Abstract
Purpose
MicroRNAs in the delta-like 1 homolog - deiodinase, iodothyronine 3 (DLK1-DIO3) cluster have been shown to be critical for embryonic development and epithelial to mesenchymal transition (EMT). DLK1-DIO3 cluster miRNAs are elevated in the serum of metastatic cancer patients. However, the biological functions of these miRNAs in the EMT and metastasis of cancer cells are poorly understood. We previously demonstrated the oncogenic and metastatic role of miR-409-3p/5p, a member of this cluster, in prostate cancer (PCa). In this study, we defined the role of miR-154* and miR-379, two key members of this cluster, in PCa progression and bone metastasis in both cell line models and clinical specimens.
Experimental design
Genetic manipulation of miR-154* and miR-379 was performed to determine their role in tumor growth, EMT and bone metastasis in mouse models. We determined the expression of miR-154* in prostate cancer clinical samples and bone metastasis samples using in situ hybridization and quantum dot labeling.
Results
Elevated expression of miR-154* and miR-379 was observed in bone metastatic PCa cell lines and tissues, and miR-379 expression correlated with PCa patient progression-free survival. Intracardiac inoculation (to mimic systemic dissemination) of miR-154* inhibitor-treated bone metastatic ARCaPM PCa cells in mice led to decreased bone metastasis and increased survival.
Conclusion
miR-154* and miR-379 play important roles in PCa biology by facilitating tumor growth, EMT and bone metastasis. This finding has particular translational importance since miRNAs in the DLK1-DIO3 cluster can be attractive biomarkers and possible therapeutic targets to treat bone metastatic PCa.
Keywords: miR-154*, miR-379, epithelial to mesenchymal transition, DLK1-DIO3 cluster
INTRODUCTION
Cancer cells often undergo epithelial to mesenchymal transition (EMT) prior to metastasizing to distant organs. Cancer cells activate embryonic programs and pathways that partially maintain stem cell identity, often referred as “stemness”. Recent studies suggest roles for microRNAs in both metastasis and cancer stem cell formation (1, 2). The microRNA members of the delta-like 1 homolog- deiodinase, iodothyronine 3 (DLK1-DIO3) clusters are activated in embryonic stem cells. During mouse embryogenesis, the microRNA (miRNA) members of the DLK1-DIO3 cluster have been shown to be elevated in the developing central nervous system, skeletal muscle, liver and lung (3). Similarly, several transgenic mouse models of prostate, liver and lung cancer also exhibit elevated levels of DLK1-DIO3 cluster miRNA members (4–8). The functions of many of these miRNAs within this imprinted region remain largely unexplored in cancer biology, specifically for EMT and cancer stemness.
Members of the DLK1-DIO3 cluster have been recently shown to be upregulated in the serum of prostate cancer (PCa) and lung cancer (LCa) patients. Members of this cluster, including miR-379, miR-154* and miR-409-3p, show increased levels in the circulating exosomes of patients with lung adenocarcinomas (9) and breast (10) and prostate (11) cancers. Our recent studies demonstrate the importance of miR-409-3p/5p in PCa growth, EMT and bone metastasis (12). In this study, we studied the role of miR-154* and miR-379, two key DLK1-DIO3 cluster miRNAs, in several EMT models of human PCa. We demonstrated that these miRNAs modulate EMT and drive metastasis. Thus, these miRNAs are promising therapeutic targets for aggressive PCa.
METHODS
Cell culture
Three human PCa bone metastatic progression models, ARCaPE and ARCaPM (13), LNCaP and C4-2 (14), were used in our study. PCa cells and 293T cells were cultured in T-medium (GibcoBRL) supplemented with 5% heat inactivated fetal bovine serum (Bio-Whittaker) with 5% FBS and 1% penicillin-streptomycin. All cells were tested for mycoplasma every three months and were negative. Small RNA preparations derived from embryonic stem cells and induced pluripotent stem cells (iPSC) were provided by Drs. Sareen and Svendsen of the Regenerative Medicine Institute at Cedars-Sinai Medical Center. Generation and characterization of these cells are described in the supplementary materials and methods section.
miRNA expression
Quantitative Real Time PCR (qRT-PCR)
Cells were trypsinized and total miRNA was extracted using a mirVana miRNA isolation kit (Ambion). miRNA expression analysis by qRT- PCR was performed separately for each miRNA using specific primer sets (Applied Biosystems) as previously described (15). RNU6B was used for normalization.
mRNA analysis
Total RNA was isolated from confluent monolayers of cells using the RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was made using Superscript®III reverse transcriptase (Life Technologies, Grand island, NY). mRNA primers were designed and synthesized at Integrated DNA Technologies (Coralville, Iowa). mRNA expression levels were determined by qRT-PCR assays and SYBR Green Dye (Applied Biosystems). Samples were analyzed using the ΔΔCt method and normalized to 18S ribosomal RNA.
MSKCC database analysis
The dataset was published by the MSKCC team (16) and was obtained from cBioPortal (17). The expression levels of miR-154 and miR-379 were analyzed along with the survival data in the dataset. For the survival analysis of miR-379, the expression levels of miR-379 in patients with non-metastatic disease were compared with the median expression level of normal individuals. The disease-free survival of patients with miR-379 expression levels higher than normal individuals (n=29) was compared with those with lower miR-379 expression levels (n=78). A Kaplan-Meier survival curve was done by log-rank test between high and low expression groups. The expression levels of miR-154* were not available in the dataset. For the analysis of miR-154, the expression levels of normal healthy individuals (n=29) were compared with the expression levels of primary (n=99) and metastatic PCa patients (n=14). Two-tailed student’s t tests were done between the normal group and two primary and metastatic groups for analysis of the differential expression of miR-154.
3`UTR assay
Stromal antigen 2 (STAG2) mutant luciferase activity
3`UTR STAG2 luciferase construct (Switchgear genomics) was used as the wild type (WT) construct and it was further mutated as described below. miR-154* mimic and control miRNA were transiently transfected along with the WT or mutant (STAG2) construct into these 293T cells and luciferase activity was determined 24 h later using the Lightswitch luciferase assay system (Switchgear Genomics).
3`UTR Mutant constructs
Mutated 3’ UTR luciferase constructs were produced by sited-directed mutagenesis. Briefly, primer pairs with two sequential base pair mutations in the miRNA seed sequence of the 3’ UTR were generated. Following polymerase chain reaction amplification, parental methylated template DNA was digested for 1 hour with Dpn I. 2 µl of the reaction was then transformed into XL-10 Gold bacteria. 16 hours post-transformation, colonies were picked for liquid culture. Plasmid DNA was isolated by the Zyppy Plasmid Miniprep Kit according to the manufacturer’s directions (Zymo Research). Mutations were confirmed by sequencing before proceeding with luciferase assays.
Primers
| miR-154* Stag2 1 | aactagaactgctgagaggactgtatatacaattttaaacctaagttgattttttttctc |
| miR-154* Stag2 2 | gagaaaaaaaatcaacttaggtttaaaattgtatatacagtcctctcagcagttctagtt |
Lentiviral transduction
ARCaPE PCa cell lines were transduced with lentivirus expressing control or miRZip-154* (miR-154*i) or miRZip-379 (miR-379i) (System Biosciences) or cluster overexpression plasmid (custom made, miR-154*, miR-379, miR-409-3p/-5p) with green fluorescent protein (GFP) or control GFP plasmid. ARCaPM PCa cell lines were transduced with lentivirus expressing cluster inhibitor plasmid (custom made) with GFP. Lentiviral preparation and transduction of cell lines were performed per the manufacturer’s instructions (System Biosciences, Mountain View, CA). GFP positive cells were FACS sorted and cultured in vitro before experiments were performed.
