Abstract
Helicobacter pylori infection is among the most prevalent infections in the world and a key cause of gastric diseases; however, its route of transmission remains unclear. This study aimed to assess the potential for fecal–oral transmission of H. pylori by leveraging its association with a disease with known etiology. Utilizing serology data from the National Health and Nutrition Examination Survey (NHANES 1999; N = 6,347), the association between H. pylori and hepatitis A virus (HAV), a sensitive indicator for fecal–oral exposure, was assessed. Survey-weighted kappa and multiple logistic regression were used to quantify the association between H. pylori and HAV after controlling for age, sex, race, poverty, birthplace, crowding, smoking, and alcohol use. Concordant serological results were found among 69.8% of participants (survey-weighted κ = 0.30, 95% confidence interval [CI] = 0.26, 0.35). The adjusted odds of H. pylori seropositivity were over two times higher after adjusting for confounders (odds ratio = 2.27, 95% CI = 1.79, 2.87). Results from this study suggest H. pylori and HAV infections are strongly associated. Since HAV is primarily transmitted through the fecal–oral route, fecal–oral transmission may be an important pathway for H. pylori spread.
Introduction
Helicobacter pylori infection is one of the most prevalent infections among humans, with seroprevalence estimates as high as 70% worldwide and up to 80% or more in developing countries.1,2 Although ample epidemiologic research on H. pylori exists, there is still uncertainty in how the pathogen is transmitted.3,4 Helicobacter pylori has been hypothesized to spread through various routes, though generally believed to spread from person to person (i.e., not through an intermediary reservoir), either through an oral-to-oral or fecal–oral route.1–6 For the purpose of this study, we defined fecal–oral transmission as transmission of pathogens in feces from one person to the oral cavity of another person either directly or through contaminated surfaces, food, or water. Prior studies examining the potential for fecal–oral transmission of H. pylori yielded mixed conclusions warranting further investigation.7–12 Better understanding of H. pylori transmission is critical in developing primary prevention interventions and reducing reliance on secondary and tertiary prevention efforts that may exacerbate H. pylori antibiotic resistance.13
Prior studies exploring potential fecal–oral transmission of H. pylori have used hepatitis A virus (HAV) seropositivity as an indicator for fecal–oral exposure.7 Since HAV is transmitted primarily by the fecal–oral route, it is inferred that HAV seropositivity is evidence for prior fecal–oral exposure. The objective of this study is to assess the relationship between fecal–oral exposure, indicated by HAV seropositivity, and H. pylori seropositivity among a representative sample of the United States. It is hypothesized that the odds of H. pylori seropositivity will be higher among HAV-positive persons than HAV-negative persons. Since individuals of lower socioeconomic status (SES) and minorities are consistently at higher risk for H. pylori infection,14 subgroup analysis of HAV and H. pylori infection by SES and race are also explored.
Materials and Methods
A cross-sectional study was conducted to assess the association between HAV and H. pylori seropositivity using data from the National Health and Nutrition Examination Survey (NHANES). NHANES is a stratified multistage probability sample of the civilian, non-institutionalized U.S. population, administered by the U.S. National Center for Health Statistics and designed specifically for population health research.15 Data from NHANES were collected through in-person interviews, clinical examinations, and laboratory testing. NHANES 1999–2000 (NHANES 1999) was the most recent survey cycle to collect H. pylori serology and was used in this study.
H. pylori and HAV serology.
Helicobacter pylori serology was measured among participants aged 3 years and older, using the Wampole Laboratories H. pylori IgG enzyme-linked immunosorbent assays (Wampole Laboratories, Cranbury, NJ), intended to detect the presence of IgG antibodies to H. pylori.16 Immune status ratio (ISR) values (range = 0–5.73) were used to categorize serostatus; ISR values < 0.90 were considered negative for H. pylori, values between 0.90 and 1.09 were equivocal, and values > 1.09 were positive.16 Participants with missing (N = 2,472) or equivocal (N = 161) H. pylori serology were excluded from analysis. HAV serology was measured in all examinees aged 2 years and older using a solid-phase competitive enzyme immunoassay, and reported as positive, negative, or missing (N = 2,321). Participants missing HAV serology were excluded.
Demographic variables.
NHANES 1999 reported race as white, black, Mexican American, or other. Birthplace was dichotomized as U.S./foreign born based on responses to the question “In what country were you born?” An income-to-poverty ratio (family income divided by federal poverty threshold) was used to approximate SES; values were categorized into six levels, from five or greater, representing the highest SES, to < 1 representing the lowest. Household crowding was approximated by number of persons living in each household divided by number of rooms in the household.
HAV immunization status, a potential source of misclassification, was available in NHANES 1999 and categorized as follows: at least 2 doses, less than 2 doses, no doses, and missing. Vaccination values of “Don't Know” or “Refused” were considered missing (N = 368). Smoking status was categorized as never, current, former, and missing. Current smokers were defined as participants who reported smoking at least 100 cigarettes, 20 cigars, or 20 pipes in their lifetime and reported currently smoking. Alcohol drinkers were defined as those having at least 12 drinks in 1 year.
Statistical analysis.
All analyses were survey weighted to account for the multistage sampling design of NHANES 1999. A survey-weighted kappa statistic was calculated to assess the magnitude of agreement between H. pylori and HAV seropositivity. The kappa statistic measures the level of agreement between binary variables and accounts for agreement by chance and has been used in prior H. pylori/HAV studies to measure serology agreement.17,18 The “svykappa” package (Lumley T, Auckland, New Zealand) in R (Foundation for Statistical Computing, Vienna, Austria) was used to calculate the survey-weighted kappa.
