Abstract
Herein we characterize the Arabidopsis thaliana AtLOX1 and tomato (Solanum lycopersicum) LOXA proteins as linoleate 9S-lipoxygenases (9-LOX), and use the enzymes to test a model that predicts a relationship between substrate binding orientation and product stereochemistry. The cDNAs were heterologously expressed in E. coli and the proteins partially purified by nickel affinity chromatography using a N-terminal (His)6-tag. Both enzymes oxygenated linoleic acid almost exclusively to the 9S-hydroperoxide with turnover numbers of 300–400/s. AtLOX1 showed a broad range of activity over the range pH 5–9 (optimal at pH 6); tomato LOXA also showed optimal activity around pH 5–7 dropping off more sharply at pH 9. Site-directed mutagenesis of a conserved active site Ala (Ala562 in AtLOX1, Ala 564 in tomato LOXA, and typically conserved as Ala in S-LOX and Gly in R-LOX), revealed that substitution with Gly led to the production of a mixture of 9S- and 13R-hydroperoxyoctadecadienoic acids from linoleic acid. To follow up on earlier reports of 9-LOX metabolism of anandamide (van Zadelhoff et al., 1998, Biochem. Biophys. Res. Commun. 248, 33), we also tested this substrate with the mutants, which produced predictable shifts in product profile, including a shift from the prominent 11S-hydroperoxy derivative of wild-type to include the 15R-hydroperoxide. These results conform to a model that predicts a head-first substrate binding orientation for 9S-LOX. We also found that linoleoyl-phosphatidylcholine is not a 9S-LOX substrate, which is consistent with this conclusion.
Keywords: Lipoxygenase, stereochemistry, linoleic acid, HODE, HPODE, anandamide, Arabidopsis, tomato, chiral analysis
Introduction
The abundance of linoleic acid (C18.2ω6) and linolenic acid (C18.3ω6) in higher plants coincides with the occurrence of multiple lipoxygenase (LOX) enzymes that specifically oxygenate these polyunsaturated fatty acids. Linoleic acid has only two available positions for LOX-catalyzed oxygenation, at C-9 or C-13, and all LOX activities in plants target one position or the other, or both. Although LOX activities abound in plant tissues, and LOX-encoding genes have been identified in dozens of plant species, the positional specificity of the vast majority of plant LOX genes remains to be determined [1]. One issue addressed here concerns the identification of a specific 9-LOX gene in Arabidopsis (AtLOX1) and tomato (LOXA). An accompanying manuscript by Andreou et al details a 9-LOX characterization from potato [2].
Currently there is no direct evidence showing how LOX enzymes catalyze their specific oxygenations [3]. The amino acid sequences in the gene family are well conserved, indicating that in general terms the arrangement of the catalytic machinery is conserved. A single atom of non-heme iron is held in place by well-conserved amino acid ligands comprised mainly of histidines and the carboxyl group of the C-terminal amino acid, usually an isoleucine. Within this conserved structural framework, individual LOX enzymes are known to oxygenate at specific positions on the fatty acid and in either the R or S stereoconfiguration. To accommodate all the known activities within the well conserved enzyme structures, one of the variables proposed in individual enzymes is a change in the head-to-tail orientation of the bound fatty acid substrate. This was proposed originally in 1972 as the logical explanation for how corn 9S-LOX and soybean 13S-LOX abstract the 11R and 11S hydrogen respectively from linoleic acid in forming their 9S-HPODE and 13S-HPODE products (Fig. 1A) [4]. Other lines of circumstantial evidence point to 13S oxygenation being associated with the “tail-first” mode of fatty acid binding. For example, 13-LOX can oxygenate very large esters such as linoleoylphosphatidylcholine in the same way as free linoleic acid (e.g. ref. [5]). This suggests that 13S oxygenation involves tail-first entry of the fatty acid into the active site, as it is hard to imagine a carboxyl esterified to phosphatidylcholine entering the LOX active site.
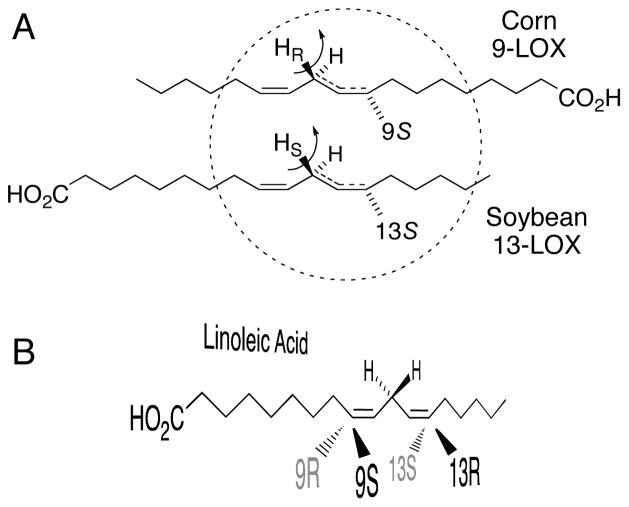
Fig. 1.
Stereochemistry of linoleic acid oxygenation. Panel A: stereospecific hydrogen abstraction and specific oxygenation of linoleic acid retain the same spatial relationship in corn 9-LOX and soybean 13-LOX if the substrates adopt reversed orientations in the respective enzyme active sites. Adapted from ref. [34]. Panel B: a drawing in perspective illustrating potential oxygenation of linoleic acid at each end of the pentadiene. Note that 9S and 13R oxygenations are on the same face of the molecule, and 9R/13S on the other face. Adapted from ref. [3].
