Abstract
The SH2B family has three members (SH2B1, SH2B2, and SH2B3) that contain conserved dimerization (DD), pleckstrin homology, and SH2 domains. The DD domain mediates the formation of homo- and heterodimers between members of the SH2B family. The SH2 domain of SH2B1 (previously named SH2-B) or SH2B2 (previously named APS) binds to phosphorylated tyrosines in a variety of tyrosine kinases, including Janus kinase-2 (JAK2) and the insulin receptor, thereby promoting the activation of JAK2 or the insulin receptor, respectively. JAK2 binds to various members of the cytokine receptor family, including receptors for GH and leptin, to mediate cytokine responses. In mice, SH2B1 regulates energy and glucose homeostasis by enhancing leptin and insulin sensitivity. In this work, we identify SH2B2β as a new isoform of SH2B2 (designated as SH2B2α) derived from the SH2B2 gene by alternative mRNA splicing. SH2B2β has a DD and pleckstrin homology domain but lacks a SH2 domain. SH2B2β bound to both SH2B1 and SH2B2α, as demonstrated by both the interaction of glutathione S-transferase-SH2B2β fusion protein with SH2B1 or SH2B2α in vitro and coimmunoprecipitation of SH2B2β with SH2B1 or SH2B2α in intact cells. SH2B2β markedly attenuated the ability of SH2B1 to promote JAK2 activation and subsequent tyrosine phosphorylation of insulin receptor substrate-1 by JAK2. SH2B2β also significantly inhibited SH2B1- or SH2B2α-promoted insulin signaling, including insulin-stimulated tyrosine phosphorylation of insulin receptor substrate-1. These data suggest that SH2B2β is an endogenous inhibitor of SH2B1 and/or SH2B2α, negatively regulating insulin signaling and/or JAK2-mediated cellular responses.
SH2-B is an adaptor protein containing an N-terminal dimerization domain (DD), a central pleckstrin homology (PH) domain, and a C-terminal SH2 domain (1, 2). The SH2-B gene encodes four isoforms (α, β, γ, and δ) via alternative mRNA splicing. All forms have the same N-terminal regions of amino acids (1–666), containing an intact DD, PH, and SH2 domain (3, 4). Genetic disruption of the SH2-B gene results in morbid obesity and type 2 diabetes, indicating that SH2-B is required for maintaining normal body weight and glucose homeostasis (5, 6). Moreover, SH2-B is also required for reproduction in mice (7).
SH2-B may regulate energy balance and body weight at least partially by enhancing Janus kinase-2 (JAK2)-mediated cytokine signaling, including leptin and/or GH signaling. Leptin is an adipose hormone that controls body weight mainly by binding to and activating the long form of the leptin receptor in the hypothalamus. GH regulates metabolism by activating the GH receptor (GHR) (8–10). JAK2, a cytoplasmic tyrosine kinase, binds to long form of the leptin receptor or GHR, mediating leptin or GH signaling, respectively (10–12). Leptin or GH stimulates the activation of JAK2, which subsequently phosphorylates multiple substrates, including insulin receptor substrate (IRS)-1, IRS2, signal transducer and activator of transcription (STAT)-3, and STAT5 (12–20). SH2-B directly binds via its SH2 domain to phosphorylated Tyr813 in JAK2, resulting in the enhancement of JAK2 activation and JAK2-mediated GH signaling (2, 21, 22). In addition, SH2-B simultaneously binds to both JAK2 and IRS proteins, specifically promoting IRS-mediated activation of the phosphatidylinositol 3-kinase pathway (23). Disruption of the SH2-B gene attenuates leptin-stimulated JAK2 activation and tyrosine phosphorylation of STAT3 and IRS2 in the hypothalamus, suggesting that SH2-B may be an important endogenous enhancer of JAK2 activation (6).
SH2-B also directly binds via its SH2 domain to phosphorylated tyrosines within the activation loop of the insulin receptor (3, 24, 25). Overexpression of SH2-B enhances insulin-stimulated tyrosine phosphorylation of IRS1 and IRS2, suggesting that SH2-B may positively modulate insulin signaling (5, 26). Consistent with this idea, SH2-B-deficient mice develop severe insulin resistance and type 2 diabetes (5, 6).