Survey-weighted multiple logistic regression was used to assess the association between H. pylori and HAV after adjusting for a priori chosen confounders (age, gender, race, birthplace, income/poverty ratio, household crowding, smoking, and alcohol consumption) known to be associated with H. pylori infection.1,3,5 Five models were additively fitted. The sequence of modeling was based on variables thought to be most associated (demographics) with H. pylori infection to least associated (behavioral). Model 1 included HAV, age, sex, and race as covariates; Model 2 added birthplace; Model 3 added income-to-poverty ratio; and Model 4 added household crowding. Model 5 included smoking and alcohol status but was restricted to participants over the age of 20 years since those under 20 years of age were not asked to provide smoking and alcohol status. A two-tailed Wald test was used to assess statistical significance of HAV (α = 0.05) for all estimates. Hosmer–Lemeshow's goodness-of-fit test was used to assess model fit (insignificant P values indicate adequate fit).19
Effect modification by race and income-to-poverty ratio were assessed using Model 4 and considered significant at α = 0.05 level. Marginal probability plots for H. pylori seropositivity with 95% confidence intervals (CI) were generated to visually assess each interaction. All logistic regression analyses and post-estimation tests of fit were done in Stata 12.1 (StataCorp LP, College Station, TX).
Sensitivity analysis.
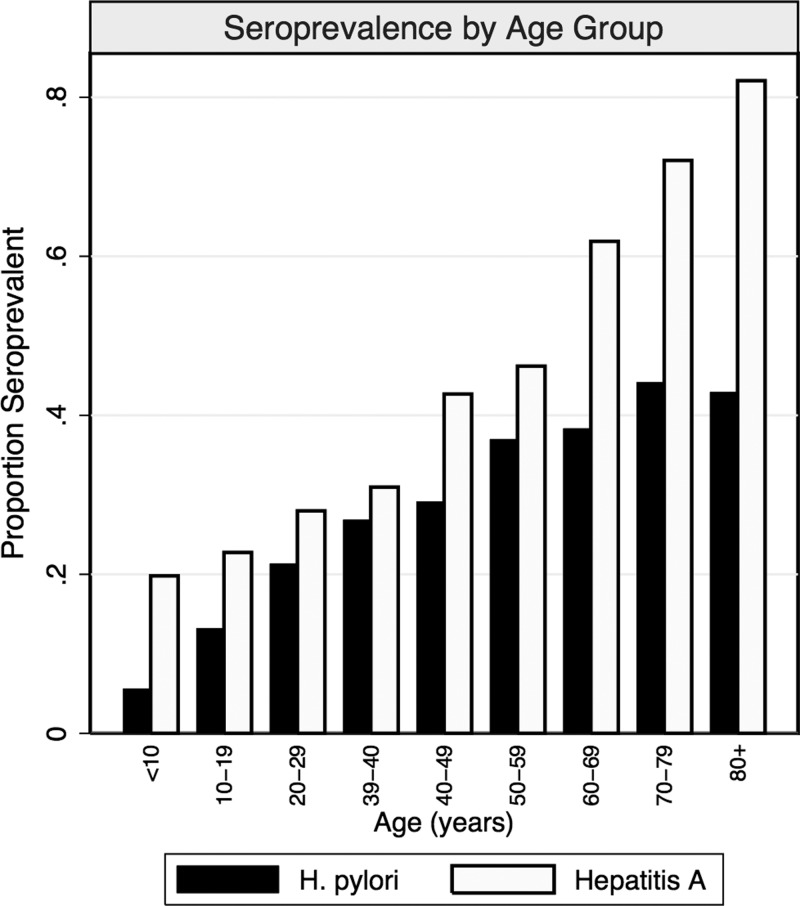
In NHANES 1999, HAV seroprevalence was higher in younger age groups (Figure 1 ), which may have been caused by uptake of the HAV vaccine made commercially available shortly before the survey. Because HAV serology cannot distinguish between naturally acquired and vaccine-acquired immunity, a sensitivity analysis restricted to participants reporting no HAV immunization was performed.
Figure 1.
Seroprevalence proportions of Helicobacter pylori and hepatitis A participants from the 1999 National Health and Nutrition Examination Survey by age group. Includes all participants with reported serology.
We also assessed the association between H. pylori serology and infections with other transmission modes available in NHANES 1999 using survey-weighted logistic regression. Serology data for sexually transmitted (herpes simplex virus 1 and 2, human immunodeficiency virus [HIV], Chlamydia) and other fecal–oral and potentially foodborne (Cryptosporidium and Toxoplasma) infections were used as indicators for alternative transmission modes. We expected to find an association between the fecal–oral transmitted infections, but not other modes. These analyses were adjusted for age, race, and gender.
Results
There were a total of 9,965 participants in the NHANES 1999 cycle. Excluding participants with missing H. pylori and HAV serology reduced the sample to 7,324. Participants under the age of 3 years (N = 996) were not required to submit serum for testing and accounted for 27.5% of exclusions. Further excluding participants with missing income-to-poverty ratio, birthplace, and household crowding values reduced the final analysis sample to 6,347 (63.7% of total).
About 36% and 25% of the sample were HAV-positive and H. pylori-positive, respectively; among H. pylori-positive participants, 62.8% were also HAV positive (Table 1). The H. pylori-positive participants were older than the H. pylori-negative participants and had a greater proportion of non-Hispanic black (17.9% versus 8.3%) and foreign born (30.7% versus 8.7%).
Table 1.