Substrate binding orientation in 9S-LOX has been deduced in different ways in the literature. In contrast to the earlier conclusion of carboxyl-first substrate binding (Fig. 1A) [4], two separate observations lead logically to a contrary conclusion [6, 7]. The first relates to a known activity of several plant 9-LOX, namely that the main product of arachidonic acid oxygenation is the 5S-hydroperoxide. van Zadelhoff et al reported that when arachidonate ethanolamide (anandamide) is metabolized by purified barley or tomato 9-LOX, the main specific oxygenation produces the 11S-hydroperoxide [6]. This suggests that the carbon chain has slipped into a different alignment in the active site, and given the presence of the bulky anandamide moiety at the carboxyl end, it is hard to see why this would force the fatty acid deeper into the active site. Therefore, the investigators drew the opposite conclusion, that the fatty acid binds tail-first in 9-LOX [6]. Butovich and Reddy observed that 1-linoleoyl-glycerol is metabolized similarly to linoleic acid by potato 9-LOX (to the 9-hydroperoxide), and as this would seem to be incompatible with carboxyl end-first binding, they also came to the logical conclusion that the fatty acid substrate binds tail-first in 9-LOX.
Herein we address the issue of substrate binding in 9-LOX by testing a model that predicts reaction specificity and the formation of R or S configuration products in LOX enzymes [8]. A single active site amino acid was found to exert a major influence over R or S oxygenation [9]. This key determinant is conserved as an Ala in S lipoxygenases and a Gly in R lipoxygenases. An Ala allows oxygenation deep in the active site cavity and gives S-hydroperoxides while a change to Gly switches the position of oxygenation across the face of the reacting pentadiene and gives R-hydroperoxide stereochemistry. The model predicts that linoleic acid 9S-LOX (which naturally have Ala in the key position), should switch to forming 13R-hydroperoxide after the appropriate Ala-to-Gly mutation (see Fig. 1B). Given that the model fits with many different LOX enzymes with different predicted orientations of fatty acid binding, the model is also only compatible with the carboxyl end-first substrate orientation in plant 9-LOX.
Experimental Procedures
Materials
Fatty acids were purchased from NuChek Prep Inc. (Elysian, MN). [1-14C]linoleic, and [1-14C]arachidonic acids were purchased from Perkin Elmer Life Sciences. Standards of racemic HPODEs and HODEs were prepared by vitamin E-controlled autoxidation [10]. 9S-H(P)ODE was prepared using the 9-LOX activity in potato tuber extracts, and 13S-H(P)ODE using soybean lipoxygenase (Sigma type V) [11]. 1-Palmitoyl-2-linoleoyl-sn-glycero-3-phosphatidylcholine (C16/LA-PC) and 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylcholine (C16/AA-PC) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL).
Construction of expression vectors
A full-length AtLOX1 cDNA (Melan et al., 1993) was kindly provided by Dr. Kaye Peterman (Wellesley College). A PCR-based strategy was used to construct an expression vector for production of AtLOX1 with an N-terminal (His)6-tag. Forward and reverse primers that amplify the cDNA contained NdeI and XhoI restriction sites, respectively. The sequence of the forward primer was 5′-GGAATTCCATATGTTCGGAGAACTTAG-3′ and that of the reverse primer 5′-CCGCTCGAGTCAGATAGAGACGCTATTT-3′. PCR amplification of AtLOX1 yielded a 2.7 -kb product that was subsequently cut with NdeI and XhoI and subcloned into the same sites of the expression vector pET-14b (Novagen, Madison, WI). The resulting construct produced a (His)6-tagged AtLOX1 protein in which 20 amino acids (MGSSHHHHHHSSGLVPRGSH) were added to the initiator Met of the LOX1 enzyme. This construct was introduced into E. coli host strain BL21 (DE3) for protein expression.
The tomato (Solanum lycopersicum) LOXA cDNA was obtained by RT-PCR using an Enhanced Avian RT-PCR kit (Sigma Aldrich). The reverse transcription reaction contained 2 μg of total RNA isolated from 9-day-old tomato seedlings (cv. Castlemart) and an oligo (dT)23 primer. The PCR reaction was performed with LOXA-specific primers 5′-CGGGATCCATGTTAGGTGAACTTGTGGGTG-3′ and 5′-CCGAGCTCACATGTGTACTCAAAACTCTTC-3′, which included BamHI and SacI restriction sites, respectively. The resulting PCR product was cloned into BamHI and SacI sites of pBlueScript. The nucleotide sequence of this tomato cDNA exactly matched the tomato LOXA sequence (GenBank accession number U09026) reported by Ferrie et al [12]. A PCR-based strategy was used to construct an expression vector for production of an N-terminally (His)6-tagged LOXA enzyme. Following amplification with primers 5′-CGGGATCCGGTGGGTGGATTAATTGGTG-3′ and 5′-CGGGATCCCTATATTGACACACTGTTT-3′, this PCR fragment was cloned into the BamHI site of pET14b. The N-terminal amino acid sequence of the resulting (His)6-tagged LOXA enzyme is MGSSHHHHHHSSGLVPRGSHMLEDPVGGL. The subcloning procedure resulted in a three amino acid change (GLD → EDP, underlined) in the N-terminus of the native LOXA protein.
Expression of recombinant LOX in E. coli
Wild-type and mutant LOX were expressed in E. coli BL21 (DE3) as (His)6-tagged proteins using the protocol described for coral 8R-LOX [13] with the following modifications. The expressed proteins were recovered using Bugbuster (Novagen) with added 1 μl/ml Benzonase (Novagen) and 1mM PMSF. The supernatants were purified on Ni-NTA agarose (Qiagen) according to manufacturer instructions. Fractions collected from the affinity column were assayed by SDS/PAGE and fractions containing recombinant lipoxygenases were dialyzed against 50 mM phosphate pH 7.0, 150 mM NaCl buffer containing 20 % glycerol.
Fractions of 0.5 ml were collected from the affinity column and 10 μl aliquots analyzed using SDS-PAGE. Protein was determined using the Bio-Rad protein assay with bovine serum albumin as standard. Fractions containing the highest concentrations of protein were pooled and dialyzed against a buffer of 50 mM Tris (pH 7.5), 300 mM NaCl to remove imidazole.
