Abstract
Melioidosis is a tropical disease of high mortality caused by the environmental bacterium, Burkholderia pseudomallei. We have collected clinical isolates from the highly endemic Northern Territory of Australia routinely since 1989, and animal and environmental B. pseudomallei isolates since 1991. Here we provide a complete record of all B. pseudomallei multilocus sequence types (STs) found in the Northern Territory to date, and distribution maps of the eight most common environmental STs. We observed surprisingly restricted geographic distributions of STs, which is contrary to previous reports suggesting widespread environmental dissemination of this bacterium. Our data suggest that B. pseudomallei from soil and water does not frequently disperse long distances following severe weather events or by migration of infected animals.
The soil and water Gram-negative bacillus, Burkholderia pseudomallei, is the etiological agent of melioidosis, a potentially life-threatening infectious tropical disease.1 Melioidosis is highly endemic in northern Australia and northeast Thailand, with increasing recognition outside these traditional regions.2,3 Melioidosis has a high mortality rate; 14% in Australia2 and up to 40% in Thailand, where it is the largest cause of infectious disease death after human immunodeficiency virus and tuberculosis.4 B. pseudomallei is classified as a Tier 1 select agent in the United States because it is intrinsically resistant to many antibiotics, there is no vaccine and aerosolization has potentially high lethality.
Burkholderia pseudomallei is one of an increasing number of bacterial species known to show robust biogeographic structure. In particular, there is a clear and robust phylogenetic division between Australian and southeast Asian strains,5–7 with almost no overlap between the environmental strains found in Australia and those in southeast Asia. An apparent exception was the presence of two multilocus sequence typing (MLST) sequence types (STs) common to both Australian and Cambodian B. pseudomallei strains (ST-105 and ST-849). However, for both these STs, isolates from Australia and Cambodia were found to be widely divergent at the whole-genome level, indicating that they were unrelated and the MLST identity represented homoplasy.8 Within Australia, some genetic population structure among different locations has been detected. In particular, there are no shared environmental STs between the Northern Territory and Queensland.9,10 Confirmation of population structure at increasingly fine scales suggests that the bacterium has limited dispersal ability. Limited dispersal is unexpected, because the species can be carried by agricultural animals and is relatively resistant to environmental stress.1 Its ability to cause infection when inhaled makes it plausible that B. pseudomallei could be dispersed in severe weather events such as cyclones. This bacterium is also able to move in the water table11 and presumably to track along waterways, to be transported in soil used for gardening, building and farming, and to live in the aerial parts of grasses.12 In addition, B. pseudomallei has been isolated from the beak of a healthy bird, suggesting another possible mode of long-distance dissemination.13 B. pseudomallei can also seed new endemic areas and cause long-term contamination of soil and water outside of the tropics.14,15 Taken together, these findings suggest that this organism has many opportunities to disperse widely. However, an important factor likely to limit dispersal is the sensitivity of B. pseudomallei to ultraviolet radiation,16 which has been exploited to eradicate the bacteria from unchlorinated bore water supplies.17