Survey-weighted summary demographics of Helicobacter pylori-positive and H. pylori-negative participants from the 1999 NHANES included in analysis, United States, 1999–2000
| Eligible population | H. pylori− | H. pylori+ | Combined |
|---|---|---|---|
| N (%) | 4,234 (74.9) | 2,113 (25.1) | 6,347 (100) |
| Age*, mean (SD) | 31.57 (17.9) | 45.02 (21.2) | 36.5 (20.2) |
| Age category, n (%) | |||
| < 19 | 2,272 (28.8) | 594 (9.5) | 2,866 (24.0) |
| 20–49 | 1,166 (49.9) | 686 (49.9) | 1,852 (49.9) |
| 50–69 | 489 (15.8) | 508 (27.2) | 997 (18.6) |
| 70+ | 307 (5.5) | 325 (13.4) | 632 (7.5) |
| Gender, n (%) | |||
| Male | 2,038 (49.2) | 1,079 (49.4) | 3,117 (49.3) |
| Female | 2,196 (50.8) | 1,034 (50.6) | 3,230 (50.7) |
| Race, n (%) | |||
| Non-Hispanic white | 1,877 (77.5) | 409 (48.5) | 2,286 (70.2) |
| Non-Hispanic black | 866 (8.3) | 547 (17.9) | 1,413 (10.7) |
| Mexican American | 1,174 (5.0) | 950 (13.4) | 2,124 (7.1) |
| Other | 317 (9.1) | 207 (20.2) | 524 (11.9) |
| Foreign born, n (%) | |||
| U.S. born | 3,714 (91.3) | 1,342 (69.3) | 5,056 (85.8) |
| Foreign born | 520 (8.7) | 771 (30.7) | 1,291 (14.2) |
| Income/poverty, n (%) | |||
| ≥ 5 | 620 (22.4) | 163 (13.6) | 783 (20.2) |
| 3–4.9 | 844 (25.7) | 277 (17.8) | 1,121 (23.7) |
| 2–2.9 | 665 (16.1) | 288 (15.8) | 953 (16.0) |
| 1–1.9 | 1,085 (21.5) | 642 (25.9) | 1,727 (22.6) |
| < 1.0 | 1,020 (14.4) | 743 (26.9) | 1,763 (17.5) |
| Household crowding†, mean (SD) | 0.58 (0.3) | 0.60 (0.4) | 0.59 (0.31) |
| Smoking status, n (%) | |||
| Never | 1,052 (37.4) | 764 (41.0) | 1,816 (38.3) |
| Current | 380 (15.8) | 332 (26.5) | 712 (18.5) |
| Former | 530 (18.1) | 422 (23.0) | 952 (19.3) |
| Missing | 2,272 (28.8) | 595 (9.5) | 1,867 (24.0) |
| Alcohol use, n (%) | |||
| No | 582 (17.7) | 505 (27.1) | 1,087 (20.1) |
| Yes | 1,313 (51.1) | 917 (58.3) | 2,230 (52.9) |
| Missing | 2,339 (31.2) | 691 (14.6) | 3,030 (27.0) |
| Hepatitis A vaccine, n (%) | |||
| At least 2 doses | 258 (5.6) | 109 (6.0) | 367 (5.7) |
| Less than 2 doses | 187 (4.0) | 128 (5.9) | 315 (4.4) |
| No doses | 3,657 (87.4) | 1,795 (84.3) | 5,452 (86.6) |
| Missing | 132 (3.1) | 81 (3.8) | 213 (3.2) |
| Hepatitis A serology, n (%) | |||
| Negative | 2,826 (72.2) | 640 (37.2) | 3,466 (63.5) |
| Positive | 1,408 (27.8) | 1,473 (62.8) | 2,881 (36.5) |
| H. pylori serology, n (%) | |||
| Negative | – | – | 4,234 (74.9) |
| Positive | – | – | 2,113 (25.1) |
NHANES = National Health and Nutrition Examination Survey; SD = standard deviation.
Age in years.
Persons per room in household.
Older age, higher household crowding, being non-white, foreign born, and having lower income levels were strongly associated with being H. pylori-positive (all P < 0.001) (Table 2). Compared with never smokers, the odds of H. pylori seropositivity were slightly elevated among current smokers (odds ratio [OR] = 1.53, P = 0.004), and there was little difference in odds between never and former smokers (P = 0.25). Alcohol drinkers had 25% lower odds of being H. pylori-positive than non-drinkers (OR = 0.75, P < 0.03). Drinking and smoking histories were not collected for participants under the age of 19 years (88.6% missing alcohol histories were of those under 19 years of age), so negative associations in the missing categories may be caused by the concentration of non-adults in these groups. The odds of being H. pylori positive did not differ by HAV immunization status, indicating vaccination did not influence H. pylori seropositivity.
Table 2.
