Abstract
Importance
This report presents evidence from spectral domain optical coherence tomography (sdOCT) and fluorescein angiography (FA) of inner foveal structural abnormalities associated with vision loss in Incontinentia pigmenti (IP).
Observations
Two children had reduced visual behavior in association with abnormalities of the inner foveal layers on sdOCT. FA showed filling defects in retinal and choroidal circulations and irregularities of the foveal avascular zones (FAZ). The foveal/parafoveal ratios were greater than 0.57 in 6 eyes of 3 patients who had extraretinal NV and/or peripheral avascular retina on FA and were treated with laser. Of these, 3 eyes of 2 patients had irregularities in FAZ and poor vision.
Conclusions and Relevance
Besides traction retinal detachment, visual loss in IP can occur with abnormalities of the inner fovea structure seen on sdOCT, consistent with prior descriptions of foveal hypoplasia. The evolution of abnormalities in the neural and vascular retina suggests a vascular cause of the foveal structural changes. More study is needed to determine any potential benefit of the foveal/parafoveal ratio in children with IP. Even with marked foveal structural abnormalities, vision can be preserved in some patients with IP with vigilant surveillance in the early years of life.
Introduction
Incontinentia pigmenti (IP), or Bloch-Sulzberger syndrome, is a rare X-linked dominant disorder mainly seen in females, because a single allele in male embryos is usually fatal.1,2 IP usually presents with a characteristic skin rash that leaves hypopigmented patches on the trunk and limbs.3 The most serious events involve the retina and central nervous system (CNS) in 18–30%.2,3,4,5 Loss of function mutations in the IKBKG/NEMO gene impair NF-kB signaling and are responsible for most cases of IP.6,7 NF-kB signaling is ubiquitous, but effects from the IKBKG/NEMO mutation do not manifest in all tissues.
Vision loss has been associated with vascular occlusions, secondary extraretinal neovascularization (NV), tractional retinal detachments,2,5,8 and foveal hypoplasia.8 Here, we report findings from spectral domain optical coherence tomography (sdOCT) in children with IP examined before 5 years of age. Poor visual behavior corresponded with inner retinal structural abnormalities.
Methods
Institutional Review Board approval was obtained from the University of Utah. All children had biopsy-proven IP and were seen by pediatric ophthalmology and retina between May 2010 and August 2014.
Data from clinical visits and examinations under anesthesia (EUA) included images from wide-angle fluorescein angiography (FA, RetCam, Clarity, Inc.) and macular sdOCTs (Bioptigen Inc, NC) (Table 1). Retinal thickness measurements were taken at the fovea from inner limiting membrane to the RPE, the parafoveal retina 1000 microns from the nasal and temporal fovea as visualized on sdOCT slices, and the choroid under the fovea (Figures 1b, 2b) using the caliper measurement tool provided with the Bioptigen software. The foveal location was estimated as 0.5 disc diameter inferior to the center of the optic nerve and 2 disc diameters temporal to it. We measured retinal thickness as reported by Vajvozic et al9 and included ILM to inner aspect of the RPE. The nasal and temporal parafoveal measurements were averaged and divided by the central foveal thickness to create a ratio.9 FAs were reviewed and analyzed qualitatively for vascular filling defects during transit phases and leakage from extraretinal neovascularization (NV) in late phases.
Table 1.
