Abstract
Previous research has shown that autobiographical episodic counterfactual thinking—i.e., mental simulations about alternative ways in which one’s life experiences could have occurred—engages the brain’s default network (DN). However, it remains unknown whether or not the DN is also engaged during impersonal counterfactual thoughts, specifically those involving other people or objects. The current study compares brain activity during counterfactual simulations involving the self, others and objects. In addition, counterfactual thoughts involving others were manipulated in terms of similarity and familiarity with the simulated characters. The results indicate greater involvement of DN during person-based (i.e., self and other) as opposed to object-based counterfactual simulations. However, the involvement of different regions of the DN during other-based counterfactual simulations was modulated by how close and/or similar the simulated character was perceived to be by the participant. Simulations involving unfamiliar characters preferentially recruited dorsomedial prefrontal cortex. Simulations involving unfamiliar similar characters, characters with whom participants identified personality traits, recruited lateral temporal gyrus. Finally, our results also revealed differential coupling of right hippocampus with lateral prefrontal and temporal cortex during counterfactual simulations involving familiar similar others, but with left transverse temporal gyrus and medial frontal and inferior temporal gyri during counterfactual simulations involving either oneself or unfamiliar dissimilar others. These results suggest that different brain mechanisms are involved in the simulation of personal and impersonal counterfactual thoughts, and that the extent to which regions associated with autobiographical memory are recruited during the simulation of counterfactuals involving others depends on the perceived similarity and familiarity with the simulated individuals.
Keywords: Counterfactual Thinking, Default Mode Network, Partial Least Squares, Mental Simulation, Self, Other
1. Introduction
We spend a substantial amount of our lives entertaining mental simulations about situations beyond our temporally and spatially present surroundings.1 Some of these situations are real but long gone, as when we remember specific episodes from our personal past. But some of these situations are hypothetical, as when we imagine ourselves in a possible future scenario—a kind of mental simulation that has come to be known as episodic future thinking (Atance & O’Neill, 2001; for reviews, see Schacter et al., 2012; Szpunar, 2010). The last decade of research in the cognitive neuroscience of both episodic memory and episodic future thinking has revealed striking commonalities between the neural mechanisms underlying both kinds of mental simulations (Okuda et al., 2003; Addis, Wong, & Schacter, 2007; Hassabis, Kumaran, & Maguire, 2007; Szpunar, Watson, & McDermott, 2007). Moreover, these studies have revealed that the brain regions commonly engaged by episodic memory and episodic future thinking are part of what it is now known as the brain’s default network (DN), a set of functionally connected brain regions including ventral medial prefrontal cortex (vMPFC), posterior cingulate cortex (PCC), inferior parietal lobule (IPL), lateral temporal cortex (LTC), dorsal medial prefrontal cortex (dMPFC), and the hippocampal formation (Buckner, Andrews-Hanna, & Schacter, 2008).
Importantly, other kinds of mental simulations about hypothetical scenarios have been shown to engage core regions of the DN as well. For instance, both mental navigation, or our capacity to mentally simulate the spatial surroundings from someone’s point of view (Maguire et al., 1998), and mentalizing, or our capacity to mentally simulate another person’s perspective (Saxe & Kanwisher, 2003; Mitchell, 2009), have shown to activate core regions of the DN (Spreng, Mar, & Kim, 2009). To account for these convergent results, Bucker and Carroll (2007) suggested that core regions of the DN may be commonly activated during these cognitive processes because the DN plays a critical functional role in the generation and support of stimulus-independent simulations in which we project ourselves onto hypothetical situations.
Further support for this view comes from studies on another kind of hypothetical thought which, up until very recently, had not received much attention in the cognitive neuroscience of mental simulation: counterfactual thinking, our tendency to think about alternative ways in which things might have occurred in the past but did not (Roese, 1997). Counterfactual thoughts play a central role in human emotion and decision-making, and have been extensively studied in philosophy and linguistics (Goodman, 1947; Lewis, 1973) as well as social psychology and behavioral economics (Roese and Olson, 1995; Mandel, Hilton and Catellani, 2005; Epstude and Roese, 2008).2 Thus, given how many of our counterfactual simulations involve projecting ourselves onto possible pasts that could have occurred but did not, it is not unreasonable to hypothesize that core regions of the DN would be engaged during counterfactual thinking, which also constitutes a kind of self-generated thought (Andrews-Hanna, Smallwood, & Spreng, 2014)
This hypothesis was recently supported by two studies (De Brigard et al., 2013; Van Hoeck et al., 2013) in which participants engaged in episodic counterfactual thinking: counterfactual simulations about alternative ways in which past personal (i.e., self-involving) events could have occurred but did not (De Brigard & Giovanello, 2012). Although both studies showed significant engagement of core regions of DN during episodic counterfactual thinking, De Brigard et al (2013) also found that the engagement of such regions was modulated by the perceived likelihood of the counterfactual thought. Specifically, they found that the more likely the counterfactual alternative was perceived, the greater the engagement of the DN. Of note, this effect was most clear in certain core regions of the DN, such as the hippocampus and the vMPFC, which were parametrically modulated by perceived likelihood of the episodic counterfactual thought.
Why is there differential engagement of DN regions during episodic counterfactual simulations? One hypothesis is that likely episodic counterfactuals were perceived by the participants as more personally relevant for social interactions. This hypothesis is consistent with much research in the social psychology of counterfactual thinking, suggesting that our tendency to engage in episodic counterfactual simulations may be a goal-oriented cognitive strategy to help us to modify future behavior in the context of social interactions (Johnson & Sherman, 1990; Markman & McMullen, 2003; Epstude & Roese, 2007). Indirect evidence in support of this hypothesis comes from a recent study in which van Hoeck and collaborators (2014) found significant overlap in brain activation during false-belief and counterfactual tasks involving possible social interactions. Critically, some of this overlap occurred in temporo-parietal junction and precuneus, which have been associated with the DN. However, this suggestive result only speaks indirectly to the above hypothesis, as they did not employ episodic counterfactual simulations based upon actual autobiographical events, and did not directly manipulate the personal relevance (for the participant) of the characters involved in the vignettes.
On the other hand, the hypothesis that involvement of the DN during autobiographically-based episodic counterfactual thoughts is associated with perceived personal relevance of the content of the simulation for social interaction is also consistent with recent proposals suggesting a critical role of the DN supporting socially relevant goal-oriented cognition (Andrews-Hanna, 2012; Andrews-Hanna, Smallwood, & Spreng, 2014). In line with these results, we conjecture that if the involvement of core DN regions during counterfactual thinking is modulated by the personal and social relevance of the simulated event, then it is likely that impersonal and non-socially relevant counterfactual simulations would engage processes outside of the DN, whereas personal and socially relevant episodic counterfactual simulations would mainly engage core regions in the DN.
To explore this general hypothesis, the current study was designed to extend our understanding of the involvement of regions of the DN during personal and socially relevant counterfactual simulations in three ways. First, this study investigates whether or not core regions of the DN are engaged during mental simulations of impersonal counterfactual thoughts pertaining to either objects or people other than oneself. Participants were asked to simulate counterfactuals that either involved themselves, other people, or objects. Given recent neuroimaging results showing significant overlap in DN regions during episodic memory and theory of mind tasks (Spreng & Grady, 2010; Mitchell, 2009), and greater involvement of DN during simulations that involve primarily autobiographical details rather than tasks involving non-autobiographical processing of objects (Addis, Wong, & Schacter, 2007, Addis et al., 2009; Hassabis et al, 2007), we expected to see greater involvement of DN during person-based (i.e., self and other) relative to object-based counterfactual simulations. Indeed, two recent fMRI studies exploring neural correlates of semantic evaluation of non-autobiographical hypothetical and counterfactual statements show relatively little involvement of DN regions (Nieuwland, 2012; Kulakova et al., 2013), further suggesting that object-based counterfactual simulations may primarily recruit processes outside the DN.
On the other hand, given previous research showing differential MPFC recruitment for self-relative to other-based mental simulations (Denny et al, 2012; Hassabis et al., in press; Wagner, Haxby, & Heatherton, 2012), we also expected to find differences in prefrontal activation between self versus other-based counterfactual simulations. Thus, a second way in which the current study seeks to investigate the involvement of DN in personal and socially relevant counterfactual simulations, is by way of contrasting the recruitment of DN regions during personal and socially relevant counterfactual thoughts (i.e., self-based) versus impersonal and non-socially relevant counterfactual simulations (i.e., object-based), on the one hand, and impersonal yet socially relevant counterfactual simulations (i.e., other-based), on the other.
Finally, since certain DN regions recruited during theory of mind tasks—e.g., MPFC, anterior cingulate cortex (ACC), and hippocampus—are differentially engaged depending on whether or not the simulated character is personally known (i.e., familiar) and/or perceived to be similar in personality by the participant (Mitchell, Macrae, & Banaji, 2006; Krienen, Tu, & Buckner, 2010), we also expected to find neural differences when other-based counterfactuals involved either familiar and/or similar characters. Thus, personal and social relevance of counterfactual simulations was manipulated in yet a third way, by asking participants to engage in three other-based counterfactual simulation tasks: they either had to imagine how things could have been different for 1) a familiar/similar character, 2) an unfamiliar/similar character, or 3) an unfamiliar/dissimilar character. Since research suggests greater recruitment of vMPFC, posterior ACC and medial temporal lobe (MTL) for similar- and familiar-others relative to self-based simulations (Mitchell, Macrae, & Banaji, 2006; Krienen, Tu, & Buckner, 2010; Perry, Hendler, & Shamay-Tsoory, 2011), we anticipated our results to be consistent with these reports. Furthermore, given previous results suggesting a tight functional coupling between the hippocampus and MPFC during mentalizing tasks involving familiar versus unfamiliar targets (Perry, Hendler, & Shamay-Tsoory, 2011; Rabin & Rosenbaum, 2012; see also Rosenbaum et al., 2007), we conducted a functional connectivity analysis seeded in the hippocampus expecting to find a similar pattern of co-activation for counterfactuals involving self and familiar-others but not unfamiliar-others. Therefore, a final aim of the current study is to explore whether differences in neural activation during counterfactual thoughts about others can be accounted for by the participant’s perceived similarity and/or familiarity with the simulated characters. We used spatiotemporal Partial Least Squares (PLS; Krishnan et al., 2011; McIntosh et al., 1996; McIntosh et al., 2004) to analyze task-related brain activation. In this approach, task conditions are analyzed simultaneously to detect covaring, as well as dissociable, patterns of activity. This multivariate method is sensitive to distributed voxel responses and is thus ideally suited to analyze distributed network activity.
2. Methods
2.1 Participants
Twenty-six healthy right-handed English-speaking young adults (M age = 20.8, SD = 2.55; 11 female) with normal or corrected-to-normal vision and no history of neurological or psychiatric conditions participated in the study. All participants provided written consent in accordance with the guidelines set by the Committee on the Use of Human Subjects in Research at Harvard University and received monetary compensation. Due to excessive motion, one subject was excluded leaving 25 participants for fMRI analysis (see below).
2.2. Pre-scan Stimulus Collection
To generate subject-specific, and therefore personally relevant counterfactual thoughts, a stimulus collection interview was conducted one week prior to scanning. Participants were asked to report 35 memories of specific decisions made in the past 10 years. Participants were asked to provide a title for each remembered decision, and to briefly state (less than 5 words) what they decided to choose. To provide retrieval support, participants were provided a list of 50 common decisions representative of their cohort determined by pilot sampling (e.g., mixing whites and colors in the laundry; telling parents about a bad grade). Participants were instructed to report only event-specific memories—i.e., vividly detailed recollections of single experienced events—as opposed to lifetime period or general event memories (Conway and Pleydell-Pearce, 2000). In addition, they were asked to report only specific memories of decisions about which they felt regret by virtue of the outcome of their choice. Finally, participants were asked to report only specific memories of regretful decisions where the outcome occurred close to having made the decision, as opposed to days or months later (e.g., missing an important appointment because they decided to take the bus instead of the subway; getting their favorite t-shirt stained because they decided to mix whites with colors in the laundry). To facilitate adherence to the instructions, examples of specific memories of past decisions were given. At the end, participants were asked to rate the degree of regret felt after the decision from 1 (“Very little regret”) to 5 (“A lot of regret”). Independently, participants were given a form to complete that included some demographic information, such as age and years of education. Importantly, two questions asked them to report their social and political views on a Likert scale ranging from 1 (Conservative) to 7 (Liberal). Following Mitchell et al. (2006), these ratings were later used to pair each participant with a similar and a dissimilar character.
