Summary
In aging, immune responses are dramatically impaired, specifically the ability to produce protective antibodies. We previously showed that with age there is a B-cell intrinsic decrease in class switch recombination (CSR) because of a decrease in activation-induced cytidine deaminase (AID). One mechanism we have demonstrated for decreased AID includes increased mRNA degradation of the transcription factor E47, critical for AID transcription. Here, we show by means of a retroviral construct containing the DsRED reporter and the 3′UTR of E47 that the 3′UTR lowers mRNA expression, and particularly in B cells from old mice. This is the first demonstration that the E47 3′UTR directly regulates its degradation. The AID mRNA was not differentially regulated by degradation in aging. Therefore, we have here further established critical components for decreased AID with age. The major aim of this study was to establish conditions for the rescue of the intrinsic defect of aged B cells with retroviral addition of the coding region of E47 in splenic B cells to restore their ability to produce optimal AID and class switch to IgG. In this study, we show that young and old primary B cells overexpressing a stable E47 mRNA up-regulate E47, AID, and CSR and improve B-cell immune responses in senescent murine B cells. Our results provide a proof of principle for the rescue of intrinsic B-cell defects and the humoral immune response in senescence.
Keywords: aging, B cells, Ig class switch, transcription factors
Introduction
It is well established that humoral, as well as cellular, immune responses are dramatically impaired in aged mice and humans (Klinman & Kline, 1997; Pawelec et al., 2002; Cancro et al., 2009; Dorshkind et al., 2009; Frasca & Blomberg, 2009; Grubeck-Loebenstein et al., 2009). In aging, both local and systemic humoral immune responses are down-regulated (Nicoletti et al., 1991, 1993; Asanuma et al., 2001; Han et al., 2003). The ability to switch the Ig class (or isotype) is very important for the quality of an immune response, e.g. in effector functions such as antibody-dependent cell cytotoxicity (ADCC). In vitro class switch recombination (CSR) can be induced by stimulating B cells with appropriate combinations of mitogens, antibodies to the B-cell receptor or to a co-stimulatory receptor (CD40) and cytokines (Snapper et al., 1988; Frasca et al., 2004b). B lymphocytes lacking E2A activity have reduced ability to undergo CSR (Quong et al., 1999; Sayegh et al., 2003; Frasca et al., 2004b). The transcription factor E47 encoded by the E2A gene is one of the key players in reduced CSR in aging with the DNA-binding complex in activated splenic B lymphocytes being E47 homodimers (Frasca et al., 2003, 2004a). Our laboratory has shown that splenic B cells from senescent mice, in contrast to young adult mice, undergo significantly limited CSR (about four-fold reduction) to multiple secondary isotypes (IgG1, G2a, G3, and E) upon stimulation in vitro with anti-CD40/IL-4 or mitogens, e.g. lipopolysaccharide (LPS) (Frasca et al., 2004b). This observation occurred with a concomitant decrease (four-fold) in levels of E47 mRNA and protein leading to a decrease in activation-induced cytidine deaminase (AID) (Frasca et al., 2004b). Activation-induced cytidine deaminase is important for CSR and somatic hypermutation (SHM) (Muramatsu et al., 2000; Diaz & Storb, 2003; Teng & Papavasiliou, 2007) and has been shown to be positively regulated by HoxC4, Pax5, and E47 (Sayegh et al., 2003; Park et al., 2009; Tran et al., 2010).We previously showed that the decrease of E47 in senescence is because of a decrease in mRNA stability (Frasca et al., 2005) and to an increase in tristetraprolin (TTP), a physiologic regulator of mRNA stability (Frasca et al., 2007). Tristetraprolin binds the 3′UTR of mRNA of cytokines and transcription factors (Lai et al., 2006; Sandler & Stoecklin, 2008). The experiments in this paper extend these studies to show directly that the 3′UTR of E47 mRNA contributes to its degradation in a B-cell line as well as in primary B cells from old mice, and thus support the mechanism of E47 mRNA regulation in aging. Defects we see in the down regulation of E47, AID, and CSR in aging are not caused by changes in numbers of follicular (FO) and marginal zone (MZ) B cells (Frasca et al., 2007). We also provide evidence that it is possible to reverse the deficient CSR seen in old mice by retrovirally transducing primary B cells with a vector containing a stable form of the E47 mRNA, without the 3′UTR. Our hypothesis is that over expression of a stable form of E47 mRNA will lead to an increase in E47 protein and increased transcription of AID. In this study, we also show that the reduced levels of AID mRNA in aging are not because of differences in its stability which is consistent with the mechanism for decreased AID being a decreased rate of its transcription. This report provides a first mechanistic proof of principle for rescue of aged B-cell immune responses in mice and humans.
Results
E47 3′UTR targets mRNA for degradation
Our previously published data show that there is an increased rate of degradation of E47 mRNA in splenic B cells of old mice (Frasca et al., 2005). E47 is a crucial transcription factor for AID, and likely because of this we also saw a decrease in AID expression in old splenic B cells. In order to directly demonstrate that the 3′UTR of E47 can be responsible for targeting degradation of E47 mRNA, we designed a retroviral construct containing DsRED with either the E47 or ku80 (Fig. 1A) 3′UTR at its 3′ terminus. The ku80 mRNA is very stable and is not differentially regulated in young and old splenic B cells (Frasca et al., 2005). After transducing the BJAB cell line (human Burkitt B-cell lymphoma) with the DsRED control vector, DsRED-E47 3′UTR or DsRED-ku80 3′UTR, we observed that the DsRED-E47 3′UTR had five-fold less DsRED mRNA than the DsRED vector as determined by qPCR (Fig. 1B). These results directly show that the E47 3′UTR confers instability of a target mRNA in a B cell line and we postulate would also be a mechanism for E47 mRNA instability in primary B cells from old mice (see also below).
Fig. 1.
Construction of DsRED vectors. (A) DsRED-E47 3′UTR or –ku80 3′UTR retroviral vector. A retroviral vector pRevTRE- was used to prepare the E47 3′UTR construct by inserting the 638-bp fragment containing the cDNA for mouse E47 3′UTR or the ku80 3′UTR construct by inserting the 235-bp fragment containing the cDNA for mouse ku80 3′UTR see Materials and Methods. The → denotes the direction of transcription. The Psi (Ψ) is the retroviral packaging signal, the promoter (⇨) is a minimal CMV promoter directly upstream from the red fluorescent protein (DsRED), denoted by (■). B. The 3′-UTR of E47 induces mRNA degradation of DsRED. BJAB cells were left untransduced, transduced with the DsRED vector control, or with DsRED-ku80 3′UTR, or DsRED-E47 3′UTR vectors. BJAB cells were then assessed for DsRED RNA and DNA by qPCR. Results are expressed as DsRED qPCR values, normalized to GAPDH first and also to DsRED DNA, as in Materials and Methods, to take into account potential differences in transduction efficiency. Results are referred to DsRED qPCR values taken as 100. Vertical bars are means ± SE from three independent experiments. The difference between DsRED vector control and DsRED-ku80 3′UTR is not statistically significant (P = 0.07). The difference between DsRED vector control and DsRED-E47 3′UTR is statistically significant (P = 0.0008).