Clinical History and sdOCT & FA Findings
| Patient No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
|
| |||||
| MRI | Not done | Thin corpus callosum and patchy subcortical and periventricular white matter disease | No MRI, normal neurologic examination | Immature myelination consistent with age, otherwise normal | Not done |
|
| |||||
| IKBKG/NEMO mutation | + i | Not done | Not done | Not done | Not done |
|
| |||||
| BCVA (age) | (13 mos) | (2 yrs) | (21 mos) | (5 yrs) | (12 mos) |
| OD | Fix & Follow | Unmaintained Fixation | Fix & follow | 20/60 | Fix & follow |
| OS | Fix & Follow | Fix & Follow | Fix & follow | 20/100 | Fix & follow |
|
| |||||
| Strabismus & amblyopia | Not noted | Present | Not noted | Present with nystagmus | Not noted |
|
| |||||
| Foveal hypoplasia | |||||
| OD | None | Present | None | Abnormal | None |
| OS | None | None | None | Present | None |
|
| |||||
| Extraretinal neovascularization | |||||
| OD | None | Present | Appeared as | Present | None |
| OS | None | Present | regressed OU | Present | None |
|
| |||||
| Vascular abnormalities noted on fluorescein angiography | FAZ normal OU; Peripheral retina vascularized OU | irregular FAZ with vessel entering FAZ OD, nl FAZ OS; peripheral nonperfusion OU; extraretinal NV OU | FAZ normal OU; peripheral nonperfusion OU; extraretinal NV OD | Irregular FAZ OS>OD; peripheral nonperfusion OU; extraretinal NV OU | FAZ normal OU; Peripheral retina vascularized OU |
|
| |||||
| Laser treatment | None | OU | OD | OU | None |
|
| |||||
| Follow-up (mos) | 11 | 31 | 17 | 61 | <1 |
|
| |||||
| Features on sdOCT | |||||
|
| |||||
| Central foveal thickness | 6 months | 3 months | 25 months | 28 months | |
| OD | 128 um | 162 um | 210 um | 656 um | ND |
| OS | 132 um | 125 um | 190 um | 808 um | |
| 15 months | 18 months | ND | 54 months | ND | |
| OD | 144 um | 228 um | 270 um | ||
| OS | 145 um | 180 um | 191 um | ||
|
| |||||
| Foveal/parafoveal ratio | 6 months | 3 months | 25 months | 28 months | ND |
| OD | 0.48 | 0.26 | 0.65 | 0.98 | |
| OS | 0.53 | 0.40 | 0.60 | 0.96 | |
| 15 months | 18 months | ND | 54 months | ND | |
| OD | 0.51 | 0.81 | 0.86 | ||
| OS | 0.52 | 0.63 | 1.00 | ||
|
| |||||
| IS/OS junction | |||||
| OD | Normal | Normal | Normal | Normal | ND |
| OS | Normal | Normal | Normal | Normal | |
|
| |||||
| Choroidal thickness | 6 months | 3 months | 25 months | 28 months | ND |
| OD | 361 um | 197 um | Unable ii | 633 um | |
| OS | 265 um | 241 um | Unable | 607 um | |
| 15 months | 18 months | 54 months | |||
| OD | 441 um | 166 um | ND | 317 um | |
| OS | 259 um | 233 um | 184 um | ||
Done at Casey Eye Institute – OHSU
Unable to discern posterior aspect of choroid to obtain accurate measurement
FAZ- foveal avascular zone; BCVA – best-corrected visual acuity;
NV - neovascularization
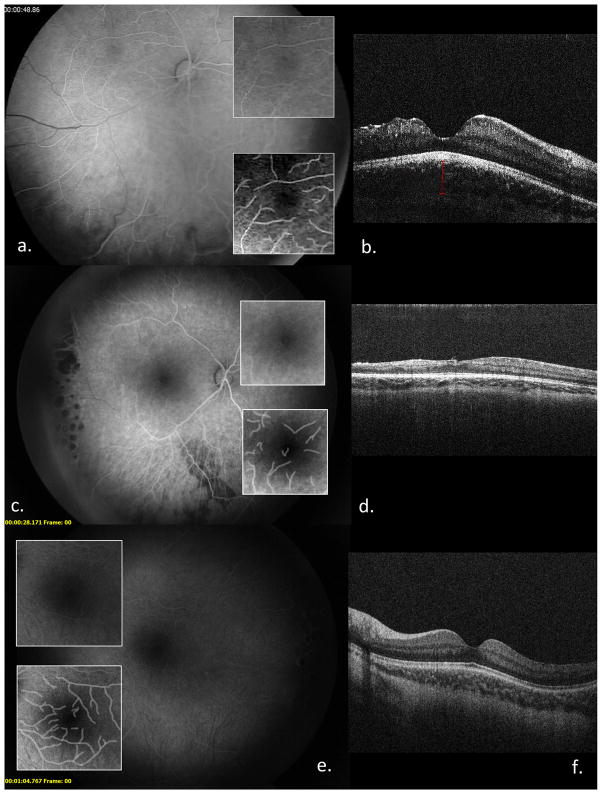
Figure 1.