Quantitation of LOX protein
The LOX proteins were quantified using a specific in-gel assay in which the LOX band on a stained SDS-PAGE gel is compared in intensity to the protein bands in a series of quantified albumin standards run in other lanes. Aliquots of the dialyzed protein from the Ni-NTA column were run on a 20–4% SDS PAGE gel, and a second gel was run with aliquots of bovine albumin in concentrations ranging from 0.25 μg to 15 μg. The gels were stained with Bio-Safe Coomassie stain (Bio-Rad) and rinsed repeatedly with water. The protein bands at ~97 kD representing the LOX protein were then quantified with the Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE), using the known amount of protein in the BSA bands to generate a standard curve.
Site Directed Mutagenesis
Site directed mutagenesis of AtLOX1 and LOXA was performed using the QuikChange(R) site directed mutagenesis kit (Stratagene) using overlapping mismatching oligonucleotides as primers designed according to the manufacturer’s instructions. The His-tagged Arabidopsis AtLOX1 and tomato LOXA in the pET14b expression vector were used as templates. The primer sequences (55-mers) were, for Arabidopsis LOX1, 5′-GTGATACGATGAATATCAATGCACTTGGTAGGCAAATCTTGATCAATGGTGGTGG, and 5′-CCACCACCATTGATCAAGATTTGCCTACCAAGTGCATTGATATTCATCGTATCAC, and for tomato LOXA, 5′-GGGACACAATGAATATTAATGCTTTGGGAAGACAGATCCTAATCAATGCTGGTGG, and 5′-CCACCAGCATTGATTAGGATCTGTCTTCCCAAAGCATTAATATTCATTGTGTCCC. PCR reactions and transformation of Escherichia coli XL1-Blue cells were performed according to the manufacturer’s instructions (Stratagene). Correctly mutated clones were identified by DNA sequencing.
Enzyme reactions, incubation and extraction
Enzymatic activity of the Nickel column-purified enzymes was determined by monitoring the increase of the signal at 235 nm using a Perkin-Elmer Lambda-35 UV-Vis spectrophotometer. Rates of reaction were calculated from the initial linear part of the curve. Rates at varying pH values were compared using 50 μM linoleic acid with phosphate buffers (pH 5.0, 6.0. 7.0 and 8.0) and borate buffer (pH 9.0). Products from reaction at pH 6 were extracted without pH change into dichloromethane or using a 1 ml Oasis HLB cartridge (Waters).
Incubations with anandamide were conducted in a 1ml quartz cuvette in 100mM sodium phosphate (pH 6) containing 0.1 mM sodium deoxycholate; anandamide (50μM final concentration) was added in 5 μl ethanol. After a 5 minute incubation at room temperature, the reactions were extracted using a 1 ml Oasis HLB cartridge (Waters).
Incubations with C16/C18.2-phosphatidylcholine were conducted in the presence of sodium deoxycholate (final concentration, 4 mM) to help solubilize the substrate. The reactions were extracted using the Bligh and Dyer procedure [14] then transesterified using sodium methoxide [5] and the methyl esters subsequently analyzed by normal phase HPLC.
HPLC analysis
Reaction products of the two LOX enzymes and mutants with [1-14C]linoleic acid or [1-14C]arachidonic acid were analyzed on an Agilent 1100 HPLC equipped with a diode array detector connected online to a Radiomatic FLO-ONE A-100 radioactive detector. Straight-phase HPLC analysis used a Beckman Ultrasphere silica column (25 × 0.46 cm) eluted at a flow rate of 1 ml/min with hexane/isopropanol/acetic acid (100/2/0.1, by volume) with UV detection at 205, 220, 235 and 270 nm. Reversed-phase HPLC used a Waters Symmetry C18 5-μm column eluted at 1 ml/min with a solvent of methanol/water/acetic acid (80/20/0.01, by volume), and finally with methanol to elute unreacted substrate. Chiral analysis was performed on HODE methyl esters using a Daicel Chiralpak AD column (25 × 0.46 cm) eluted with hexane/methanol (100/2, by volume) at a flow rate of 1 ml/min, with UV detection at 235 nm [15].
Results
Expression and purification
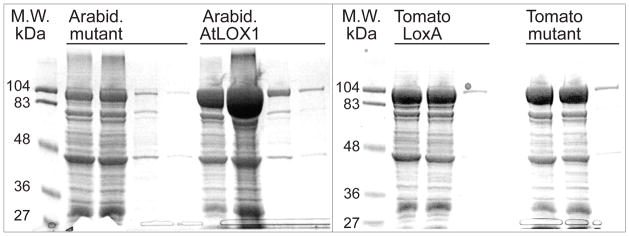
The cDNAs in the bacterial expression vector were prepared with an added N-terminal (His)6--tag as described in Methods. The two LOX enzymes expressed well in E. coli (2–5 mg LOX protein per 100 ml culture) and were partially purified by nickel affinity chromatography (Fig. 2). The Arabidopsis Ala562Gly mutant expressed at lower levels, although sufficient for evaluation of its activity and for analysis of products. In other SDS-PAGE analyses in which the LOX protein band was not overloaded (in contrast to Fig. 2), each LOX protein band at ~97 kD was quantified by in-gel assay (Odyssey Imaging Systems, see Methods), and this specifically quantified LOX protein was used in the calculation of activity turnover numbers.
Fig. 2.
SDS-PAGE gel analysis of LOX enzymes expressed in E. coli. The mutants refer to the AtLOX1 Ala562Gly and tomato LOXA Ala564Gly mutants. The three lanes for each sample represent aliquots (10 μl) from the first three 0.5 ml fractions collected from the nickel NTS column purification. Proteins were detected using Coomassie staining. Abbreviation: MW, molecular weight.