AID mRNA is not differentially degraded in B cells of young and old mice
To evaluate the mechanism of AID down regulation in old vs. young B cells, we measured AID mRNA stability. These initial studies were critical for the overall objective of this report, to possibly rescue the defects in IgG and AID in aged murine B cells. Splenic B cells from young and old mice were first activated for 5 days with LPS + IL-4 and then treated with Actinomycin D for 0, 10, 45, and 90 min prior to RNA extraction. Quantitative PCR (qPCR) for AID was then performed. Activation-induced cytidine deaminase mRNA has a short half-life of 10 min, but is not differentially degraded in young and old B cells (Fig. 2). These results are consistent with the transcription regulation of AID and the molecular regulation of E47 with age being primarily responsible for the decreased levels of AID as we have seen a 1:1 correlation between E47 and AID in aged human B cells (Frasca et al., 2008a) as well as E47 being the major regulator of AID in aged murine B cells (Frasca et al., 2004b, 2010b). In order to obtain a direct correlation in this paper, we over-expressed E47 to test if it would be sufficient to up-regulate AID in aged primary B cells.
Fig. 2.
Activation-induced cytidine deaminase (AID) mRNA stability in young and old splenic B cells is similar. Splenic B cells from young and old mice were stimulated with lipopolysaccharide (LPS) + IL-4 for 5 days. Cells were then treated with Actinomycin D for 0, 10, 45, and 90 min, and harvested for RNA extraction and qPCR. Results are expressed as AID RNA expression normalized to GAPDH ± SE from three pairs of young (□) and old (◆) mice. Both young and old untreated samples are expressed as 100% at time 0, but the actual value of the old is 23 ± 5%when compared to the young. The difference between young and old mice is not significant at any Actinomycin D time point, as determined by the two-tailed Student’s t test.
Transduction efficiency is comparable in young and old B cells and the E47 vector rescues E47protein levels in old B cells
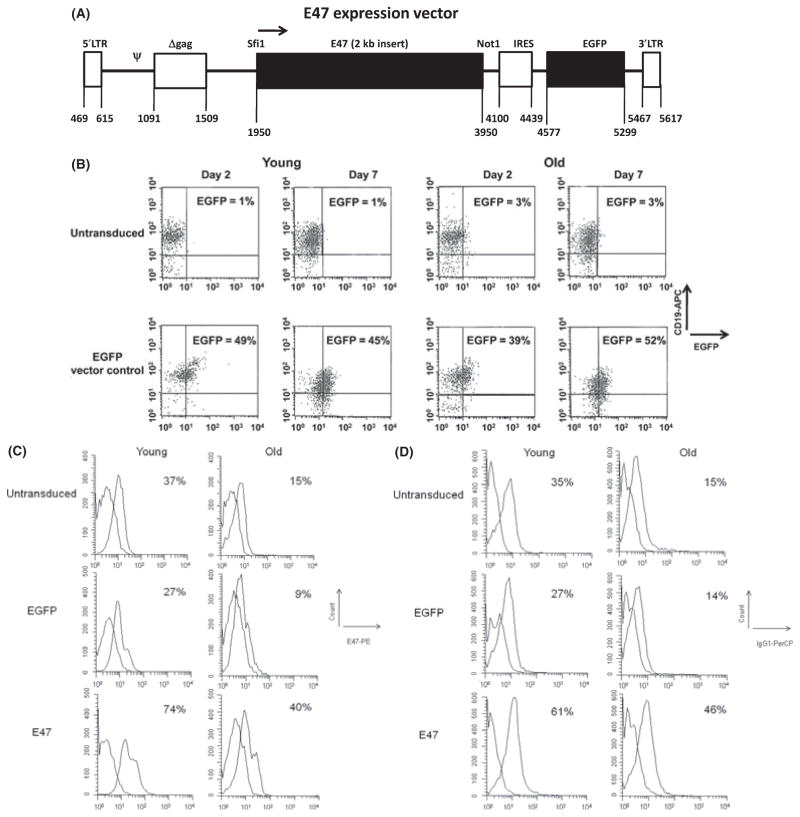
Next, we wanted to investigate whether a retroviral vector containing only the stable portion of the E47 mRNA would be able to rescue levels of E47, AID, and CSR after transduction of splenic B cells from old mice and from young mice as a control. A diagram of the E47 vector used in these experiments is shown in Fig. 3A. To check the effectiveness of the transduction, the following experiment was performed: Splenic B cells from young and old mice were first activated for 24 h with LPS + IL-4, transduced and then cultured for an additional 1–7 days with LPS + IL-4. Figure 3B is a representative figure for the FACS analysis of CD19 positive cells at day 2, with 26–55% (41% average) of the young or 39–56% (46% average) of the old cells transduced with the enhanced green fluorescent protein (EGFP) vector control being EGFP positive. There were no significant differences between young and old B cells in transduction frequency. At day 7, the percentage of EGFP positive B cells was 40–73% (54% average) in the young or 25–70% (52% average) in the old cells. It is important to note that at least up to day 7 post-transduction, the cells retained expression, and EGFP positive cells were equally expressed in the expanding population (Fig. 3B).
Fig. 3.