FAs, FAZ, and computer-enhanced FAZ (a, c, e); sdOCTs (b, d, e) of OD (a–d) and OS (e, f) of Patient 2 at 3 months (a, b, e, f) and 18 months of age (c, d, e, f).
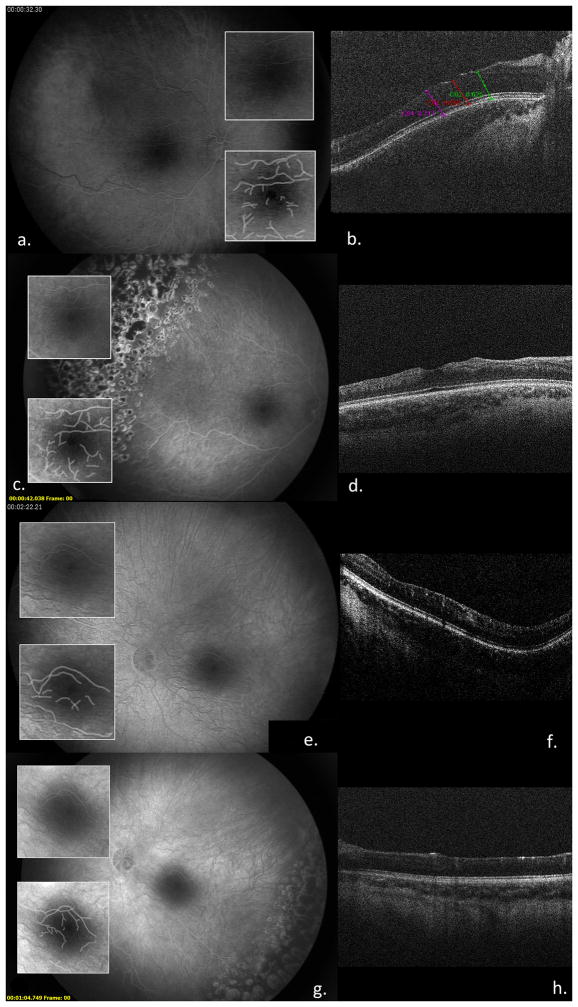
Figure 2.
FAs and FAZ, and computer-enhanced FAZ (a, c, e); sdOCTs (b, d, e) of OD (a–d) and OS (e, f) of Patient 4 at 28 months (a, b, e, f) and 54months of age (c, d, e, f)
PubMed searches were done without limitations on date using the terms incontinentia pigmenti, eye, ocular, optical, optical coherence tomography, incontinentia pigmenti, and fovea to ascertain current literature on sdOCT methods, IP and foveal development.
Results
Patients were females examined by age 5 (Table 1). Two had poor visual development, inner foveal structural abnormalities and retinal thinning that increased through 2 years of follow-up. Both had MRI abnormalities consistent with IP and extraretinal NV requiring laser. In these 2 patients and in Patient 3 with extraretinal NV, foveal/parafoveal ratios on sdOCT were >0.57.9
Case Reports
Patient #2
A caucasian female diagnosed by skin biopsy at 2 months of age underwent EUA at 2.5 months. FA transit OD showed patchy choroidal filling defects, delayed filling of retinal veins and irregularities in the foveal avascular zone (FAZ). There was avascular peripheral retina but no extraretinal NV. One month later, extraretinal NV developed OD, and the peripheral avascular retina was treated with laser. sdOCT OD showed irregularity of the contour of the nasal right fovea with thinning of the inner retinal layers and a normal appearing outer retina and IS/OS line. sdOCT OS was normal (Figure 1). On subsequent EUAs between 6 and 27 months (Table 1), laser was performed for extraretinal NV OS, and sdOCT OD showed thinning of temporal retinal layers, irregularity of the foveal contour, and loss of definition of the inner retinal layers throughout the fovea. The foveal/parafoveal ratio was increased (Table 1)9. sdOCT, foveal/parafoveal ratio, and FAZ were normal OS. At 23 months of age, the child was patched for intermittent exotropia and a fixation preference OS. A brain MRI/MRA at 6 months of age showed a reduced corpus callosum volume and patchy white matter lesions.