Unadjusted odds ratios of Helicobacter pylori seropositivity by demographic variables for participants from the 1999 NHANES included in analysis (N = 6,347), United States, 1999–2000
| OR | 95% CI | P | ||
|---|---|---|---|---|
| Age* | 1.03 | 1.02 | 1.03 | < 0.001 |
| Age category | ||||
| < 19 | Ref | |||
| 20–49 | 3.04 | 2.36 | 3.93 | < 0.001 |
| 50–69 | 5.24 | 4.03 | 6.82 | < 0.001 |
| 70+ | 7.44 | 5.52 | 10.02 | < 0.001 |
| Gender | ||||
| Male | Ref | |||
| Female | 0.99 | 0.88 | 1.12 | 0.87 |
| Race | ||||
| White | Ref | |||
| Black | 3.43 | 2.75 | 4.28 | < 0.001 |
| Mexican | 4.29 | 3.40 | 5.43 | < 0.001 |
| Other | 3.54 | 2.75 | 4.55 | < 0.001 |
| Birthplace | ||||
| U.S. born | Ref | |||
| Foreign born | 4.68 | 3.79 | 5.79 | < 0.001 |
| Income/poverty ratio | ||||
| ≥ 5.0 | Ref | |||
| 3–4.9 | 1.14 | 0.83 | 1.58 | 0.40 |
| 2–2.9 | 1.62 | 1.14 | 2.30 | 0.01 |
| 1–1.9 | 1.99 | 1.44 | 2.76 | < 0.001 |
| < 1.0 | 3.09 | 2.27 | 4.20 | < 0.001 |
| Household crowding* | 1.51 | 1.07 | 2.15 | 0.02 |
| Smoking | ||||
| Never | Ref | |||
| Current | 1.53 | 1.18 | 1.99 | 0.004 |
| Former | 1.16 | 0.89 | 1.50 | 0.25 |
| Missing | 0.30 | 0.23 | 0.39 | < 0.001 |
| Alcohol | ||||
| No | Ref | |||
| Yes | 0.75 | 0.58 | 0.96 | 0.03 |
| Missing | 0.31 | 0.23 | 0.41 | < 0.001 |
| Hepatitis A vaccine | ||||
| At least 2 doses | Ref | |||
| Less than 2 doses | 1.37 | 0.71 | 2.64 | 0.33 |
| No doses | 0.89 | 0.55 | 1.44 | 0.62 |
| Missing | 1.14 | 0.56 | 2.32 | 0.70 |
| Hepatitis A serostatus | ||||
| Negative | Ref | |||
| Positive | 4.39 | 3.38 | 5.68 | < 0.001 |
CI = confidence interval; NHANES = National Health and Nutrition Examination Survey; OR = odds ratio.
Continuous variable.
About 15% of participants were both HAV positive and H. pylori positive (N = 1,473) and 2,826 (54.1%) were both seronegative. Overall, there was 69.8% serology concordance, which was statistically significant (κ = 0.30, 95% CI = 0.26, 0.35).
The odds of H. pylori seropositivity were more than four times higher among HAV-positive participants than HAV negative (OR = 4.39, 95% CI = 3.38, 5.68). After adjusting for age, gender, and race, the H. pylori/HAV association attenuated by 41% (OR = 2.57, 95% CI = 2.03, 3.25) (Table 3, Model 1) and attenuated again after adjusting for birthplace (OR = 2.27, 95% CI = 1.79, 2.87) (Table 3, Model 2) with no change in significance. Adjusting for income-to-poverty ratio attenuated the association slightly and including household crowding did not change estimates (Table 3, Model 4). Model 5 resulted in a slight increase in the OR for H. pylori seropositivity (OR = 2.30, 95% CI = 1.75, 3.03) (Table 3, Model 5). Among the fitted models, Models 2 (P = 0.53) and 5 (P = 0.23) had the best fit based on Hosmer–Lemeshow's goodness-of-fit test (insignificant P values indicate adequate fit)19 and reported as preferred estimates. These results were replicated using the same methods with data from the first phase of NHANES III from 1988 to 1991 (Supplemental Table 1), further supporting this association. There was no significant difference in the H. pylori/HAV association between survey cycles (i.e., no interaction by survey cycle) (P = 0.93).
Table 3.
Survey-weighted multiple logistic regression results for Helicobacter pylori seropositivity among participants in the 1999 NHANES included in analysis, United States, 1999–2000
| Model 1 (N = 6,342) | Model 2 (N = 6,342) | Model 3 (N = 6,342) | Model 4 (N = 6,342) | Model 5 (N = 3,480) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Hepatitis A serostatus | |||||||||||||||
| Negative | Ref | Ref | Ref | Ref | |||||||||||
| Positive | 2.57 | 2.03, 3.25 | < 0.001 | 2.27 | 1.79, 2.87 | < 0.001 | 2.17 | 1.71, 2.76 | < 0.001 | 2.17 | 1.71, 2.76 | < 0.001 | 2.30 | 1.75, 3.03 | < 0.001 |
| Age category | |||||||||||||||
| ≤ 19 | Ref | Ref | Ref | Ref | Omitted | ||||||||||
| 20–49 | 3.42 | 2.47, 4.73 | < 0.001 | 3.08 | 2.19, 4.32 | < 0.001 | 3.63 | 2.54, 5.19 | < 0.001 | 3.74 | 2.58, 5.41 | < 0.001 | Ref | ||
| 50–69 | 6.02 | 4.47, 8.11 | < 0.001 | 5.66 | 4.25, 7.52 | < 0.001 | 7.09 | 5.26, 9.55 | < 0.001 | 7.55 | 5.42, 10.52 | < 0.001 | 1.93 | 1.59, 2.33 | < 0.001 |
| 70+ | 7.97 | 5.38, 11.82 | < 0.001 | 7.74 | 5.12, 11.70 | < 0.001 | 8.68 | 5.54, 13.61 | < 0.001 | 9.39 | 5.84, 15.08 | < 0.001 | 2.63 | 1.78, 3.87 | < 0.001 |
| Gender | |||||||||||||||
| Male | Ref | Ref | Ref | Ref | Ref | ||||||||||
| Female | 0.91 | 0.81, 1.03 | 0.12 | 0.93 | 0.83, 1.05 | 0.23 | 0.88 | 0.78, 1.00 | 0.06 | 0.89 | 0.78, 1.01 | 0.07 | 0.98 | 0.85, 1.14 | 0.81 |
| Race | |||||||||||||||
| White | Ref | Ref | Ref | Ref | Ref | ||||||||||