E47 retroviral vector and E47 rescue in aged splenic B cells. (A) A bicistronic retroviral vector pMXs-IG was used to prepare our construct pMXs-E47-IG (E47 vector) by inserting the 2-kb fragment containing the cDNA for mouse E47 without its 5′ and 3′UTR into the Sfi I and Not I sites. The → denotes the direction of transcription. The promoter is within the 5′ long terminal repeat (LTR), Psi (Ψ) is the packaging signal, Δgag is a truncated gag sequence, E47 and the enhanced green fluorescent protein (EGFP) are denoted by (■). (B) Transduction efficiency in young and old B cells is comparable. Splenic B cells were activated with LPS and IL-4 for 24 h and then left untransduced (top panels) or transduced with the EGFP vector control (bottom panels). Days indicate days after transduction. The y axis shows Flow cytometric expression for CD19-APC and the x axis shows EGFP expression. The % EGFP+ for both the CD19+ and CD19− populations is given (in the CD19+ quadrant). Data are representative of cells from five young and six old mice, and averages are given in the text. (C) Retroviral transduction with E47 elevates E47 in young and old B cells. Splenic B cells for (C) and (D) were treated as in (B) but with the addition of the E47-vector-transduced group. This is a representative figure from five young and six old mice showing the E47 intracellular staining at day 2 post-transduction (peak). Isotype controls were used to set the gate for the E47 staining and subtracted from the E47 percentage before young and old fold difference comparison (see Results). Cell counts (y axis) and E47 expression (x axis) are shown. The%E47 is from all cells derived from the CD19+ cultured cells. Untransduced and EGFP-transduced young or old B cells are not statistically different, as determined by the two-tailed Student’s t test, n = 5. The difference between young and old untransduced is significant at P = 0.01. The difference between young vector control and young E47 vector transduced is significant at P = 0.001. The difference between old vector control and old E47 vector transduced is significant at P = 0.01. The difference between young untransduced and old E47 transduced is not significant showing the rescue of the depleted old phenotype for E47. (D). E47 transduction elevates IgG in young and old B cells. This is a representative figure of IgG1 membrane staining at day 7 from five young and six old mice. Isotype controls were used to set the gate for the IgG1 staining, and subtracted from the IgG1 percentage before young and old fold difference comparison (see Results). Cell count (y axis) and IgG1 (x axis) expression are shown. Untransduced and EGFP-transduced young or old B cells are not significantly different as determined by the two-tailed Student’s t test. The difference between young and old untransduced is significant at P = 0.02. The difference between young vector control and young E47 vector transduced is significant at P = 0.01. The difference between old vector control and old E47 vector transduced is significant at P = 0.03. The difference between young vector control and old E47 transduced is not significant supporting a rescue in class switch recombination (CSR) and IgG expression.
In the representative Fig. 3C, we demonstrate the effectiveness of the E47 vector by FACS cytoplasmic E47 staining at day 2 (peak for E47 expression) which is increased in B cells of both young and old mice when compared to the untransduced controls. The average of five young and six old mice showed no significant differences between untransduced and transduced EGFP vector control splenic B cells here and for all subsequent experiments, and therefore our reported fold differences use the untransduced control for comparisons. These results demonstrate that untransduced levels of E47 in old B cells are lower, here about 2.3-fold (1.6–4.7), from that in young. The levels of both E47 in young and old E47-transduced B cells were increased over their vector controls in average by 2.1-fold (1.9–2.7) in the young and by 2.9-fold (2.0–4.4) in the old. Most importantly, the E47 protein levels in the E47-transduced old B cells were increased/rescued to young-like untransduced levels.
The representative Fig. 3D shows that at day 7 post-transduction (peak for IgG), the increase in E47 leads to an increase of surface IgG1 in young and old B cells transduced with the E47 vector vs. the untransduced control. Both the levels of E47 and IgG1in old have been elevated to levels similar or higher than that of the young untransduced controls. The average percent of IgG1 membrane expression for both young and old untransduced control primary splenic B cells is 2.3-fold (1.7–2.9) lower in the old when compared to young. The levels of IgG1 in both young and old E47-transduced B cells were significantly increased over their vector control by about 1.9-fold (1.5–2.7) in the young and by about 2.9-fold (2.0–5.2) in the old. Most importantly, the membrane IgG1 levels in the E47-transduced old B cells were elevated/rescued to young-like levels.
Transduction of both young and old B cells with E47 significantly increases E47 and AID mRNA levels
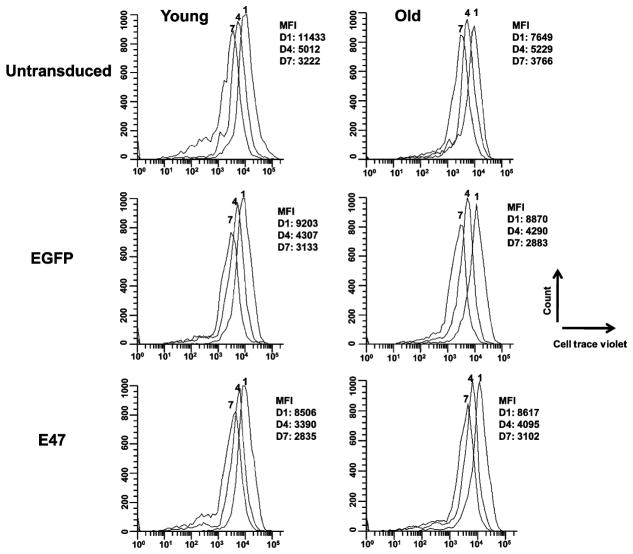
Quantitative PCR (qPCR) was used to measure mRNA levels for both E47 and AID in untransduced and transduced primary splenic B cells. E47 qPCR confirmed the increased levels of E47 mRNA. Activation-induced cytidine deaminase qPCR was critical to demonstrate its involvement in the molecular pathway by which increased E47 and AID lead to increased IgG observed in the old E47-transduced B cells. The E47 qPCR results show a 2.3- or a 3.6-fold increase in E47 mRNA expression in young or old E47-transduced cells when compared to the EGFP vector control, respectively (Fig. 4A). The EGFP vector control was subsequently used as the control. These results show that the mRNA levels of E47 in the old transduced B cells have been increased/rescued to levels comparable to those of young untransduced B cells, consistent with flow protein data (Fig. 3C). The exogenous E47 expressed in the old E47 transduced splenic B cells is more stable than that of untransduced cells as shown by treatment with Actinomycin D (Fig. 4B). The exogenous E47 levels were determined by subtracting the E47 mRNA levels of the untransduced samples from the E47-vector-transduced samples. The young untransduced and E47-vector-transduced B cells have completely overlapping curves of degradation with a t1/2 of 24 and 28 min respectively, while the old untransduced had a t1/2 of 7 min and the old E47-vector-transduced had a t1/2 of 36 min. Consistent with increased E47 mRNA/protein levels after transduction with the E47 vector, we observed about a 2.0-fold increase in both young and old AID qPCR at day 7 when compared to the untransduced control B cells (Fig. 5). The increase in AID is consistent with its regulation by E47 in these experiments. In order to rule out that the relatively modest differences between the transduced and untransduced cells was not because of simply differences in either cell division or apoptosis caused by E47 overexpression, we performed the following experiments. Figure 6 shows that the rate of proliferation (as measured by CellTrace Violet) is not significantly different between the untransduced, EGFP- or E47-vector-transduced cells. The proliferation rates seen here are less than those we and others have previously seen with CFSE loading at the same time as LPS + IL-4 stimulation. This is likely due to two things: (i) The CellTrace violet dye is less efficient than CFSE as we have observed (data not shown), but was necessary because cells were EGFP positive and (ii) The time of loading the dye at day 2 after stimulation was suboptimal, but we chose this time to avoid any variations and interference with the transduction. In Table 1, we show that the rate of apoptosis (measured by Annexin V) is not significantly different between the untransduced, EGFP- or E47 vector transduced cells. Therefore, the above increases in AID are not simply because of increased cell proliferation or increased viability of the E47-transduced cells.
Fig. 4.