Cell viability assay and invasion assays
Cell viability assays were performed using trypan blue staining. Cancer cell invasion was assayed in Companion 24-well plates (Becton Dickinson Labware) with 8 µm porosity polycarbonate filter membranes as described previously (18).
Western analysis
Whole cell lysates from cell lines were prepared using a modified RIPA lysis buffer (50 mM Tris HCl, 1% NP-40, 0.5% Na-deoxycholate, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, 10% glycerol) supplemented with 1:100 dilution of the protease inhibitor cocktail and the tyrosine phosphatase inhibitor (Sigma). Proteins were then separated on 4–20% or 10% acrylamide gels and transferred to nitrocellulose membrane. Membranes were probed with STAG2 (Cell Signaling Technology) antibody. β-actin (Sigma) was used as the normalization control.
In situ hybridization (ISH)-Quantum dots (QD)
Mouse tibia was formalin-fixed and paraffin-embedded. The miRNA ISH protocol was followed per the manufacturer’s instructions (Exiqon, MA). The scramble and miR154* probes were 5’-biotin labeled. The probes were linked to streptavidin-conjugated QD. Tissue sections were deparaffinized, treated with proteinase-K and dehydrated. ISH was performed for 1 h at 55°C, followed by washes and streptavidin blocking and a reaction with streptavidin-conjugated QD at a specified wavelength. The QD staining procedure was followed as previously described (19). Single QD labeling was performed and scramble or miR-154* probes were labeled with 625 nm QDs. Images were taken at 40×. H&E staining was performed on subsequent tissue sections.
Human tissue array
A Gleason score tissue array was obtained from Vancouver Prostate Center. The use of tissue specimens was approved by the institutional review board of the Cedars-Sinai Medical Center (IRB# Pro21228). The tissues consisted of BPH (N=4), Gleason score 6 (N=12) and Gleason 7 (N=7). Each tissue had two sample cores. The tissue array was stained for H&E and graded by a pathologist to confirm the Gleason score. Single QD labeling was performed as previously mentioned (19). miR-154* was labelled with 625 nm QD and signals were quantified as previously mentioned (19). The QD fluorescence intensity of each tissue section was determined and analyzed. Human prostate caner bone tissues were stained following the same procedure, except multiplexed ISH-QD was performed, where miR-154* (red) was stained first followed by miR-409-3p (green) or miR-409-5p (green) which was labeled with 565 nm QD.
In vivo metastasis study
All animal experiments were IACUC approved and done in accordance with institutional guidelines. Luciferase tagged ARCaPM control and ARCaPM-154*i cells were injected intra-cardially as previously described (20) in SCID/beige mice (Charles River Laboratories) (N=5). Mice were imaged using X-ray and bioluminescence via the IVIS® Lumina Imaging system. Mice were given NIR dye (IR783) 48 h before euthanasia. The tumor-specific NIR dye was used to detect metastatic tumors in the mice.
Statistical analysis
Values were expressed as means ± standard deviation. All experiments were done in triplicates at least two independent times. Statistical analysis was performed using Student's t-test or ANOVA. Values of p<0.05 were considered to be statistically significant.
RESULTS
miR-154* and miR-379 of the DLK1-DIO3 cluster are overexpressed in bone metastatic EMT models of human PCa
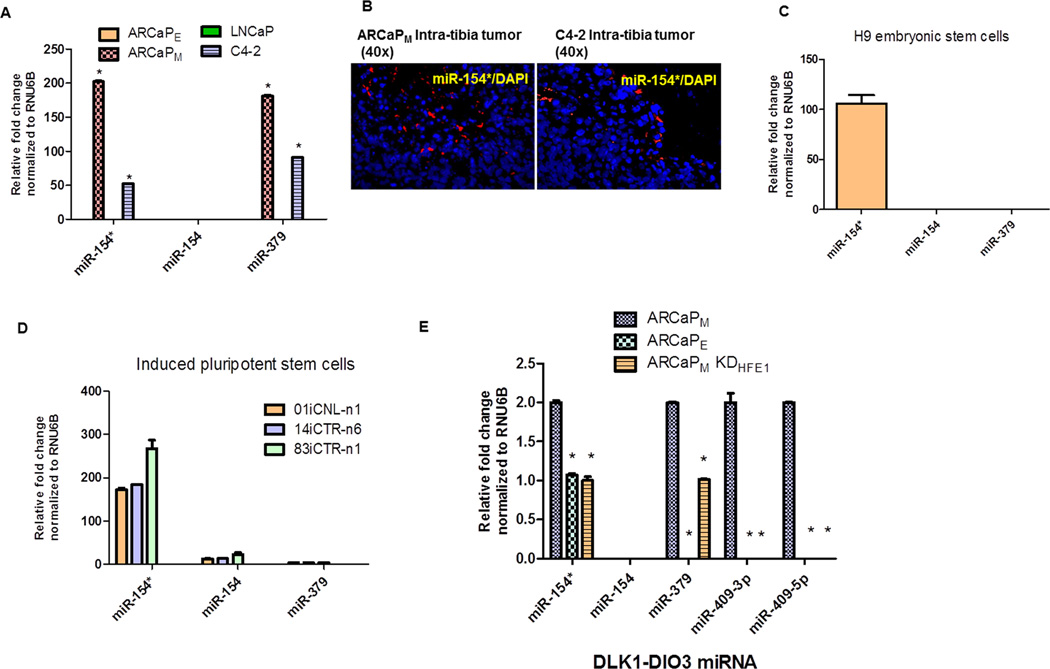
We determined the levels of miR-154/154* and miR-379 in two bone metastatic models of PCa, ARCaP and LNCaP. Mesenchymal-type ARCaPM cells upon intracardiac inoculation or orthotopic implantation have 100% bone metastatic capability compared to their isogenic epithelial-type counterpart ARCaPE cells (13). Similarly, C4-2 PCa cells have a high metastatic ability compared to their lineage-related LNCaP cells (21). Both miR-154* and miR-379 were elevated in ARCaPM cells compared to ARCaPE cells, and in C4-2 cells compared to LNCaP cells (Figure 1A). We determined the expression of miR-154* in intra-tibial prostate cancer bone metastasis tissues by ISH-QD and observed higher expression in the metastatic prostate cancer cells (Figure 1B). Previous studies demonstrated that several members of the DLK1-DIO3 miRNA cluster are elevated in human embryonic stem cells and induced pluripotent cells (iPSCs) (22, 23). We measured the relative levels of these miRNAs in H9 embryonic stem cells and in iPSCs. Both H9 human embryonic stem cells and patient-derived iPS cells had elevated expression of miR-154* (Figure 1C and 1D) but not miR-379 (23). We previously demonstrated that one of the predominant signaling pathways activated in PCa bone metastasis is the β2-microglobulin/HFE pathway (20, 24). Inhibition of either of these proteins results in reversal of EMT (20). We observed that ARCaPM HFE knockdown cells (ARCaPM KDHFE1 cells) had significantly decreased expression of miR-154* and miR-379 similar to the epithelial ARCaPE PCa cells (Figure 1E). Additionally, other members, such as miR-409-3p/-5p of the DLK1-DIO3 cluster, were also decreased in ARCaPM HFE knockdown cells. These data together demonstrate that miR-154* and miR-379 are elevated in PCa EMT and bone metastatic models.