| Black | 4.41 | 3.26, 5.96 | < 0.001 | 4.23 | 3.06, 5.86 | < 0.001 | 3.63 | 2.64, 4.98 | < 0.001 | 3.59 | 2.64, 4.88 | < 0.001 | 3.43 | 2.54, 4.64 | < 0.001 |
| Mexican | 4.82 | 3.77, 6.18 | < 0.001 | 3.82 | 2.89, 5.06 | < 0.001 | 3.17 | 2.35, 4.27 | < 0.001 | 3.02 | 2.33, 3.92 | < 0.001 | 3.52 | 2.49, 4.97 | < 0.001 |
| Other | 3.46 | 2.66, 4.51 | < 0.001 | 2.39 | 1.75, 3.26 | < 0.001 | 2.03 | 1.48, 2.78 | < 0.001 | 1.99 | 1.47, 2.70 | < 0.001 | 2.14 | 1.61, 2.84 | < 0.001 |
| Birthplace | |||||||||||||||
| U.S. born | – | – | – | Ref | Ref | Ref | Ref | ||||||||
| Foreign born | – | – | – | 1.97 | 1.51, 2.58 | < 0.001 | 2.01 | 1.50, 2.70 | < 0.001 | 1.96 | 1.42, 2.71 | 0.001 | 1.91 | 1.34, 2.72 | 0.001 |
| Income/poverty ratio | |||||||||||||||
| ≥ 5 | – | – | – | – | – | – | Ref | Ref | Ref | ||||||
| 3–4.9 | – | – | – | – | – | – | 1.26 | 0.87, 1.82 | 0.20 | 1.24 | 0.85, 1.80 | 0.248 | 1.22 | 0.81, 1.82 | 0.31 |
| 2–2.9 | – | – | – | – | – | – | 1.44 | 1.02, 2.04 | 0.04 | 1.40 | 0.97, 2.02 | 0.066 | 1.38 | 0.97, 1.95 | 0.07 |
| 1–1.9 | – | – | – | – | – | – | 1.61 | 1.29, 2.01 | < 0.001 | 1.55 | 1.22, 1.97 | 0.002 | 1.35 | 1.05, 1.72 | 0.02 |
| < 1 | – | – | – | – | – | – | 2.89 | 2.13, 3.90 | < 0.001 | 2.75 | 1.95, 3.87 | < 0.001 | 2.14 | 1.48, 3.10 | 0.001 |
| Household crowding* | – | – | – | – | – | – | – | – | – | 1.22 | 0.86, 1.74 | 0.248 | 1.13 | 0.77, 1.66 | 0.50 |
| Smoking | |||||||||||||||
| Never | – | – | – | – | – | – | – | – | – | – | – | – | Ref | ||
| Current | – | – | – | – | – | – | – | – | – | – | – | – | 2.11 | 1.59, 2.82 | < 0.001 |
| Former | – | – | – | – | – | – | – | – | – | – | – | – | 1.38 | 1.07, 1.78 | 0.02 |
| Missing | – | – | – | – | – | – | – | – | – | – | – | – | Omitted | ||
| Alcohol | |||||||||||||||
| No | – | – | – | – | – | – | – | – | – | – | – | – | Ref | ||
| Yes | – | – | – | – | – | – | – | – | – | – | – | – | 0.93 | 0.73, 1.18 | 0.53 |
| Missing | – | – | – | – | – | – | – | – | – | – | – | – | 1.18 | 0.79, 1.78 | 0.39 |
CI = confidence interval; NHANES = National Health and Nutrition Examination Survey; OR = odds ratio.
Model 1: hepatitis A + age + gender + race, Hosmer–Lemeshow goodness-of-fit (P = 0.21); Model 2: Model 1 + birthplace, Hosmer–Lemeshow goodness-of-fit (P = 0.53); Model 3: Model 2 + income-to-poverty, Hosmer–Lemeshow goodness-of-fit (P = 0.003); Model 4: Model 3 + household crowding, Hosmer–Lemeshow goodness-of-fit (P = 0.003); Model 5: Model 4 + smoking + alcohol (restricted to 20 years of age and older), Hosmer–Lemeshow goodness-of-fit (P = 0.23).
Household crowding modeled as a continuous variable (persons per room in household).
The H. pylori/HAV association did not differ by poverty subgroup (P = 0.24) indicating no interaction by SES (Figure 2 ). Similarly, no significant differences in the H. pylori/HAV association were observed by race (P = 0.43). As a post hoc analysis, interaction by birthplace was found to be significant (P = 0.048); the odds of H. pylori seropositivity was 1.9 times higher in HAV-positives than negatives (OR = 1.91, 95% CI = 1.51, 2.43) among U.S. born, and over five times higher (OR = 5.19, 95% CI = 2.45, 11.00) among foreign born.
Figure 2.
Marginal probabilities of Helicobacter pylori seropositivity by hepatitis A status for each (A) income-to-poverty ratio category, (B) race category, and (C) birthplace category. There was no significant interaction by income-to-poverty ratio (P = 0.24) or race (P = 0.43), however interaction by birthplace was found to be significant (P = 0.048).
The sensitivity analysis restricted to non-HAV-immunized participants (N = 5,452) resulted in similar OR estimates from the full sample (OR = 2.32, 95% CI = 1.73, 3.12), indicating little influence of immunization on the estimated H. pylori/HAV association. Full results are given in Supplemental Table 2.
In addition to HAV, we found significant positive associations between H. pylori and other fecal–oral (Cryptosporidium, P < 0.001) and potentially foodborne (Toxoplasma, P < 0.001) infections, but not sexually transmitted (HIV, P = 0.36; Chlamydia, P = 0.80) infections (Supplemental Table 3).
Discussion
After adjusting for confounders, the odds of being H. pylori positive were over two times higher among HAV-positive individuals in this analysis, supporting the existence of a strong association between H. pylori and HAV. Similar results were also found in the earlier NHANES III sample (1989–1991) indicating this association may persist over time (Supplemental Table 1). Since HAV is primarily transmitted through the fecal–oral route and is a marker for prior fecal–oral exposure, the association between H. pylori and HAV found in this study supports the potential for fecal–oral transmission of H. pylori.