(A) Transduction of both young and old B cells with E47 significantly increases E47 mRNA levels. Splenic B cells from young and old mice were treated as in Fig. 3C. At day 2 after transduction, cells were harvested and qPCR was performed. Results are E47 mRNA expression normalized to GAPDH ± SE from five young and six old mice, untransduced (white bars), EGFP transduced (striped bars) or E47 transduced (black bars). Results are normalized to young untransduced taken as 100%. Untransduced and EGFP-transduced B cells (either for young or old) are not statistically different, as determined by the two-tailed Student’s t test. The difference between young and old untransduced is significant at P = 0.0001. The difference between young untransduced and young E47 transduced is significant at P = 0.036. The difference between old untransduced and old E47 transduced is significant at P = 0.005. The difference between young untransduced and old E47 transduced is not significant, consistent with the flow data (Fig. 3C) B. Exogenous E47 mRNA stability is increased in old E47-vector-transduced-splenic B cells. Splenic B cells from young and old mice were stimulated with LPS + IL-4 and either left untransduced or transduced with the E47-vector. Two days post-transduction, cells were treated with Actinomycin D for 0, 30 and 90 min, and harvested for RNA extraction and qPCR. Here, the endogenous E47 is represented by (◆) and the exogenous E47 is represented by (□). The exogenous E47 levels were determined by subtracting the E47 mRNA levels of the untransduced samples from the E47-vector-transduced samples. Results are expressed as E47 mRNA normalized to GAPDH ± SE from three pairs of young and old mice. Both young and old untreated samples are expressed as 100% at time 0, but the actual % expressed relative to the young untransduced values were: for the untransduced young 95 ± 2, the E47-vector-transduced-young 235 ± 26, the untransduced old 42 ± 5, and the E47-vector-transduced-old 130 ± 7. The difference of stability between endogenous and exogenous E47 levels in the young is not significant at any Actinomycin D time point, as determined by the two-tailed Student’s t test. Difference in stability between endogenous and exogenous E47 levels in the old is significant at all Actinomycin D time points, as determined by the two-tailed Student’s t test at 0 min P = 0.0004, 30 min P = 0.01, and 90 min P = 0.04.
Fig. 5.
Transduction of both young and old B cells with E47 increases AID mRNA levels. Splenic B cells from young and old mice were treated as in Fig. 3C. At day 7 post-transduction, cells were harvested and qPCR was performed. Results are expressed as AID mRNA qPCR normalized to GAPDH±SE from five young and six old mice, untransduced (white bars), EGFP transduced (striped bars) or E47 transduced (black bars). Young untransduced are taken as 100%. The difference between young and old untransduced is significant at P = 0.02. The difference between young untransduced and young E47-vector transduced is significant at P = 0.005. The difference between old untransduced and old E47-vector transduced is significant at P = 0.01. The difference between young untransduced and old E47 transduced is not significant, again showing rescue, here of AID, in the old B cells with E47 transduction.
Fig. 6.
Transduction of young and old splenic B cells does not change the cell proliferation rates. Splenic B cells were activated with lipopolysaccharide and IL-4 for 24 h and then left untransduced or transduced with enhanced green fluorescent protein vector control or E47-vector. The cells were loaded with CellTrace Violet 24 h post-transduction and assayed after 1, 4, and 7 days by flow cytometry. This is a representative figure of stimulated B cells from three young and three old mice.
Table 1.
Transduction does not affect apoptosis in splenic B cells
| % of Annexin V positive cells | Day 1 (%) | Day 4 (%) | Day 7 (%) |
|---|---|---|---|
| Young untransduced | 5 ± 0.23 | 5.6 ± 0.1 | 12.1 ± 0.82 |
| Young EGFP | 4.9 ± 0.32 | 5.2 ± 0.15 | 13 ± 0.74 |
| Young E47 | 5.6 ± 0.23 | 5.9 ± 0.2 | 11 ± 0.71 |
| Old untransduced | 5.4 ± 0.15 | 5.6 ± 0.2 | 15 ± 1.35 |
| Old EGFP | 5.3 ± 0.26 | 5.3 ± 0.07 | 15 ± 0.78 |
| Old E47 | 5.8 ± 0.51 | 5.9 ± 0.44 | 14 ± 1 |
This is an average from three pairs of young and old mice ± SEM. (For the young untransduced vs. young E47 transduced at day 1, P = 0.03, day 4, P = 0.11, and day 7, P = 0.13 and for the old untransduced vs. old E47 transduced at day 1: P = 0.4; day 4: P = 0.5; and day 7: P = 0.3). CD19+ B cells from the spleens of young and old mice were isolated, cultured with LPS + IL-4, and either left untransduced, transduced with the EGFP vector control or the E47-vector. At days 1, 4, and 7 post-transduction, cells were harvested and stained with Annexin V-APC and assayed by flow cytometry.
IgG1 secretion is increased in both young and old B cells transduced with E47
To confirm the function of the E47 vector-transduced B cells and their antibody secreting progeny, ELISAs for IgG1 were performed. IgG1 production was reduced in the supernatants of cultured EGFP vector control transduced splenic B cells from old when compared to young mice (Fig. 7). After transduction with the E47 vector, splenic B cells of young and old mice showed about a 4.2- and a 4.5-fold increase in IgG1 production, respectively, when compared to the controls at day 7 (Fig. 7). These results show proof of principle for rescue of old B cell function.
Fig. 7.
Production of IgG1 measured by ELISA was up-regulated in both young and old B cells after transduction with E47. Splenic B cells from young and old mice were treated as in Fig. 3C. At day 7 post-transduction, cell supernatants were collected and assayed by ELISA to evaluate IgG1 production. Vertical bars are means ± SE from five young and six old mice, EGFP transduced (striped bars) or E47 transduced (black bars). The EGFP vector control is represented here as the control, because the untransduced and EGFP controls are not statistically different and only the vector control supernatants were assayed here. The difference between young and old EGFP transduced is significant at P = 0.008. The difference between young EGFP transduced and young E47 transduced is significant at P = 0.007, as determined by the two-tailed Student’s t test. The difference between old EGFP transduced and old E47 transduced is significant at P = 0.0014. The difference between young EGFP transduced and old E47 transduced is not significant P = 0.08, again showing the rescue of the old phenotype.
Discussion
The purpose of this study was to establish conditions to reverse/rescue defects in the humoral immune system with age, here in mice. The humoral immune system in humans and mice is deficient with advanced age, showing progressive decline at > 18 months in mice and over 65 years of age in humans. This deficiency leads to increased susceptibility to infections and poor response to vaccines, among others. Our laboratory has identified key molecular markers for aging B cells; decreases in E47, AID, CSR, and IgG in mice (Frasca et al., 2003, 2004a,b) and humans (Frasca et al., 2008a,b). Decreased levels of E47 and AID in aged mice as well as a two-fold reduction of E47 in E2A heterozygous (±) knockout mice and a four-fold reduction in the E2A ± old mice are correlated with reduced CSR (Frasca et al., 2004b, 2010b).