2.3. Instruction session, stimuli and experimental conditions
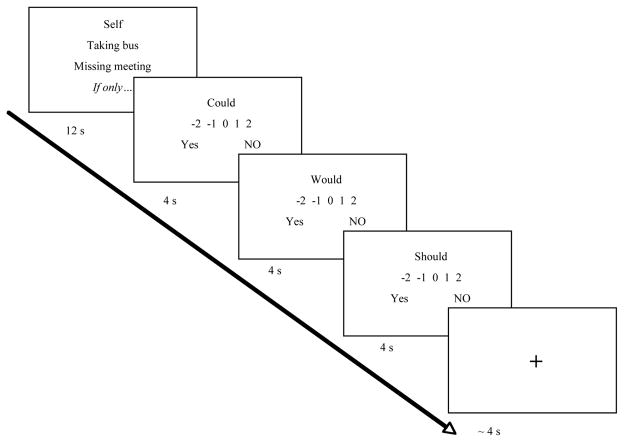
From the reported memories, the 28 that received the highest ratings of regret were selected as stimuli. The remaining memories were used for practice during the instruction session prior to scanning. The purpose of this instruction session was to explain the tasks and to familiarize participants with the stimuli and three target characters that would feature in the experimental tasks. Participants were told that all stimuli had the same structure, and that they would see a screen displaying a heading indicating the task, and three lines of text below (Figure 1). Then, participants received instruction on the Self condition. They were informed that they would see a display with the heading “Self”, followed by the title of one of their reported decisions, the choice they made, and a line reading “If only”. Participants were instructed to mentally complete the thought, “If only…”, by imagining how things would have been better for the person referred to in the heading (i.e., themselves) in the situation referred by the title and the choice (e.g., “If only I had taken the T instead of the bus this morning”; “If only I had separated the whites from the colors when doing laundry that one time”). They were told that the screen would be displayed for 12 seconds, and were encouraged to use the whole time to come up with a very vivid counterfactual simulation.
Figure 1.
Experimental design.
Next, participants were instructed to complete a short form asking them to think of a relative or close friend with whom they were very familiar, to whom they considered themselves similar and who was of the same gender and roughly their age. They were asked to briefly list the reasons why they thought this person was similar and familiar to them, and were asked to rate how similar and how familiar they were to this person on a scale from 1 (Very dissimilar/unfamiliar) to 10 (Very similar/familiar). Participants were then told that in the second task—the Familiar/Similar (FamSim) condition—they would see a heading with the name of the friend or relative that they just identified (e.g., “Morgan”), followed by a previously reported decision-title and choice, as well as the line “If only”. As with the Self condition, participants were instructed to mentally complete the thought “If only…” by imagining how things would have been better for the person referred to in the heading (i.e., Morgan) in the situation referred by the title and the choice (e.g., “If only Morgan had taken the T instead of the bus this morning”; “If only Morgan had separated the whites from the colors when doing laundry that one time”). They were told that the screen would be displayed for 12 seconds, and were encouraged to use the entire time to come up with a very vivid counterfactual simulation.
For the third condition, participants were presented with a fictional unfamiliar character designed to be similar to the participants. Two such characters were designed: one female (“Cathy”), for female participants, and one male (“Clark”), for male participants. These characters depicted young undergraduate students in Boston, with fairly liberal social and political beliefs, and with interests common among the participant’s population (for those participants who gave conservative ratings during the pre-scan stimulus collection session, Cathy and Clark also depicted young undergraduates in Boston, but with rather conservative social and political beliefs). A photograph downloaded from the Internet accompanied the description. Participants were told that these characters described real people and were asked to rate how similar they were to this person on a scale from 1 (Very dissimilar) to 10 (Very similar). Participants were then told that in the third task—the Unfamiliar/Similar (UnfSim) condition—they would see a heading with the name of one of these characters (i.e., “Cathy” or “Clark”), a decision title, a choice, and the line “If only”. As before, participants were instructed to mentally complete the thought “If only…” by imagining how things would have been better for the person referred to in the heading (i.e., “Cathy” or “Clark”) in the situation indicated by the title and the choice (e.g., “If only Clark had taken the T instead of the bus this morning”; “If only Cathy had separated the whites from the colors when doing laundry that one time”). The fourth condition—the Unfamiliar/Dissimilar (UnfDis) condition—was parallel to the previous one, except participants were presented with fictional unfamiliar characters designed to be dissimilar to participants. One female (“Susan”) and one male character (“Sean”) were created. Each depicted individuals in their 50s, living in rural Texas, with rather conservative social and political beliefs, and with personal interests very much unlike those of the common undergraduate in Boston (for those participants who gave conservative ratings during the pre-scan stimulus collection session, Susan and Sean also depicted individuals in their 50s, but living in Portland and with rather liberal social and political beliefs). Photographs also accompanied these descriptions and participants were asked to rate how similar they were to this person on a scale from 1 (Very dissimilar) to 10 (Very similar). As before, participants were instructed to mentally complete the thought “If only…” by imagining how things would have been better for the person referred to in the heading (i.e., “Susan” or “Sean”) in the situation indicated by the title and the choice (e.g., “If only Susan had taken the T instead of the bus this morning”; “If only Sean had separated the whites from the colors when doing laundry that one time”). Also as before, the screen appeared for 12 seconds, and participants were encouraged to use the entire time to vividly imagine the counterfactual simulation.
Finally, for the Object condition, participants saw the heading “Object”, followed by the name of an ordinary object, one of its features, and the line “If only”. Participants were instructed to mentally complete the thought “If only” by imagining how things would have been better for the object referred to in the screen if the displayed feature had been different. For instance, if the object was “Skateboard” and the feature was “Four wheels”, participants were asked to imagine a change in the feature that they thought would have made the object better (e.g., If only the wheels could rotate in a 360 angle). As before, the screen was displayed for 12 seconds, and participants were encouraged to use the entire time to come up with a vivid counterfactual simulation. The list of 28 objects and their properties was chosen as follows. From the Medical Research Council (MRC) Psycholingustic Database (Coltheart, 1981), the names of 50 common and highly imaginable concrete objects were chosen, and each object was paired with its most salient property. Next, a pilot norming survey with an independent sample of 20 subjects was conducted, by asking them to assess how common were these objects in their past, how easily mutable they found the properties to be, and how easy it was to imagine a variation in the property that could, in their option, make the object better. The 28 objects and the properties that received the highest ranking in this pilot survey were chosen for the stimuli included in the Object condition.
Following the 12 seconds with the slide for the counterfactual simulation, participants were asked to give three ratings: 1) Could the event/object have occurred/been in the way you just simulated? 2) Would the event/object have occurred/been in the way you just simulated? 3) Should the event/object have occurred/been in the way you just simulated? Participants were told that “could” ratings were supposed to reflect their assessment of the plausibility of simulation regardless of the character’s willingness to bring about the change; “would” ratings were supposed to reflect their assessment of the plausibility of the simulation given their judgments on the character’s willingness to bring about the change; and “should” ratings were supposed to reflect their normative assessment on the goodness of the simulated change. To further clarify the ratings we provided examples of counterfactual events in which modal judgments such as “could”, “would” and “should” diverge (e.g., FamSim: “I guess although Morgan could have separated colors and whites, and given how much she cares about her clothes she should have done it, knowing how penny-pinching she is and how much she hates to do laundry she probably wouldn’t have done it”; Object: “Although stop signs could have been green, I am not sure they would have been, and I am pretty sure they should not have been green”). All ratings varied across a 5 point scale anchored at “No” and “Yes”. Each rating slide was displayed for 4 seconds, and the order was counterbalanced per run (Figure 1).
2.4. Scanning session
In the scanner, participants completed seven runs with 20 trials per run consisting of 4 trials per condition. Since all 28 decisions and choices would appear once per condition for the Self, FamSim, UnfSim, and UnfDis conditions, they were pseudo-randomized so that each choice and decision would appear only once per run. Each run was 10 minutes long, and included 20 s (10 TRs) of fixation at the beginning and at the end that were dropped during the analysis. Images were acquired on a 3T Siemens Magnetom TimTrio Scanner, equipped with a 12-channel head coil. Participants’ heads were held in place with cushions. An initial localizer was followed by a high-resolution magnetization-prepared rapid gradient echo sequence (MPRAGE; 176 × 1 mm sagittal slices, TE = 1.64 ms, TR = 2530 ms, flip angle = 7.0 deg., voxel size = 1 × 1 × 1 mm). Functional scans were collected during 7 runs using a whole brain, 2T* gradient-echo, EPI sequence (TR = 2 s, TE = 30 ms, FOV = 216 mm, flip angle = 80 degrees) Interleaved slices (31 × 5 mm slices; 0.5 mm skip) parallel to the AC/PC plane, as identified by the T1 structural scan. Stimuli were projected in black letters onto a screen at the head of the bore. Participants saw the screen on a mirror placed on the head coil. E-Prime Software (psychology Software Tools, Inc., Pittsburgh, PA) was used for stimuli presentation and to collect behavioral responses, for which participants used a five-button MR compatible response box with their right hand.
2.5. Post-scan interview
Immediately following the scanning session, participants were asked to complete a post scan interview. They were presented with all the trials they completed in the scanner, in the same order in which they appeared on the scanner, and with the same display, and they were asked to report what they thought of while in the scanner by way of completing the sentence “If only…” for each trial. Participants took about 40 minutes to finish this post-scan interview. Participants were then debriefed and paid for their participation.
2.6. Data preprocessing and analysis
Analyses of variance (ANOVA) and t-tests were used to analyze ratings and scores of the post-scan interviews. Cronbach’s alpha values were calculated to verify inter-rater reliability in scoring of post-scan interview data. Functional MRI data were preprocessed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (Mathworks, Natick, MA). Images were realigned, co-registered, segmented, normalized to MNI template, spatially smoothed using a 8 mm full-with at half maximum isotropic Gaussian kernel, and re-sliced (2 × 2 × 2 mm voxels).
The neuroimaging data were then analyzed in three stages with spatiotemporal PLS (Krishnan et al., 2011; McIntosh et al., 1996; McIntosh et al., 2004). Spatiotemporal PLS is a multivariate functional neuroimaging analysis tool designed to identify whole brain patterns of activity that are correlated with tasks. PLS is a robustly validated (Krishnan et al., 2011; McIntosh et al., 1996; McIntosh et al., 2004; McIntosh & Lobaugh, 2004) and widely used analysis technique (e.g., Addis et al, 2012; Gerlach et al., 2011; Grady et al., 2010; Martin et al., 2011; Hassabis et al., 2014) that is sensitive to distributed voxel responses rather than to the activity of individual voxels per se. PLS assesses the covariance between brain voxels (BOLD signal) and the experimental design to identify a limited number of orthogonal components (Latent Variables, LVs) that optimally relate the two. This data-driven approach is similar to independent component analysis in that it determines orthogonal whole brain patterns of activity. Unlike independent component analysis, the number of latent structures is constrained by the experimental conditions. Unlike standard univariate analyses that examine the activity of any single voxel independently, PLS detects brain-wide systems that covary with the experimental design.