The SH2B family contains three members, SH2-B, APS, and Lnk, which have a conserved structure of a DD, PH, and SH2 domain. Recently members of the SH2B family were renamed by the HUGO Gene Nomenclature Committee as SH2B1 for SH2-B, SH2B2 for APS, and SH2B3 for Lnk. SH2B2 binds via its SH2 domain to JAK2 and the insulin receptor in a similar fashion as SH2B1 (27–30). SH2B2 also enhances insulin signaling in cultured cells (26). In addition, SH2B2 is phosphorylated by the insulin receptor and mediates insulin-stimulated activation of the Cbl/TC10 pathway in cultured adipocytes (30–34). Surprisingly, insulin sensitivity is modestly increased in SH2B2-deficient mice, suggesting that the SH2B2 gene product(s) may negatively regulate insulin sensitivity in animals (35, 36).
In this study, we identified a novel isoform of SH2B2 (designated as SH2B2β, with the previously reported SH2B2 as SH2B2α). SH2B2β contains a DD and PH domain but lacks a SH2 domain. The DD domain has been reported to mediate both homodimerization of SH2B1 or SH2B2 and heterodimerization of SH2B1 with SH2B2 (2, 37, 38). Consistent with those reports, we demonstrated that SH2B2β bound to both SH2B1 and SH2B2α. Moreover, SH2B2β inhibited the ability of SH2B1 to promote JAK2 activation and insulin signaling. Our results suggest that SH2B2β may function as an endogenous inhibitor for SH2B1- and/or SH2B2α-mediated cellular responses.
Materials and Methods
Reagents
Lipofectamine 2000 was purchased from Invitrogen Life Technologies (Carlsbad, CA). Nonidet P-40 was purchased from Calbiochem (La Jolla, CA). Monoclonal antiphosphotyrosine antibody (PY20) was purchased from Upstate Biotechnology Inc. (Lake Placid, NY). Mouse SH2B2β cDNA was inserted into the prk5-Flag expression vector and used for transient transfection experiments. Mouse SH2B2β cDNA was inserted into pGEX-4X-1 (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). Glutathione S-transferase (GST)-SH2B2β fusion proteins were purified from bacteria and used as antigens to prepare polyclonal anti-SH2B2β antibodies.
RT-PCR
Total RNA was extracted from mouse tissues using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. First-strand cDNA was synthesized from total RNA (3 μg) using oligo dT (12–18) primer and Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI), and subjected to PCR amplification. RT-PCR products were separated by agarose gels, stained with ethidium bromide, and visualized by UV light. Two sets of primers were designed to specifically amplify SH2B2β (designated as P1-P4 in Fig. 1C): set 1, SH2B2β sense (P1), 5′-GAACGTTTCCGCCTGGAGTTC-3′, SH2B2β antisense (P4), 5′-GAAGGAGTGACTTTATTCAGCAG-3′; set 2, SH2B2β sense (P2), 5′-ATGTGGAGCCTCAGAAGTGGTG-3′, SH2B2β antisense (P3), 5′-ATAGCCTTGAACCCATGCAG-3′; β-actin sense, 5′-AAATCGTGCGTGACATCAAA-3′, β-actin antisense, 5′-AAGGAAGGCTGGAAAAGAGC-3′.
Fig. 1.