Figure 1. Members of the DLK1-DIO3 miRNA cluster (miR-154/154* and miR-379) are overexpressed in aggressive bone metastatic EMT models of human PCa.
(A) miRNA expression of miR-154/154* and miR-379 in PCa models (mesenchymal cells ARCaPM compared to ARCaPE and LNCaP verses C4-2 PCa cells) by qRT-PCR analysis. (B) miRNA stained or miR-154* stained PCa mouse bone metastatic models (C4-2 and ARCaPM cells) assayed by ISH-QD analysis (Magnification 40X). miR-154* stained in red, nucleus stained with DAPI. (C), (D) miRNA expression of miR-154/154* and miR-379 in H9 embryonic stem cells and iPS cells by qRT- PCR. (E) miRNA expression in EMT models in PCa, mesenchymal cells-ARCaPM, epithelial cells-ARCaPE and ARCaPM KDHFE1, assayed by qRT-PCR. *: p<0.05 was considered to be statistically significant by one way ANOVA-Tukey analysis.
Inhibition of miR-154* or miR-379 results in reversal of EMT (MET) of PCa cells
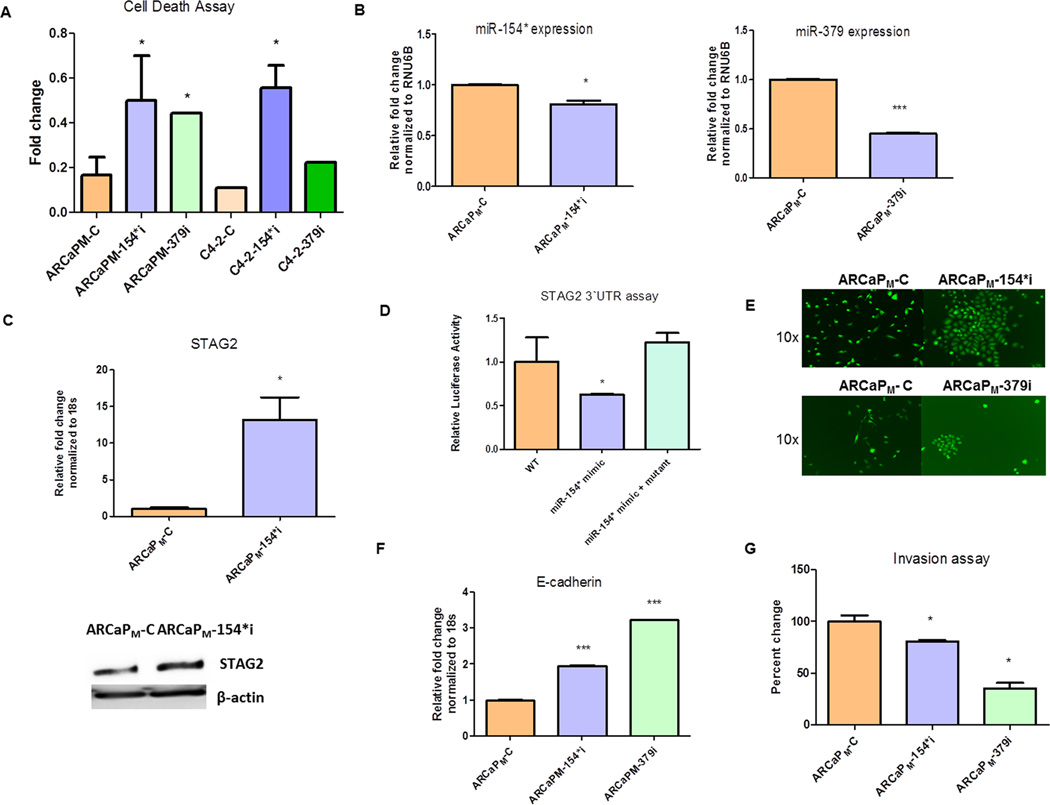
To test the hypothesis whether miR-154* and miR-379 is involved in PCa EMT, we transiently depleted miR-154* and miR-379 in ARCaPM and C4-2 cells using siRNA and determined the cell viability by the trypan blue exclusion test. Both miR-154* knockdown cells and miR-379 inhibited cells underwent increased cell death compared to control transfected cells in both of the PCa cell line models studied (Figure 2A). Next, we introduced a shRNA in the mesenchymal-type ARCaPM PCa cells to generate miR-154* knockdown (ARCaPM-154*i) or miR-379 knockdown (ARCaPM-379i) cells. In addition we used a scrambled shRNA to generate a control shRNA vector (ARCaPM-C). Reduced expression of miR-154* was detected in the ARCaPM-154*i cells by qRT-PCR analysis compared to the shRNA control vector ARCaPM-C. Reduced expression of miR-379 was also observed in the ARCaPM-379i cells by qPCR analysis compared to the ARCaPM-C cells (Figure 2B). RNU6B was used for normalization. The miR-154* target gene, stromal antigen 2 (STAG2), was measured in the knockdown cells (TargetScan v6.2). STAG2 is a tumor suppressor protein (25). The mRNA and protein expression of STAG2 was increased in the ARCaPM-154*i cells compared to the control ARCaPM cells (Figure 2C). We further tested if miR-154* directly binds to the 3`UTR of STAG2 using a luciferase reporter assay. Compared to control miRNA treated cells, miR-154* mimic-treated cells had reduced basal luciferase activity (Figure 2D). We mutated the 3`UTR of STAG2 at the miR-154* binding site and demonstrated restoration of luciferase activity in response to the miR-154* mimic (Figure 2D). Interestingly, inhibition of miR-154* or miR-379 led to reversion of mesenchymal ARCaPM cells to an epithelial phenotype (Figure 2E) accompanied by increases in E-cadherin mRNA expression in ARCaPM-154*i and ARCaPM-379i cells but not in ARCaPM-C cells (Figure 2F). To determine if functional reversal of EMT (MET) occurred in addition to morphological changes, we performed invasion assays on these cell lines. ARCaPM-154*i and ARCaPM-379i cells had significantly decreased invasive capacity compared to ARCaPM-C cells (Figure 2G). Taken together, these results demonstrate that miR-154* and miR-379 play an important role in the EMT and invasive capacity of PCa cells.
Figure 2. Inhibition of miR-154* or miR-379 results in mesenchymal to epithelial transition of PCa cells.
(A) Cell death in ARCaPM PCa cells in response to a miR-154* and miR-379 inhibitor using trypan blue exclusion assay. (B) Expression of miR-154* and miR-379 assayed by qRT- PCR in ARCaPM-C control PCa cells and ARCaPM-154*i (miR-154* inhibitor transfected cells) and ARCaPM-379i (miR-379 inhibitor) transfected cells. Data is normalized to RNU6B. (C) STAG2 (mir-154* target) protein and mRNA expression in ARCaPM-C and ARCaPM-154*i PCa cells assayed by western analysis and qRT-PCR. (D) 3`UTR binding luciferase assay using wild type (WT) and mutant 3`UTR (STAG2) construct and miR-154* mimics in 293T cells. (E) Morphological changes in ARCaPM-154*i and ARCaPM-379i compared to ARCaPM-C control PCa cells. (F) E-cadherin mRNA assayed in ARCaPM-C, ARCaPM-154*i and ARCaPM-379i expressing PCa cells by qRT-PCR, normalized to 18s RNA. (G) Invasion assay of ARCaPM-C, ARCaPM-154*i and ARCaPM-379i expressing PCa cells. *p<0.05, or ***p< 0.001 were considered to be statistically significant by t-test or ANOVA- Tukey test.