Activity at each time point, relative to trial onset, for each voxel is averaged across trials of a given condition and normalized to activity in the first TR of the trial and the data matrix is then expressed as voxel-by-voxel deviation from the grand mean across the entire experiment. This matrix is then analyzed with singular value decomposition to derive the optimal effects in the data. Here, we applied PLS analysis to event-related fMRI data and the results provide a set of brain regions wherein activity is reliably related to the task conditions at 12 post-stimulus time points (i.e., 12 TRs = 24 s) for each LV. Each brain voxel is given a singular value weight, known as a salience (akin to a component loading in principle components analysis), which is proportional to the covariance of activity with the task contrast at each time point on each LV. Multiplying the salience by the BOLD signal value in that voxel and summing the product across all voxels gives a “brain score” for each participant for each time point on a given LV (like a component score in principal components analysis). These brain scores can be used to examine differences in brain activity across conditions, as greater activity in brain areas with positive (or negative) weights on a latent variable will yield positive (or negative) mean scores for a given condition over each time point. The significance of each LV as a whole is determined by permutation testing, using 500 permutations. In a second, independent step, the reliability of the saliences for the brain voxels across subjects, characterizing each pattern identified by a LV, is determined by bootstrap resampling, using 300 iterations, to estimate the standard errors for each voxel. Clusters larger than 100 mm3 comprising voxels with a ratio of the salience to the bootstrap standard error values (i.e., the “bootstrap ratio”; BSR) greater than 3.2 (p < .00024) were reported. The local maximum for each cluster was defined as the voxel with a BSR higher than any other voxel in a 2-cm cube centered on that voxel. PLS identifies whole brain patterns of activity in a single analytic step, thus, no correction for multiple comparisons is required.
In the first PLS analysis, a data-driven “mean-centered” approach was taken to examine the maximal effects across conditions. In a second analysis, we conducted a “non-rotated” analysis to specifically assess person-based counterfactual conditions, and contrasted Self versus FamSim, UnfSim and UnfDis. The Object condition was not included in this analysis. As such, activity from trials in the Self condition was weighted against trials from each one of the other three person-based conditions, with the other two person-based conditions weighted as 0. For this analysis only participants for whom the self-other manipulation was clearly successful were included. That is, we excluded participants who, contrary to the experimental objective of the current study, provided only moderate endorsements of similarity with the characters in the UnfSim condition and only moderate endorsements of dissimilarity with the characters in the UnfDis condition (see behavioral results below for further details). Thus, data from only those participants who gave extreme ratings of similarity to the characters (1, 2, or 3 and 8, 9 or 10) were included in the analyses (N = 18).
In the final PLS analysis, we tested the hypothesis that the hippocampus and the MPFC may be differentially coupled during tasks involving counterfactual simulations for familiar versus unfamiliar characters. To do so, we conducted a task-related functional connectivity analysis using seed PLS (McIntosh 1999; Burianova et al., 2010; Krishnan et a;., 2011). Seed PLS is a multivariate task-related functional connectivity analysis technique used to investigate the relationship between the activity of a seed region and the activity in the rest of the brain (McIntosh, 1999). Using right hippocampus as a seed, we assessed the task-related functional connectivity of this region with the rest of the brain during Self, FamSim, UnfSim and UnfDis over the simulation interval (first 6 TRs). BOLD signal values from right hippocampus—centered on the peak activation voxel of hippocampal activity associated with person-based counterfactuals, as revealed by the mean-centered analysis above (LV1; MNI x,y,z = 34 −16 −18)—and its 26 adjacent voxels were extracted and averaged from TR 4 after stimulus onset.3 Seed values were correlated with activity in all brain voxels, across participants. This matrix was then analyzed with singular value decomposition, assessed for statistical significance by permutation testing, and for reliability by bootstrap resampling, as described above.
3. Results
3.1. Behavioral results
During the stimulus collection interview, on average participants rated their political (M = 4.84, SD = 1.11) and social (M = 5.52, SD = 1.29) views as slightly liberal. There was no significant difference between these ratings (p > .05) and both were strongly correlated (r = .61). During the instruction session, participants rated the characters in the FamSim (M = 8.28, SD = .98) and the UnfSim conditions (M = 6.84, SD = .90) as more similar to them than the characters in the UnfDis condition (M = 2.16, SD = 1.10; smallest t(48) = 16.4, p < .001). However, characters in the FamSim condition were deemed more similar than those in the UnfSim condition (t(48) = 5.42, p < .005). (This difference was reduced, but not eliminated (t(34) = 3.89, p < 01), for participants in the non-rotated analysis, whose ratings of similarity were on average slightly higher (M = 7.28; SD = 67) for the UnfSim.)
The behavioral results collected during the scanning session can be found in Table 1. Average Ratings were analyzed using a 3 (Judgment: Could, Should, Would) x 5 (Condition: Self, FamSim, UnfSim, UnfDis, Object) repeated measures ANOVA, which revealed main effects of Judgment (F(2, 24) = 58.81, p < .001, η2 = .831) and Condition (F(4, 22) = 25.70, p < .001, η2 = .82) qualified by a Judgment by Condition interaction (F(8, 18) = 3.10, p < .05, η2 = .58). Direct comparisons showed that ratings for “Could” were significantly higher than those of “Should” and “Would” across all conditions (largest p < .005, corrected), which indicates that participants complied with the task, as they were asked to imagine plausible counterfactuals. As for differences between conditions, “Could” judgments for self-based counterfactuals received higher ratings than for other-based counterfactuals (largest p < .01, corrected), and all in turn received higher ratings that object-based counterfactuals (largest p < .01, corrected). However, there were no differences among FamSim, UnfSim, and UnfDis (p > .05). “Should” judgments were significantly higher for Self and UnfDis (p < .01, corrected) and Object (p < .001). Finally, “Would” judgments were significantly different for person-based and object based counterfactuals (largest p < .001), but not among person-based counterfactuals.
Table 1.
Behavioral results. Left: percentage of counterfactual modifications of “choice”, “situation” and “other” during post-scan interview (N = 17). Right: Mean ratings collected online in the scanner (N = 26). Numbers in parenthesis indicate standard deviations.
| Condition | Modification | Rating | ||||
|---|---|---|---|---|---|---|
| Choice | Situation | Other | Could | Should | Would | |
| Self | 93.49% | 6.23% | 0.28% | 4.75 (0.29) | 4.22 (0.70) | 3.19 (0.85) |
| Fam_Sim | 83.12% | 15.76% | 0.98% | 4.49 (0.46) | 3.85 (0.68) | 3.45 (0.65) |
| Unf_Sim | 82.07% | 17.23% | 0.70% | 4.50 (0.47) | 3.95 (0.67) | 3.56 (0.49) |
| Unf_Dis | 76.54% | 21.71% | 1.75% | 4.28 (0.63) | 3.65 (0.82) | 3.28 (0.58) |
| Object | 0.07% | 96.29% | 3.54% | 4.04 (0.65) | 2.57 (0.65) | 2.62 (0.71) |
Seventeen participants completed post-scan interviews4, which were scored following Girotto et al.’s (2007) approach. Counterfactuals that undid features of the protagonist’s choice (e.g., “If Cathy had chosen a different meal”) were coded as “choice” modifications. Counterfactuals that undid features of the situation (e.g., “If there had been more options on the menu”) were coded as “situation” modifications. The remaining counterfactuals were coded as “other”. Across conditions inter-rater reliability was good (lowest Cronbach’s α = .93). A 5 (Condition: Self, FamSim, UnfSim, UnfDis, Object) × 3 (Modification: Choice, Situation, Other) repeated measures ANOVA revealed a main effect of Modification (F(2, 15) = 2478.39, p <.001, η2 = .997) with a significant Modification by Condition interaction (F(8, 9) = 331.41, p < .001, η2 = .997). Overall, person-based counterfactuals modified features of the choice, whereas object-based counterfactuals modified features of the situation (p < .001, corrected). Given that objects do not really have choices, this result supports the expectation that essentially all object-based modifications would be coded as modifications of the situation. Within person-based counterfactuals, participants modified more features of the choice for Self-based counterfactuals relative to counterfactuals involving unfamiliar dissimilar characters (p < .05, corrected). No other effects were apparent.
3.2. fMRI results
3.2.1. Mean-centered PLS analysis
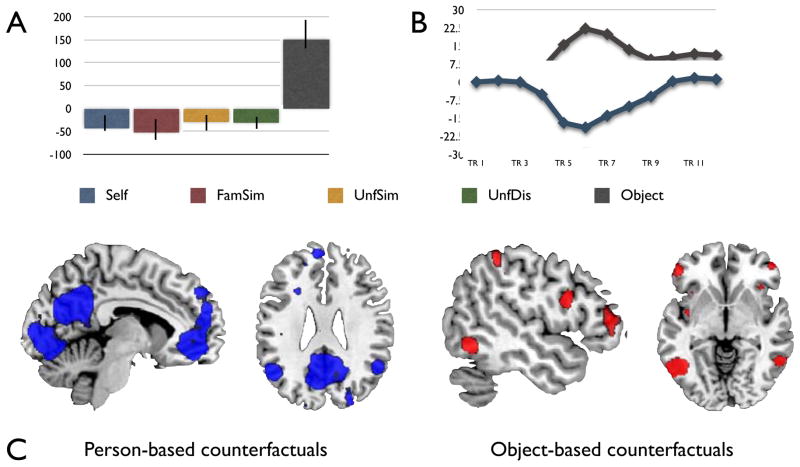
The first analysis showed that brain regions engaged during person-based counterfactual simulations [Self + FamSim + UnfSim + UnfDis] were dissociated from those engaged during object-based counterfactual simulations, as revealed by the identification of a significant latent variable (LV1, p < .0001, accounting for 69.19% of the crossblock covariance. Figure 2A). During the window of maximal neural differentiation (TR 3 – 5, Figure 2B) only two regions associated with the DN were engaged during object-based counterfactual simulations: inferior parietal lobule (IPL; BA 40) and inferior frontal gyrus toward the rostropolar cortex (BA 9/10). In contrast, the set of activated regions engaged by person-based counterfactuals during this time window included all of the regions previously associated with the DN: vMPFC and ACC (including BA 24, posterior, medial and rostral aspects of BA 10, and BA 32), posterior cingulate/retrosplenial cortex (BA 23/31), IPL toward superior temporal and supramarginal gyrus (BA 39/40), lateral temporal cortex at the middle temporal gyrus (BA 21), dMPFC (BA 24, BA 9/10, BA 32), and right hippocampus. Finally, object-based counterfactuals engaged left parahippocampal gyrus whereas person-based based counterfactuals engaged right parahippocampal gyrus. (Figure 2C. For a complete list of brain regions associated with LV1 see tables 2a-b).
Figure 2.
Results from mean-centered PLS analysis: Latent Variable 1 (LV 1). (A) Plot of brain scores with confidence intervals. (B) Plot of temporal brain scores indicating weighed average of activation across all voxels in all participants during the length of the task. (C) Regions with negative saliences (blue) were engaged by person-based counterfactuals, whereas regions with positive saliences (red) were engaged by object-based counterfactuals. All regions are shown at a threshold of p < .001.