Catalytic activity and pH-activity profile
The pH dependence of AtLOX1 reacting with linoleic acid as substrate showed very similar rates over the pH range of 5 – 9, maximal at pH 6 (310 turnovers/sec). For tomato LOXA activity was optimal around pH 6 (390 turnovers/sec) and was maintained at least 30% and 15% of this optimal activity at pH 8 and pH 9, respectively. SP-HPLC analysis of the products from linoleic acid at pH 6 showed that 9S-HPODE accounted for over 95% of the products of both enzymes (Table 1, Fig. 3A, Fig. 4C). α-Linolenic acid reacted at similar rates and similarly formed almost exclusively the 9-hydroperoxide. For comparison to the reactions at the optimal pH 6, the distribution of products from a reaction with linoleic acid conducted at pH 9 was also analyzed by SP-HPLC. Remarkably, the product pattern was indistinguishable from the reaction products at pH 6, i.e. 9S-HPODE accounted for >95% of the products (data not shown). This is in contrast to the properties of soybean LOX-1, which is specific for 13S oxygenation at its optimal pH 9–10, but changes at lower pH (pH 6 or pH 7) to producing a mixture of 9- and 13-hydroperoxide products with both trans-trans and cis-trans conjugated dienes [16].
Table 1.
Linoleic acid products formed by A. thaliana AtLOX1 and tomato LOXA
| ENZYME | 9-HPODE (%) | 13-HPODE (%) |
|---|---|---|
| A. thaliana WT | 98.8 | 1.2 |
| A. thaliana A562G | 68.9 | 31.1 |
| Tomato WT | 99.1 | 0.9 |
| Tomato A564G | 59.9 | 40.1 |
| Chiral Analysis (%) | ||||
|---|---|---|---|---|
| 9R | 9S | 13R | 13S | |
| A. thaliana WT | 0.4 | 99.6 | 71.7* | 28.3 |
| A. thaliana A562G | 0.7 | 99.3 | 98.4 | 1.6 |
| Tomato WT | 0.3 | 99.7 | 67.7* | 32.3 |
| Tomato A564G | 0.9 | 99.1 | 98.6 | 1.4 |
Note that, as shown above, WT produces 13-HPODE as only ~1% of products
Fig. 3.
Normal phase HPLC analysis of linoleic acid products. Panel A: wild-type tomato LOXA product profile. Panel B: tomato LOXA Ala564Gly product profile. A Beckman Ultrasphere silica column (5μ, 25 × 0.46 cm) was eluted at 1 ml/min with hexane/isopropanol/glacial acetic acid (100/2/0.1, by volume) with UV detection at 235 nm. Abbreviation: TPP, triphenylphosphine
Fig. 4.
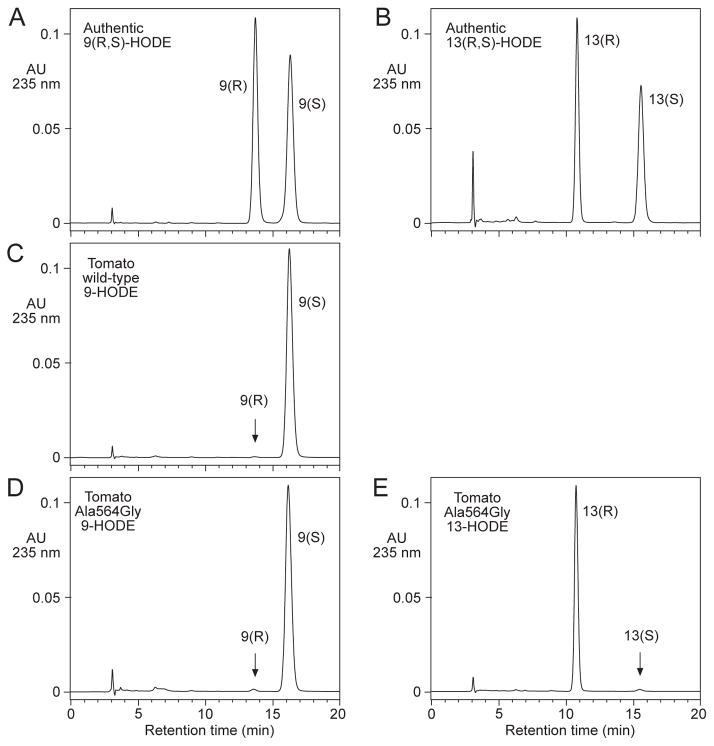
Chiral phase HPLC analysis of HODE methyl ester derivatives. Panels A and B: resolution of 9RS-HODE and 13RS-HODE, respectively. Panel C: 9-HODE from wild-type tomato LOXA. Panel D: 9-HODE from tomato LOXA Ala564Gly. Panel E: 13-HODE from tomato LOXA Ala564Gly. A Diacel Chiralpak AD column (5μ, 25 × 0.46 cm) was eluted at 1 ml/min with hexane/methanol (100/2, by volume) with UV detection at 235 nm.
Effects of active site Ala-Gly mutation
By alignment with other LOX enzymes, the residue equivalent to the Ala that has a major influence on reaction stereospecificity [9] is Ala562 in Arabidopsis 9-LOX and Ala564 in tomato 9-LOX. This Ala was changed to Gly by site-directed mutagenesis and the expressed enzymes were reacted with linoleic acid. Following reduction of the hydroperoxides, two similar-sized peaks of hydroxy product were obtained by normal phase HPLC (Fig. 3B), identified as 13-HODE and 9-HODE by co-chromatography with standards and by their characteristic identical UV spectra with lambda max 234 nm (cf. ref [17]). Chiral analysis showed the two products were almost purely the 13R-HODE and 9S-HODE enantiomers, respectively (Fig. 4, panels D and E).