E47 is a key regulator of B-cell development, AID transcription, and CSR (Quong et al., 1999; Sayegh et al., 2003; Frasca et al., 2004b, 2005b, 2007; Cancro et al., 2009). In aged murine splenic B cells, E47 is decreased because of decreased mRNA stability (Frasca et al., 2005). Our previous preliminary data are consistent with the increased rate of decay of the E47 mRNA in B cells from old mice being because of increased TTP binding to the E47 3′UTR (Frasca et al., 2007). We have directly tested and confirmed here that the 3′UTR of E47 can cause mRNA to be targeted for degradation by using the reporter DsRED retroviral vector with the E47 3′UTR, as opposed to little or no degradation induced with the ku80 3′UTR. Because of this repressive mechanism of E47 in splenic B cells of old mice, our hypothesis here was that E47 without a 3′UTR would indeed be a more stable mRNA capable of rescuing levels of E47, AID, and CSR in aging. We also show here that exogenous E47 without the 3′UTR had increased mRNA stability at least in the old E47-vector-transduced B cells. This increased stability of the exogenous retroviral E47 in splenic B cells from old mice is likely due to the absence of the 3′UTR which cannot be degraded by the increased amount of the active, unphosphorylated TTP. The young E47-vector-transduced B cells have increased levels of E47 mRNA because of increased transcription, but no difference in the mRNA stability between endogenous and exogenous E47, likely because they have lower levels of TTP.
The decrease of E47 in B cells of old mice leads to a reduction in the transcription of AID. We show here that the decrease seen in AID is not because of increased decay of AID mRNA in aging as the rate of AID mRNA decay is comparable in young and old splenic B cells.
Using the E47 retroviral expression vector (without the 3′UTR), we detected successful transduction of stimulated B cells by measuring EGFP. Expression of E47 was confirmed by flow analyses as well as E47 qPCR of transduced young and old B cells. The qPCR experiments showed optimal levels of AID mRNA at day 7 after LPS+IL-4 stimulation (kinetic data not shown). Others’ previous results have shown an increase in AID upon transduction with E47 (Sayegh et al., 2003). The current results have significant additional information: here, we emphasize correcting the defect in old mouse B cells, the previous study used only cells from young mice and at suboptimal times for AID (29 h post-transduction). It was not intuitively obvious that increasing E47 would ‘rescue’ the elderly B-cell defects of AID, CSR, Ig expression, and secretion. These results are the first to show the importance of being able to correct/rescue the aged B-cell defects and are an essential precursor/proof of principle for future studies in mice and humans to improve immune responses in compromised individuals including the elderly.
As a consequence of transduction with the E47 vector, both young and old E47-transduced splenic B cells showed a significant increase in AID and IgG1. This increase in AID and IgG1 is not because of increased proliferation or increased viability of the E47-vector-transduced B cells as we have shown here with the CellTrace Violet cell proliferation assay and by Annexin V staining. The ELISA results clearly show an age-related decrease in IgG1 production in splenic B cells as we have previously shown with different stimulation protocols (Frasca et al., 2004b). The amount of IgG1 secreted by old splenic B cells transduced with the E47 vector was about 4.5-fold higher than that of the vector control but also 1.8-fold higher than that of young vector control, showing that we could rescue the deficient old response to that equivalent to or above that of the young cells. The relatively higher increased values of IgG1 when compared to AID in E47-transduced cells may reflect additional positive effects of E47 on Ig heavy chain transcription as the intronic and 3′ enhancers contain sites for E47 (Ephrussi et al., 1985; Meyer et al., 1995).The experiments presented here show dramatic effects of restoring the intrinsic E47 defect in old B cells to improve antibody class switch and Ig expression/secretion.
The benefits of rescuing levels of E47 and AID should not only lead to increases in CSR but also in SHM as AID is critical for SHM (Muramatsu et al., 2000; Honjo et al., 2002; Diaz & Storb, 2003; Teng & Papavasiliou, 2007). Somatic hypermutation is key for diversity allowing the B-cell receptor to recognize and react with high affinity to subsequent antigenic encounter. The results presented here are significant because CSR is critical for changing the effector function of the Ig, regulating among other things ADCC, macrophage phagocytosis, and antigenic presentation. Because B cells in aged mice and humans have an intrinsic defect in CSR and AID, we hypothesize that this will contribute to deficits in vaccine responses and titers in the elderly. Our recent results show that the serum antibody response to influenza vaccination, both seasonal (Frasca et al., 2010a) and H1N1 (manuscript in preparation), correlates with the ability of B cells in vitro to make a good AID response both to CpG at time 0 (before vaccination) and to the vaccine at day 28 after vaccination. In conclusion, our results offer a model system for strategies to improve the immune response in the elderly.
Experimental procedures
Mice
Young (2–4 months of age) and old (24–27 months of age) male and female BALB/c mice were purchased from the NIA/NIH. All old mice used in the study were phenotypically old determined by bone marrow phenotyping for low numbers/percentages of pre-B cells (Van der Put et al., 2004). This correlates with their B-cell function (Frasca et al., 2003), but the number of mature B cells are similar in young and old mice (Frasca et al., 2007). There were no differences observed between female and male mice. All studies adhered to the principles of laboratory animal care guidelines and were IACUC approved.
Cell lines
The BJAB (human Burkitt B-cell lymphoma) cell line was cultured in complete RPMI 1640 medium, supplemented with 10% heat-inactivated fetal calf serum (FCS), 10 μg mL−1 penicillin/streptomycin, 2 × 10−5 M 2-ME, 2 mM L-glutamine, and 1 mM sodium-pyruvate (Invitrogen-GIBCO; Carlsbad, CA, USA).
The PT67 retroviral packaging cell line (BD Biosciences, San Jose, CA, USA), a cell line encoding the polytropic envelope, was cultured in complete Dulbecco’s Modified Eagle Medium (DMEM) supplemented as above but with tetracycline-free FCS (BD Biosciences). This cell line was needed in order to produce the retrovirus for use with human cell lines and is the optimal packaging cell line sold for the pRev-TRE retroviral system (see the following paragraph for DsRED retroviral constructs).
The ΦNXE ecotropic retroviral packaging cell line based on 293T cells (Pear et al., 1993) (from Dr. G. Nolan, Stanford University, Palo Alto, CA, USA) was cultured in complete DMEM medium supplemented as described earlier. This cell line has been characterized and optimized for use with the pMXs retro-viral system.