Table 2.
| Table 2a. Regions associated with object versus person-centered counterfactuals (LV 1).
| |||||||
|---|---|---|---|---|---|---|---|
| Region of Activation | Hemisphere | BA | Voxels | MNI Coordinates
|
BSR* | ||
| X | Y | Z | |||||
| TR 3 (4–6s after stimulus onset) | |||||||
| Inferior Parietal Lobule | L - R | 40 | 160 | 44 | −36 | 44 | 5.0526 |
| Inf. Temporal/Sup. Occipital | L | 19 | 59 | −46 | −56 | −8 | 4.4147 |
| Middle Frontal Gyrus | R | 9 | 37 | 38 | 48 | 34 | 4.2102 |
| Middle Frontal Gyrus | L | 46 | 27 | −50 | 46 | 10 | 4.0837 |
| Poscentral Gyrus | R | 1 | 40 | 36 | −38 | 70 | 3.9205 |
| Inferior Frontal Gyrus | R | 47 | 36 | 36 | 24 | −10 | 3.8221 |
| TR 4 (6–8s after stimulus onset) | |||||||
| Middle Occipital Gyrus | L | 19 | 485 | −50 | −60 | −10 | 6.6009 |
| Middle Frontal Gyrus | L | 6 | 390 | −24 | 6 | 50 | 6.4317 |
| Middle Frontal Gyrus | L | 46 | 458 | −46 | 34 | 18 | 5.7596 |
| Inferior Frontal Gyrus | L - R | 44 | 231 | −48 | 8 | 24 | 5.7297 |
| Inferior Parietal Lobule | L - R | 40 | 560 | −60 | −32 | 36 | 5.4495 |
| Inferior Frontal Gyrus | L - R | 47 | 107 | 32 | 24 | −8 | 5.3401 |
| Inferior Frontal Gyrus | R | 10 | 222 | 50 | 46 | 0 | 5.2602 |
| Fusiform Gyrus | L | 20 | 58 | −30 | −36 | −20 | 5.0943 |
| Insula | L - R | 13 | 64 | −42 | −2 | −4 | 4.6021 |
| Parahippocampal Gyrus | L | 35 | 26 | −32 | −24 | −24 | 4.3676 |
| Middle Temporal Gyrus | R | 37 | 21 | 54 | −56 | −4 | 3.9002 |
| TR 5 (8–10s after stimulus onset) | |||||||
| Inferior Frontal Gyrus | L - R | 46 | 931 | −46 | 34 | 16 | 8.4174 |
| Middle Frontal Gyrus | L | 6 | 620 | −24 | 6 | 54 | 8.4078 |
| Middle/Superior Occipital Gyrus | L | 19 | 924 | −50 | −62 | −10 | 7.7207 |
| Inferior Frontal Gyrus | L - R | 9 | 497 | −50 | 8 | 26 | 7.6433 |
| Inferior Parietal Lobule | L - R | 40 | 926 | −60 | −30 | 38 | 6.982 |
| Inferior Temporal Gyrus | R | 37 | 361 | 56 | −54 | −6 | 5.7416 |
| Fusiform Gyrus | L - R | 37 | 90 | −30 | −36 | −16 | 4.6959 |
| Angular Gyrus | R | 39 | 20 | 48 | −78 | 30 | 4.5254 |
| Parahippocampal Gyrus | L | 36 | 47 | −32 | −26 | −28 | 4.4103 |
| Insula | L - R | 13 | 53 | −40 | −2 | −6 | 4.3383 |
| Superior Parietal | L | 7 | 22 | −10 | −66 | 54 | 4.0527 |
| Middle Frontal Gyrus | L | 11 | 17 | −34 | 36 | −12 | 3.8869 |
| TR 6 (10–12s after stimulus onset) | |||||||
| Middle Temporal Gyrus | L - R | 37 | 2717 | −51 | −64 | 7 | 9.187 |
| Inferior Parietal | L - R | 40 | 3292 | −57 | −27 | 35 | 8.5406 |
| Inferior Frontal Gyrus | L - R | 45/46 | 995 | −50 | 37 | 7 | 995 |
| Inferior Frontal Gyrus | R | 9 | 388 | 61 | 15 | 27 | 5.9657 |
| Insula | L | 13 | 289 | −42 | −2 | −3 | 5.6149 |
| Superior Parietal | R | 7 | 130 | 12 | −55 | 56 | 4.5479 |
| Parahippocampal Gyrus | L | 19 | 228 | −30 | −43 | −5 | 4.2567 |
| Middle Frontal Gyrus | L - R | 11 | 27 | −32 | 38 | −14 | 3.9525 |
| Table 2b. Regions associated with person versus object-centered counterfactuals (LV 1).
| |||||||
|---|---|---|---|---|---|---|---|
| Region of Activation | Hemisphere | BA | Voxels | MNI Coordinates
|
BSR* | ||
| X | Y | Z | |||||
| TR 3 (4–6s after stimulus onset) | |||||||
| Lingual Gyrus | L | 18 | 964 | −14 | −82 | −12 | −6.156 |
| Cuneus | R | 17 | 574 | 14 | −90 | 6 | −6.0214 |
| Fusiform Gyrus | L | 20 | 70 | −42 | −26 | −16 | −5.8435 |
| Middle Frontal Gyrus | L | 11 | 503 | −20 | 38 | −6 | −5.7647 |
| Caudate | L | 104 | −24 | −20 | 30 | −5.2151 | |
| Cingulate Gyrus | R | 31 | 25 | 20 | −48 | 30 | −5.0058 |
| Anterior Cingulate | R | 32 | 196 | 16 | 30 | −8 | −4.9764 |
| Middle Frontal Gyrus | L | 8 | 158 | −20 | 32 | 44 | −4.8455 |
| Superior Frontal Gyrus | L - R | 9 | 188 | −12 | 50 | 26 | −4.679 |
| Midde Frontal/Precentral Gyrus | L | 6/9 | 42 | −38 | 2 | 50 | −4.1788 |
| Precuneus | L | 7 | 74 | −4 | −58 | 38 | −4.0608 |
| Inferior Frontal Gyrus | R | 11 | 16 | 10 | 40 | −16 | −3.8600 |
| Parahippocampal Gyrus | R | 35 | 14 | 18 | −26 | −16 | −3.6952 |
| TR 4 (6–8s after stimulus onset) | |||||||
| Middle Occipital Gyrus | L | 18 | 1154 | −14 | −90 | 14 | −11.049 |
| Medial Frontal Gyrus | R | 11 | 4814 | 6 | 48 | −12 | −8.3631 |
| Superior Temporal Gyrus | R - L | 39 | 926 | 54 | −56 | 24 | −7.3121 |
| Middle Temporal Gyrus | L | 21 | 719 | −50 | −10 | −16 | −7.0508 |
| Middle Frontal Gyrus | L - R | 8 | 634 | −44 | 10 | 46 | −6.1981 |
| Middle Frontal Gyrus | R | 9 | 253 | 22 | 36 | 42 | −5.3237 |
| Postcentral Gyrus | R – L | 3 | 23 | 30 | −28 | 40 | −5.1331 |
| Insula | L – R | 13 | 26 | −42 | −24 | 26 | −4.9015 |
| Middle Frontal Gyrus | R | 10 | 65 | 34 | 54 | 0 | −4.8875 |
| Cingulate Gyrus | L | 23 | 173 | 0 | −16 | 30 | −4.8625 |
| Hippocampus | R | 24 | 34 | −16 | −18 | −4.5669 | |
| Superior Temporal Gyrus | R | 41 | 14 | 40 | −40 | 6 | −4.5663 |
| Parahippocampal Gyrus | R | 36 | 100 | 44 | −30 | −10 | −4.4147 |
| Parahippocampal Gyrus | R | 30 | 12 | 16 | −42 | 6 | −3.763 |
| TR 5 (8–10s after stimulus onset) | |||||||
| Cuneus | L | 18 | 6133 | −16 | −86 | 12 | −10.449 |
| Middle Frontal Gyrus | R | 8 | 1232 | 22 | 36 | 44 | −8.3357 |
| Medial Frontal Gyrus | L | 11 | 9190 | −6 | 44 | −12 | −8.2585 |
| Supramarginal Gyrus | R | 40 | 3715 | 54 | −54 | 26 | −6.7694 |
| Insula | L | 13 | 1121 | −40 | −24 | 26 | −6.5106 |
| Precentral gyrus | R - L | 6 | 296 | 22 | −18 | 52 | −5.9258 |
| Superior Temporal Gyrus | L – R | 38 | 40 | −38 | 24 | −24 | −5.6552 |
| Postcentral gyrus | R | 3 | 168 | 50 | −16 | 22 | −5.5824 |
| Inferior Frontal Gyrus | R - L | 47 | 36 | 34 | 28 | −22 | −4.8434 |
| Putamen | R | 180 | 24 | 8 | 12 | −5.3571 | |
| Cingulate Gyrus | L | 24 | 34 | −24 | −20 | 46 | −4.1696 |
| Superior Frontal Gyrus | L | 8 | 11 | −28 | 26 | 58 | −3.6629 |
| TR 6 (10–12s after stimulus onset) | |||||||
| Cuneus | R | 17 | 3648 | 14 | −85 | 8 | −6.5017 |
| Posterior Cingulate | L | 31 | 1609 | −4 | −55 | 25 | −6.2999 |
| Anterior Cingulate | L - R | 32 | 666 | −16 | 41 | −4 | −5.6542 |
| Superior Frontal Gyrus | L - R | 8 | 87 | 16 | 45 | 11 | −5.3763 |
| Supramarginal Gyrus | R | 40 | 493 | 57 | −53 | 27 | −4.8681 |
| Superior Temporal Gyrus | L | 39 | 62 | −46 | −57 | 27 | −4.3977 |
| Medial Frontal Gyrus | L | 6 | 27 | −16 | 31 | 35 | −4.0638 |
Note: All activations reported survived a threshold of p < .0002 (BSR = 3.2), with a cluster size > 10. BA = approximate Brodmann area. L = Left; R = Right.
The bootstrap ratio (BSR) is the parameter estimate for that voxel over its standard error. It is proportional to a z score.
3.2.2. Non-rotated PLS analysis
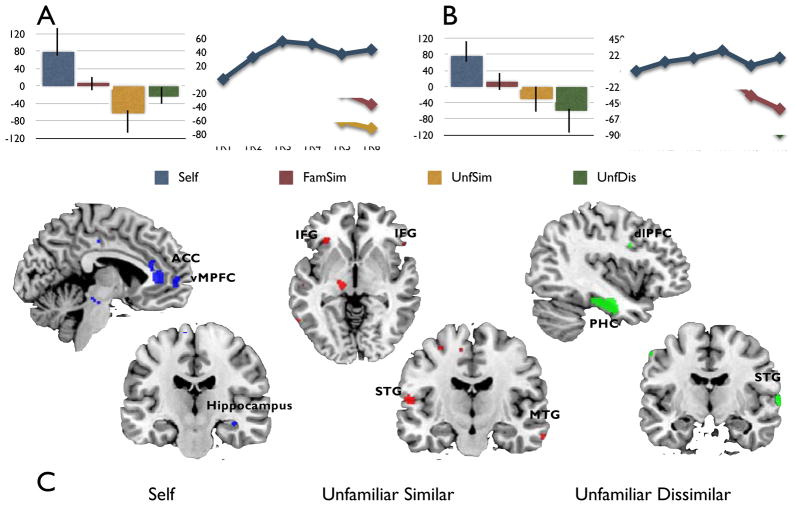
The results of this second analysis revealed that although person-based counterfactual simulations engaged core areas of the brain’s DN, certain regions were preferentially recruited depending on whether the counterfactual involved oneself, an unfamiliar yet similar other, or an unfamiliar and dissimilar other. Specifically, as revealed by the identification of a significant latent variable (LV 2, p < .018, 38.62% crossblock, see Figure 3A) the contrast Self > UnfSim revealed preferential recruitment of ACC (BA 32, BA 24), vmPFC (BA 10), IPL toward the supramarginal gyrus (BA 40) and right hippocampus. In contrast, UnfSim > Self revealed greater involvement of lateral middle and superior temporal gyri (BA 21; BA 22) as well as dorsal and lateral aspects of the MPFC (BA 10, BA 9, see Figure 3C. For a complete list of brain regions associated with LV 2 see tables 3a–b).
Figure 3.
Results from non-rotated PLS analysis: Latent Variables 2 and 3 (LV 2, LV 3). (A) Plot of brain scores with confidence intervals and temporal brain scores for the contrast Self > UnfSim from LV 2 (B) Plot of brain scores with confidence intervals and temporal brain scores for the contrast Self > UnfDis from LV 3. (C) Regions in blue were preferentially associated with Self, those in red were preferentially associated with UnfSim, and those in green were preferentially associated with UnfDis. All regions are shown at a threshold of p < .001.