Inhibition of miR-154* results in decreased bone and soft tissue metastasis of PCa cells
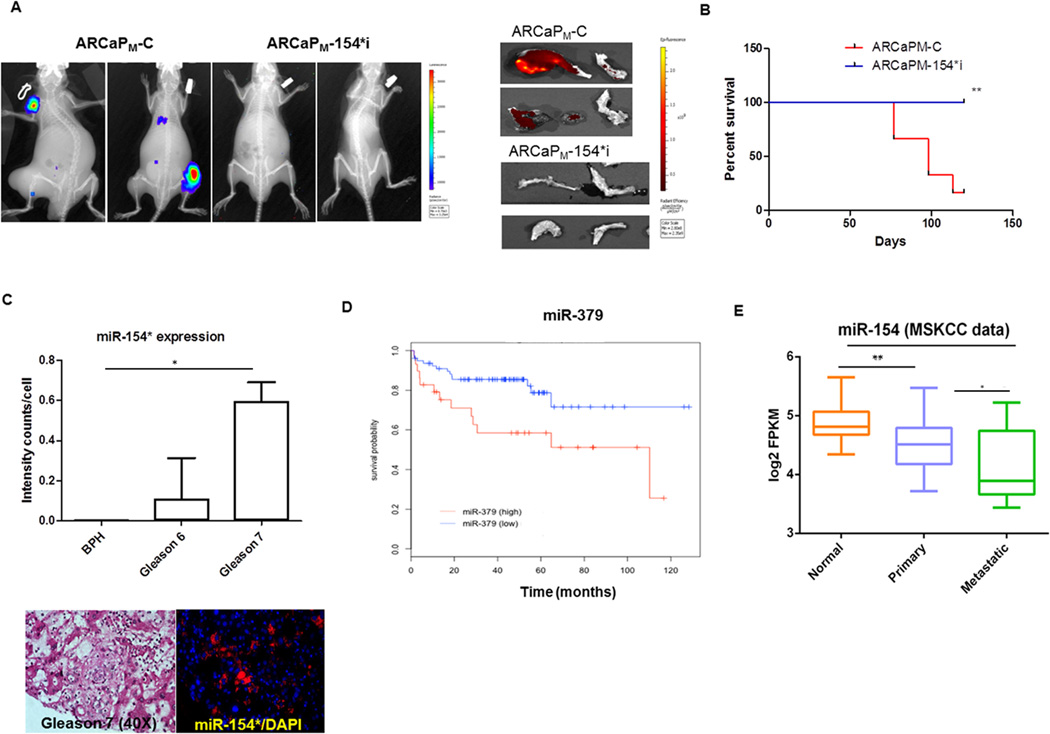
Cancer cells gain their metastatic potential by undergoing EMT. Previous studies from our laboratory using the ARCaP model demonstrate the close association between EMT and PCa bone metastasis (26). Since miR-154* levels are elevated in metastatic cancer cells, we determined whether inhibition of miR-154* would lead to decreased metastasis in vivo. Consistent with our hypothesis, we found that inhibition of miR-154* resulted in increased cell death, mesenchymal to epithelial transition (MET) and decreased invasion in vitro. To determine if miR-154* plays a role in cancer metastasis in vivo, we inoculated luciferase-tagged ARCaPM-C control cells or ARCaPM-154*i (miR-154* inhibited) cells via the intracardiac route into SCID/Beige mice (N=5/group) to mimic in vivo metastasis. Tumor growth was monitored by luciferase imaging. Mice that received ARCaPM-154*i cells had a significantly decreased incidence of metastasis (0/5) compared to mice that received ARCaPM-C control cells (4/5) at 15 weeks post-inoculation. X-ray and luciferase imaging of representative mice from both groups are shown in Figure 3A. The tumors were detected by IR783 (near infrared dye) in all mice (Figure 3A). The control mice developed metastatic tumors at 1–5 sites in the body, while ARCaPM-154*i injected mice did not develop any tumors. Bone tumors sites included the tibia, femur, humerus and mandible, and had mixed osteoblastic and osteolytic lesions (27). Mice inoculated with ARCaPM-C control cells had decreased survival compared to those inoculated with ARCaPM-154*i cells as shown in the Kaplan Meier survival curve (Figure 3B). Taken together, these studies demonstrate that miR-154* is essential for the development of bone metastasis of human PCa cells and that knockdown of miR-154* reduces bone metastasis and increases the survival of mice.
Figure 3. Inhibition of miR-154* results in decreased metastasis of bone and soft tissue of PCa cells.
(A) Representative metastatic lesions observed by X-ray/luciferase imaging of ARCaPM–C cell and ARCaPM-154*i cell tumors in SCID/Beige mice (n=5/group) following intra-cardial injection. Images of bone with tumor in the ARCaPM–C cells and ARCaPM-154*i injected mice using near infra-red dye (IR783). (B) Kaplan Meier’s curve of ARCaPM–C cells and ARCaPM-154*i injected SCID/Beige mice (n =5/group). (C) miR-154* expression in PCa clinical samples from BPH, Gleason 6 and 7 tissues assayed by ISH-QD labeling. Data were plotted as intensity counts/cell in tissues. Representative image of Gleason 7 tissue with miR-154* staining in red (magnification 40×). Nuclei stained by DAPI. (D) Kaplan-Meier disease free survival (DFS) curves for the PCa patients, based on miR-379 expression in the MSKCC dataset. The y-axis is disease free survival probability, and the x-axis is survival in months. Blue line represents the DFS of patients with miR-379 lower than the median of the normal individuals (n=78). Red line represents the DFS of patients with miR-379 higher than the median of the normal individuals (n=29). Data was analyzed using log-rank test (p=0.0117). (E) miR-154 expression in normal, primary and metastatic PCa patients. Significant differential expression of miR-154 was noted between normal individual (n=29), primary (n=99) and metastatic (n=14) PCa patients. *: p<0.05, or ** p < 0.005 were considered to be statistically significant by t-test or ANOVA- Tukey test.
Elevated expression of miR-154* and miR-379 in human PCa clinical samples
We next determined the expression of miR-154* in human PCa tissues using in situ hybridization and quantum dot analysis. miR-154* probes were biotin-labeled (Exiqon) and further labeled to a streptavidin conjugated QD at a specified wavelength. The tissues were separated into three groups, benign prostatic hyperplasia (BPH) (N=4), Gleason 6 (N=12) and Gleason 7 (N=7). Each tissue sample has two sections. Gleason 7 tumor tissues had significantly higher staining of miR-154* compared to BPH (Figure 3C). A representative image of Gleason 7 demonstrates higher staining in the tumor tissues (Figure 3C). We also determined the expression of miR-154* in other cancers and found increased expression in other urological cancers (Supplementary Figure S1). Using the publicly available database (MSKCC), we demonstrated that elevated expression of miR-379 is associated with progression-free survival and miR-154 (the opposite strand of miR-154*) is downregulated in both primary and metastatic PCa tissues compared with normal individuals (Figure 3D & E) (28–30). These results are consistent with previous studies demonstrating that miR-154 is decreased in prostate cancer cell lines and clinical samples (28–30). These databases did not have information on miR-154*. Nevertheless, our observations in bone metastatic PCa cell line models are clinically relevant and our experimental observations correlated well with tumor progression. These results collectively demonstrate that miR-154* and miR-379 are highly expressed in PCa and miR-379 expression correlates with progression-free survival in patients.