Arachidonic acid metabolism
Plant 9-LOX tend to form a mixture of chiral hydroperoxides when arachidonic acid (an unnatural substrate for a plant) is used as substrate (e.g. ref [18, 19]). 9-LOX purified from potato tubers and one of the recombinant potato LOX isozymes give 5S-HPETE as the major hydroperoxide [20, 21]. Some other plant 9-LOX are reported to give 11S-HPETE as the major product [22]. As measured by the radioactive products formed from [14C]arachidonic acid (after NaBH4 reduction of the HPETEs to HETEs), we found that AtLOX1 formed 11-HETE as the major product (accounting for 47% of the total HETEs), together with half as much 5-HETE (23%), and in order of abundance also 9-HETE (11%), 12-HETE (9%), and 8-HETE and 15-HETE (4.5% each). Tomato LOXA formed 11-HETE and 5-HETE in essentially equal amounts (38–39% each), and also 8-HETE (11%), 12-HETE (4%), 15-HETE (3%) and 9-HETE (2%). The pattern of hydroperoxide products for the tomato LOXA is similar to that illustrated previously for a 9-LOX purified from tomato [6]. We also found that the product profiles for each wild-type 9-LOX with arachidonic acid as substrate were essentially the same at pH 7 and pH 9; this contrasts with the results shown for a recombinant rice 9-LOX isozyme in which the 5-HPETE product almost disappeared at high pH [23].
Catalytic activity with other substrates
Our results using arachidonate ethanolamide (anandamide) as substrate with the two recombinant 9-LOX enzymes are summarized in Table 2. The chiral analyses were performed after reduction to the hydroxy form, HANA, on a reversed-phase Chiralpak AD-RH column in which H(P)ETEs and their methyl ester derivatives are known to run with the R enantiomer eluting before the S enantiomer [24]. The same appeared to be true for anandamide derivatives, as confirmed using an authentic standard of 15S-HPANA prepared using soybean LOX-1, and from the fact that 11S-HPANA is the known product of purified 9-LOX from barley and tomato [6]. The most significant findings were that wild-type AtLOX1 converted anandamide mainly to 11S-HPANA (99.4% 11S, and accounting for 91% of the total HPANA products). The AtLOX1 Ala562Gly mutant formed 11S-HPANA (98.3% 11S) plus an additional prominent product, 15R-HPANA (93% 15R) in a 3:2 ratio in favor of 11-HPANA. Wild-type tomato LOXA also formed mainly 11S-HPANA (99.0% 11S, accounting for 71% of total HPANA products) plus 16% 5S-HPANA (89% 5S). The tomato LOXA Ala564Gly mutant formed two additional prominent products, 15R-HPANA (93% 15R, accounting for 34% of HPANA products) and 9-HPANA (19% of products). On chiral analysis the 9-HANA product chromatographed as one main peak with no definite minor enantiomer, so it was not possible to determine order of elution and infer chirality.
Table 2.
Distribution of 9-LOX products from anandamide (2A) and the chirality of selected products (2B)
| 2A | ||||||
|---|---|---|---|---|---|---|
| Enzyme | H(P)ANA products (% of total) | |||||
| 15 | 11 | 12 | 8 | 9 | 5 | |
| Tom WT | 5.5 | 71.8 | 1.8 | 3.6 | 1.3 | 15.9 |
| Tom Mut | 19.6 | 18.2 | 2.5 | 1.3 | 18.9 | 39.5 |
| Arab WT | 4.7 | 90.8 | 0.6 | 0.2 | 0.9 | 2.9 |
| Arab Mut | 34.1 | 59.4 | 1.1 | 0.3 | 1.7 | 3.5 |
| 2B | ||||||
|---|---|---|---|---|---|---|
| Enzyme | Chirality of 15-, 11- and 5-HANA (%) | |||||
| 15R | 15S | 11R | 11S | 5R | 5S | |
| Soybean | 2.5 | 97.5 | 15.5 | 84.5 | -a | - |
| Tomato WT | 53 | 47 | 1 | 99 | 11 | 89 |
| Tom Mut | 85 | 15 | 4 | 96 | 1.3 | 98.7 |
| Arab WT | 62 | 38 | 0.6 | 99.4 | 2.5 | 97.5 |
| Arab Mut | 93 | 7 | 1.7 | 98.3 | 6.6 | 93.4 |
not a product with soybean LOX1.
We also tested 1-palmitoyl-2-linoleoylphosphatidycholine as a potential substrate for AtLOX1 using the bile salt deoxycholate as detergent, a condition that achieves reaction with plant 13-LOX [25], coral 8R-LOX [9], as well as several mammalian arachidonate 12-LOX and 15-LOX [26–29]. However, we could detect no reaction with the recombinant 9-LOX, in agreement with a result reported for a purified tomato 9-LOX [19].
Discussion
The Arabidopsis genome contains six LOX genes (Table 3) and there are at least five LOX genes in tomato (Table 4). Although tomato has long been used as a source of 9-LOX [30], to our knowledge, the positional specificity, as determined by direct characterization of LOX reaction products, has not been reported for any recombinant Arabidopsis or tomato LOX. Our experimental analysis of purified recombinant AtLOX1 and tomato LOXA showed that both proteins function as specific linoleate 9-LOX. This finding is consistent with phylognetic analysis showing that AtLOX1 and tomato LOXA are closely related members of the plant LOX family (Fig. 5), and further predicts that other members of this subgroup are 9-LOXs as well. In an accompanying paper, Andreou et al characterize the activity of a related sequence from potato also as a linoleate 9-LOX [2].
Table 3.
Arabidopsis thaliana LOX genes
| Designation | GenBank Access. No. | AGI No.a | #amino acids | MW (Da) | N-terminal sequence | Positional specificity | References |
|---|---|---|---|---|---|---|---|
| AtLOX1 | NM_104376 | AT1G55020 | 859 | 98045 | MFGELRDLLT | 9 | [35], this paper |
| AtLOX2 | NM_114383 | AT3G45140 | 870 | 99036 | MYCRESLSSL | 13 | [36] |
| AtLOX3 | NM_101603b | AT1G17420 | 919 | 103710 | MALAKELMGY | 13 | c Feussner, 2nd 13-LOX (GenBank AJ249794) |
| AtLOX4 | AJ302042 | AT1G72520 | 926 | 104813 | MALANEIMGS | 13 | c Feussner, 3rd 13-LOX, chloroplastic, leaves |
| AtLOX5 | NM_113137d | AT3G22400 | 886 | 101058 | MIHTDIAEIL | 9 | c Feussner 2nd 9-LOX |
| AtLOX6 | NM_105423 | AT1G67560 | 917 | 104514 | MFVASPVKTN | 13 | c Feussner, plastid 13-LOX |
Gene designation at www.arabidopsis.org
GenBank entry AC007843 has 7 less N-terminal amino acids
Designations given in the GenBank entry
GenBank entry AJ302043 is the same sequence with 32 less N-terminal amino acids.