Table 3.
| Table 3a. Regions associated with counterfactual simulations for self versus unfamiliar similar characters (contrast Self > UnfSim; LV 2).
| |||||||
|---|---|---|---|---|---|---|---|
| Region of Activation | Hemisphere | BA | Voxels | MNI Coordinates
|
BSR* | ||
| X | Y | Z | |||||
| TR 2 (2–4s after stimulus onset) | |||||||
| Thalamus | R | 24 | 6 | −24 | 16 | 5.0434 | |
| Insula | L | 13 | 17 | −34 | −46 | 12 | 5.0022 |
| Cuneus | R | 30 | 24 | 28 | −76 | 4 | 4.8008 |
| Cerebellum | R - L | 12 | 16 | −88 | −30 | 4.1521 | |
| Caudate | L | 11 | −32 | −36 | 4 | 4.0698 | |
| Inferior Frontal Gyrus | R | 47 | 10 | 48 | 44 | −14 | 3.8596 |
| TR 3 (4–6s after stimulus onset) | |||||||
| Caudate | R | 22 | 36 | −18 | −14 | 5.2110 | |
| TR 4 (6–8s after stimulus onset) | |||||||
| Superior Frontal Gyrus | R | 6 | 10 | 12 | −16 | 78 | 4.3748 |
| Anterior Cingulate | R | 32 | 15 | 16 | 38 | 8 | 3.8885 |
| Middle Temporal Gyrus | R | 37 | 11 | 58 | −68 | 2 | 3.8080 |
| TR 5 (8–10s after stimulus onset) | |||||||
| Midde Frontal Gyrus | R | 47 | 90 | 56 | 40 | −2 | 4.6608 |
| Anterior Cingulate | R | 32 | 51 | 16 | 36 | 8 | 4.5010 |
| Middle Temporal Gyrus | R | 21 | 11 | 72 | −24 | −8 | 4.4932 |
| Medial Frontal Gyrus | L | 10 | 134 | −10 | 38 | −4 | 4.1791 |
| Cerebellum | L | 30 | −14 | −40 | −14 | 4.1601 | |
| Anterior Cingulate | L | 10 | 29 | −8 | 52 | 2 | 3.9347 |
| Inferior Parietal/Supramarginal | L | 40 | 65 | −50 | −60 | 34 | 3.8954 |
| Hippocampus | R | 17 | 28 | −14 | −18 | 3.6760 | |
| TR 6 (10–12s after stimulus onset) | |||||||
| Anterior Cingulate | L | 10 | 107 | −12 | 52 | 2 | 5.0406 |
| Anterior Cingulate | L | 24 | 185 | −2 | 36 | 6 | 4.5184 |
| Middle Frontal Gyrus | L | 9 | 11 | −30 | 28 | 36 | 3.9999 |
| Precuneus | L | 19 | 13 | −40 | −78 | 36 | 3.9580 |
| Table 3b. Regions associated with counterfactual simulations for self versus unfamiliar similar characters (contrast UnfSim > Self; LV 2).
| |||||||
|---|---|---|---|---|---|---|---|
| Region of Activation | Hemisphere | BA | Voxels | MNI Coordinates
|
BSR* | ||
| X | Y | Z | |||||
| TR 2 (2–4s after stimulus onset) | |||||||
| Medial Frontal Gyrus | L | 6 | 103 | 0 | 28 | 40 | −5.8197 |
| Precentral Gyrus | L | 43 | 48 | −56 | −12 | 12 | −5.1224 |
| Middle Temporal Gyrus | L | 21 | 16 | −60 | −60 | 0 | −4.9599 |
| Cerebellum | L | 28 | −32 | −74 | −40 | −4.7568 | |
| Inferior Frontal Gyrus | L | 47 | 26 | −30 | 28 | −2 | −4.7047 |
| Middle Occipital Gyrus | L | 19 | 27 | −46 | −80 | 12 | −4.6413 |
| Middle Frontal Gryus | L - R | 10 | 39 | −38 | 38 | 28 | −4.4662 |
| Superior Parietal Gyrus | R | 7 | 12 | 36 | −76 | 46 | −4.2154 |
| Superior Temporal Gyrus | R - L | 38 | 10 | 52 | 16 | −22 | −4.1903 |
| Precuenus | R - L | 7/19 | 23 | 38 | −78 | 36 | −3.9878 |
| Postcentral Gyrus | R | 3 | 10 | 36 | −34 | 48 | −3.8161 |
| TR 3 (4–6s after stimulus onset) | |||||||
| Superior Parietal Lobule | R - L | 7 | 11 | 32 | −80 | 46 | −5.2946 |
| Postcentral Gyrus | R | 2 | 43 | 62 | −26 | 50 | −4.9791 |
| Middle Frontal Gyrus | R | 9 | 15 | 54 | 30 | 34 | −4.1390 |
| Precentral Gyrus | R | 43 | 10 | 50 | −10 | 14 | −3.8734 |
| Middle Frontal Gyrus | L | 10 | 25 | −40 | 44 | 24 | −3.8263 |
| Superior Temporal Gyrus | L | 13 | 12 | −42 | −24 | 8 | −3.7964 |
| Middle Frontal Gyrus | L | 46 | 12 | −42 | 24 | 22 | −3.7338 |
| TR 4 (6–8s after stimulus onset) | |||||||
| Inferior Frontal Gyrus | L | 9 | 47 | −48 | 4 | 22 | −5.9282 |
| Middle Frontal Gyrus | R | 46 | 43 | 50 | 40 | 20 | −5.8548 |
| Middle Frontal Gyrus | L | 10 | 24 | −42 | 56 | 14 | −5.2298 |
| Precentral Gyrus | R | 6 | 17 | 62 | 4 | 30 | −4.9403 |
| Inferior Frontal Gyrus | R | 47 | 23 | 36 | 32 | −10 | −4.9312 |
| Middle Temporal Gyrus | R | 21 | 37 | 66 | −2 | −20 | −4.3198 |
| Cerebellum | L - R | 17 | −10 | −62 | −46 | −4.2825 | |
| Superior Frontal Gyrus | L | 10 | 16 | −38 | 48 | 28 | −4.1949 |
| Superior Temporal Gyrus | L | 22 | 42 | −50 | −18 | 2 | −3.9873 |
| Superior Frontal Gyrus | R | 8 | 13 | 8 | 28 | 52 | −3.7728 |
| Superior Parietal Lobule | R | 7 | 40 | 14 | −70 | 56 | −3.7318 |
| TR 5 (8–10s after stimulus onset) | |||||||
| Insula | R - L | 13 | 113 | 42 | −2 | 18 | −5.4646 |
| Postcentral Gyrus | L | 3 | 134 | −20 | −26 | 50 | −4.9002 |
| Precentral Gyrus | L - R | 6 | 62 | −20 | −12 | 58 | −4.3559 |
| Middle Frontal Gyrus | L | 10 | 17 | 40 | 60 | 12 | −4.3218 |
| Fusiform Gyrus | L | 20 | 10 | −38 | −10 | −28 | −4.2720 |
| Postcentral Gyrus | R | 43 | 84 | 62 | −10 | 18 | −4.2109 |
| Inferior Frontal Gyrus | R | 45 | 46 | 46 | 12 | 18 | −4.1887 |
| Medial Frontal Gyrus | R | 8 | 16 | 6 | 28 | 48 | −3.9781 |
| TR 6 (10–12s after stimulus onset) | |||||||
| Inferior Frontal Gyrus | R | 45 | 639 | 58 | 24 | 14 | −6.9500 |
| Fusiform Gyrus | R - L | 20 | 10 | 38 | −14 | −32 | −6.3246 |
| Middle Frontal Gyrus | R | 10 | 17 | 40 | 58 | 14 | −5.0839 |
| Medial Frontal Gyrus | L | 32 | 42 | −12 | 12 | 48 | −4.8589 |
| Postcentral Gyrus | L | 1 | 49 | −56 | −20 | 48 | −4.7748 |
| Insula | L - R | 13 | 116 | −38 | −28 | 2 | −4.7426 |
| Precentral Gyrus | R | 4 | 50 | 62 | −14 | 32 | −4.7294 |
| Superior Temporal Gyrus | L | 41/42 | 87 | −36 | −34 | 16 | −4.6962 |
| Middle Frontal Gyrus | R | 9 | 66 | 58 | 18 | 36 | −4.3255 |
| Superior Frontal Gyrus | R | 8 | 17 | 6 | 30 | 52 | −4.2335 |
| Middle Occipital Gyrus | R | 18 | 46 | 26 | −82 | −10 | −4.2309 |
| Precentral Gyrus | R | 6 | 11 | 24 | −16 | 52 | −4.2296 |
| Superior Temporal Gyrus | R - L | 22 | 37 | 58 | −2 | −2 | −4.2217 |
| Precuneus | L | 7 | 36 | 0 | −70 | 38 | −3.9491 |
| Postcentral Gyrus | L | 40 | 65 | −30 | −40 | 60 | −3.9175 |
Note: All activations reported survived a threshold of p < .0002 (BSR = 3.2), with a cluster size > 10. BA = approximate Brodmann area. L = Left; R = Right.
The bootstrap ratio (BSR) is the parameter estimate for that voxel over its standard error. It is proportional to a z score.
The contrast Self > UnfDis also showed preferential recruitment of ACC (BA 32; BA 24), vMPFC (BA 10), IPL (BA 40) and right hippocampus, as revealed by the identification of a second significant latent variable (LV 3, p <.028, 35.74% crossblock, see Figure 3B). In contrast, UnfDis > Self revealed greater involvement of lateral temporal cortices (BA 20; BA 21; BA 22) as well as dorso-lateral MPFC, both right (BA 9) and left (BA 9). This contrast also revealed greater involvement of bilateral fusiform (BA 20) and parahippocampal gyri (BA 36; see Figure 3C. For a complete list of brain regions associated with LV 3 see tables 4a–b). Finally, there were no significant results for the contrast Self versus FamSim.
Table 4.
| Table 4a. Regions associated with counterfactual simulations for self versus unfamiliar dissimilar characters (contrast Self > UnfDis; LV 3).
| |||||||
|---|---|---|---|---|---|---|---|
| Region of Activation | Hemisphere | BA | Voxels | MNI Coordinates
|
BSR* | ||
| X | Y | Z | |||||
| TR 2 (2–4s after stimulus onset) | |||||||
| Cerebellum | R - L | 27 | 12 | 12 | −88 | 5.0691 | |
| Middle Frontal Gyrus | L | 10 | 12 | −38 | 64 | 4 | 4.9039 |
| Caudate | L | 27 | −20 | −20 | 28 | 4.3201 | |
| Posterior Cingulate | R | 31 | 23 | 26 | −66 | 18 | 4.3064 |
| TR 3 (4–6s after stimulus onset) | |||||||
| Cerebellum | L - R | 143 | −40 | −78 | −40 | 6.0486 | |
| Hippocampus | R | 11 | 32 | −44 | 4 | 3.9246 | |
| Cingulate Gyrus | R | 31 | 10 | 16 | −42 | 44 | 3.7024 |
| TR 4 (6–8s after stimulus onset) | |||||||
| Cerebellum | L | 28 | −40 | −76 | −26 | 4.6130 | |
| TR 5 (8–10s after stimulus onset) | |||||||
| Medial Frontal Gyrus | L - R | 10 | 149 | −10 | 38 | −6 | 5.1137 |
| Hippocampus | R | 31 | 34 | −46 | 2 | 5.0039 | |
| Cerebellum | L | 12 | −44 | −54 | −50 | 4.5965 | |
| Anterior Cingulate | R | 24/32 | 46 | 2 | 30 | 10 | 4.4893 |
| Superior Frontal Gyrus | L | 6 | 10 | −10 | 22 | 66 | 4.1087 |
| Inferior Parietal Lobule | R | 40 | 30 | 54 | −62 | 40 | 3.9198 |
| TR 6 (10–12s after stimulus onset) | |||||||
| Medial Frontal Gyrus | L | 10 | 128 | −12 | 52 | −2 | 4.5654 |
| Anterior Cingulate | R - L | 32 | 64 | 6 | 48 | −2 | 4.1537 |
| Inferior Parietal Lobule | L | 40 | 17 | −50 | −62 | 46 | 4.1055 |
| Table 4b. Regions associated with counterfactual simulations for unfamiliar dissimilar characters versus self (contrast UnfDis > Self; LV 3).