These results agree with past clinical and observational studies supporting the fecal–oral transmission hypothesis of H. pylori.7,20 Parsonnet and others10 reported isolating H. pylori from human feces in 22% of subjects through induced catharsis. Though not immediately generalizable to human models, Cellini and others11 demonstrated fecal–oral transmission of H. pylori among mice. A recent case–control study among health-care workers found exposure to patient feces increased the risk of H. pylori infection and exposure to oral secretions was not associated with H. pylori infection, suggesting fecal–oral transmission may be as viable or more viable as a transmission model than oral–oral or gastro–oral transmisson.12 However, Parsonnet and others10 reported successful isolation of H. pylori from induced vomit (using ipecac) from all infected patients in the their study (N = 16) and from air sampled during vomiting from 38% (N = 6) and concluded that vomiting (gastro–oral) transmission may be the most viable mode of transmission. As such, fecal–oral transmission may not be the only mode of H. pylori transmission.
HAV is not the only pathogen associated with H. pylori. Moreira and others18 reported a significant positive association between H. pylori and Giardia lamblia—an infection primarily transmitted through the fecal–oral route. In our supplemental analysis, we found positive associations with Cryptosporidium and Toxoplasma. Cryptosporidium is primarily transmitted through the fecal–oral route via contaminated water. Toxoplasma is transmitted primarily through infected cat feces. Similarly, past studies have noted cats may become infected with H. pylori21 and that H. pylori has also been isolated from cat feces,22 raising the possibility for zoonotic infection and explaining the association between H. pylori and toxoplasma.
Results in this study contrast with prior studies by Chen and others23 examining a small adolescent population in Taiwan (N = 91), Egemen and others24 in a sample of children in Izmir, Turkey (N = 102), Lin and others25 with a sample of primary school students in Taipei (N = 289), and Furuta and others26 with a clinic-based sample of adults in Japan (N = 1,043). These studies reported weak nonsignificant associations between H. pylori and HAV serology, but were limited in size and scope (all studied non-adult populations, except for Furuta and others) and not generalizable to broader populations. In contrast, our study is the first to use a large nationally representative dataset to study the serologic association between H. pylori and HAV, and did find a strong, significant, and consistent association. Another large nationwide study by Stroffolini and others27 reported a strong association between H. pylori and HAV among a sample of 1,695 military students in Italy and concluded the association was driven by higher exposure to poorer regional hygienic conditions.
It is possible that fecal–oral H. pylori transmission may be less common in children than in adults and prior studies focusing solely on children may have missed the association.28 For instance, in a school-based study examining both kindergarten students and adult teachers, investigators found a significant association between HAV and H. pylori seropositivity among teachers, but not students.29 Further, acute infection with H. pylori during childhood is characterized by vomiting and thus gastro–oral transmission may be more common among children.30 One of the limitations of our study was the high level of excluded children (53.3% of children < 10 years old excluded) due to missing serology, which may have biased OR estimates upward.
Kappa analysis results indicated concordant seropositivity of infections were more likely than chance, but the magnitude of agreement was only fair.31 These results agree with prior studies reporting low kappa statistics for H. pylori and HAV concurrence,17,18 and have been used to argue against a common mode of transmission.32,33 However, the low magnitude of agreement between infections may be due to differences in immunologic responses and detection.
HAV IgG antibodies are generally detectable for life, whereas H. pylori IgG titers decline to undetectable levels shortly after eradication treatment or infection clearance.34–37 Although targeted H. pylori treatment was uncommon before 1999, use of incidental antibiotics has been found to reduce the prevalence of H. pylori infection.38 Moreover, natural history studies have noted high rates of spontaneous clearance.39,40 The decline in detectable H. pylori antibodies after infection clearance may result in misclassification of true prior H. pylori exposure and reduce concurrence with HAV seropositivity. Because H. pylori infection is thought to occur early in life and clearance or treatment may occur later in life, this may explain the divergence between H. pylori and HAV seroprevalence in older age groups (Figure 1) and the low agreement in positive serology. Prior studies have cited this divergence in seroprevalence as support against a common mode of transmission, but have not considered the differences in immunologic responses.17,32 There does appear to be high agreement in negative serology between the infections, which has also been reported in prior studies.18,25
It has been argued that H. pylori/HAV serology studies fail to account for HAV vaccination and that observed associations between the infections are confounded by vaccination.7 However, HAV vaccination was not widespread during the NHANES 1999 such that this bias was unlikely; furthermore, our sensitivity analyses of the non-immunized sample suggested vaccination had no effect on the association between H. pylori and HAV.
Although there was no heterogeneity in the H. pylori/HAV association by poverty or race, there was significant interaction by birthplace with odds of H. pylori seropositivity being much higher among HAV-positive foreign born than U.S. born. The persistently high burden of infection observed in minority racial and ethnic groups living in the United States is often attributed to low SES, crowded households, and immigration from high burden countries.41 Indeed, the risk of H. pylori infection is generally higher in foreign countries, particularly in developing or low-resource settings.42 Differential sanitation standards in developing countries may explain the higher rates of fecal–oral transmitted diseases in those countries and the greater risk of exposure and susceptibility to H. pylori and HAV infection among foreign-born immigrants in the United States.43
The association between H. pylori and HAV found in this study provides insight into the potential mechanism of H. pylori transmission; the strong, persistent association with HAV supports the possibility that the infections share a common mode of transmission. Since HAV is primarily transmitted through the fecal–oral route, fecal–oral transmission may be an important—but not isolated—pathway for H. pylori spread.