Splenic B-cell enrichment
B cells were isolated from the spleens of young and old mice. Briefly, cells were washed twice with medium (RPMI 1640) and incubated for 20 min at 4 °C with anti-CD19 Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the Mini-Macs protocol (Miltenyi Biotec) (20 μL Microbeads + 180 μL PBS, for 107 cells). Cells were then purified using magnetic columns. At the end of the purification procedure, cells were found to be almost exclusively (95–98%) B220-positive by cytofluorimetric analysis.
B cell culture
B cells were cultured in complete RPMI 1640 medium, as above with addition of 1% non-essential amino acids (Invitrogen-GIBCO). Cells (106 mL−1) were stimulated in flat-bottom 6-well culture plates with LPS (10 μg mL−1) (Sigma-Aldrich, St. Louis, MO, USA) and IL-4 (50 ng mL−1) (R&D Systems, Minneapolis, MN, USA), for 1–8 days. At the end of each stimulation time, B cells were counted in trypan blue to evaluate viability which was found comparable in cultures of young and old B cells (within10%). The cell numbers at day 7 in young and old stimulated B cells are not significantly different and do not account for the reduction in E47/AID/IgG in old (data not shown).
ELISA
Young and old untransduced, E47- or EGFP-vector-control-transduced splenic B cells were stimulated with LPS and IL-4 for 7 days and culture supernatants were collected. IgG1 in the collected supernatants was analyzed by ELISA according to a sandwich protocol previously described (Frasca et al., 2004b).
Flow cytometry
Samples of 1 × 105 E47- or EGFP-vector-control-transduced splenic B cells were washed once with PBS and stained with 20 μL of 1/40 diluted APC-conjugated anti-CD19 (550992; BD Biosciences), and biotin-conjugated anti-IgG1 (553441; BD Biosciences), subsequently revealed using PerCP-Streptavidin (554064; BD Biosciences). After 20 min at 4 °C, cells were fixed and permeabilized for intracellular staining with PE-conjugated anti-E47 (552511; BD Biosciences). Cells were then fixed in 0.25% paraformaldehyde, washed with FACS buffer, resuspended and analyzed on the LSRI (BD Biosciences) using logarithmic amplification. Cells were also analyzed by flow cytometry for EGFP expression. Single color controls were used for compensation purposes. Analysis of EGFP, E47 (peak at day 2 post-transduction), and IgG1 (peak at day 7 post-transduction, day 8 post-stimulation with LPS + IL-4) was performed gated on live cells. Percentages of E47 and IgG1 seen in E47-transduced cells reflect both endogenous and E47-vector-induced expression.
For cell proliferation and apoptosis studies, one day after transduction, samples of 8 × 106 untransduced, E47- or EGFP-vector-control-transduced splenic B cells were loaded with 5 μM CellTrace Violet (Invitrogen-GIBCO) in PBS for 20 min at 37 °C, protected from light. The reaction was then quenched by adding five times the original staining volume of complete culture medium to the cells and incubating them for 5 min. Cells were then washed and cultured up to seven additional days stimulated with LPS + IL-4. Samples were harvested at 1, 4, and 7 days after stimulation, washed twice with PBS, and stained with 100 μL of 1/20 diluted Annexin V-APC (eBiosciences, San Diego, CA, USA). Cells were acquired by the LSR II(BD Biosciences) and analyzed separately by WinList (Verity Software House, Topsham ME, USA).
Preparation of the 3′UTR DsRED retroviral constructs and transient transfection of PT67 packaging cells
A retroviral Tetracycline ON (Tet-on) response vector system was utilized to determine the importance of the 3′UTR in generating reduced levels of E47. This was key in confirming the importance of the 3′UTR in E47 mRNA stability as well as for generating the best subsequent E47 constructs for possible improvement/rescue of the aged B-lymphocyte phenotype. The pRevTRE retroviral vector (BD Biosciences) was used to build our control construct pRevTRE-DsRED (will be referred to as DsRED vector) by inserting the 700-bp DsRED fragment into the Sal I and Cla I sites. We also added a NotI site previous to the ClaI site allowing us to later introduce the 3′UTR of E47 or ku80 between the NotI site and the ClaI site. The pRevTRE-DsRED-3′UTR- ku80 was constructed by inserting the 235-bp fragment containing the cDNA for mouse ku80 3′UTR at the 3′ end of the DsRED coding region (will be referred to as DsRED-ku80 3′UTR). The pRevTRE-DsRED-3′UTR- E47 was constructed by inserting the 638-bp fragment containing the cDNA for mouse E47 3′UTR at the 3′ end of the DsRED coding region (will be referred to as DsRED-E47 3′UTR). Desired fragments were purified using the QiaQuickGelExtraction-Kit (Qiagen, Valencia, CA, USA). Ligation of the two fragments was carried out with a molar insert: vector ratio of 3:1 at 16 °C overnight using the Quick Ligation Kit (NEB, Ipswich, MA, USA). Transformation of supercompetent E. coli (Agilent Technologies- Stratagene, La Jolla, CA, USA) was performed according to the manufacturer’s protocol. Transformed bacteria were grown on LB-Plates with ampicillin (100 μg mL−1) and colonies were obtained for plasmid preparation. Plasmid DNA was tested by restriction digest and PCR. In addition to our pRevTRE constructs, we also used the helper vector, pTet-on (also from BD Biosciences), in order to turn on the Tet responsive element on the pRev-TRE vector by addition of Tetracycline or Doxycycline (1 μg mL−1).
The DsRED retroviral constructs including DsRED, DsRED-E47 3′UTR, and DsRED-ku80 3′UTR were each co-transfected with the pTet-on vector into the PT67 packaging cell line. The transfections were performed in 10 cm (six well) culture plates, seeded at 2.8 × 106 cells/well, 24 h prior to transfection. Cells were transfected using 60 μL of Lipofectamine 2000 reagent (Invitrogen-GIBCO) with a total amount of 24 μg DNA/500 μL of Opti-MEM media, according to the manufacturer’s instructions. Twenty-four hours post-transfection, supernatants were discarded and fresh complete RPMI medium was added to the cultures. The 48-h (after transfection) supernatants were collected and used for retroviral transduction.
3′UTR DsRED retroviral transductions
Supernatants from the PT67 packaging cell line were harvested, filtered, and used immediately for DsRED retroviral transduction of the BJAB cell line using the centrifugation method (Kotani et al., 1994). In brief, 7 × 106 cells were harvested and resuspended in 2 mL retroviral supernatant with 8 μg mL−1 final concentration of polybreme (Sigma-Aldrich). The mixture was centrifuged in 6-well plates for 1.5 h at 1200 rpm (290 g) at 32 °C (Kotani et al., 1994) in order to achieve close contact between the retroviral particles and the cells. After 24 h, the media was replaced with 5 mL fresh RPMI complete medium plus 1 μg mL−1 Doxycycline, and cells were cultured at 37 °C and 5% CO2.