The Northern Territory of Australia, with an area of 1,346,200 km2, lies between latitudes 11 and 26°S. The most northerly tropical region of the Northern Territory, known as the “Top End,” is subject to monsoonal weather patterns. In contrast, the southerly and western regions are characterized by hot semi-arid and arid deserts. The Northern Territory has a population of approximately 245,000 people and has been sparsely occupied throughout its history from first settlement over 40,000 years ago, and since European settlement in the early nineteenth century. Just over half the current population is concentrated in the Northern Territory's capital city, Darwin (12°S), on the northern coastline. Most of the remainder of the population is settled in smaller towns and remote indigenous communities throughout the Territory and on its northern offshore islands. Although melioidosis is highly endemic in the tropical Top End, sporadic cases are occasionally seen in the normally arid Central Australia, south of latitude 20°S, such as occurred following unusually heavy rain in 2011.18
Herein we report the geographical distribution of B. pseudomallei STs within the Northern Territory. We use data from over 3,000 environmental strains collected since 1991 at the Menzies School of Health Research in Darwin, Australia. We have mapped the geographical distribution of the most common B. pseudomallei STs in environmental samples in the Northern Territory; specifically, those that have been found in 10 or more unique environmental samples. We also report the complete list of environmental, animal, and human clinical B. pseudomallei STs recorded in our sampling efforts across the Northern Territory, up until January 28, 2015. The earliest clinical sample in our data set is from 1980, with all other clinical samples collected prospectively from melioidosis cases since the commencement of the Darwin Prospective Melioidosis Study on October 1, 1989.19
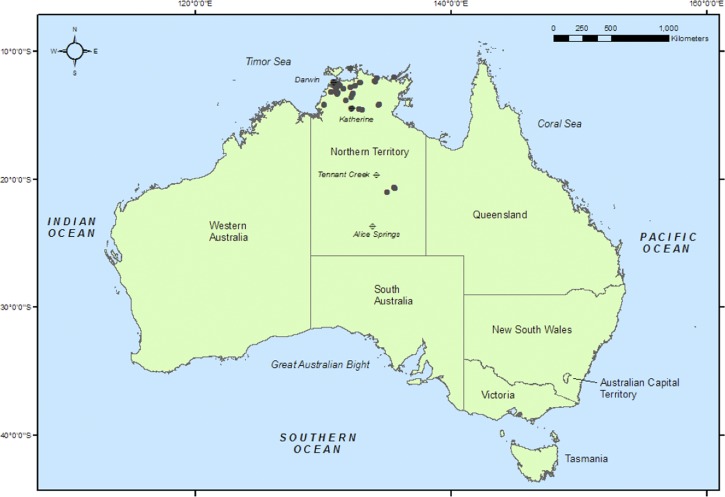
Our environmental samples are taken mostly from soil or water, and include both disturbed urban sites such as near housing or building sites and undisturbed sites. Environmental sampling in the Northern Territory is logistically challenging due to the long travel distances between population centers, the large number of unsealed roads, the lack of road access to most locations, and extreme isolation outside of the few major towns and cities. These difficulties are reflected by the concentration of samples in Darwin and its surrounding areas (Figure 1 ). Our most southerly B. pseudomallei-positive environmental samples were found in a water hole south of Tennant Creek, at a remote location at latitude 21°S. Although sporadic clinical cases of melioidosis have occurred further south than this location,18 we have not recovered B. pseudomallei from environmental samples south of 21°S in the Northern Territory.
Figure 1.
Map of Australia showing the Northern Territory. Dots indicate all locations in the Northern Territory where Burkholderia pseudomallei has been cultured from environmental samples.
Phylogenetic analysis shows that Australian B. pseudomallei strains are ancestral to Southeast Asian strains.6 Accordingly, our results demonstrate the considerable diversity of B. pseudomallei in the Northern Territory (Table 1). In total, 379 different MLST STs have been documented from the Northern Territory among all the environmental, animal, and clinical samples from the past 35 years of sampling that have been subjected to MLST.
Table 1.