Supplementary Material
Footnotes
Authors' addresses: David Bui, Heidi E. Brown, Robin B. Harris, and Eyal Oren, Division of Epidemiology and Biostatistics, University of Arizona, Tucson, AZ, E-mails: davidbui@email.arizona.edu, heidibrown@email.arizona.edu, rbharris@email.arizona.edu, and eoren@email.arizona.edu.
References
- 1.Goh KL, Chan WK, Shiota S, Yamaoka Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2011;16:1–9. doi: 10.1111/j.1523-5378.2011.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9:33–39. [PubMed] [Google Scholar]
- 3.Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283–297. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 4.Goodman KJ, Correa P. The transmission of Helicobacter pylori. A critical review of the evidence. Int J Epidemiol. 1995;24:875–887. doi: 10.1093/ije/24.5.875. [DOI] [PubMed] [Google Scholar]
- 5.Boyanova L. Epidemiology of Helicobacter pylori infection. In: Boyanova L, editor. Helicobacter pylori. Wymondham, England: Caister Academic Press; 2011. pp. 135–159. [Google Scholar]
- 6.Megraud F. Aliment Pharmacol Ther. 1995;9:85–91. [PubMed] [Google Scholar]
- 7.BinSaeed AA. Is there a link between seropositivity to Helicobacter pylori and hepatitis A virus? A systematic review. Int J Infect Dis. 2010;14:E567–E571. doi: 10.1016/j.ijid.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Webb PM, Knight T, Newell DG, Elder JB, Forman D. Helicobacter pylori transmission: evidence from a comparison with hepatitis A virus. Eur J Gastroenterol Hepatol. 1996;8:439–441. [PubMed] [Google Scholar]
- 9.Hazell SL, Mitchell HM, Hedges M, Shi X, Hu PJ, Li YY, Lee A, Reisslevy E. Hepatitis-A and evidence against the community dissemination of Helicobacter pylori via feces. J Infect Dis. 1994;170:686–689. doi: 10.1093/infdis/170.3.686. [DOI] [PubMed] [Google Scholar]
- 10.Parsonnet J, Shmuely H, Haggerty T. Fecal and oral shedding of Helicobacter pylori from healthy infected adults. JAMA. 1999;282:2240–2245. doi: 10.1001/jama.282.23.2240. [DOI] [PubMed] [Google Scholar]
- 11.Cellini L, Dainelli B, Angelucci D, Grossi L, Di Bartolomeo S, Di Campli E, Marzio L. Evidence for an oral-faecal transmission of Helicobacter pylori infection in an experimental murine model. APMIS. 1999;107:477–484. [PubMed] [Google Scholar]
- 12.De Schryver A, Van Winckel M, Cornelis K, Moens G, Devlies G, De Backer G. Helicobacter pylori infection: further evidence for the role of feco-oral transmission. Helicobacter. 2006;11:523–528. doi: 10.1111/j.1523-5378.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 13.Boyanova L, Mitov I. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther. 2010;8:59–70. doi: 10.1586/eri.09.113. [DOI] [PubMed] [Google Scholar]
- 14.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol. 2012;175:54–59. doi: 10.1093/aje/kwr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR. National Health and Nutrition Examination Survey: Analytic Guidelines, 1999–2010. Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention C-Reactive Protein (CRP). NHANES: 1999–2000 Data Documentation, Codebook, and Frequencies. 2008. http://www.cdc.gov/nchs/nhanes/nhanes1999-2000/LAB11.htm Available at. Accessed October 3, 2013.
- 17.Malaty HM, Tanaka E, Kumagai T, Ota H, Kiyosawa K, Graham DY, Katsuyama T. Seroepidemiology of Helicobacter pylori and hepatitis A virus and the mode of transmission of infection: a 9-year cohort study in rural Japan. Clin Infect Dis. 2003;37:1067–1072. doi: 10.1086/378276. [DOI] [PubMed] [Google Scholar]
- 18.Moreira ED, Nassri VB, Santos RS, Matos JF, de Carvalho WA, Silvani CS, Sant'Ana CSE. Association of Helicobacter pylori infection and giardiasis: results from a study of surrogate markers for fecal exposure among children. World J Gastroenterol. 2005;11:2759–2763. doi: 10.3748/wjg.v11.i18.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosmer DW, Hosmer T, leCessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;16:965–980. doi: 10.1002/(sici)1097-0258(19970515)16:9<965::aid-sim509>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Rudi J, Toppe H, Marx N, Zuna I, Theilmann L, Stremmel W, Raedsch R. Risk of infection with Helicobacter pylori and hepatitis A virus in different groups of hospital workers. Am J Gastroenterol. 1997;92:258–262. [PubMed] [Google Scholar]
- 21.Fox JG, Batchelder M, Marini R, Yan L, Handt L, Li X, Shames B, Hayward A, Campbell J, Murphy JC. Helicobacter pylori-induced gastritis in the domestic cat. Infect Immun. 1995;63:2674–2681. doi: 10.1128/iai.63.7.2674-2681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handt LK, Fox JG, Dewhirst FE, Fraser GJ, Paster BJ, Yan LL, Rozmiarek H, Rufo R, Stalis IH. Helicobacter pylori isolated from the domestic cat—public-health implications. Infect Immun. 1994;62:2367–2374. doi: 10.1128/iai.62.6.2367-2374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen LK, Hwang SJ, Wu TC, Chu CH, Shaw CK. Helicobacter pylori and hepatitis A virus infection in school-aged children on two isolated neighborhood islands in Taiwan. Helicobacter. 2003;8:168–172. doi: 10.1046/j.1523-5378.2003.00140.x. [DOI] [PubMed] [Google Scholar]