| |||||||
|---|---|---|---|---|---|---|---|
| Region of Activation | Hemisphere | BA | Voxels | MNI Coordinates
|
BSR* | ||
| X | Y | Z | |||||
| TR 2 (2–4s after stimulus onset) | |||||||
| Cingulate Gyrus | L | 24 | 13 | −14 | 0 | 34 | −4.7253 |
| Precentral Gyrus | R | 4 | 16 | 34 | −16 | 40 | −4.3838 |
| Insula | L | 13 | 29 | −32 | 10 | 16 | −4.2240 |
| Middle Occipital Gyrus | R | 19 | 12 | 50 | −60 | −10 | −4.0880 |
| Inferior Temporal Gyrus | R | 20 | 20 | 64 | −14 | −24 | −4.0879 |
| Postcentral Gyrus | R | 40 | 22 | 66 | −20 | 14 | −4.0552 |
| TR 3 (4–6s after stimulus onset) | |||||||
| Superior Temporal Gyrus | R | 22 | 47 | 68 | −12 | 2 | −4.8699 |
| Fusiform Gyrus | L | 20 | 16 | −40 | −38 | −18 | −4.3375 |
| Middle Temporal Gyrus | L | 39 | 29 | −36 | −76 | 26 | −4.1297 |
| TR 4 (6–8s after stimulus onset) | |||||||
| Middle Temporal Gyrus | R | 21 | 39 | 70 | −4 | −22 | −5.2524 |
| Parahippocampal Gyrus | R | 36 | 18 | 38 | −32 | −26 | −4.9821 |
| Parahippocampal Gyrus | L | 28 | 16 | −18 | −16 | −16 | −4.0265 |
| Middle Frontal Gyrus | L | 9 | 14 | −26 | 36 | 40 | −3.9633 |
| TR 5 (8–10s after stimulus onset) | |||||||
| Parahippocampal Gyrus | L | 36 | 198 | −42 | −22 | −24 | −5.8533 |
| Inferior Frontal Gyrus | R - L | 45/46 | 28 | 52 | 22 | 14 | −4.5282 |
| Inferior Frontal Gyrus | L | 47 | 38 | −34 | 28 | −22 | −4.5136 |
| Inferior Frontal Gyrus | R | 9 | 33 | 48 | 2 | 22 | −4.3926 |
| Middle Frontal Gyrus | L | 6 | 23 | −24 | −18 | 66 | −4.2517 |
| Middle Temporal Gyrus | R | 21 | 11 | 50 | −20 | −22 | −3.8416 |
| Superior Temporal Gyrus | R | 22 | 11 | 48 | −14 | −2 | −3.7562 |
| TR 6 (10–12s after stimulus onset) | |||||||
| Fusiform Gyrus | L - R | 20 | 26 | −44 | −8 | −22 | −6.1059 |
| Inferior Frontal Gyrus | L | 9 | 60 | −56 | 16 | 28 | −5.2990 |
| Inferior Frontal Gyrus | R | 45 | 296 | 54 | 24 | 16 | −5.0419 |
| Inferior Occipital Gyrus | L | 18 | 35 | −40 | −92 | −8 | −5.0395 |
| Middle Frontal Gyrus | R | 11 | 34 | 38 | 42 | −14 | −4.6795 |
| Postcentral Gyrus | R | 43 | 56 | 56 | −18 | 16 | −4.4379 |
| Precuneus | L | 7 | 34 | −8 | −56 | 40 | −4.4024 |
| Insula | R | 13 | 87 | 44 | −10 | 0 | −4.1583 |
| Inferior Frontal Gyrus | L - R | 47/46 | 48 | −34 | 32 | 0 | −4.1374 |
| Middle Occipital Gyrus | L | 37 | 21 | −52 | −68 | −12 | −3.9145 |
| Middle Temporal Gyrus | R | 21 | 27 | 50 | −14 | −20 | −3.8270 |
Note: All activations reported survived a threshold of p < .0002 (BSR = 3.2), with a cluster size > 10. BA = approximate Brodmann area. L = Left; R = Right.
The bootstrap ratio (BSR) is the parameter estimate for that voxel over its standard error. It is proportional to a z score.
3.2.3. Seed PLS analysis
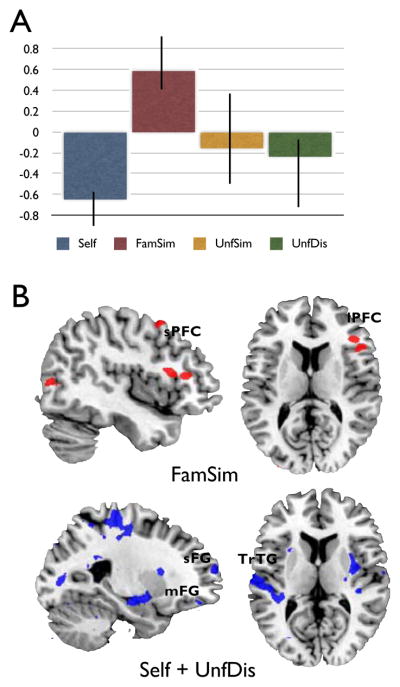
This analysis resulted in two differentiated patterns of task-related functional connectivity between the right hippocampal seed and correlated brain regions, as revealed by the identification of LV 4 (p < .034, 40.28% crossblock, see Figure 4A). One pattern of functional connectivity, identified only for the FamSim condition, revealed a significant correlation between the hippocampal seed and lateral temporal gyrus (BA 21/22), bilateral superior frontal gyrus (BA 8), right inferior frontal gurys (BA 46), left IPL (BA 40), and bilateral lingual gyrus (BA 18/19). A second pattern of functional connectivity, associated with the Self and the UnfDis conditions, revealed a significant correlation between the right hippocampal seed and left transverse temporal gyrus (BA 41), ventral aspects of the superior (BA 10) and medial frontal gyrus (BA 6), and bilateral inferior and middle temporal gyri (BA 19/37; BA 21), among other regions (see Figure 4B. For a complete list of brain regions associated with LV 4 see tables 5a-b).
Figure 4.
Results from seed PLS analysis: Latent Variable 3 (LV 4). (A) Plot of brain scores with confidence intervals. (B) Regions with negative saliences (blue) co-vary with the hippocampal seed during the Self and UnfDis conditions. Regions with positive saliences (red) co-vary with the hippocampal seed during the FamSim condition.
Table 5.
| Table 5a .Peak regions functionally connected with a right hippocampal seed (y = −16) during counterfactual simulation involving a self and unfamiliar dissimilar characters versus familiar similar (LV 4).
| |||||||
|---|---|---|---|---|---|---|---|
| Region of Activation | Hemisphere | BA | Voxels | MNI Coordinates
|
BSR* | ||
| X | Y | Z | |||||
| Transverse Temporal Gyrus | L | 41 | 1935 | −36 | −34 | 11 | −7.5046 |
| Lingual Gyrus | R | 261 | 32 | −73 | 7 | −6.8901 | |
| Claustrum | R | 1641 | 34 | 6 | 7 | −6.4067 | |
| Superior Frontal Gyrus | L | 10 | 93 | −30 | 59 | 14 | −6.2620 |
| Medial Frontal Gyrus | R | 6 | 3280 | 4 | −24 | 64 | −6.1991 |
| Inferior Temporal Gyrus | R - L | 19/37 | 327 | 55 | −70 | −2 | −5.8427 |
| Cerebellum | L | 39 | −12 | −91 | −26 | −5.7620 | |
| Cuneus | L | 30 | 104 | −26 | −75 | 7 | −5.5897 |
| Inferior Parietal Lobule | L | 40 | 127 | −53 | −30 | 31 | −5.217 |
| Precuneus | R - L | 7/19 | 133 | 16 | −48 | 50 | −5.1355 |
| Thalamus | L | 22 | −18 | −28 | 16 | −5.0887 | |
| Postcentral Gyrus | R - L | 2/3 | 352 | 44 | −24 | 27 | −4.9190 |
| Middle Temporal Gyrus | R | 21 | 19 | 67 | −16 | −4 | −4.3742 |
| Anterior Cingulate | R | 33 | 51 | 6 | 9 | 20 | −4.3301 |
| Middle Frontal Gyrus | L | 11 | 48 | −30 | 42 | −12 | −4.1377 |
| Table 5b. Peak regions functionally connected with a right hippocampal seed (y = −16) during counterfactual simulations involving familiar similar characters versus self and unfamiliar dissimilar characters (LV 4).
| |||||||
|---|---|---|---|---|---|---|---|
| Region of Activation | Hemisphere | BA | Voxels | MNI Coordinates
|
BSR* | ||
| X | Y | Z | |||||
| Superior Frontal Gyrus | 44 | 18 | 51 | 4.3811 | |||
| Inferior Parietal Lobule | L | 40 | 19 | −59 | −44 | 45 | 4.1423 |
| Inferior Frontal Gyrus | R | 46 | 28 | 44 | 30 | 10 | 3.9195 |
| Middle Temporal Gyrus | R | 22 | 10 | −67 | −46 | 6 | 3.7095 |
| Cerebellum | L | 11 | −24 | −28 | −19 | 3.6580 | |
| Lingual Gyrus | R - L | 18/19 | 10 | 18 | −72 | 0 | 3.5915 |
Note: All activations reported survived a threshold of p < .0002 (BSR = 3.2), with a cluster size > 10. BA = approximate Brodmann area. L = Left; R = Right.
The bootstrap ratio (BSR) is the parameter estimate for that voxel over its standard error. It is proportional to a z score.).
4. Discussion
Counterfactual thinking is a critical psychological capacity that enables us to simulate alternative ways things could have been by flexibly manipulating stored knowledge (see footnote 2 above). Here we examined the neural basis of self, other and object-based counterfactual thinking. First, we observed that there are different patterns of brain activation during person-based (whether involving oneself or other people) relative to object-based counterfactual simulations. Moreover, this analysis showed that person-based counterfactual simulations engaged all of the core regions associated with the DN (Buckner et al., 2008), whereas object-based counterfactual simulations only recruited lateral aspects of two such regions (i.e., IPL and iFG).
These results add to a growing body of evidence suggesting that ordinary occurrences of self-generated thoughts, of which counterfactual simulations form a large subset (Roese & Olson, 1995; Epstude & Roese, 2008; Markman, Klein, Suhr, 2009), are supported by the activity of the brain’s DN (Andrews-Hanna, 2012, Andrews-Hanna, Smallwood, & Spreng, 2014). However, our results also help to qualify this hypothesis by showing that not all self-generated counterfactual thoughts engage the DN to the same degree, as core regions of the DN were only associated with the generation of counterfactual thoughts involving people rather than objects. This difference may be due to the fact that DN activity has been primarily associated with personally and/or socially relevant self-generated thoughts (Andrews-Hanna, Smallwood, & Spreng, 2014). Thinking about alternative forms for inanimate objects does not have the same kind of personal and/or social relevance as thoughts about alternative ways in which person-based events could have occurred.
A related hypothesis, put forth by Buckner and Carroll (2007), suggests that the brain’s DN is preferentially recruited during cognitive tasks that require self-projection. However, the results of our first analysis speak against this hypothesis, as all core areas of the DN were recruited during mental simulations that did not require projecting oneself but rather projecting others onto counterfactual scenarios. This claim is also consistent with recent studies showing common recruitment of core regions of the brain’s DN during counterfactual and theory of mind tasks that are other-rather than self-centered (Van Hoeck et al., 2014). Nonetheless, it is important to acknowledge that although our experimental design tried to keep constant the autobiographical component of the simulations, by asking participants to imagine alternative ways in which situations could have unfolded during events for which participants had autobiographical knowledge, it is possible that the use of autobiographical information to construct a mental simulation is sufficient to engage the DN.
