Kowalczewska M, Fenollar F, Lafitte D, Raoult D. Identification of candidate antigen in Whipple's disease using a serological proteomic approach. Proteomics. 2006;6:3294–3305. doi: 10.1002/pmic.200500171. [DOI] [PubMed] [Google Scholar]
- 19.Renesto P, Azza S, Dolla A, Fourquet P, Vestris G, Gorvel JP, Raoult D. Rickettsia conorii and R. prowazekii proteome analysis by 2DE-MS: a step toward functional analysis of rickettsial genomes. Ann N Y Acad Sci. 2005;1063:90–93. doi: 10.1196/annals.1355.014. [DOI] [PubMed] [Google Scholar]
- 20.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 21.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Fotso Fotso A, Mediannikov O, Diatta G, Almeras L, Flaudrops C, Parola P, Drancourt M. MALDI-TOF mass spectrometry detection of pathogens in vectors: The Borrelia crocidurae/Ornithodoros sonrai paradigm. PLoS Negl Trop Dis. 2014;8:e2984. doi: 10.1371/journal.pntd.0002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haitham E, Henry M, Diatta G, Mediannikov O, Sokhna C, Tall A, Socolovschi C, Cutler SJ, Bilcha KD, Ali J, Campelo D, Barker SC, Raoult D, Drancourt M. Multiplex real-time PCR diagnostic of relapsing fevers in Africa. PLoS Negl Trop Dis. 2013;7:e2042. doi: 10.1371/journal.pntd.0002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgdorfer W. Hemolymph test. A technique for detection of rickettsiae in ticks. Am J Trop Med Hyg. 1970;19:1010–1014. [PubMed] [Google Scholar]
- 25.Parola P, Raoult D. Ticks and tick borne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 26.Mediannikov O, Socolovschi C, Bassene H, Diatta G, Ratmanov P, Fenollar F, Sokhna C, Raoult D. Borrelia crocidurae infection in acutely febrile patients, Senegal. Emerg Infect Dis. 2014;20:1335–1338. doi: 10.3201/eid2008.130550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parola P, Diatta G, Socolovschi C, Mediannikov O, Tall A, Bassene H, Trape JF, Raoult D. Tick-borne relapsing fever borreliosis, rural Senegal. Emerg Infect Dis. 2011;17:883–885. doi: 10.3201/eid1705.100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai Q, Restrepo BI, Porcella SF, Raffel SJ, Schwan TG, Barbour AG. Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids. Mol Microbiol. 2006;60:1329–1343. doi: 10.1111/j.1365-2958.2006.05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lescot M, Audic S, Robert C, Nguyen TT, Blanc G, Cutler SJ, Wincker P, Couloux A, Claverie JM, Raoult D, Drancourt M. The genome of Borrelia recurrentis, the agent of deadly louse-borne relapsing fever, is a degraded subset of tick-borne Borrelia duttonii. PLoS Genet. 2008;4:e1000185. doi: 10.1371/journal.pgen.1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwan TG, Schrumpf ME, Hinnebusch BJ, Anderson DE, Konkel ME. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J Clin Microbiol. 1996;34:2483–2492. doi: 10.1128/jcm.34.10.2483-2492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haitham E, Gimenez G, Sokhna C, Bilcha K, Ali J, Barker SC, Cutler S, Raoult D, Drancourt M. Multispacer sequence typing relapsing fever borreliae in Africa. PLoS Negl Trop Dis. 2012;6:e1652. doi: 10.1371/journal.pntd.0001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trape JF, Diatta G, Arnathau C, Bitam I, Sarih M, Belghyti D, Bouattour A, Elguero E, Vial L, Mané Y, Baldé C, Prugnolle F, Chauvancy G, Mahé G, Granjon L, Duplantier JM, Durand P, Renaud F. The epidemiology and geographic distribution of relapsing fever borreliosis in West and North Africa, with a review of the Ornithodoros erraticus complex (Acari: Ixodida) PLoS One. 2013;8:e78473. doi: 10.1371/journal.pone.0078473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vial L, Diatta G, Tall A, Ba El H, Bouganali H, Durand P, Sokhna C, Rogier C, Renaud F, Trape JF. Incidence of tick-borne relapsing fever in west Africa: longitudinal study. Lancet. 2006;368:37–43. doi: 10.1016/S0140-6736(06)68968-X. [DOI] [PubMed] [Google Scholar]
- 34.Cohen-Bacrie S, Ninove L, Nougaire'de A, Charrel R, Richet H, Minodier P, Badiaga S, Noël G, La Scola B, De Lamballerie X, Drancourt M, Raoult D. Revolutionizing clinical microbiology laboratory organization in hospitals with in situ point-of-care. PLoS One. 2011;6:e22403. doi: 10.1371/journal.pone.0022403. [DOI] [PMC free article] [PubMed] [Google Scholar]