Summary of sequence types (ST) of Burkholderia pseudomallei found in the Northern Territory (1980–2015), and the sources from which they have been isolated
| ST | n (soil) | n (water) | n (other environment) | n (human) | Total | Animal |
|---|---|---|---|---|---|---|
| 36 * | 28 | 0 | 0 | 53 | 81 | Y |
| 109 * | 68 | 41 | 0 | 87 | 196 | Y |
| 114 | 1 | 0 | 0 | 2 | 3 | N |
| 116 | 0 | 0 | 0 | 4 | 4 | Y |
| 121 | 2 | 2 | 0 | 2 | 6 | N |
| 126 | 0 | 1 | 0 | 6 | 7 | N |
| 131 | 2 | 6 | 0 | 8 | 16 | Y |
| 132 * | 28 | 14 | 0 | 54 | 96 | Y |
| 144 * | 22 | 0 | 0 | 12 | 34 | Y |
| 149 | 2 | 0 | 0 | 3 | 5 | N |
| 238 | 2 | 0 | 0 | 1 | 3 | N |
| 266 * | 6 | 10 | 2 | 9 | 27 | Y |
| 275 | 1 | 0 | 0 | 4 | 5 | N |
| 279 | 5 | 0 | 0 | 14 | 19 | N |
| 281 | 1 | 0 | 0 | 1 | 2 | N |
| 296 | 0 | 1 | 0 | 0 | 1 | Y |
| 320 * | 8 | 3 | 0 | 9 | 20 | Y |
| 325 * | 0 | 15 | 0 | 7 | 22 | N |
| 326 * | 7 | 9 | 0 | 1 | 17 | N |
| 327 | 3 | 2 | 0 | 10 | 15 | N |
| 328 | 0 | 1 | 0 | 0 | 1 | Y |
| 329 | 3 | 3 | 0 | 4 | 10 | N |
| 332 | 2 | 1 | 0 | 2 | 5 | N |
| 333 | 5 | 10 | 0 | 5 | 20 | Y |
| 334 | 0 | 1 | 0 | 1 | 2 | N |
| 335 | 0 | 1 | 0 | 19 | 20 | N |
| 337 | 0 | 2 | 0 | 1 | 3 | N |
| 437 | 0 | 1 | 0 | 1 | 2 | N |
| 456 | 1 | 0 | 0 | 5 | 6 | N |
| 464 | 1 | 0 | 0 | 13 | 14 | Y |
| 466 | 0 | 1 | 0 | 8 | 9 | N |
| 468 | 1 | 0 | 0 | 4 | 5 | N |
| 473 | 0 | 0 | 0 | 1 | 1 | Y |
| 480 | 4 | 0 | 0 | 4 | 8 | N |
| 483 | 2 | 0 | 0 | 16 | 18 | Y |
| 553 | 8 | 0 | 0 | 34 | 42 | N |
| 559 | 0 | 4 | 0 | 1 | 5 | Y |
| 562 | 4 | 0 | 6 | 18 | 28 | N |
| 566 | 2 | 0 | 0 | 4 | 6 | N |
| 572 | 0 | 0 | 0 | 1 | 1 | Y |
| 616 | 0 | 0 | 0 | 1 | 1 | Y |
| 617 | 1 | 0 | 0 | 1 | 2 | Y |
| 639 | 1 | 0 | 0 | 1 | 2 | N |
| 673 | 0 | 6 | 0 | 0 | 6 | Y |
| 674 | 0 | 3 | 0 | 0 | 3 | Y |
| 682 | 1 | 0 | 0 | 1 | 2 | N |
| 766 | 1 | 0 | 0 | 1 | 2 | N |
| 883 | 1 | 0 | 0 | 1 | 2 | N |
| 885 | 1 | 0 | 0 | 1 | 2 | N |
| 970 | 1 | 0 | 0 | 3 | 4 | N |
| STs found only in human samples | 103, 104, 105, 106, 107, 108, 111, 112, 113, 115, 117, 118, 120, 122, 124, 125, 127, 128, 129, 130, 134, 135, 136, 137, 138, 141, 142, 143, 145, 146, 147, 150, 239, 241, 242, 243, 244, 245, 249, 255, 259, 261, 262, 264, 269, 270, 278, 294, 322, 331, 427, 428, 429, 434, 435, 439, 440, 452, 453, 454, 455, 457, 458, 459, 461, 462, 463, 465, 467, 469, 470, 471, 472, 478, 479, 481, 482, 552, 554, 555, 556, 557, 560, 561, 563, 564, 565, 571, 573, 574, 575, 576, 577, 578, 579, 580, 581, 582, 583, 584, 585, 586, 587, 588, 