Overexpression of miRNA members of the DLK1-DIO3 cluster promotes EMT in PCa cells
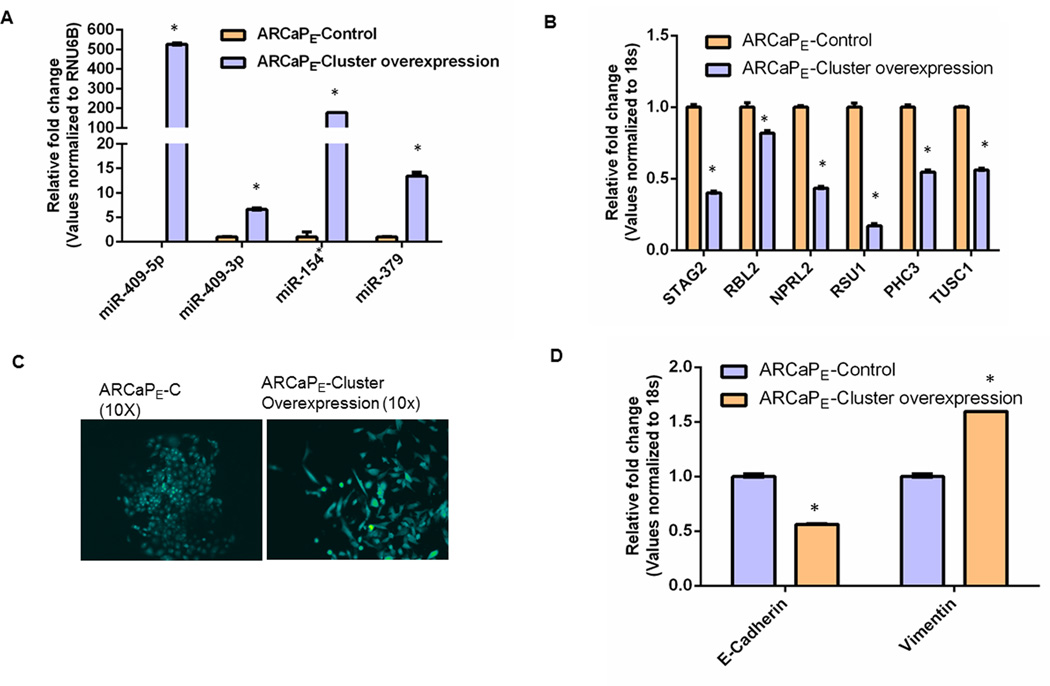
Previously published studies by our lab demonstrate that miR-409-3p/5p, two key miRNAs in the DLK1-DIO3 cluster, are elevated in PCa cells (12). Since all four members (miR-409-3p/5p, miR-154* and miR-379) of the DLK1-DIO3 cluster are elevated in PCa bone metastatic cells, we stably transduced ARCaPE cells, a marginally metastatic epithelial cell line, using a lentivirus that carries a GFP control vector (ARCaPE-C) or a lentiviral overexpression vector that carries GFP and a combination of all four miRNAs- miR-409-3p, -5p, miR-154* and miR-379 (ARCaPE-cluster overexpression). The miRNA expression of the members of the DLK1-DIO3 cluster was determined by qRT-PCR assay (Figure 4A). In the ARCaPE-cluster overexpression cells, miR-409-5p and miR-154* were highly expressed (100 fold) followed by moderate expression of miR-379 and miR-409-3p (5–10 fold) when compared to ARCaPE-C PCa cells (Figure 4A). Next, we measured the expression levels of the target genes of these miRNAs by qRT-PCR. Several of the target genes of these four miRNAs include tumor suppressors that are shared by these miRNAs (Prediction software: Targetscan v6.2 June 2012 and Pictar). Overexpression of these miRNAs resulted in a decrease in mRNA levels of several of the target genes that are commonly shared by these miRNAs. STAG2 (target gene of miR-154* and miR-409-5p) and Ras suppressor protein 1 (RSU1) (target gene of miR-409-3p and miR-409-5p) were significantly decreased in the ARCaPE-cluster overexpression cells compared to control (Figure 4B). miR-409-5p targets including STAG2, retinoblastoma-like 2 (RBL2), nitrogen permease regulator-like 2 (NPRL2) and RSU1 were decreased in the cluster overexpression cells (Figure 4B). Targets of miR-409-3p including RSU1, polyhomeotic homolog 3 (PHC3) and tumor suppressor candidate 1 (TUSC1) were decreased in cluster overexpression cells (Figure 4B). These results demonstrate that overexpression of miRNA members of the DLK1-DIO3 microRNA mega-cluster results in decreased expression of several tumor suppressor genes. We also observed EMT changes in ARCaPE-cluster overexpression PCa cells. The cells appeared spindle shaped and had decreased expression of E-cadherin and increased expression of vimentin compared to ARCaPE-C cells (Figure 4C). To evaluate the combinatorial effects of knockdown of all four miRNAs (miR-409-3p, miR-409-5p, miR-379 and miR-154*) in metastatic ARCaPM prostate cancer cells, we introduced shRNA that targets all four miRNAs and found that successful shRNA-mediated knockdown of miR-154* and miR-409-3p (the knockdown of miR-409-5p and miR-379 was not effective in this experiment) led to reversal of EMT. ARCaPM cells underwent morphological, biochemical and functional EMT changes upon knockdown of miR-154* and miR-409-3p (Supplementary Fig. S2, A–D).
Figure 4. Overexpression of miR-154*, miR-409-3p/-5p and miR-379 induces EMT in PCa cells.
(A) Expression of miR-409-5p/-3p, miR-154* and miR-379 assayed by real time PCR in ARCaPE-C control PCa cells and ARCaPE-cluster overexpressing cells normalized to RNU6B. (B) RNA expression of miR-409-5p/-3p and miR-154* targets in ARCaPM PCa cells assayed by qRT-PCR. (miR-154* mRNA targets: STAG2, miR-409-5p mRNA targets: STAG2, RBL2, NPRL2 and RSU1, miR-409-3p mRNA targets: RSU1, PHC3 and TUSC1). (C) Morphological EMT changes in ARCaPE-C control PCa cells and ARCaPE-cluster overexpressing cells; magnification 10× (D) RNA expression of EMT markers, E-cadherin and vimentin in ARCaPE-C control PCa cells and ARCaPE-cluster overexpressing cells assayed by qRT-PCR. *: p<0.05 was considered to be statistically significant by t-test.