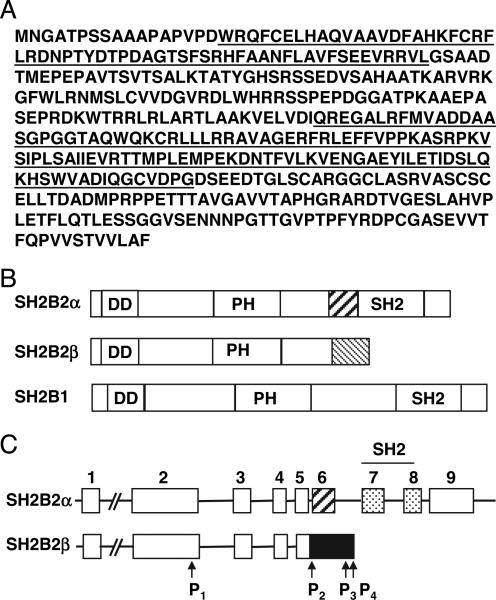
Sequence analysis of SH2B2β. A, Amino acid sequences of mouse SH2B2β. An N-terminal DD and a C-terminal PH domain are underlined. B, A schematic representation of SH2B2α, SH2B2β, and SH2B1 proteins. C, A schematic representation of alternative splicing of SH2B2α and SH2B2β. Individual boxes represent exons. Exons 7 and 8, which are absent in SH2B2β, encode a SH2 domain. P1, P2, P3, and P4 indicate the positions of primers used in RT-PCR.
Cell culture and transfection
HEK293 cells were grown at 37 C in 5% CO2 in DMEM supplemented with 25 mm glucose, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% calf serum. Cells were split at 2 × 105 cells/well in 6-well culture dishes 24 h before transfection and transfected with indicated plasmids using Lipofectamine 2000 reagents according to the manufacturer's instruction. Experiments were performed on the transfected cells 48 h later.
GHR cDNA was generated from mouse liver by RT-PCR, confirmed by DNA sequencing, and subcloned into a retroviral vector [pBabe(hygro)-GHR]. Similarly, murine JAK2 cDNA was inserted into a retroviral vector [pBabe(puro)-JAK2]. pBabe(hygro)-GHR or pBabe(puro)-JAK2 was transiently cotransfected into 293T cells with pC/ECO to generate recombinant GHR or JAK2 retroviruses, respectively. Human γ2A fibroblasts were infected sequentially with recombinant JAK2 and GHR retroviruses and selected by 2 μm/ml puromycin (for JAK2) and 0.2 mg/ml hygromycin (for GHR) for a week to generate γ2AGHR/JAK2 cells, which stably express GHR and JAK2. γ2AGHR/JAK2 cells were grown at 37 C in 5% CO2 in DMEM supplemented with 25 mm glucose, 100 U/ml penicillin, 100 μg/ml streptomycin, and 6% fetal calf serum.
Immunoprecipitation and immunoblotting assays
Confluent cells were deprived of serum overnight (~16 h) in DMEM containing 0.6% BSA, and treated with or without insulin as indicated in figure legends. Cells were rinsed two times with ice-cold PBS, solubilized in lysis buffer [50 mm Tris (pH 7.5), 1% Nonidet P-40, 150 mm NaCl, 2 mm EGTA, 1 mm Na3VO4, 100 mm NaF, 10 mm Na4P2O7, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin], and centrifuged at 9000 × g for 25 min at 4 C. Protein concentrations in the supernatant (cell extracts) were measured using a protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA). Cell extracts were incubated with indicated antibody at 4 C for 2 h. The immune complexes were collected on protein A-agarose during 1 h incubation at 4 C. The beads were washed three times with washing buffer [50 mm Tris (pH 7.5), 1% Nonidet P-40, 150 mm NaCl, 2 mm EGTA] and boiled for 5 min in SDS-PAGE sample buffer [50 mm Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate, 2% β-mercaptoethanol, 10% glycerol, 0.005% bromophenol blue]. The solubilized proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes (Amersham International Plc, Amersham, UK), and detected by immunoblotting with indicated antibody using ECL or Odyssey detection system. Some membranes were subsequently incubated at 55 C for 30 min in stripping buffer [100 mm β-mercaptoethanol, 2% sodium dodecyl sulfate, 62.5 mm Tris-HCl (pH 6.7)] to prepare them for reprobing.
Brown adipose tissue (BAT) extracts (1 mg protein in 100 ml volume) were prepared from male mice (8–9 wk), and incubated with either preimmune serum or αSH2B2 (20 μl) on ice for 2 h. The mixtures were incubated with protein-A agarose (50 μl) for 1.5 h in 4 C and centrifuged for 2 min at 4 C. The supernatants were again incubated with protein-A agarose (30 μl) at 4 C for 1 h and centrifuged for 2 min to remove residual antibodies. Preimmune serum- or αSH2B2-cleared BAT extracts were immunoblotted with preimmune serum, αSH2B2, or anti-β-actin antibody.