Table 4.
Tomato (Lycopersicon esculentum/Solanum lycopersicum) LOX genesa
| Designation | GenBank Access. No. | #amino acids | MW (Da) | N-terminal sequence | Positional specificity | References |
|---|---|---|---|---|---|---|
| LOXA | U09026 | 860 | 96764 | MLGQLVGGLI | 9S | cloned by [12], this paper |
| LOXB | U09025 | 859 | 97122 | MSLGGIVDAI | 13S | cloned by [12] |
| LOXC | U37839 | 896 | 101744 | MLKPQFQQST | n.s.b | [37] |
| LOXD | U37840 | 908 | 102292 | MALAKEIMGI | n.s.b | [37] |
| LOXE | AY008278 | 862 | 97505 | MILNKIVDSI | n.s. | [38] |
Genome sequencing currently in progress (2008)
n.s.: not specified
Fig. 5.
Phylogenetic tree of selected plant LOX enzymes. The enzymes include the six LOX in the Arabidopsis, the current five reported for tomato, ten soybean enzymes, and five sequences from potato. The enzymes studied in the present paper are shown in bold type. The asterisk marks a potato LOX studied in an accompanying paper [2]. GenBank accession numbers are shown.
The notion that AtLOX1 and tomato LOXA are homologous proteins is supported by similarities in their expression pattern. Both genes, for example, are expressed to relatively high levels in germinating seedlings [12, 31]. Our results thus support the hypothesis that 9-LOX activity plays a physiological role during the early stages of seedling growth. Plant 9-LOX has been implicated in a variety of developmental and stress-related processes [1, 32]. Recently, oxylipins derived from the 9-LOX pathway were shown to play a role in lateral root development and pathogen defense in Arabidopsis [33]. Our data are in agreement with the proposal by Vellosillo and co-workers that 9-LOX activity in Arabidopsis roots can be attributed to LOX1/LOX5 [33].
Using the AtLOX1 and tomato LOXA we re-examined the debated issue of the mode of substrate binding orientation in the active site of 9-LOX, and how this relates to formation of specific chiral products. According to the model we outlined in the Introduction [34], linoleic acid should bind with a carboxyl end-first orientation in the active site of linoleate 9S-LOX, the same conclusion as originally inferred from the hydrogen abstraction results of 1972 with corn 9S-LOX (cf. Fig. 1A) [4]. The model predicts that the Ala-to-Gly switch in 9S-LOX should produce a new product, 13R-HPODE, formed on the same face of the molecule as 9S-HPODE (cf. Fig. 1B). This is exactly what was observed (Fig. 2, Table 1).
We also re-examined the 9-LOX metabolism of anandamide, and also the consequences of the Ala-to-Gly mutation on the distribution of products. As noted in the Introduction, purified barley and tomato 9-LOX were reported to convert anandamide mainly to 11S-HPANA (11S-hydroperoxyanandamide), in contrast to their products with arachidonic acid, which were largely 5-HPETE for barley 9-LOX and 5-HPETE/11-HPETE mixtures for tomato 9-LOX. And, because it appears that the additional functional moiety at the carboxyl end has pushed oxygenation further down the carbon chain, these observations were used to infer that the substrate binding orientation in plant 9-LOX is tail end-first [6]. Although this is a reasonable conclusion, we offer a different interpretation. Whereas van Zadelhoff et al placed an emphasis on the conversion of arachidonic acid to 5S-HPETE by the linoleate 9-LOX [6], we note that formation 11S-HPETE is equally common with several of the wild-type enzymes (cf. refs. [6, 19, 22] and the Results section above). With anandamide, the balance of oxygenation products tips in favor of 11S-HPANA and in some cases 5S-HPANA remains prominent. Significantly, the Ala-to-Gly mutation induces the formation of 15R-HPANA along with the 11S-HPANA, and 9-HPANA along with 5-HPANA (Results, Table 3). These new findings fit perfectly with the orientation model that predicts carboxyl end-first binding of the substrate. Admittedly, this will require the “extra” ethanolamide moiety at C-1 to be accommodated in the 9-LOX active site, but our surmise is that this is the most straightforward interpretation of the available data.
Butovich and Reddy used the observation that linoleate 9-LOX can metabolize 1-linoleoyl-glycerol to the 9-hydroperoxide to propose the tail-first binding orientation [7]. As an extension of this conjecture, we tested 1-palmitoyl-2-linoleoyl-phosphatidylcholine and could find no reaction. While caution is warranted in interpretation of negative results, this lack of reaction classifies the linoleate 9-LOX along with other LOX enzymes that are predicted to exhibit carboxyl end-first binding and that show no reaction with very large phospholipid esters (arachidonate 8S-LOX and 5S-LOX) [9, 27]. (By contrast, all the LOX enzymes that have predicted tail-first substrate binding do react specifically with phospholipid ester substrates [5, 9, 27]). Again, our interpretation is that the 9S-LOX active site can accommodate substrates with C-1 appendages of the size of ethanolamide or glycerol, and that overall, the carboxyl end-first binding is the most satisfactory model for oxygenations by linoleate 9S-LOX enzymes.
Acknowledgments
We thank Kaye Peterman (Wellesley College) for kindly proving the AtLOX1 cDNA. This work was supported by NIH grants GM-53638 and GM-074888 (to ARB) and by the U.S. Department of Energy Grant DE-FG02-91ER20021 (to GAH).