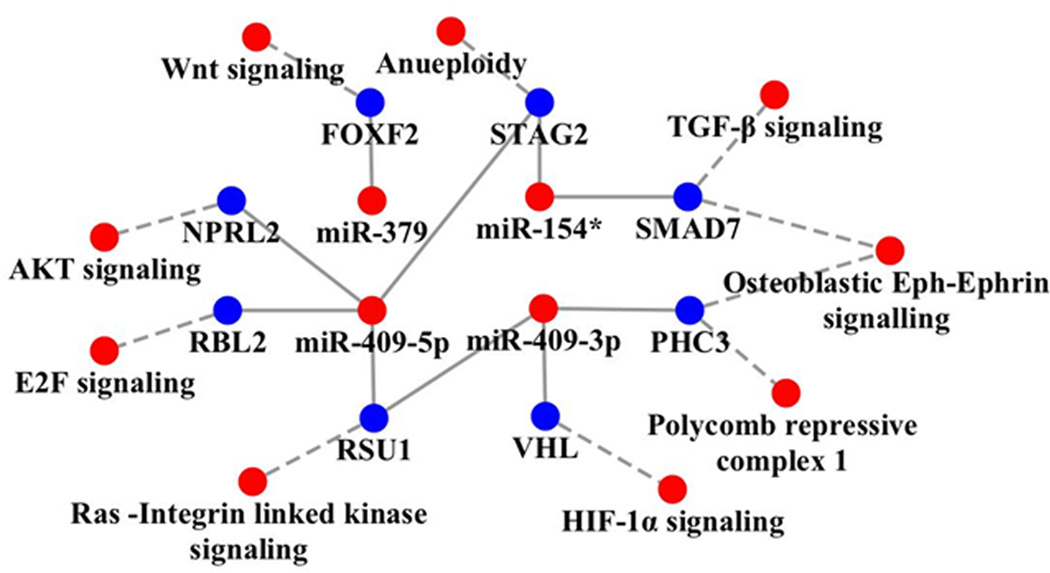
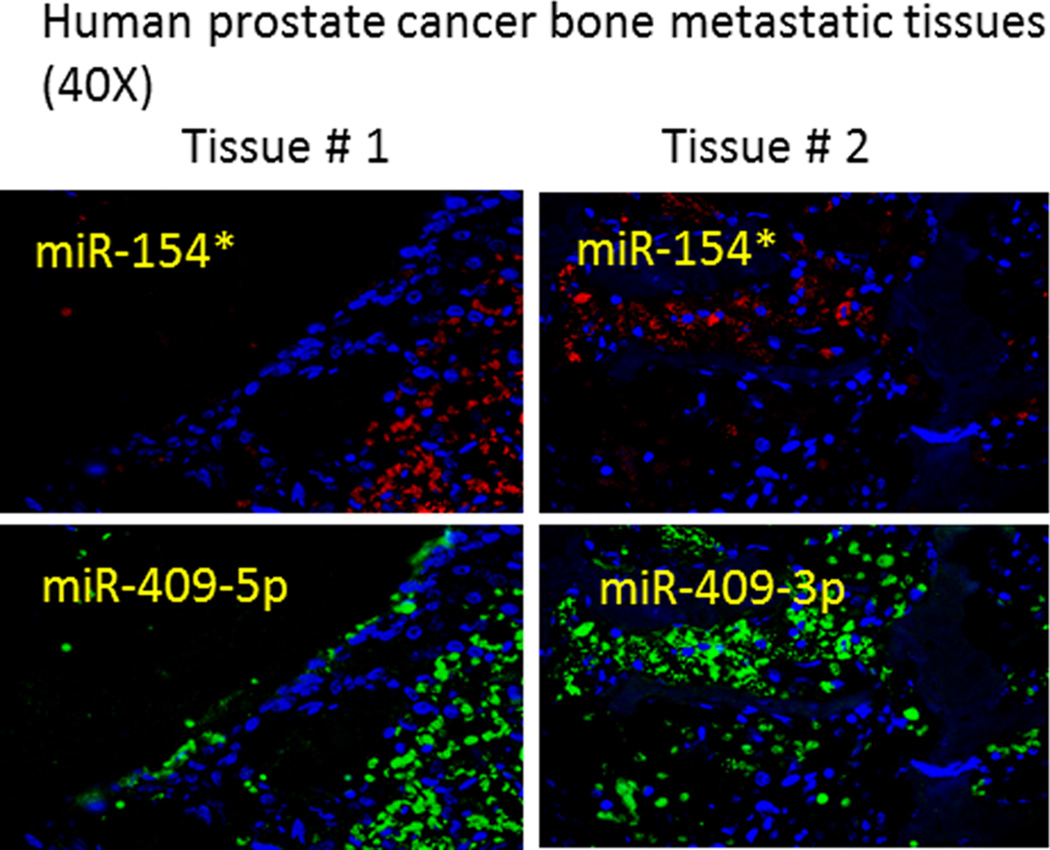
Analyses of the target pathways regulated by these miRNAs include oncogenic pathways such as E2F signaling, the Ras pathway, hypoxia inducible factor signaling, and the WNT and transforming growth factor-β (TGF-β) pathways. These pathways also activate EMT and the cancer stem cell phenotype (Figure 5). miR-154* targets STAG2 and SMAD7. STAG2 is known to induce aneuploidy (25, 31). SMAD7 is an inhibitor of the TGF-β pathway (32). miR-379 is predicted to inhibit forkhead box F2 (FOXF2) which has been shown to inhibit the WNT pathway in colon cancer development (33). We previously demonstrated that miR-409-3p and miR-409-5p inhibit RSU1, which is a known inhibitor of the oncogenic Ras pathway (12, 34, 35). Other targets of miR-409-3p include von Hippel-Lindau tumor suppressor, E3 ubiquitin protein ligase (VHL) and PHC3. VHL is a tumor suppressor and degrades HIF-1α (36). Inhibition of VHL results in the stabilization of HIF-1α, which is known to induce resistance to radiation and chemotherapeutic agents (37). PHC3 is a tumor suppressor protein and is lost in osteosarcoma (38). PHC3 and Ephrin receptors have been predicted to exhibit protein-protein interactions (www.biograph.be/). miR-409-5p has been shown to target STAG2, RSU1, NPRL2 and RBL2 (12). NPRL2 activates AKT pathway (39, 40) and RBL2 activates the E2F pathway (41). Thus, these miRNA activate oncogenic proteins by targeting tumor suppressors. Since these miRNAs are elevated in PCa bone metastatic models, we determined the levels of these miRNAs in human PCa bone metastatic samples using multiplexed ISH-QD labeling. We observed increased miR-154*, miR-409-3p and miR-409-5p staining in human metastatic tumor tissues in the bone (Figure 6). Taken together, these results demonstrate that miR-154*, miR-409-3p/-5p and miR-379 induce EMT in PCa cells, show upregulated expression in human PCa bone metastasis tissues and correlate with progression-free survival in PCa patients.
Figure 5.
Cytoscape analysis of target genes and signaling pathways altered by miR-409-3p/5p, miR-154* and miR-379 in the DLK1-DIO3 cluster. The miRNA in this cluster target tumor suppressors which block several pathways in human cancer. Red dots represent activation of miRNA and oncogenic pathways and blue dots represent inhibition of tumor suppressor genes.
Figure 6.
miR-154* (red), miR-409-3p (green) and miR-409-5p (green) staining of human prostate bone metastatic tissue using multiplexed ISH-QD labeling. Two human prostate bone metastatic tissues were used, and multiplexed for probes against miR-154* and miR-409-3p or miR-409-5p.