In vitro kinase assays
HEK293 cells were transiently transfected with indicated plasmids. Forty-eight hours later, cells were solubilized in lysis buffer. JAK2 was immunoprecipitated with αJAK2, and JAK2 immunoprecipitates were washed extensively with a kinase reaction buffer [50 mm HEPES (pH 7.6), 10 mm MnCl2, 100 mm NaCl, 0.5 mm dithiothreitol, 1 mm Na3VO4] and incubated with [γ-32P]ATP (10 μCi) in the kinase reaction buffer supplemented with 20 μm cold ATP, 10 μg/ml aprotinin, and 10 μg/ml leupeptin at room temperature for 30 min. The precipitates were then washed with lysis buffer, boiled for 5 min in the SDS-PAGE sample buffer, and resolved by SDS-PAGE. 32P-labeled JAK2 was visualized by autoradiography. Proteins on SDS-PAGE gels were subsequently transferred to a nitrocellulose membrane and immunoblotted with appropriate antibodies.
Results
Identification of SH2B2β as a new isoform of SH2B2
We searched the National Center for Biotechnology Information database (Bethesda, MD) for SH2B2 homologs to identify potential new isoforms of SH2B2. A novel species of mRNA (accession no. BC049777 and AK017604) was identified, and designated as SH2B2β. The previously reported APS is renamed SH2B2α. SH2B2β mRNA is predicted to encode a protein of 420 amino acids that contains an N-terminal DD and a C-terminal PH domain (Fig. 1A). The N-terminal regions (amino acids 1–388) are identical between SH2B2α and SH2B2β, including the DD and PH domains; however, SH2B2β completely lacks a C-terminal SH2 domain (Fig. 1B).
To determine whether SH2B2α and SH2B2β are derived from the SH2B2 gene via alternative mRNA splicing, the intron/exon structure was examined by analyzing the genomic sequence of the SH2B2 gene (accession no. AC083890). SH2B2α has nine exons with the first exon being a noncoding exon and exons 7 and 8 encoding a SH2 domain (Fig. 1C). In contrast, SH2B2β has only five exons. The first four exons are identical between SH2B2α and SH2B2β. Exon 5 of SH2B2β is derived from both exons 5 and 6 of SH2B2α, plus two additional DNA fragments (the intron between exons 5 and 6 and the intron between exons 6 and 7 of SH2B2α) (Fig. 1C). The insertion of intronic sequence between exons 5 and 6 causes a shift in reading frame, resulting in a unique C-terminal 32 amino acids in SH2B2β (Fig. 1A).
To determine tissue distribution of SH2B2β, total RNA was prepared from multiple tissues in mice and subjected to RT-PCR to detect SH2B2β mRNA. Two sets of SH2B2β-specific primers (designated as P1-P4) were designed to specifically amplify a portion of SH2B2β, which is absent in SH2B2α (Fig. 1C). Total RNA was prepared in multiple tissues from wild-type and SH2B2 knockout mice and reversely transcribed to cDNA. Using 5′ P1 and 3′ P4 primers in RT-PCR analysis, SH2B2β mRNA was easily detected in BAT, the brain, white adipose tissue, and hypothalamus from wild-type mice but undetectable in SH2B2 knockout mice (Fig. 2A). Similarly, SH2B2β mRNA was detected in the brain, BAT, hypothalamus, white adipose tissue, skeletal muscle, spleen, and embryos (E15) but not in heart, lung, and ovary, using 5′ P2 and 3′ P3 primers (Fig. 2B). The RT-PCR products derived from 5′ P1 and 3′ P4 primers were purified and subjected to DNA sequencing analysis, confirming the identity of SH2B2β (Fig. 2C).
Fig. 2.