Abbreviations
- HPODE
hydroperoxyoctadecadienoic acid
- HODE
hydroxyoctadecadienoic acid
- HPETE
hydroperoxyeicosatetraenoic acid
- HETE
hydroxyeicosatetraenoic acid
- HPANA
hydroperoxyanandamide
- HANA
hydroxyanandamide
- HPLC
high pressure liquid chromatography
- LOX
lipoxygenase(s)
- UV
ultraviolet
- CPM
counts per minute
- WT
wild-type
References
- 1.Feussner I, Wasternack C. The Lipoxygenase Pathway. Annu Rev Plant Biol. 2002;53:275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- 2.Andreou A, Hornung E, Rosahl S, Feussner I. On the Substrate Binding of a 9-LOX from Potato. Lipids. 2008 doi: 10.1007/s11745-008-3264-4. submitted. [DOI] [PubMed] [Google Scholar]
- 3.Schneider C, Pratt DA, Porter NA, Brash AR. Control of Oxygenation in Lipoxygenase and Cyclooxygenase Catalysis. Chem Biol. 2007;14:473–488. doi: 10.1016/j.chembiol.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egmond MR, Vliegenthart JFG, Boldingh J. Stereospecificity of the Hydrogen Abstraction at Carbon Atom n-8 in the Oxygenation of Linoleic Acid by Lipoxygenases from Corn Germs and Soya Beans. Biochem Biophys Res Commun. 1972;48:1055–1060. doi: 10.1016/0006-291x(72)90815-7. [DOI] [PubMed] [Google Scholar]
- 5.Brash AR, Ingram CD, Harris TM. Analysis of a Specific Oxygenation Reaction of Soybean Lipoxygenase-1 with Fatty Acids Esterified in Phospholipids. Biochemistry. 1987;26:5465–5471. doi: 10.1021/bi00391a038. [DOI] [PubMed] [Google Scholar]
- 6.van Zadelhoff G, Veldink GA, Vliegenthart JFG. With Anandamide as Substrate Plant 5-Lipoxygenases Behave Like 11-Lipoxygenases. Biochem Biophys Res Commun. 1998;248:33–38. doi: 10.1006/bbrc.1998.8910. [DOI] [PubMed] [Google Scholar]
- 7.Butovich IA, Reddy CC. Enzyme-Catalyzed and Enzyme-Triggered Pathways in Dioxygenation of 1-Monolinoleoyl-rac-Glycerol by Potato Tuber Lipoxygenase. Biochim Biophys Acta. 2001;1546:379–398. doi: 10.1016/s0167-4838(01)00162-5. [DOI] [PubMed] [Google Scholar]
- 8.Coffa G, Imber AN, Maguire BC, Laxmikanthan G, Schneider C, Gaffney BJ, Brash AR. On the Relationships of Substrate Orientation, Hydrogen Abstraction and Product Stereochemistry in Single and Double Dioxygenations by Soybean Lipoxygenase-1 and Its Ala542Gly Mutant. J Biol Chem. 2005;280:38756–38766. doi: 10.1074/jbc.M504870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffa G, Brash AR. A Single Active Site Residue Directs Oxygenation Stereospecificity in Lipoxygenases: Stereocontrol Is Linked to the Position of Oxygenation. Proc Natl Acad Sci USA. 2004;101:15579–15584. doi: 10.1073/pnas.0406727101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peers KF, Coxon DT. Controlled Synthesis of Monohydroperoxides by α-Tocopherol Inhibited Autoxidation of Polyunsaturated Lipids. Chem Phys Lipids. 1983;32:49–56. [Google Scholar]
- 11.Brash AR, Song W-C. Detection, Assay, and Isolation of Allene Oxide Synthase. Methods Enzymol. 1996;272:250–259. doi: 10.1016/s0076-6879(96)72030-x. [DOI] [PubMed] [Google Scholar]
- 12.Ferrie BJ, Beaudoin N, Burkhart W, Bowsher CG, Rothstein SJ. The Cloning of Two Tomato Lipoxygenase Genes and Their Differential Expression During Fruit Ripening. Plant Physiol. 1994;106:109–118. doi: 10.1104/pp.106.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutaud O, Brash AR. Purification and Catalytic Activities of the Two Domains of the Allene Oxide Synthase-Lipoxygenase Fusion Protein of the Coral Plexaura homomalla. J Biol Chem. 1999;274:33764–33770. doi: 10.1074/jbc.274.47.33764. [DOI] [PubMed] [Google Scholar]
- 14.Bligh EG, Dyer WJ. A Rapid Method of Total Lipid Extraction and Purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 15.Schneider C, Boeglin WE, Brash AR. Enantiomeric Separation of Hydroxy-Eicosanoids by Chiral Column Chromatography: Effect of the Alcohol Modifier. Anal Biochem. 2000;287:186–189. doi: 10.1006/abio.2000.4847. [DOI] [PubMed] [Google Scholar]
- 16.Gardner HW. Soybean Lipoxygenase-1 Enzymically Forms Both (9S)- and (13S)-Hydroperoxides from Linoleic Acid by a pH-Dependent Mechanism. Biochim Biophys Acta. 1989;1001:274–281. doi: 10.1016/0005-2760(89)90111-2. [DOI] [PubMed] [Google Scholar]
- 17.Ingram CD, Brash AR. Characterization of HETEs and Related Hydroxy-Dienes by UV Spectroscopy. Lipids. 1988;23:340–344. doi: 10.1007/BF02537345. [DOI] [PubMed] [Google Scholar]
- 18.Heydeck D, Wiesner R, Kühn H, Schewe T. On the Reaction of Wheat Lipoxygenase with Arachidonic Acid and Its Oxygenated Derivatives. Biomed Biochim Acta. 1991;50:11–15. [PubMed] [Google Scholar]