DISCUSSION
To understand the biology of the miRNAs of the DLK1-DIO3 mega cluster in EMT and cancer bone metastasis and to identify novel biomarkers and/or therapeutic targets, we probed for specific miRNAs of this megacluster in unique EMT models of human PCa developed in our laboratory. Specifically, the miRNAs miR-154* and miR-379, located within the DLK1-DIO3 cluster, were highly upregulated in two PCa cell lines with a mesenchymal phenotype and with bone metastatic potential (Figure 1). The miRNA members of the DLK1-DIO3 cluster have been shown to be important for totipotency during embryogenesis and induced pluripotent stem cell formation. We uncovered a surprising role for miR-154* and miR-379, which is expressed by embryonic stem cells and pluripotent stem cells, promoting PCa development and metastasis. Specifically, we show that 1) miR-154* and miR-379 are elevated in human PCa tumor tissues and miR-379 expression correlate with PCa patient progression-free survival, 2) miR-154* and miR-379 can promote EMT of PCa cells in vitro, and 3) inhibition of miR-154* results in decreased bone metastatic tumor growth and increased survival. Thus, miR-154* and miR-379 appear to be promising new biomarkers for cancer detection and attractive new therapeutic targets for PCa treatment.
The primary functions of miR-154* are to repress tumor suppressors and modulate the expression of EMT and stemness to mediate downstream convergent signal axes. Inhibition of miR-154* resulted in increased E-cadherin and decreased invasion in vitro. miR-154*-depleted PCa cells had decreased bone metastasis and increased survival. miR-154* mediates its effects by downregulating its target gene, STAG2, which is a tumor suppressor. STAG2 plays a critical role in the cohesion complex and a decrease in STAG2 has been correlated with aneuploidy and cancer (25, 31). Other miR-154* target genes may be involved such as SMAD7, which inhibits the TGF-β pathways (32) involved in EMT (42). Interestingly, the sense strand of miR-154* is undetectable or decreased in PCa models and in PCa patients (28, 29, 30). miR-154* is upregulated in both lung squamous cell carcinoma and lung adenocarcinoma patients (9). miR-379, another member of the DLK1-DIO3 cluster, located upstream of the miR-154 gene cluster, is moderately elevated in PCa cells (Figure 1). Inhibition of miR-379 also resulted in reversal of EMT (MET), increased E-cadherin and decreased invasion. miR-379 expression in clinical specimens correlates with disease-free survival of prostate cancer patients. Consistent with our observations, previous reports demonstrate that the miRNAs of the DLK1-DIO3 cluster are elevated in the serum of cancer patients compared to healthy patients (9, 11). Consistent with our studies, it was shown that miR-379 is also elevated in metastatic PCa patients compared to patients with localized disease (43). Interestingly, other members of the DLK1-DIO3 megacluster share similar mRNA target genes. STAG2 is targeted by both miR-154* and miR-409-5p, whereas RSU1 is targeted by both miR-409-5p and miR-409-3p. Thus the members of the cluster work synergistically and are elevated in the mesenchymal type PCa cells to promote EMT and metastasis (Figure 5). We conclude that the miRNA members of the DLK1-DIO3 cluster promote EMT, stemness, and bone metastasis in PCa. These findings have potential clinical implications in both the biomarker field and the therapeutic arena.
Supplementary Material
Translational relevance.
Currently there are no good biomarkers or therapy for prostate cancer (PCa) bone metastasis. MicroRNAs in the delta-like 1 homolog - deiodinase, iodothyronine 3 (DLK1-DIO3) cluster located in human chrosomome 14 have been shown to be critical for embryonic development and epithelial to mesenchymal transition (EMT) of embryonic cells. In this study, we identified a novel role for miR-154* and miR-379, two members of the DLK1-DIO3 cluster, in prostate tumor growth, epithelial to mesenchymal transition (EMT), stemness and bone metastasis. Further, we demonstrate that miR-154* and other miRNA members are elevated in cell lines and bone metastasis tissues from prostate cancer patients. Overexpression of miR-154* and miR-379 in benign PCa cells promotes EMT, and inhibition of miR-154* and miR-379 results in decreased EMT and bone metastasis in experimental models. Thus miR-154* and miR-379, already implicated in embryonic development, have a role in PCa metastasis and could serve both as a novel biomarker and as a therapeutic target.
Acknowledgements
We would like to thank Drs. Svendsen and Sareen for sharing the iPSC and embryonic stem cell cultures and Mr. Gary Mawyer for manuscript editing. Grant support came from P01-CA98912, DAMD-17-03-02-0033 and RO1-CA122602 (L.W.K. Chung).
REFERENCES
- 1.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han Z, He H, Zhang F, Huang Z, Liu Z, Jiang H, et al. Spatiotemporal expression pattern of Mirg, an imprinted non-coding gene, during mouse embryogenesis. J Mol Histol. 2012;43:1–8. doi: 10.1007/s10735-011-9367-x. [DOI] [PubMed] [Google Scholar]
- 4.Lim L, Balakrishnan A, Huskey N, Jones KD, Jodari M, Ng R, et al. MiR-494 within an oncogenic MicroRNA megacluster regulates G1/S transition in liver tumorigenesis through suppression of MCC. Hepatology. 2014;59(1):202–215. doi: 10.1002/hep.26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luk JM, Burchard J, Zhang C, Liu AM, Wong KF, Shek FH, et al. DLK1-DIO3 genomic imprinted microRNA cluster at 14q32.2 defines a stemlike subtype of hepatocellular carcinoma associated with poor survival. J Biol Chem. 2011;286:30706–30713. doi: 10.1074/jbc.M111.229831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haga CL, Phinney DG. MicroRNAs in the imprinted DLK1-DIO3 region repress the epithelial-to-mesenchymal transition by targeting the TWIST1 protein signaling network. J Biol Chem. 2012;287:42695–42707. doi: 10.1074/jbc.M112.387761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lempiainen H, Couttet P, Bolognani F, Muller A, Dubost V, Luisier R, et al. Identification of Dlk1-Dio3 imprinted gene cluster noncoding RNAs as novel candidate biomarkers for liver tumor promotion. Toxicol Sci. 2013;131:375–386. doi: 10.1093/toxsci/kfs303. [DOI] [PubMed] [Google Scholar]
- 8.Valdmanis PN, Roy-Chaudhuri B, Kim HK, Sayles LC, Zheng Y, Chuang CH, et al. Upregulation of the microRNA cluster at the Dlk1-Dio3 locus in lung adenocarcinoma. Oncogene. 2013 Dec 9; doi: 10.1038/onc.2013.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cazzoli R, Buttitta F, Di Nicola M, Malatesta S, Marchetti A, Rom WN, et al. microRNAs Derived from Circulating Exosomes as Noninvasive Biomarkers for Screening and Diagnosing Lung Cancer. J Thorac Oncol. 2013;8:1156–1162. doi: 10.1097/JTO.0b013e318299ac32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Meng H, Zhou F, Zhai L, Zhang L, Gu F, et al. MicroRNA-132 is frequently down-regulated in ductal carcinoma in situ (DCIS) of breast and acts as a tumor suppressor by inhibiting cell proliferation. Pathol Res Pract. 2013;209:179–183. doi: 10.1016/j.prp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen HC, Xie W, Yang M, Hsieh CL, Drouin S, Lee GS, et al. Expression differences of circulating microRNAs in metastatic castration resistant prostate cancer and low-risk, localized prostate cancer. The Prostate. 2013;73:346–354. doi: 10.1002/pros.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josson S, Gururajan M, Hu P, Shao C, Chu GC, Zhau HE, et al. miR-409-3p/-5p promotes tumorigenesis, epithelial to mesenchymal transition and bone metastasis of human prostate cancer. Clin Cancer Res. 2014 Jun 24; doi: 10.1158/1078-0432.CCR-14-0305. pii: clincanres.0305.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Wang R, Xie ZH, Odero-Marah V, Pathak S, Multani A, et al. Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. The Prostate. 2006;66:1664–1673. doi: 10.1002/pros.20488. [DOI] [PubMed] [Google Scholar]