SH2B2β tissue distribution. A, Total RNA was prepared from multiple tissues from wild-type and SH2B2 knockout mice and subjected to RT-PCR to detect SH2B2β using 5′ P1 and 3′ P4 primers (upper panel) or β-actin (lower panel). The positions of primers P1, P2, P3, and P4 were indicated in Fig. 1C. WAT, White adipose tissue. B, Total RNA was prepared from various tissues from a wild-type mouse and subjected to RT-PCR to detect SH2B2β using 5′ P2 and 3′ P3 primers (upper panel) or β-actin (lower panel). Experiments in A and B were repeated more than three times with similar results. C, SH2B2β cDNA was prepared from mouse brain by RT-PCR using 5′ P1 and 3′ P4 primers and subjected to DNA sequencing analysis. D, SH2B2α and SH2B2β were transiently coexpressed in HEK293 cells. Cell extracts were immunoblotted with αSH2B2. E, BAT extracts (1 mg protein) were prepared from three mice (1, 2, and 3) and incubated with αSH2B2 or preimmune serum. αSH2B2-and preimmune serum-cleared BAT extracts were immunoblotted with preimmune serum (top panel). The same blot was reprobed with αSH2B2 (middle panel) and αactin (bottom panel), respectively. Molecular markers (kilodal-tons) were indicated at the left in both D and E.
To demonstrate endogenous SH2B2β protein, αSH2B2 was generated using GST-SH2B2β as antigen. αSH2B2 specifically recognized both recombinant SH2B2α and SH2B2β in immunoblotting analysis (Fig. 2D). BAT extracts were prepared from three mice and immunoprecipitated with preimmune serum or αSH2B2. αSH2B2, but not preimmune serum, is predicted to remove both SH2B2α and SH2B2β from the BAT extracts. Preimmune serum- or αSH2B2-cleared BAT extracts were immunoblotted with preimmune serum, αSH2B2, or anti-β-actin antibodies, respectively. Preimmune serum was unable to detect either SH2B2α or SH2B2β as expected (Fig. 2E, top panel). In contrast, αSH2B2 specifically detected two proteins in preimmune serum- but not αSH2B2-cleared BAT extracts (Fig. 2E, middle panel). These two proteins have molecular masses approximate for SH2Bα and SH2B2β, respectively, suggesting that they are endogenous SH2B2α and SH2B2β. Moreover, SH2B2α and SH2B2β protein levels were similar in BAT (Fig. 2E).
SH2B2β binds to both SH2B1 and SH2B2α
Because SH2B2β contains an intact DD, we determined whether SH2B2β binds to SH2B1 and/or SH2B2α. N-terminal Myc-tagged SH2B1β was transiently expressed in HEK293 cells, and cell extracts were prepared and incubated with either immobilized GST or GST-SH2B2β fusion proteins. Precipitates were immunoblotted with anti-Myc (αMyc). GST-SH2B2β, but not GST, bound to SH2B1β (Fig. 3A).
Fig. 3.