Preparation of the E47 coding region retroviral construct and transient transfection of the ΦNXE packaging cells
The bicistronic retroviral vector pMXs-IG (referred to as the EGFP vector) with the 5′LTR of murine moloney leukemia virus for its promoter received from Dr. Thomas Malek, University of Miami (Onishi et al., 1996; Gong & Malek, 2007) was used to prepare our construct pMXs-E47-IG (referred to as the E47 vector). We inserted the 2-kb fragment containing the cDNA for mouse E47, excluding the 5′UTR and 3′UTR, into the SfiI and NotI sites, upstream of internal ribosome entry site/EGFP. The E47 vector without the E47 3′UTR should express a more stable E47 mRNA that would not be targeted for degradation by TTP (Frasca et al., 2007), and results herein. The vector and insert were purified, ligated, and transformed, and colonies were screened for the insert (as described earlier).
The E47 retroviral construct and the EGFP control retroviral construct were transiently transfected into the ΦNXE packaging cell line, optimized for this vector system, using Lipofectamine 2000, cells seeded and supernatants collected for retroviral transduction as described earlier.
E47 Retroviral transduction
Supernatants from the ΦNXE packaging cells were harvested, filtered through a 0.45-μm-syringe filter and used immediately or frozen (for use within a week) for retroviral transduction of activated/stimulated primary splenic B cells. Retroviral transductions of stimulated B cells (10 μg mL−1 LPS and 50 ng mL−1 IL-4, 24 h before transduction) were performed using the centrifugation method (Kotani et al., 1994) as described earlier with addition of 5 mL fresh complete RPMI media plus LPS and IL-4, and cells were cultured at 37 °C and 5% CO2 for up to 7 days post-transduction.
RNA extraction and RT–PCR reactions
Total RNA was isolated from 106 transduced/untransduced BJAB cells or splenic activated B cells using the TRIzol Reagent (Invitrogen-GIBCO) according to the manufacturer’s protocol. RNA was eluted from the provided columns (PureLink Micro-to-Midi Total RNA Purification System; Invitrogen-GIBCO) with 20 μL distilled diethyl pyrocarbonate (DEPC)-water and stored at −80 °C until use.
For the mRNA stability study, B cells were either transduced and stimulated with LPS + IL-4 for 2 days (for stability of the exogenous E47) or only stimulated with LPS + IL-4 for 5 days (for AID stability) and treated with 10 μg/106 cells of Actinomycin D for 10, 30, 45, or 90 min prior to RNA extraction. Reverse transcriptase PCR (RT–PCR) was performed in a MasterCycler Eppendorf machine. RNA (2 μL) at a concentration of 0.5 μg μL−1 was used as template for reverse transcription to produce cDNA in a total of 20 μL. Quantitative PCR (qPCR) was performed using the TaqMan primers and master mix from Applied Biosystems (ABI, Foster City, CA, USA) to evaluate E47, AID, and GAPDH RNA expression with the following primers: E47, Tcfe2 # Mm0117557_g1; AID, Aicda #Mm00507774_m1; and GAPDH #Mm99999915_g1. The SYBR Green primers and master mix were used to evaluate DsRED RNA expression (DsRED forward primer 5′-CGCCACCATGGTGCG, DsRED reverse primer 5′-TAGAGTCGCGGCCGCTAC). Both Taqman and SYBR Green reactions were conducted in MicroAmp 96-well plates (N8010560), and run in the ABI 7300 machine. Calculations were made with ABI software. Briefly, we determined the cycle number at which transcripts reached a significant threshold (Ct) for E47, AID, DsRED and GAPDH as control. A value of the target gene, relative to GAPDH, was calculated and expressed as ΔCt. For DsRED experiments only, after normalization to GAPDH, we also normalized to gene copy number within the cells, to take into account potential differences in transduction efficiency by taking the DNA fraction from the Trizol extraction to PCR for DsRED (DsRED transduction efficiency differences among experiments was not > 10%). This gene copy number normalization was crucial to determine that the decreased DsRED in the DsRED-E47 3′UTR transduced cells was not because of decreased transduction efficiency. Because of the addition of either ku80 or E47 3′UTR to the DsRED, the mRNA of the DsRED may be more susceptible to mRNA degradation and is no longer a reporter indicative of transduction efficiency. This extra calculation/normalization is not necessary for the E47 retrovirus because it carries a separate reporter, EGFP, and EGFP vector transductions also gave similar transduction efficiencies (see text).
Acknowledgments
We thank Jim Philips for his invaluable help with the flow cytometer, and Michelle Perez for her secretarial help. This work was supported by National Institutes of Health AG-17618 and AG-23717 (to B.B.B.) and by National Institutes of Health AG-025256 and AI-064591 (to R.L.R.).
References
- Asanuma H, Hirokawa K, Uchiyama M, Suzuki Y, Aizawa C, Kurata T, Sata T, Tamura S. Immune responses and protection in different strains of aged mice immunized intranasally with an adjuvant-combined influenza vaccine. Vaccine. 2001;19:3981–3989. doi: 10.1016/s0264-410x(01)00129-3. [DOI] [PubMed] [Google Scholar]
- Cancro MP, Hao Y, Scholz JL, Riley RL, Frasca D, Dunn-Walters DK, Blomberg BB. B cells and aging: molecules and mechanisms. Trends Immunol. 2009;30:313–318. doi: 10.1016/j.it.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M, Storb U. A novel cytidine deaminase AIDs in the delivery of error-prone polymerases to immunoglobulin genes. DNA Repair (Amst) 2003;2:623–627. doi: 10.1016/s1568-7864(02)00240-9. [DOI] [PubMed] [Google Scholar]
- Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- Ephrussi A, Church GM, Tonegawa S, Gilbert W. B lineage – specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985;227:134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB. Effects of aging on B cell function. Curr Opin Immunol. 2009;21:425–430. doi: 10.1016/j.coi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Nguyen D, Riley RL, Blomberg BB. Decreased E12 and/or E47 transcription factor activity in the bone marrow as well as in the spleen of aged mice. J Immunol. 2003;170:719–726. doi: 10.4049/jimmunol.170.2.719. [DOI] [PubMed] [Google Scholar]
- Frasca D, Nguyen D, Riley RL, Blomberg BB. Effects of aging on DNA-binding activity of the E47 transcription factor in splenic B cells. Mech Ageing Dev. 2004a;125:111–112. doi: 10.1016/j.mad.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Frasca D, Van der Put E, Riley RL, Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol. 2004b;172:2155–2162. doi: 10.4049/jimmunol.172.4.2155. [DOI] [PubMed] [Google Scholar]