Reduced activation of DN regions during object-versus person-based counterfactual simulations is consistent with findings in sentence-comprehension tasks involving counterfactual statements, which tend to recruit processes outside of DN (Nieuwland, 2012). Interestingly, Kulakova et al (2013) found involvement of one core DN region (right cuneus) with an activation peak that was almost identical to our finding in LV1 for the person-based > object-based contrast. In their study, Kulakova and collaborators had participants semantically evaluate hypothetical and counterfactual sentences presented either visually or aurally. They found that independent of the modality of presentation, right cuneus was more active during sentence comprehension of counterfactual relative to hypothetical statements. Although they admonish not to rule out the possibility that such activation may simply reflect linguistic processing, Kulakova et al. do suggest that the activation in cuneus may be related to scene construction that could have occurred during sentence comprehension (referencing Hassabis et al, 2007). This interpretation is also consistent with our findings, as object-based simulations actively precluded scene construction, while person-based counterfactual simulations were likely to require the construction and maintenance of complex visual scenes.
Second, we examined whether there are significant differences in the recruitment of DN regions during self-relative to other-based counterfactual thoughts. Since a number of previous results suggested such differential recruitment (Denny et al, 2012; Wagner, Haxby, & Heatherton, 2012), we hypothesized that different patterns of brain activation within the DN would emerge depending on whether the counterfactual simulation involved a familiar and/or a dissimilar character. This hypothesis was confirmed when we contrasted self-based against other-based counterfactual simulations.
A region that showed preferential recruitment during self-based as opposed to other-based counterfactual simulation was rostral ACC. This result replicates those obtained by Krienen, Tu, and Buckner (2010), who found activity in the rACC to be reliably greater for simulations involving oneself relative to strangers, even when the strangers were perceived as being similar by the subject. It is important to note that ACC has been previously associated with feelings of regret, which normally accompany upward counterfactuals (i.e., imagining better outcomes for past decisions or events). Since we employed upward counterfactuals in the current study, it is possible that at least part of this increased activation in rACC is accounted for by the regret producing nature of the counterfactual simulation. Although this is certainly a possibility, Canessa et al (2009) compared brain activation between self-based and other-based counterfactual simulations using a regret-producing task and found equal engagement of rACC between conditions. This finding suggests that the increase in rACC activity found in the current study during self-based relative to other-based counterfactual simulation cannot be fully accounted by regret. However, further research is needed to determine the extent to which this increase in rACC activity is due to the self-referential nature of the counterfactual simulation above and beyond regret.
Anterior right hippocampus was also recruited during self relative to other-based counterfactual simulations. This result dovetails with recent evidence associating hippocampal activation with the construction of mental simulations involving self-projection on to imagined scenarios in the possible future (Addis, Wong, & Schacter, 2007; Gaesser et al., 2013; Hassabis et al., 2007; Addis & Schacter, 2012; Schacter et al., 2012) as well as actual (Squire, 1992; Tulving, 1985) and possible pasts (Addis et al, 2009; De Brigard et al., 2013; De Brigard & Giovanello, 2012; Van Hoeck et al., 2013).
We next examined differential recruitment of DN regions as a function of how similar and/or familiar participant’s perceived the simulated characters to be (Mitchell, Macrae, and Banaji, 2006; Krienen, Tu, and Buckner, 2010). Recruitment of the MPFC is modulated by the participant’s familiarity with the character featured in their counterfactual simulations. As noted, self-based counterfactual simulations preferentially recruited the ventral aspect of the MPFC, a region that has been consistently reported as showing greater involvement during internally-generated simulations that are self-referential (D’Argembeau at al., 2007; Denny et al., 2012; Wagner, Haxby, & Heatherton, 2012). In contrast, lateral and dorsal aspects of the MPFC were preferentially recruited during mental simulations of counterfactual thoughts involving unfamiliar characters regardless of their perceived similarity. These results are consistent with a recent proposal by Krienen and collaborators (2010) according to which regions of the PFC along the midline are sensitive to mental simulations involving characters that are perceived as personally relevant and socially close rather than merely similar to oneself.
Unlike self-based counterfactual simulations, those involving unfamiliar characters preferentially recruited lateral aspects of the superior temporal gyrus. This result is consistent with the suggestion that lateral regions of the superior temporal gyrus may enable the retrieval of semantic and conceptual knowledge during the construction of self-generated mental simulations (Andrews-Hanna, Smallwood, and Spreng, 2014; Spreng & Grady, 2010). Given the lack of episodic information about unfamiliar characters—regardless of the degree of perceived similarity—participants may have latched onto general and stereotypical semantic and conceptual information about the simulated characters in order to generate their counterfactual simulations. This view agrees with the recent semantic scaffolding hypothesis, according to which information from semantic memory facilitates the construction of mental simulations by providing a conceptual scaffold or structure into which to integrate further episodic details (Greenberg & Verfaellie, 2010; Irish et al, 2012; for a related proposal see Ranganath & Ritchey, 2012). By contrast, self-based counterfactual simulations may comparatively require less semantic scaffolding, as the main components of such mental simulations are primarily provided by episodic memory (i.e., the constructive episodic simulation hypothesis; Addis, Wong, & Schacter, 2007; Schacter, Addis, & Buckner, 2007; Schacter & Addis, 2007). This view finds stronger support in recent results showing strong interdependence between the hippocampus and the ventral aspect of the MPFC during simulations involving oneself and close others, but not so with individuals that are not perceived as close, similar or familiar (Muscatell, Addis, & Kensinger, 2010; Perry, Hendler, & Shamay-Tsoory, 2011).
At this point, it is important to acknowledge a potential challenge with the interpretation of the current results. Given our interest in investigating whether or not the relatively greater involvement of DN during likely relative to unlikely episodic counterfactual simulations may be due to the fact that likely as opposed to unlikely counterfactuals are perceived as more socially and personally relevant by the subject, we purposefully designed the current experiment so that participants would only construct counterfactual simulations they considered likely. To that extent, we succeeded, as participants “could” ratings, which presumably tapped at their subjective assessment of perceived likelihood, were on average above 4 (1 = “No”; 5 = “Yes”), and no participant rated his or her simulations below 3. However, as our behavioral results indicate, “could” ratings for self-based simulations were slightly higher than for other-based, and these in turn were higher than for object-based counterfactual simulations. As such, it remains a possibility that the initial finding by De Brigard et al (2013), showing greater involvement of DN for likely relative to unlikely episodic counterfactual thoughts, actually reflects a difference in participants’ subjective assessments of comparative likelihood among counterfactual thoughts (i.e., possible event A is more/less likely than possible event B) rather than a categorical judgment sharply dividing counterfactuals into likely versus unlikely. Since the current study cannot rule out that interpretation, it may be possible that al last some of the variance in the current results can be accounted for by a difference in subjective assessments of comparative likelihood for self-, other- and object-based counterfactual simulations. A future study directly comparing self-, other- and object-based likely versus unlikely counterfactual simulations should be able to resolve this potential confound.5
Finally, to further understand the role of the hippocampus and its relation to other regions of the DN during self-relative to other-based counterfactual simulation, a functional connectivity analysis revealed that the right hippocampal seed was functionally coupled with ventrolateral PFC, lateral temporal gyrus and lingual gyrus during counterfactual simulations involving familiar similar characters. The fact that this functional coupling occurred for familiar similar as opposed to self-based counterfactual simulations is consistent with recent evidence from Rabin and Rosenbaum (2012) showing involvement in the areas during theory of mind tasks involving familiar characters relative to autobiographical recollection. Perry et al. (2011) also showed functional coupling between hippocampus and MPFC during autobiographical and theory-of-mind processes involving familiar others. These findings have been interpreted as suggesting that episodic memory details are recruited during simulations involving close similar others to a greater extent than simulations involving those we do not know or with whom we do not share personality traits. Our activation patterns are consistent with this observation. However, differential patterns of functional connectivity convey a different story for the Self and UnfDis conditions. This pattern revealed functional coupling between the hippocampal seed and a number of regions, including superior frontal (BA 10) and middle frontal gyrus (BA 11). The extent to which this functional coupling may be driven by the Self rather than the UnfDis condition is unclear. Different functional connectivity profiles between the hippocampus and prefrontal and lateral temporal areas depend on whether the simulation involves familiar similar or self and unfamiliar dissimilar others. Further research is needed to understand the way in which the hippocampus may contribute to the generation of mental simulations of counterfactual past and possible future events from episodic and semantic details stored in memory (for discussion, see Schacter et al, in press).
Taken together, the results of the analyses pertaining to person-based counterfactual simulations dovetail with a recent proposal put forth by Andrews-Hanna and collaborators (Andrews-Hanna et al., 2010; Andrews-Hanna, Smallwood, & Spreng, 2014) according to which there are different identifiable subsystems within the DN. One such subsystem, the medial temporal subsystem, is preferentially active during internally-generated mental simulations involving self-referential and autobiographical components, such as self-based counterfactual thoughts. But there is another subsystem, the dorsal medial subsystem, which tends to be recruited during internally-generated mental simulations constructed out of narratives involving general and stereotypical social knowledge, among which one could classify mental simulations of counterfactual events involving unfamiliar others. The differential recruitment of these two subsystems during the generation of person-based counterfactual simulations may help explain the effect in counterfactual mutation found in our behavioral results, as well as those reported by Girotto et al (2007) and Pighin et al, (2011), where participants mutated different aspects of a decision depending on whether they were actors or readers of the situation. That is, mental simulations generated to evaluate personal counterfactuals may preferentially recruit autobiographical details from episodic memory whereas those generated to evaluate counterfactuals featuring unfamiliar characters may preferentially recruit stereotypical social knowledge from semantic memory.
Finally, although the focus of the current study was to explore differences in brain activation when entertaining counterfactuals about objects and people we are differently related to, we also found intriguing differences in three modal judgments (i.e., could, would, and should) across all counterfactual conditions. Given previously reported results showing behavioral (De Brigard, Szpunar & Schacter, 2013; Szpunar & Schacter, 2013) and brain differences (Weiler et al, 2010; De Brigard et al, 2013) in perceived likelihood between episodic future and counterfactual thinking, it is worth exploring the extent to which perceived likelihood influences modal judgments on counterfactual simulations. Similarly, we believe that exploring ways in which other factors, such as desirability or vividness, affect our modal judgments on different person-based counterfactual simulations is a fruitful and important avenue for future research. After all, the results reported here strongly suggest that the kinds of hypothetical simulations upon which modal judgments are based are complex, and that they draw on different brain systems depending on the contents of the simulation. Considering how often people’s actions are judged on the basis of whether we think they could or should have done otherwise, and how frequently such judgments carry profound legal and moral implications, understanding the precise cognitive mechanisms underlying modal judgments during counterfactual simulations remains an issue of upmost importance for future research.
Supplementary Material
Acknowledgments
Thanks to Konstantina Psomopoulou and Greg Stewart for their help coding the interviews. We also thank Randy Buckner and Talia Konkle for helpful suggestions. This research was supported by a grant from the National Institute of Mental Health (NIMH MH060941) awarded to D.L.S.
Footnotes
The notion of ‘simulation’ has been traditionally employed as an alternative to the so-called “‘theory’-theory” in the literature on mentalizing. However, nowadays the term has acquired a wider scope, becoming essentially a shorthand to refer to the cognitive process of generating coherent imaginations involving scenes (for discussion see, Schacter, Addis, & Buckner, 2008). In a recent comprehensive volume on mental simulation, and in line with this more general definition, Markman, Klein and Suhr (2008) defined ‘simulation’ simply as “the act of imagination and generation of alternative realities” (p. vii). Our use of ‘simulation’ is consistent with this broader definition. We thank an anonymous reviewer for inviting us to clarify this issue.