643, 675, 678, 679, 680, 681, 683, 709, 710, 711, 712, 713, 715, 716, 717, 718, 720, 721, 722, 723, 724, 726, 727, 728, 729, 730, 731, 732, 734, 736, 737, 738, 739, 740, 741, 742, 743, 744, 745, 746, 747, 748, 749, 750, 751, 752, 753, 754, 755, 756, 757, 758, 759, 760, 761, 762, 763, 764, 765, 767, 768, 769, 770, 771, 772, 773, 774, 775, 776, 777, 778, 779, 780, 781, 785, 786, 787, 788, 790, 791, 792, 793, 794, 795, 796, 797, 798, 800, 801, 806, 807, 809, 810, 811, 812, 813, 838, 839, 840, 841, 842, 843, 844, 845, 853, 868, 869, 870, 884, 886, 887, 888, 889, 890, 891, 892, 893, 894, 901, 902, 903, 908, 965, 966, 967, 968, 969, 971, 972, 973, 974, 975, 977, 978, 979, 980, 981, 982, 983, 984, 985, 986, 987, 988, 989, 990, 991, 992, 993, 1000, 1055, 1123 | |||||
| STs found only in environmental samples | 123, 151, 260, 268, 281, 295, 324, 330, 336, 339, 475, 558, 594, 635, 636, 637, 638, 640, 641, 642, 644, 646, 714, 719, 789, 799, 802, 803, 804, 805, 846, 847, 848, 849, 850, 851, 852, 860, 861, 862, 863, 864, 865, 866, 876, 877, 878, 895, 896, 906, 976, 995, 996, 998, 999, 1016, 1019, 1020, 1022, 1025, 1026, 1027, 1028, 1029, 1030, 1031, 1032, 1033, 1044 | |||||
| STs found only in animal samples | 446, 612, 725, 783, 784 | |||||
MLST = multilocus sequence typing.
Counts refer to the number of times an ST has been recovered from a given type of sample. STs recovered multiple times from the same environmental sample, or the same patient, have only been counted once. Samples mapped in Figure 3 are denoted by an asterisk (*). Please refer to the B. pseudomallei MLST database (http://bpseudomallei.mlst.net) for the MLST genotype corresponding to each ST.
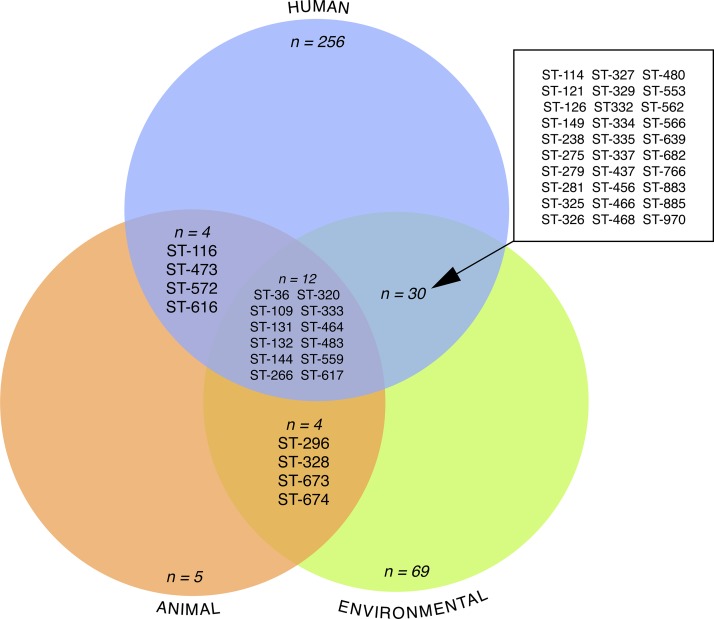
The most commonly recovered STs from environmental sampling in the Northern Territory are listed in Table 1 and are marked with an asterisk (*). Each of these STs has been recovered from at least 10 different environmental samples. All eight of these common environmental STs have also been recovered from either human or animal samples, indicating that all are capable of causing disease (Figure 2 ). All eight of the common STs except for ST-325 have been found in soil, with all but ST-36 and ST-144 also found in bore water (groundwater pumped from artesian aquifers), a water source commonly used for household supply in rural areas outside of Darwin. None of these eight STs has been identified in environmental samples outside the Northern Territory.