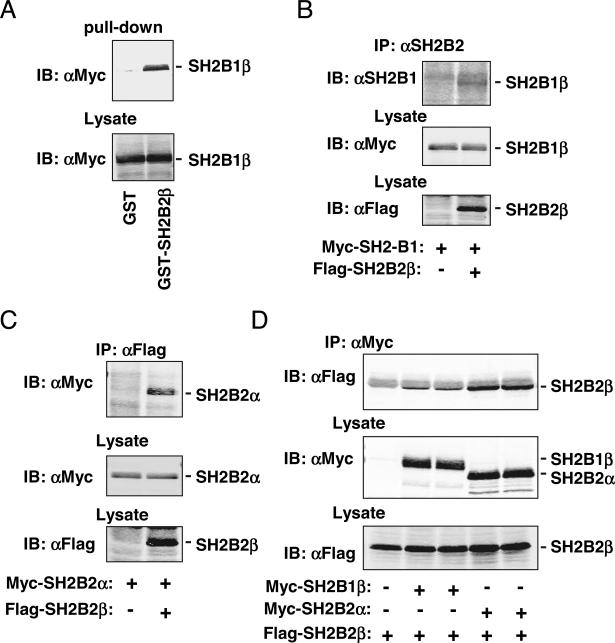
SH2B2β binds to both SH2B1 and SH2B2α. A, Myc-tagged SH2B1β was transiently overexpressed in HEK293 cells. Cell extracts were prepared and incubated with immobilized GST or GST-SH2B2β fusion proteins. GST- or GST-SH2B2β-bound SH2B1β was immunoblotted (IB) with αMyc. B, Myc-tagged SH2B1β was transiently coexpressed with or without Flag-tagged SH2B2β in HEK293 cells. Cell extracts were immunoprecipitated with αSH2B2 and immunoblotted with αSH2B1. In parallel experiments, cell extracts were immunoblotted with αMyc or αFlag to detect SH2B1β or SH2B2β, respectively. C, Myc-tagged SH2B2α was transiently coexpressed with or without Flag-tagged SH2B2β in HEK293 cells. Cell extracts were immunoprecipitated with αFlag and immunoblotted with αMyc. In parallel experiments, cell extracts were immunoblotted with αMyc or αFlag to detect SH2B2α or SH2B2β, respectively. D, Flag-tagged SH2B2β was transiently coexpressed with Myc-tagged SH2B1β or SH2B2α in HEK 293 cells. Cells extracts were immunoprecipitated with αMyc and immunoblotted with αFlag (top panel). In parallel experiments, cell extracts were immunoblotted with αMyc (middle panel) or αFlag (bottom panel). Similar results (A–D) were obtained in more than three independent experiments.
To determine whether SH2B2β binds to SH2B1 in cells, SH2B2β was transiently coexpressed with SH2B1β in HEK293 cells. SH2B2β was immunoprecipitated with αSH2B2 and immunoblotted with αSH2B1. SH2B2β was coimmunoprecipitated with SH2B1β (Fig. 3B). Similarly, SH2B2β was also coimmunoprecipitated with SH2B2α (Fig. 3C). To determine whether SH2B1β or SH2B2α reciprocally coimmunoprecipitated with SH2B2β, Flag-tagged SH2B2β was coexpressed with Myc-tagged SH2B1β or SH2B2α. SH2B1β or SH2B2α was immunoprecipitated with αMyc and immunoblotted with αFlag. Both SH2B1β and SH2B2α were coimmunoprecipitated with SH2B2β (Fig. 3D). Together, these data indicate that SH2B2β binds to both SH2B1β and SH2B2α, presumably via their DD domains.
SH2B2β inhibits SH2B1-enhanced JAK2 activation
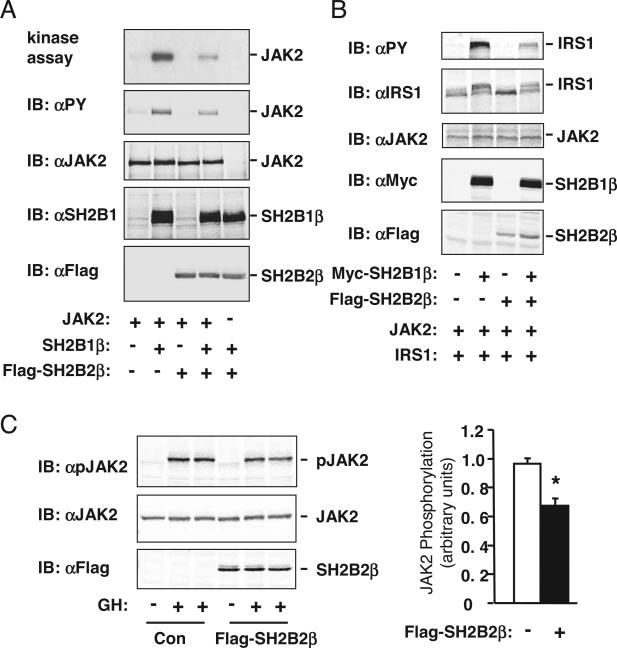
SH2B1 is a potent enhancer of JAK2 activation (21). An SH2-terminal truncated SH2B2α binds via its DD domain to SH2B1 and inhibits SH2B1-promoted JAK2 activation (2). To determine whether SH2B2β inhibits SH2B1-promoted JAK2 activation in a similar fashion, JAK2 was transiently coexpressed with SH2B1β in the presence or absence of the overexpresion of SH2B2β. JAK2 was immunoprecipitated with anti-JAK2 (αJAK2) and subjected to in vitro kinase assays or immunoblotting with anti-phospho-tyrosine (αPY). SH2B1β significantly enhanced JAK2 kinase activity (Fig. 4A, panel 1) and JAK2 tyrosine phosphorylation (Fig. 4A, panel 2) as expected. Overexpression of SH2B2β dramatically inhibited JAK2 activation and phosphorylation (Fig. 4A). The expression of SH2B1β and Flag-tagged SH2B2β was confirmed by immunoblotting cell extracts with αSH2B1 or αFlag, respectively (Fig. 4A, panels 4 and 5). JAK2 protein levels were similar with or without coexpression of SH2B1 and/or SH2B2β (Fig. 4A, panel 3).