- 14.Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. The Prostate. 2000;44:91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. Jul 1;44(2). [DOI] [PubMed] [Google Scholar]
- 15.Josson S, Sung SY, Lao K, Chung LW, Johnstone PA. Radiation modulation of microRNA in prostate cancer cell lines. The Prostate. 2008;68:1599–1606. doi: 10.1002/pros.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver B, et al. Integrative genomic profiling of human prostate cancer. Cancer cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science signaling. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nomura T, Huang WC, Zhau HE, Wu D, Xie Z, Mimata H, et al. Beta2-microglobulin promotes the growth of human renal cell carcinoma through the activation of the protein kinase A, cyclic AMP-responsive element-binding protein, and vascular endothelial growth factor axis. Clin Cancer Res. 2006;12:7294–7305. doi: 10.1158/1078-0432.CCR-06-2060. [DOI] [PubMed] [Google Scholar]
- 19.Hu P, Chu GC, Zhu G, Yang H, Luthringer D, Prins G, et al. Multiplexed quantum dot labeling of activated c-Met signaling in castration-resistant human prostate cancer. PloS one. 2011;6:e28670. doi: 10.1371/journal.pone.0028670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josson S, Nomura T, Lin JT, Huang WC, Wu D, Zhau HE, et al. beta2-microglobulin induces epithelial to mesenchymal transition and confers cancer lethality and bone metastasis in human cancer cells. Cancer research. 2011;71:2600–2610. doi: 10.1158/0008-5472.CAN-10-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer research. 1994;54:2577–2581. [PubMed] [Google Scholar]
- 22.Liu L, Luo GZ, Yang W, Zhao X, Zheng Q, Lv Z, et al. Activation of the imprinted Dlk1-Dio3 region correlates with pluripotency levels of mouse stem cells. J Biol Chem. 2010;285:19483–19490. doi: 10.1074/jbc.M110.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang WC, Wu D, Xie Z, Zhau HE, Nomura T, Zayzafoon M, et al. beta2-microglobulin is a signaling and growth-promoting factor for human prostate cancer bone metastasis. Cancer research. 2006;66:9108–9116. doi: 10.1158/0008-5472.CAN-06-1996. [DOI] [PubMed] [Google Scholar]
- 25.Solomon DA, Kim T, Diaz-Martinez LA, Fair J, Elkahloun AG, Harris BT, et al. Mutational inactivation of STAG2 causes aneuploidy in human cancer. Science. 2011;333:1039–1043. doi: 10.1126/science.1203619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhau HE, Odero-Marah V, Lue HW, Nomura T, Wang R, Chu G, et al. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin Exp Metastasis. 2008;25:601–610. doi: 10.1007/s10585-008-9183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odero-Marah VA, Wang R, Chu G, Zayzafoon M, Xu J, Shi C, et al. Receptor activator of NF-kappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res. 2008;18:858–870. doi: 10.1038/cr.2008.84. [DOI] [PubMed] [Google Scholar]
- 28.Formosa A, Markert EK, Lena AM, Italiano D, Finazzi-Agro E, Levine AJ, et al. MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c, miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p, mapped to the 14q32.31 locus, regulate proliferation, apoptosis, migration and invasion in metastatic prostate cancer cells. Oncogene. 2013 Oct 28; doi: 10.1038/onc.2013.451. [DOI] [PubMed] [Google Scholar]
- 29.Zhu C, Li J, Cheng G, Zhou H, Tao L, Cai H, et al. miR-154 inhibits EMT by targeting HMGA2 in prostate cancer cells. Mol Cell Biochem. 2013;379:69–75. doi: 10.1007/s11010-013-1628-4. [DOI] [PubMed] [Google Scholar]
- 30.Zhu C, Shao P, Bao M, Li P, Zhou H, Cai H, et al. miR-154 inhibits prostate cancer cell proliferation by targeting CCND2. Urol Oncol. 2014 Jan 32;(1):31.e9–31.e16. doi: 10.1016/j.urolonc.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Kim MS, Kim SS, Je EM, Yoo NJ, Lee SH. Mutational and expressional analyses of STAG2 gene in solid cancers. Neoplasma. 2012;59:524–529. doi: 10.4149/neo_2012_067. [DOI] [PubMed] [Google Scholar]
- 32.Edlund S, Bu S, Schuster N, Aspenstrom P, Heuchel R, Heldin NE, et al. Transforming growth factor-beta1 (TGF-beta)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-beta-activated kinase 1 and mitogen-activated protein kinase kinase 3. Molecular biology of the cell. 2003;14:529–544. doi: 10.1091/mbc.02-03-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nik AM, Reyahi A, Ponten F, Carlsson P. Foxf2 in intestinal fibroblasts reduces numbers of Lgr5(+) stem cells and adenoma formation by inhibiting Wnt signaling. Gastroenterology. 2013;144:1001–1011. doi: 10.1053/j.gastro.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Nieves R, Desantis AI, Cutler ML. Rsu1 contributes to regulation of cell adhesion and spreading by PINCH1-dependent and - independent mechanisms. J Cell Commun Signal. 2013 Dec 7;(4):279–293. doi: 10.1007/s12079-013-0207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dougherty GW, Jose C, Gimona M, Cutler ML. The Rsu-1-PINCH1-ILK complex is regulated by Ras activation in tumor cells. Eur J Cell Biol. 2008;87:721–734. doi: 10.1016/j.ejcb.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 37.Palayoor ST, Burgos MA, Shoaibi A, Tofilon PJ, Coleman CN. Effect of radiation and ibuprofen on normoxic renal carcinoma cells overexpressing hypoxia-inducible factors by loss of von Hippel-Lindau tumor suppressor gene function. Clin Cancer Res. 2004;10:4158–4164. doi: 10.1158/1078-0432.CCR-04-0005. [DOI] [PubMed] [Google Scholar]
- 38.Iwata S, Takenobu H, Kageyama H, Koseki H, Ishii T, Nakazawa A, et al. Polycomb group molecule PHC3 regulates polycomb complex composition and prognosis of osteosarcoma. Cancer Sci. 2010;101:1646–1652. doi: 10.1111/j.1349-7006.2010.01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurata A, Katayama R, Watanabe T, Tsuruo T, Fujita N. TUSC4/NPRL2, a novel PDK1-interacting protein, inhibits PDK1 tyrosine phosphorylation and its downstream signaling. Cancer Sci. 2008;99:1827–1834. doi: 10.1111/j.1349-7006.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, et al. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maddika S, Ande SR, Panigrahi S, Paranjothy T, Weglarczyk K, Zuse A, et al. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat. 2007;10:13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Lenferink AE, Cantin C, Nantel A, Wang E, Durocher Y, Banville M, et al. Transcriptome profiling of a TGF-beta-induced epithelial-to-mesenchymal transition reveals extracellular clusterin as a target for therapeutic antibodies. Oncogene. 2010;29:831–844. doi: 10.1038/onc.2009.399. [DOI] [PubMed] [Google Scholar]
- 43.Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B, et al. Changes in circulating microRNA levels associated with prostate cancer. British journal of cancer. 2012;106:768–774. doi: 10.1038/bjc.2011.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.