- Frasca D, Van der Put E, Landin AM, Gong D, Riley RL, Blomberg BB. RNA stability of the E2A-encoded transcription factor E47 is lower in splenic activated B cells from aged mice. J Immunol. 2005;175:6633–6644. doi: 10.4049/jimmunol.175.10.6633. [DOI] [PubMed] [Google Scholar]
- Frasca D, Landin AM, Alvarez JP, Blackshear PJ, Riley RL, Blomberg BB. Tristetraprolin, a negative regulator of mRNA stability, is increased in old B cells and is involved in the degradation of E47 mRNA. J Immunol. 2007;179:918–927. doi: 10.4049/jimmunol.179.2.918. [DOI] [PubMed] [Google Scholar]
- Frasca D, Landin AM, Lechner SC, Ryan JG, Schwartz R, Riley RL, Blomberg BB. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008a;180:5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- Frasca D, Landin AM, Riley RL, Blomberg BB. Mechanisms for decreased function of B cells in aged mice and humans. J Immunol. 2008b;180:2741–2746. doi: 10.4049/jimmunol.180.5.2741. [DOI] [PubMed] [Google Scholar]
- Frasca D, Diaz A, Romero M, Landin AM, Phillips M, Lechner SC, Ryan JG, Blomberg BB. Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine. 2010a;18:1591–1595. doi: 10.1016/j.vaccine.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Romero M, Landin AM, Diaz A, Riley RL, Blomberg BB. Protein phosphatase 2A (PP2A) is increased in old murine B cells and mediates p38 MAPK/tristetraprolin dephosphorylation and E47 mRNA instability. Mech Ageing Dev. 2010b;131:306–314. doi: 10.1016/j.mad.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Malek TR. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J Immunol. 2007;178:242–252. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B, Della Bella S, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res. 2009;21:201–209. doi: 10.1007/BF03324904. [DOI] [PubMed] [Google Scholar]
- Han S, Yang K, Ozen Z, Peng W, Marinova E, Kelsoe G, Zheng B. Enhanced differentiation of splenic plasma cells but diminished long-lived high-affinity bone marrow plasma cells in aged mice. J Immunol. 2003;170:1267–1273. doi: 10.4049/jimmunol.170.3.1267. [DOI] [PubMed] [Google Scholar]
- Honjo T, Kinoshita K, Muramatsu M. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu Rev Immunol. 2002;20:165–196. doi: 10.1146/annurev.immunol.20.090501.112049. [DOI] [PubMed] [Google Scholar]
- Klinman NR, Kline GH. The B-cell biology of aging. Immunol Rev. 1997;160:103–114. doi: 10.1111/j.1600-065x.1997.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Kotani H, Newton PB, III, Zhang S, Chiang YL, Otto E, Weaver L, Blaese RM, Anderson WF, McGarrity GJ. Improved methods of retroviral vector transduction and production for gene therapy. Hum Gene Ther. 1994;5:19–28. doi: 10.1089/hum.1994.5.1-19. [DOI] [PubMed] [Google Scholar]
- Lai WS, Parker JS, Grissom SF, Stumpo DJ, Blackshear PJ. Novel mRNA targets for tristetraprolin (TTP) identified by global analysis of stabilized transcripts in TTP-deficient fibroblasts. Mol Cell Biol. 2006;26:9196–9208. doi: 10.1128/MCB.00945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KB, Skogberg M, Margenfeld C, Ireland J, Pettersson S. Repression of the immunoglobulin heavy chain 3′ enhancer by helix-loop-helix protein Id3 via a functionally important E47/E12 binding site: implications for developmental control of enhancer function. Eur J Immunol. 1995;25:1770–1777. doi: 10.1002/eji.1830250643. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Nicoletti C, Borghesi-Nicoletti C, Yang XH, Schulze DH, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. II. Phosphorylcholine-antibody in young and aged mice differ in both VH/VL gene repertoire and in specificity. J Immunol. 1991;147:2750–2755. [PubMed] [Google Scholar]
- Nicoletti C, Yang X, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. III. Phosphorylcholine antibody from young and aged mice differ in structure and protective activity against infection with Streptococcus pneumoniae. J Immunol. 1993;150:543–549. [PubMed] [Google Scholar]
- Onishi M, Kinoshita S, Morikawa Y, Shibuya A, Phillips J, Lanier LL, Gorman DM, Nolan GP, Miyajima A, Kitamura T. Applications of retrovirus-mediated expression cloning. Exp Hematol. 1996;24:324–329. [PubMed] [Google Scholar]
- Park SR, Zan H, Pal Z, Zhang J, Al-Qahtani A, Pone EJ, Xu Z, Mai T, Casali P. HoxC4 binds to the promoter of the cytidine deaminase AID gene to induce AID expression, class-switch DNA recombination and somatic hypermutation. Nat Immunol. 2009;10:540–550. doi: 10.1038/ni.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Barnett Y, Forsey R, Frasca D, Globerson A, McLeod J, Caruso C, Franceschi C, Fulop T, Gupta S, Mariani E, Mocchegiani E, Solana R. T cells and aging, January 2002 update. Front Biosci. 2002;7:d1056–d1183. doi: 10.2741/a831. [DOI] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quong MW, Harris DP, Swain SL, Murre C. E2A activity is induced during B-cell activation to promote immunoglobulin class switch recombination. EMBO J. 1999;18:6307–6318. doi: 10.1093/emboj/18.22.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler H, Stoecklin G. Control of mRNA decay by phosphorylation of tristetraprolin. Biochem Soc Trans. 2008;36:491–496. doi: 10.1042/BST0360491. [DOI] [PubMed] [Google Scholar]
- Sayegh CE, Quong MW, Agata Y, Murre C. E-proteins directly regulate expression of activation-induced deaminase in mature B cells. Nat Immunol. 2003;4:586–593. doi: 10.1038/ni923. [DOI] [PubMed] [Google Scholar]
- Snapper CM, Finkelman FD, Stefany D, Conrad DH, Paul WE. IL-4 induces co-expression of intrinsic membrane IgG1 and IgE by murine B cells stimulated with lipopolysaccharide. J Immunol. 1988;141:489–498. [PubMed] [Google Scholar]
- Teng G, Papavasiliou FN. Immunoglobulin somatic hypermutation. Annu Rev Genet. 2007;41:107–120. doi: 10.1146/annurev.genet.41.110306.130340. [DOI] [PubMed] [Google Scholar]
- Tran TH, Nakata M, Suzuki K, Begum NA, Shinkura R, Fagarasan S, Honjo T, Nagaoka H. B cell-specific and stimulation-responsive enhancers derepress Aicda by overcoming the effects of silencers. Nat Immunol. 2010;11:148–154. doi: 10.1038/ni.1829. [DOI] [PubMed] [Google Scholar]
- Van der Put E, Frasca D, King AM, Blomberg BB, Riley RL. Decreased E47 in senescent B cell precursors is stage specific and regulated posttranslationally by protein turnover. J Immunol. 2004;173:818–827. doi: 10.4049/jimmunol.173.2.818. [DOI] [PubMed] [Google Scholar]