Patient #4
A caucasian female was diagnosed with IP on day 4 of life. Clinical examination at 2 weeks of life was normal, but foveal reflexes were reduced and a brain MRI showed an immature myelin pattern at 4.5 months. Ensuing visits with pediatric ophthalmology were performed for poor visual development OS more than OD and treated with patching therapy. EUA with FA at 21 months revealed peripheral avascular retina OU with extraretinal NV OS, managed with laser. Follow-up EUAs with sdOCT and FA at age 27 and 29 months showed disorganized inner retinal layers OU, irregularities of both FAZ (Figure 2) and extraretinal NV OD that was treated with laser. At age 5 years, visual acuity was 20/60 OD and 20/100 OS. The foveal reflex was reduced OD and nearly absent OS, and sdOCT showed loss of the foveal depression with irregularities and thinning of the inner nuclear, inner plexiform and nerve fiber/ganglion cell layers in both eyes. Outer retinas and IS/OS lines appeared normal. Foveal/parafoveal ratios were elevated (Table 1).
Discussion
We present the first sdOCTs of children with IP highlighting two with abnormal inner foveal structure, abnormal FAZ and decreased vision. The 3 infants with extraretinal NV did not all have foveal layer disorganization, but all had increased foveal/parafoveal ratios (>0.579) in at least one eye.
Prior to wide-angle FA and hand-held sdOCT, vision loss in IP was associated with vitreous hemorrhage, tractional retinal detachment and retinal ischemia from vascular occlusions. Foveal hypoplasia was reported on fundoscopic photographs and FAs as lack of a foveolar pit, pigmentary changes, and abnormal FAZ.3 The genetic mutation in IP involves the gene expressing NF-kB. NF-kB signaling affects all cells, but our patients had inner retinal layer disorganization and thinning reflected in increased foveal/parafoveal ratios and did not manifest outer retinal abnormalities. These findings suggest that their pathophysiology involved vascular events and neural structural changes rather than primary defects in neural architecture from abnormal NF-kB signaling. Loss of vascular support in vein occlusions can lead to thinning of the parafoveal retina,10 but the structural changes in our patients did not respect the horizontal raphe, suggesting a broader effect than in an occlusion of a branch retinal arteriole or vein.
The most serious manifestation of IP involves the CNS. Patients 2 and 4 both had abnormal MRI findings that are reported in IP.11 The cause of the CNS anomalies in IP patients is unknown but believed to be from vaso-occlusive events.12,13 Goldberg emphasized that CNS imaging can be life-saving in patients with IP and retinal manifestations, because stroke-like events can occur.11,14
In summary, we present inner foveal abnormalities in children with biopsy-proven IP and vision loss. Coordination among ophthalmologists, dermatologists and pediatric neurologists is important. EUA with FA to detect extraretinal NV, laser to treat avascular peripheral retina, sdOCT to assess the macular structure, and clinical examination to identify and treat strabismus and amblyopia are important. Extraretinal NV can occur in the absence of foveal structural abnormalities, and more study is warranted regarding the potential importance of the foveal/parafoveal ratio in association with peripheral retinal abnormalities. In patients with retinal involvement, monthly examinations for the first 3–4 months of life and then every 3 to 4 months are recommended for at least the first year.15 Neurologic evaluations in all patients with IP and CNS imaging with retinal involvement are recommended.11 With vigilant follow-up, vision can be preserved in patients with IP.