Fig. 4.
SH2B2β inhibits SH2B1-promoted JAK2 activation. A, JAK2 was transiently coexpressed with SH2B1β in the presence or absence of SH2B2β in HEK293 cells. JAK2 was immunoprecipitated with αJAK2 and subjected to in vitro kinase assays in the presence of [γ-32P-]ATP. 32P-labeled JAK2 was visualized by autoradiography (panel 1), immunoblotted (IB) with αPY (panel 2) or αJAK2 (panel 3). In parallel experiments, cell extracts were immunoblotted with αSH2B1 (panel 4) or αFlag (panel 5). Similar results were obtained from two independent experiments. B, JAK2 was transiently coexpressed with Myc-tagged SH2B1β and IRS1 in the presence or absence of Flag-tagged SH2B2β in HEK293 cells. Cell extracts were immunoblotted with αPY, αIRS1, αJAK2, αMyc, or αFlag as indicated. C, γ2AGHR/JAK2 cells were deprived of serum overnight and stimulated with 4 U/ml GH for 10 min. Cell extracts were immunoblotted with αpJAK2, αJAK2, or αFlag as indicated (left panels). Phosphorylated JAK2 in GH-treated cells was quantified and normalized to total JAK2 protein levels (right panels). Con, Control. *, P < 0.05.
To determine whether SH2B2β inhibits SH2B1-promoted tyrosine phosphorylation of IRS1, a JAK2 substrate, SH2B1β was transiently coexpressed with or without SH2B2β in HEK293 cells expressing both JAK2 and IRS1. Cell extracts were immunoblotted with αPY and reprobed with αIRS1. SH2B1β alone markedly enhanced JAK2-mediated tyrosine phosphorylation of IRS1 (Fig. 4B). SH2B2β dramatically inhibited SH2B1β-promoted tyrosine phosphorylation of IRS1 (Fig. 4B). The expression of Myc-tagged SH2B1β and Flag-tagged SH2B2β was confirmed by immunoblotting cell extracts with αMyc and αFlag, respectively (Fig. 4B). JAK2 protein levels were similar for each experimental condition (Fig. 4B).
To determine whether SH2B2β inhibits hormone-stimulated JAK2 activation, SH2B2β was introduced by adenoviral-mediated gene transfer in γ2AGHR/JAK2 cells (human fibroblasts) that stably express both mouse GHR and JAK2. Cells were treated with GH, and cell extracts were immunoblotted with α-phospho-JAK2 (αpJAK2). αpJAK2 specifically recognizes active JAK2 that is phosphorylated on Tyr1007/1008. GH robustly stimulated phosphorylation and activation of JAK2, and GH-stimulated JAK2 activation was inhibited by SH2B2β (Fig. 4C).