Figure 2.
Venn diagram of sequence types (STs), determined by multilocus sequence typing (MLST) found in Burkholderia pseudomallei isolates from environmental, human, and animal samples from the Northern Territory. Refer to Table 1 for the unlisted isolates found only in human, animal or environmental samples.
None of the eight commonest Darwin region STs were single-locus or double-locus variants of each other according to eBURST analysis (http://bpseudomallei.mlst.net). This finding is consistent with an ancient origin for B. pseudomallei in this region, rather than recent divergence from one or more common ancestor(s).6
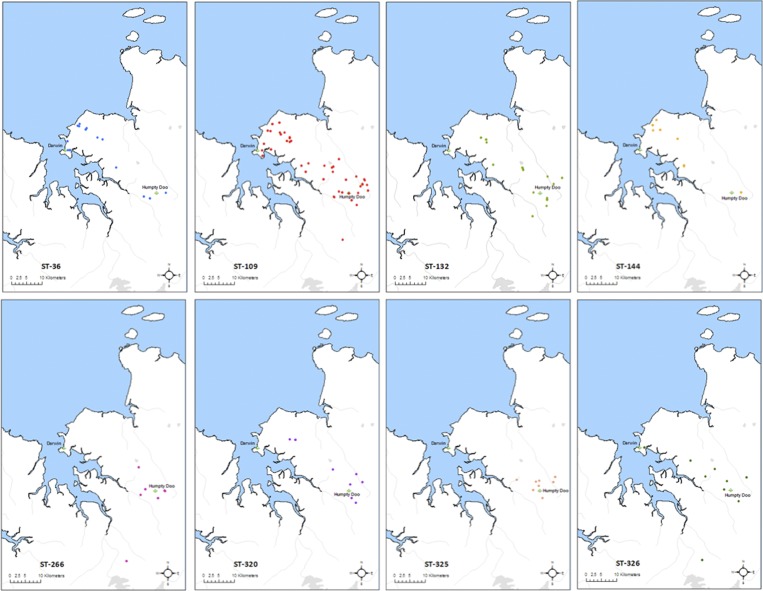
ST-109 is the most frequently identified B. pseudomallei ST in environmental samples from the Northern Territory, and is also the most widely dispersed. ST-109 has been found in environmental samples from Darwin south to Livingstone, over a maximum linear distance of approximately 45 km (Figure 3 ). It is also the most common ST recovered from clinical samples over the past 26 years. ST-326 is found over a similar distance but is recovered much less frequently. Some STs show restricted distributions, and in particular are absent from the Darwin urban region, despite being among the most common STs. For example, ST-320 and ST-132 are only found in the rural regions of greater Darwin and further south, whereas ST-109, ST-36, ST-144, and ST-326 all occur in Darwin city proper. ST-266 and ST-325 have the most restricted distributions, having only been found in Humpty Doo, south of Darwin. The rate of new STs being recovered in Darwin and surrounds in response to increased sampling effort is levelling off, suggesting that only a limited number of environmental strains are yet to be discovered in this specific region.10 Melioidosis also occurs in remote indigenous communities on the offshore islands of the Top End, but none of our most common STs have yet been found in environmental strains from any of these islands.
Figure 3.
Geographic distribution of the eight most common Burkholderia pseudomallei multilocus sequence type (ST) genotypes cultured from environmental samples in the Northern Territory.