Acknowledgments
We thank David Dries MD and Robert Hoffman MD for clinical input regarding patients 2 and 4. “I had full access to all of the data and take responsibility for the integrity of the data and the accuracy of the data analysis.” – M. Elizabeth Hartnett
An Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah and NEI R01 EY015130 (PI: MEH) provided partial support for the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflicts of Interest: No conflicting relationship exists for any author
J.B and M.E.H. wrote the manuscript. M.P.Y provided critical review of the data and manuscript. M.E.H. and M.P.Y. provided patients and clinical input. J.B., T.C.M and R. H performed OCT evaluations and measurements. G.J. performed all OCTs and created figures.
References
- 1.Ardelean D, Pope E. Incontinentia pigmenti in boys: a series and review of the literature. Pediatric dermatology. 2006;23:523–7. doi: 10.1111/j.1525-1470.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- 2.Landy SJ, Donnai D. Incontinentia pigmenti (Bloch-Sulzberger syndrome) Journal of medical genetics. 1993;30:53–9. doi: 10.1136/jmg.30.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg MF, Custis PH. Retinal and other manifestations of incontinentia pigmenti (Bloch-Sulzberger syndrome) Ophthalmology. 1993;100:1645–54. doi: 10.1016/s0161-6420(93)31422-3. [DOI] [PubMed] [Google Scholar]
- 4.Carney R., Jr Incontinentia Pigmenti: A World Statistical Analysis. Archives of Dermatology. 1976;112:535–542. [PubMed] [Google Scholar]
- 5.O’Doherty M, Mc Creery K, Green AJ, Tuwir I, Brosnahan D. Incontinentia pigmenti--ophthalmological observation of a series of cases and review of the literature. The British journal of ophthalmology. 2011;95:11–6. doi: 10.1136/bjo.2009.164434. [DOI] [PubMed] [Google Scholar]
- 6.Conte MI, Pescatore A, Paciolla M, et al. Insight into IKBKG/NEMO locus: report of new mutations and complex genomic rearrangements leading to incontinentia pigmenti disease. Human mutation. 2014;35:165–77. doi: 10.1002/humu.22483. [DOI] [PubMed] [Google Scholar]
- 7.Smahi A, Courtois G, Vabres P, et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium Nature. 2000;405:466–72. doi: 10.1038/35013114. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg MF. The blinding mechanisms of incontinentia pigmenti. Ophthalmic genetics. 1994;15:69–76. [PubMed] [Google Scholar]
- 9.Vajzovic L, Hendrickson AE, O’Connell RV, et al. Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. American journal of ophthalmology. 2012;154:779–89 e2. doi: 10.1016/j.ajo.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim CS, Shin KS, Lee HJ, Jo YJ, Kim JY. Sectoral retinal nerve fiber layer thinning in branch retinal vein occlusion. Retina (Philadelphia, Pa) 2014;34:525–30. doi: 10.1097/IAE.0b013e3182a2e746. [DOI] [PubMed] [Google Scholar]
- 11.Lee AG, Goldberg MF, Gillard JH, Barker PB, Bryan RN. Intracranial assessment of incontinentia pigmenti using magnetic resonance imaging, angiography, and spectroscopic imaging. Archives of pediatrics & adolescent medicine. 1995;149:573–80. doi: 10.1001/archpedi.1995.02170180103019. [DOI] [PubMed] [Google Scholar]
- 12.Berlin AL, Paller AS, Chan LS. Incontinentia pigmenti: a review and update on the molecular basis of pathophysiology. Journal of the American Academy of Dermatology. 2002;47:169–87. doi: 10.1067/mjd.2002.125949. quiz 88–90. [DOI] [PubMed] [Google Scholar]
- 13.Francis JS, Sybert VP. Incontinentia pigmenti. Seminars in cutaneous medicine and surgery. 1997;16:54–60. doi: 10.1016/s1085-5629(97)80036-3. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg MF. The skin is not the predominant problem in incontinentia pigmenti. Archives of dermatology. 2004;140:748–50. doi: 10.1001/archderm.140.6.748. [DOI] [PubMed] [Google Scholar]
- 15.Holmstrom G, Thoren K. Ocular manifestations of incontinentia pigmenti. Acta ophthalmologica Scandinavica. 2000;78:348–53. doi: 10.1034/j.1600-0420.2000.078003348.x. [DOI] [PubMed] [Google Scholar]