Although related, the expression “counterfactual” as it is used in psychology does not square precisely with the way in which the notion of “counterfactual” is used in philosophy and linguistics. Philosophers and linguists tend to be interested in the semantics of counterfactual statements; that is, they seek to understand how to assign truth values to conditional statements whose antecedents are false by virtue of referring to (or, less controversially, expressing) events that are contrary-to-fact. Psychologists, on the other hand, understand “counterfactual” as a psychological term, employed in reference to the cognitive process of thinking about alternative ways in which a thought-to-be-true fact could have occurred differently. As such, it is possible for a counterfactual thought, understood psychologically, to be semantically factual. If I think “Had I left the door open, the dog wouldn’t have left”, because I wrongly believe that I closed the door, I am entertaining a counterfactual thought that may not qualify as a counterfactual, in the semantic sense, because the antecedent could very well be true, namely if I did, in fact, leave the door open. Moreover, early canonical uses of the term “counterfactual simulation” restricted its use to imagined alternative ways in which past events could have occurred (Kahneman and Miller, 1986; Roese, 1997; McMullen, 1997). Now, though, psychologists tend to use the term “counterfactual simulation” in a more encompassing way, referring to the process of actively constructing and maintaining a mental image or scene in which one or several known facts are altered. Our use of the term “counterfactual simulation” is consistent with this latter construal, although we are sensitive to the fact that, semantically, counterfactual simulations may best be called hypothetical (De Brigard, 2014). We thank an anonymous reviewer for inviting us to clarify this issue.
This step—which is tantamount to the use of a spherical ROI in SPM—centers in the peak voxel and selects a cube around all of the voxels in its neighborhood, i.e., all of the voxels directly adjacent to the peak voxel.
Since the post-scan interview took about one hour after an already long scanning session, many participants opted out, leaving only 17 completed interviews.
We thank an anonymous reviewer for bringing this issue to our attention.
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. The Hippocampus and imagining the future: Where do we stand? Front Hum Neurosci. 2012;5:Article 173. doi: 10.3389/fnhum.2011.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Knapp K, Roberts RP, Schacter DL. Routes to the past: Neural substrates of direct and generative autobiographical memory retrieval. NeuroImage. 2012;59:2908–2922. doi: 10.1016/j.neuroimage.2011.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann NY Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atance CM, O’Neill DK. Episodic future thinking. Trends Cogn Sci. 2001;5:533–539. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self projection and the brain. Trends Cogn Sci. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burianova H, McIntosh AR, Grady CL. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. NeuroImage. 2010;49(1):865–874. doi: 10.1016/j.neuroimage.2009.08.066. [DOI] [PubMed] [Google Scholar]
- Canessa N, Motterlini M, Di Dio C, Perani D, Scifo P, Cappa SF, Rizzolatti G. Understanding Other’s Regret: A fMRI Study. Plos One. 2009;4(10):e7402. doi: 10.1371/journal.pone.0007402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart M. The MRC Psycholinguistic Database. Q J Exp Psychol. 1981;33A:497–505. [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107(2):261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective-taking. J Cogn Neurosci. 2007;19:935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- De Brigard F. Is memory for remembering? Recollection as a form of episodic hypothetical thinking. Synthese. 2014;191(2):155–185. [Google Scholar]
- De Brigard F, Addis D, Ford JH, Schacter DL, Giovanello KS. Remembering what could have happened: Neural correlates of episodic counterfactual thinking. Neuropsychologia. 2013;51(12):2401–2414. doi: 10.1016/j.neuropsychologia.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brigard F, Szpunar KK, Schacter DL. Coming to grips with reality: Effect of repeated simulation on the perceived plausibility of episodic counterfactual thoughts. Psychol Sci. 2013;24(7):1329–1334. doi: 10.1177/0956797612468163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brigard F, Giovanello KS. Influence of outcome valence in the subjective experience of episodic past, future and counterfactual thinking. Conscious Cogn. 2012;21(3):1085–1096. doi: 10.1016/j.concog.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Denny B, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. 2012;24(8):1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstude K, Roese N. The functional theory of counterfactual thinking. Personal Soc Psychol Rev. 2008;12:168–192. doi: 10.1177/1088868308316091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser B, Spreng RN, McLelland VC, Addis DR, Schacter DL. Imagining the future: Evidence for a hippocampal contribution to constructive processing. Hippocampus. 2013;23:1150–1161. doi: 10.1002/hipo.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Gilmore AW, Schacter DL. Solving future problems: Default network and executive activity associated with goal-directed mental simulations. NeuroImage. 2011;55:1816–1824. doi: 10.1016/j.neuroimage.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotto V, Ferrante D, Pighin S, Gonzalez M. Post-decisional counterfactual thinking by actors and readers. Psychol Sci. 2007;18:510–515. doi: 10.1111/j.1467-9280.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- Grady C, Protzner A, Kovacevic N, Strother S, Afshin-Pour B, et al. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20:1432–47. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DL, Verfaellie M. Interdependence of episodic and semantic memory: evidence from neuropsychology. J Int Neuropsychol Soc. 2010;16:748–753. doi: 10.1017/S1355617710000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann DS, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci USA. 2007;104:1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. J Neurosci. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Spreng RN, Rusu AA, Robbins CA, Mar RA, Schacter DL. Imagine all the people: How the brain creates and uses personality models to predict behavior. Cereb Cortex. 2014;24:1979–1987. doi: 10.1093/cercor/bht042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M, Addis DR, Hodges JR, Piguet O. Considering the role of semantic memory in episodic future thinking: evidence from semantic dementia. Brain. 2012;135:2178–2191. doi: 10.1093/brain/aws119. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Sherman SJ. Handbook of motivation and cognition: Foundations of social behavior. Vol. 2. Guilford Press; New York: 1990. Constructing and reconstructing the past and the future in the present; pp. 482–526. [Google Scholar]
- Kahneman D, Miller DT. Norm theory: Comparing reality to its alternatives. Psych Rev. 1986;93(2):136–153. [Google Scholar]
- Krienen FM, Tu PC, Buckner RL. Clan mentality: evidence that medial prefrontal cortex responds to close others. J Neurosci. 2010;30(41):13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Williams L, McIntosh A, Abdi H. Partial least squares (PLS) methods for neuroimaging: a tutorial and review. NeuroImage. 2010;56:455–75. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Kulakova E, Aichhorn M, Schurz M, Kronbichler M, Perner J. Processing counterfactual and hypothetical conditionals: An fMRI investigation. NeuroImage. 2013;72:265–271. doi: 10.1016/j.neuroimage.2013.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O’Keefe J. Knowing where and getting there: a human naviation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Markman KD, McMullen MN. A reflection and evaluation model of comparative thinking. Pers Soc Psychol Rev. 2003;7:244–267. doi: 10.1207/S15327957PSPR0703_04. [DOI] [PubMed] [Google Scholar]
- Markman KD, Klein WMP, Suhr JA. Handbook of Imagination and Mental Simulation. NY: Psychology Press; 2009. [Google Scholar]
- Martin VC, Schacter DL, Corballis MC, Addis DR. A role for the hippocampus in encoding future simulations. Proc Natl Acad Sci USA. 2011;108:13858–13863. doi: 10.1073/pnas.1105816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using Partial Least Squares. NeuroImage. 1996;3(3):143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh A, Chau W, Protzner A. Spatiotemporal analysis of event-related fMRI data using partial least squares. NeuroImage. 2004;23:764–75. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- McIntosh A, Lobaugh N. Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage. 2004;23:S250–63. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McIntosh A, Misic B. Multivariate Statistical Analyses for Neuroimaging Data. Annual Review of Psychology. 2013;64:499–525. doi: 10.1146/annurev-psych-113011-143804. [DOI] [PubMed] [Google Scholar]
- McMullen MN. Affective contrast and assimilation in counterfactual thinking. J Exp Soc Psych. 1997;33:77–100. [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Inferences about other minds. Philos Trans R Soc B. 2009;364:1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Addis DR, Kensinger EA. Self-involvement modulates the effective connectivity of the autobiographical memory network. Soc Cogn Affect Neurosci. 2010;5:68–76. doi: 10.1093/scan/nsp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwland MS. Establishing propositional truth-value in counterfactual and real-world contexts during sentence comprehension: differential sensitivity of the left and right inferior frontal gyri. NeuroImage. 2012;59(4):3433–3440. doi: 10.1016/j.neuroimage.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, et al. Thinking of the future and the past: The roles of the frontal pole and the medial temporal lobes. NeuroImage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Perry D, Hendler T, Shamay-Tsoory SG. Projecting memories: the role of the hippocampus in emotional mentalizing. NeuroImage. 2011;54:1669–1676. doi: 10.1016/j.neuroimage.2010.08.057. [DOI] [PubMed] [Google Scholar]
- Pighin S, Byrne RM, Ferrante D, Gonzalez M, Girotto V. Counterfactual thoughts by experienced, observed and narrated events. Think Reason. 2011;17:197–211. [Google Scholar]
- Rabin JS, Rosenbaum RS. Familiarity modulates the functional relationship between theory of mind and autobiographical memory. NeuroImage. 2012;62:520–529. doi: 10.1016/j.neuroimage.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13(10):713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Roese NJ, Olson JM. What might have been: The social psychology of counterfactual thinking. Mahwah, NJ: Erlbaum; 1995. [Google Scholar]
- Roese NJ. Counterfactual Thinking. Psych Bull. 1997;66:805–818. doi: 10.1037/0033-2909.121.1.133. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Stuss DT, Levine B, Tulving E. Theory of mind is independent of episodic memory. Science. 2007;318:1257. doi: 10.1126/science.1148763. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: The role of the temporo-parietal junction in “theory of mind”. NeuroImage. 19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Phil Trans R Soc B. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: The prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: concepts, data, and applications. The Year in Cognitive Neuroscience, Annals of the New York Academy of Sciences. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Benoit R, De Brigard F, Szpunar KK. Episodic future thinking and episodic counterfactual thinking: Intersections between memory and decisions. Neurobiol Learn Mem. doi: 10.1016/j.nlm.2013.12.008. In press. doi.org/10.1016/j.nlm.2013.12.008. [DOI] [PMC free article] [PubMed]
- Spreng RN, Mar RA, Kim ASN. The common basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: A quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady C. Patterns of brain activity supporting autobiographical memory, prospection and theory-of-mind and their relationship to the default mode network. J Cogn Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proc Natl Acad Sci USA. 2007;104:642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK. Episodic future thought: An emerging concept. Persp Psychol Sci. 2010;5:142–162. doi: 10.1177/1745691610362350. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Schacter DL. Get real: Effects of repeated simulation and emotion on the perceived plausibility of future experiences. J Exp Psychol: General. 2013;142:323–327. doi: 10.1037/a0028877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and Consciousness. Can Psychol. 1985;26(1):1–12. [Google Scholar]
- Van Hoeck N, Ma N, Ampe L, Baetens K, Vandekerckhove M, Van Overwalle F. Counterfactual thinking: An fMRI study on changing the past for a better future. Soc Cogn Affect Neurosci. 2013;8(5):556–564. doi: 10.1093/scan/nss031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoeck N, Begtas E, Steen J, Kestmont J, Vandekerckhove M, Van Overwalle F. False belief and counterfactual reasoning in a social environment. NeuroImage. 2014;90:315–325. doi: 10.1016/j.neuroimage.2013.12.043. [DOI] [PubMed] [Google Scholar]
- Wagner DD, Haxby JV, Heatherton TF. The Representation of Self and Person Knowledge in the Medial Prefrontal Cortex. WIREs: Cogn Sci. 2012;3:451–47. doi: 10.1002/wcs.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. Foreseeing the future: occurrence probability of imagined future events modulates hippocampal activation. Hippocampus. 2010;20:685–690. doi: 10.1002/hipo.20695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.