- 24.Egemen A, Yilmaz O, Akil I, Altuglu I. Evaluation of association between hepatitis A and Helicobacter pylori infections and routes of transmission. Turk J Pediatr. 2006;48:135–139. [PubMed] [Google Scholar]
- 25.Lin H-Y, Chuang C-K, Lee H-C, Chin N-C, Lin S-P, Yeung C-Y. A Seroepidemiologic study of Helicobacter pylori and hepatitis A virus infection in primary school students in Taipei. J Microbiol Immunol Infect. 2005;38:176–182. [PubMed] [Google Scholar]
- 26.Furuta T, Kamata T, Takashima M, Futami H, Arai H, Hanai H, Kaneko E. Study of transmission routes of Helicobacter pylori in relation to seroprevalence of hepatitis A virus. J Clin Microbiol. 1997;35:1891–1893. doi: 10.1128/jcm.35.7.1891-1893.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroffolini T, Rosmini F, Ferrigno L, Fortini M, D'Amelio R, Matricardi PM. Prevalence of Helicobacter pylori infection in a cohort of Italian military students. Epidemiol Infect. 1998;120:151–155. doi: 10.1017/s0950268897008625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tosun SY, Kasirga E, Ertan P, Aksu S. Evidence against the fecal-oral route of transmission for Helicobacter pylori infection in childhood. Med Sci Monit. 2003;9:CR489–CR492. [PubMed] [Google Scholar]
- 29.Lin DB, Tsai TP, Yang CC, Wang HM, Nieh WT, Ling UP, Changlai SP, You SL, Ho MS, Chen CJ. Association between seropositivity of antibodies against hepatitis A virus and Helicobacter pylori. Am J Trop Med Hyg. 2000;63:189–191. doi: 10.4269/ajtmh.2000.63.189. [DOI] [PubMed] [Google Scholar]
- 30.Leung W-K, Siu KLK, Kwok CKL, Chan S-Y, Sung R, Sung JJY. Isolation of Helicobacter pylori from vomitus in children and its implication in gastro-oral transmission. Am J Gastroenterol. 1999;94:2881–2884. doi: 10.1111/j.1572-0241.1999.01431.x. [DOI] [PubMed] [Google Scholar]
- 31.Landis JR, Koch GG. Measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 32.Luzza F, Imeneo M, Maletta M, Paluccio G, Giancotti A, Perticone F, Foca A, Pallone F. Seroepidemiology of Helicobacter pylori infection and hepatitis A in a rural area: evidence against a common mode of transmission. Gut. 1997;41:164–168. doi: 10.1136/gut.41.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang YJ, Wang SM, Chen CT, Huang MC, Chang CJ, Liu CC. Lack of evidence for fecal-oral transmission of Helicobacter pylori infection in Taiwanese. J Formos Med Assoc. 2003;102:375–378. [PubMed] [Google Scholar]
- 34.Stapleton JT. Host immune-response to hepatitis A virus. J Infect Dis. 1995;171:S9–S14. doi: 10.1093/infdis/171.supplement_1.s9. [DOI] [PubMed] [Google Scholar]
- 35.Kosunen TU, Seppala K, Sarna S, Sipponen P. Diagnostic-value of decreasing IgG, IgA, and IgM antibody-titers after eradication of Helicobacter pylori. Lancet. 1992;339:893–895. doi: 10.1016/0140-6736(92)90929-w. [DOI] [PubMed] [Google Scholar]
- 36.Cutler AF, Prasad VM, Santogade P. Four-year trends in Helicobacter pylori IgG serology following successful eradication. Am J Med. 1998;105:18–20. doi: 10.1016/s0002-9343(98)00134-x. [DOI] [PubMed] [Google Scholar]
- 37.Cutler AF, Prasad VM. Long-term follow-up of Helicobacter pylori serology after successful eradication. Am J Gastroenterol. 1996;91:85–88. [PubMed] [Google Scholar]
- 38.Broussard CS, Goodman KJ, Phillips CV, Smith MA, Fischbach LA, Day RS, Aragaki CC. Antibiotics taken for other illnesses and spontaneous clearance of Helicobacter pylori infection in children. Pharmacoepidemiol Drug Saf. 2009;18:722–729. doi: 10.1002/pds.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duque X, Vilchis J, Mera R, Trejo-Valdivia B, Goodman KJ, Mendoza ME, Navarro F, Roque V, Moran S, Torres J, Correa P. Natural history of Helicobacter pylori infection in Mexican schoolchildren: incidence and spontaneous clearance. J Pediatr Gastroenterol Nutr. 2012;55:209–216. doi: 10.1097/MPG.0b013e318248877f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luzza F, Suraci E, Larussa T, Leone I, Imeneo M. High exposure, spontaneous clearance, and low incidence of active Helicobacter pylori infection: the Sorbo San Basile study. Helicobacter. 2014;19:296–305. doi: 10.1111/hel.12133. [DOI] [PubMed] [Google Scholar]
- 41.Zajacova A, Dowd JB, Aiello AE. Socioeconomic and race/ethnic patterns in persistent infection burden among U.S. adults. J Gerontol A Biol Sci Med Sci. 2009;64:272–279. doi: 10.1093/gerona/gln012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peleteiro B, Bastos A, Ferro A, Lunet N. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci. 2014;59:1698–1709. doi: 10.1007/s10620-014-3063-0. [DOI] [PubMed] [Google Scholar]
- 43.Baaten GG, Sonder GJ, Van Der Loeff MF, Coutinho RA, Van Den Hoek A. Fecal-orally transmitted diseases among travelers are decreasing due to better hygienic standards at travel destination. J Travel Med. 2010;17:322–328. doi: 10.1111/j.1708-8305.2010.00442.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.