We provide the first description of the frequency and distribution of B. pseudomallei strains in the environment in the Northern Territory, which in the heavy 2009–2010 monsoonal wet season had the highest yet recorded incidence of melioidosis (50.2 cases per 100 000 population).20 Our study is based on a comprehensive data set of B. pseudomallei strains available for the region, representing 35 years of sampling data from environmental, animal and human clinical sources. We show that the abundance of B. pseudomallei varies among different STs, but geographic distributions are restricted, suggesting limited dispersal of the organism within the Australian environment. However, we note the limitations within our data set and that our findings may not directly apply to the situation of B. pseudomallei dispersal in southeast Asia. Furthermore, despite over two decades of sampling, much of the Northern Territory remains unsampled. More intensive sampling efforts in these remote areas will be important for our understanding of B. pseudomallei ecology, and particularly the ability of the organism to disperse within the environment by anthropogenic or natural means. More comprehensive distribution data would also provide a better foundation for determining whether particular STs are more transmissible and/or virulent than others, or are better able to flourish in crucial infrastructure such as water supplies. These data would also provide an improved basis for epidemiological tracking of strain source, such as in the case of deliberate release of an Australian B. pseudomallei strain.
ACKNOWLEDGMENTS
We thank Glenda Harrington, Ian Harrington, and Vanessa Theobald for support with sample collection and culturing, and Daniel Godoy for support with MLST. We also thank the microbiology laboratory staff at Royal Darwin Hospital for support and expertise in identification of B. pseudomallei from clinical cases. This work was supported by project grants from the Australian National Health and Medical Research Council.
Footnotes
Authors' addresses: Stephanie N. J. Chapple, Erin P. Price, Derek S. Sarovich, Evan McRobb, Mark Mayo, and Mirjam Kaestli, Global and Tropical Health Division, Menzies School of Health Research, Casuarina, Northern Territory, Australia, E-mails: stephanie.chapple@menzies.edu.au, erin.price@menzies.edu.au, derek.sarovich@menzies.edu.au, evan.mcrobb@menzies.edu.au, mark.mayo@menzies.edu.au, and mirjam.kaestli@menzies.edu.au. Brian G. Spratt, Department of Infectious Disease Epidemiology, Faculty of Medicine, Imperial College London, United Kingdom, E-mail: b.spratt@imperial.ac.uk. Bart J. Currie, Royal Darwin Hospital and Menzies School of Health Research, Casuarina, Darwin, Northern Territory, Australia 0811, E-mail: bart.currie@menzies.edu.au.
References
- 1.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 3.Dance DAB. Melioidosis in Puerto Rico: the iceberg slowly emerges. Clin Infect Dis. 2015;60:251–253. doi: 10.1093/cid/ciu768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limmathurotsakul D, Wuthiekanun V, Chantratita N, Wongsuvan G, Amornchai P, Day NPJ, Peacock SJ. Burkholderia pseudomallei is spatially distributed in soil in northeast Thailand. PLoS Negl Trop Dis. 2010;4:e694. doi: 10.1371/journal.pntd.0000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vesaratchavest M, Tumapa S, Day NPJ, Wuthiekanun V, Chierakul W, Holden MTG, White NJ, Currie BJ, Spratt BG, Feil EJ, Peacock SJ. Nonrandom distribution of Burkholderia pseudomallei clones in relation to geographical location and virulence. J Clin Microbiol. 