- 19.Regdel D, Kühn H, Schewe T. On the Reaction Specificity of the Lipoxygenase from Tomato Fruits. Biochim Biophys Acta. 1994;1210:297–302. doi: 10.1016/0005-2760(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu T, Rådmark O, Samuelsson B. Enzyme with Dual Lipoxygenase Activities Catalyzes Leukotriene A4 Synthesis from Arachidonic Acid. Proc Natl Acad Sci USA. 1984;81:689–693. doi: 10.1073/pnas.81.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Reddanna P, Reddy GR, Kidd R, Hildenbrandt G, Reddy CC. Expression, Purification, and Characterization of a Recombinant 5-Lipoxygenase from Potato Tuber. Biochem Biophys Res Commun. 1998;243:438–443. doi: 10.1006/bbrc.1998.8115. [DOI] [PubMed] [Google Scholar]
- 22.Reddy GR, Reddanna P, Reddy CC, Curtis WR. 11-Hydroperoxyeicosatetraenoic Acid Is the Major Dioxygenation Product of Lipoxygenase Isolated from Hairy Root Cultures of Solanum Tuberosum. Biochem Biophys Res Commun. 1992;189:1349–1352. [PubMed] [Google Scholar]
- 23.Zhang LY, Hamberg M. Specificity of Two Lipoxygenases from Rice: Unusual Regiospecificity of a Lipoxygenase Isoenzyme. Lipids. 1996;31:803–809. doi: 10.1007/BF02522975. [DOI] [PubMed] [Google Scholar]
- 24.Schneider C, Yu Z, Boeglin WE, Zheng Y, Brash AR. Enantiomeric Separation of Hydroxy and Hydroperoxy Eicosanoids by Chiral Column Chromatography. Methods Enzymol. 2007;433:145–157. doi: 10.1016/S0076-6879(07)33008-5. [DOI] [PubMed] [Google Scholar]
- 25.Eskola L, Laakso S. Bile Salt-Dependent Oxygenation Phospholipids by Soybean Lipoxygenase-1. Biochim Biophys Acta. 1983;751:305–311. [Google Scholar]
- 26.Schewe T, Halangk W, Hiebsch C, Rapoport SM. A Lipoxygenase in Rabbit Reticulocytes Which Attacks Phospholipids and Intact Mitochondria. FEBS Lett. 1975;60:149–153. doi: 10.1016/0014-5793(75)80439-x. [DOI] [PubMed] [Google Scholar]
- 27.Jung G, Yang DC, Nakao A. Oxygenation of Phosphatidylcholine by Human Polymorphonuclear Leukocyte 15-Lipoxygenase. Biochem Biophys Res Commun. 1985;130:559–566. doi: 10.1016/0006-291x(85)90453-x. [DOI] [PubMed] [Google Scholar]
- 28.Murray JJ, Brash AR. Rabbit Reticulocyte Lipoxygenase Catalyzes Specific 12(S) and 15(S) Oxygenation of Arachidonyl-Phosphatidylcholine. Arch Biochem Biophys. 1988;265:514–523. doi: 10.1016/0003-9861(88)90156-7. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi Y, Glasgow WC, Suzuki H, Taketani Y, Yamamoto S, Anton M, Kühn H, Brash AR. Investigation of the Oxygenation of Phospholipids by the Porcine Leukocyte and Human Platelet Arachidonate 12-Lipoxygenases. Eur J Biochem. 1993;218:165–171. doi: 10.1111/j.1432-1033.1993.tb18362.x. [DOI] [PubMed] [Google Scholar]
- 30.Matthew JA, Chan HW-S, Galliard T. A Simple Method for the Preparation of Pure 9-D-Hydroperoxide of Linoleic Acid and Methyl Linoleate Based on the Positional Specificity of Lipoxygenase in Tomato Fruit. Lipids. 1977;12:324–326. doi: 10.1007/BF02533358. [DOI] [PubMed] [Google Scholar]
- 31.Melan MA, Enriquez A, Peterman TK. The Lox1 Gene of Arabidopsis Is Temporally and Spatially Regulated in Germinating Seedlings. Plant Physiol. 1994;105:383–393. doi: 10.1104/pp.105.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howe GA, Schilmiller AL. Oxylipin Metabolism in Response to Stress. Curr Op Plant Biol. 2002;5:230–236. doi: 10.1016/s1369-5266(02)00250-9. [DOI] [PubMed] [Google Scholar]
- 33.Vellosillo T, Martínez M, López MA, Vicente J, Cascón T, Dolan L, Hamberg M, Castresana C. Oxylipins Produced by the 9-Lipoxygenase Pathway in Arabidopsis Regulate Lateral Root Development and Defense Responses through a Specific Signaling Cascade. Plant Cell. 2007;19:831–846. doi: 10.1105/tpc.106.046052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coffa G, Schneider C, Brash AR. A Comprehensive Model of Positional and Stereo Control in Lipoxygenases. Biochem Biophys Res Commun. 2005;338:87–92. doi: 10.1016/j.bbrc.2005.07.185. [DOI] [PubMed] [Google Scholar]
- 35.Melan MA, Dong X, Endara ME, Davis KR, Ausubel FM, Peterman TK. An Arabidopsis Thaliana Lipoxygenase Gene Can Be Induced by Pathogens, Abscisic Acid, and Methyl Jasmonate. Plant Physiol. 1993;101:441–450. doi: 10.1104/pp.101.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell E, Mullet JE. Characterization of an Arabidopsis Lipoxygenase Gene Responsive to Methyl Jasmonate and Wounding. Plant Physiol. 1993;103:1133–1137. doi: 10.1104/pp.103.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heitz T, Bergey DR, Ryan CA. A Gene Encoding a Chloroplast-Targeted Lipoxygenase in Tomato Leaves Is Transiently Induced by Wounding, Systemin, and Methyl Jasmonate. Plant Physiol. 1997;114:1085–1093. doi: 10.1104/pp.114.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen G, Hackett R, Walker D, Taylor A, Lin Z, Grierson D. Identification of a Specific Isoform of Tomato Lipoxygenase (TomloxC) Involved in the Generation of Fatty Acid-Derived Flavor Compounds. Plant Physiol. 2004;136:2641–2651. doi: 10.1104/pp.104.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]