2006;44:2553–2557. doi: 10.1128/JCM.00629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson T, Giffard P, Beckstrom-Sternberg S, Auerbach R, Hornstra H, Tuanyok A, Price EP, Glass MB, Leadem B, Beckstrom-Sternberg JS, Allan GJ, Foster JT, Wagner DM, Okinaka RT, Sim SH, Pearson O, Wu ZN, Chang J, Kaul R, Hoffmaster AR, Brettin TS, Robison RA, Mayo M, Gee JE, Tan P, Currie BJ, Keim P. Phylogeographic reconstruction of a bacterial species with high levels of lateral gene transfer. BMC Biol. 2009;7:78. doi: 10.1186/1741-7007-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale J, Price EP, Hornstra H, Busch JD, Mayo M, Godoy D, Wuthiekanun V, Baker A, Foster JT, Wagner DM, Tuanyok A, Warner J, Spratt BG, Peacock SJ, Currie BJ, Keim P, Pearson T. Epidemiological tracking and population assignment of the non-clonal bacterium, Burkholderia pseudomallei. PLoS Negl Trop Dis. 2011;5:e1381. doi: 10.1371/journal.pntd.0001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Smet B, Sarovich DS, Price EP, Mayo M, Theobald V, Kham C, Heng SH, Thong P, Holden MTG, Parkhill J, Peacock SJ, Spratt BG, Jacobs JA, Vandamme P, Currie BJ. Whole-genome sequencing confirms that Burkholderia pseudomallei multilocus sequence types common to both Cambodia and Australia are due to homoplasy. J Clin Microbiol. 2015;53:323–326. doi: 10.1128/JCM.02574-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng AC, Ward L, Godoy D, Norton R, Mayo M, Gal D, Spratt BG, Currie BJ. Genetic diversity of Burkholderia pseudomallei isolates in Australia. J Clin Microbiol. 2008;46:249–254. doi: 10.1128/JCM.01725-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McRobb E, Kaestli M, Price EP, Sarovich DS, Mayo M, Warner J, Spratt BG, Currie BJ. Distribution of Burkholderia pseudomallei in northern Australia, a land of diversity. Appl Environ Microbiol. 2014;80:3463–3468. doi: 10.1128/AEM.00128-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker A, Tahani D, Gardiner C, Bristow KL, Greenhill AR, Warner J. Groundwater seeps facilitate exposure to Burkholderia pseudomallei. Appl Environ Microbiol. 2011;77:7243–7246. doi: 10.1128/AEM.05048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaestli M, Schmid M, Mayo M, Rothballer M, Harrington G, Richardson L, Hill A, Hill J, Tuanyok A, Keim P, Hartmann A, Currie BJ. Out of the ground: aerial and exotic habitats of the melioidosis bacterium Burkholderia pseudomallei in grasses in Australia. Environ Microbiol. 2011;14:2058–2070. doi: 10.1111/j.1462-2920.2011.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampton V, Kaestli M, Mayo M, Choy JL, Harrington G, Richardson L, Benedict S, Noske R, Garnett ST, Godoy D, Spratt BG, Currie BJ. Melioidosis in birds and Burkholderia pseudomallei dispersal, Australia. Emerg Infect Dis. 2011;17:1310–1312. doi: 10.3201/eid1707.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mollaret HH. L'affaire du jardin des plantes ou comment la mélioïdose fit son apparition en France. Med Mal Infect. 1988;18:643–654. [Google Scholar]
- 15.Currie B, Smith-Vaughan H, Golledge C, Buller N, Sriprakash KS, Kemp DJ. Pseudomonas pseudomallei isolates collected over 25 years from a nontropical endemic focus show clonality on the basis of ribotyping. Epidemiol Infect. 1994;113:307–312. doi: 10.1017/s0950268800051736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagripanti JL, Levy A, Robertson J, Merritt A, Inglis TJJ. Inactivation of virulent Burkholderia pseudomallei by sunlight. Photochem Photobiol. 2009;85:978–986. doi: 10.1111/j.1751-1097.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- 17.McRobb E, Kaestli M, Mayo M, Price EP, Sarovich DS, Godoy D, Spratt BG, Currie BJ. Melioidosis from contaminated bore water and successful UV sterilization. Am J Trop Med Hyg. 2013;89:367–368. doi: 10.4269/ajtmh.13-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yip TW, Hewagama S, Mayo M, Price EP, Sarovich DS, Bastian I, Baird RW, Spratt BG, Currie BJ. Endemic melioidosis in residents of desert region after atypically intense rainfall in central Australia, 2011. Emerg Infect Dis. 2015;21:1038–1040. doi: 10.3201/eid2106.141908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin Prospective Study. PLoS Negl Trop Dis. 2010;4:e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parameswaran U, Baird RW, Ward LM, Currie BJ. Melioidosis at Royal Darwin Hospital in the big 2009-2010 wet season: comparison with the preceding 20 years. Med J Aust. 2012;196:345–348. doi: 10.5694/mja11.11170. [DOI] [PubMed] [Google Scholar]