SH2B2β inhibits SH2B1- and SH2B2α-promoted insulin signaling
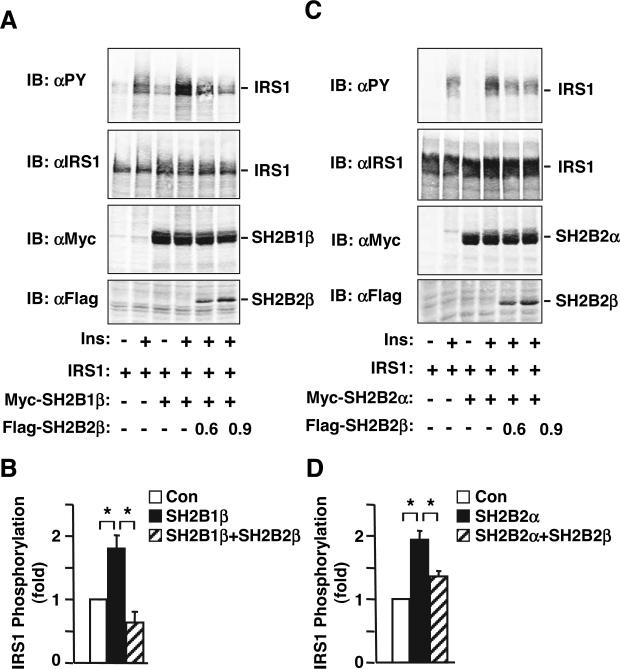
Both SH2B2α and SH2B1 are reported to bind via their respective SH2 domains to the activation loop of the insulin receptor and enhance insulin signaling (5, 26). To determine whether SH2B2β attenuates the ability of SH2B1 to promote insulin signaling, SH2B1β and IRS1 were transiently coexpressed with or without SH2B2β in HEK293 cells. Cells were deprived of serum overnight and treated with insulin (50 nm) for 10 min. Cell extracts were immunoblotted with αPY and reprobed with αIRS1. SH2B1β markedly enhanced insulin-stimulated tyrosine phosphorylation of IRS1 (Fig. 5, A and B). SH2B2β inhibited SH2B1β-promoted tyrosine phosphorylation of IRS1 in a dose-dependent manner (Fig. 5, A and B). The expression of Myc-tagged SH2B1β and Flag-tagged SH2B2β were confirmed by immunoblotting cell extracts with αMyc and αFlag, respectively (Fig. 5A, bottom two panels). In similar experiments, SH2B2α enhanced insulin-stimulated tyrosine phosphorylation of IRS1, which was significantly inhibited by SH2B2β (Fig. 5, C and D).
Fig. 5.
SH2B2β inhibits SH2B1- and SH2B2α-mediated enhancement of insulin (Ins)-stimulated tyrosine phosphorylation of IRS1. A, IRS1 was transiently coexpressed with Myc-tagged SH2B1β in the presence or absence of Flag-tagged SH2B2β in HEK293 cells as indicated. Cells were treated with insulin (50 nm) for 10 min, and cell extracts were immunoblotted (IB) with αPY, αIRS1, αMyc, or αFlag as indicated. B, HEK293 cells were transiently transfected with empty vector [control (Con)], SH2B1, or SH2B1 plus SH2B2β and treated with insulin. IRS1 phosphorylation was quantified and normalized to IRS1 protein levels. IRS1 phosphorylation in SH2B1- or SH2B1/SH2B2β-transfected cells was normalized to IRS1 phosphorylation in control cells (mean ± sem, n = 3). C, IRS1 was transiently coexpressed with Myc-tagged SH2B2α in the presence or absence of Flag-tagged SH2B2β in HEK293 cells as indicated. Cells were treated with insulin (50 nm) for 10 min, and cell extracts were immunoblotted with αPY, αIRS1, αMyc, or αFlag as indicated. D, HEK293 cells were transiently transfected with empty vector (control), SH2B2α, or SH2B2α plus SH2B2β and treated with insulin. IRS1 phosphorylation was quantified and normalized to IRS1 protein levels. IRS1 phosphorylation in SH2B2α- or SH2B2α/SH2B2β-transfected cells was normalized to IRS1 phosphorylation in control cells (mean ± sem, n = 4). *, P < 0.05.
Discussion
In this study, we identified SH2B2β as a novel isoform of SH2B2. SH2B2α and SH2B2β appear to be derived from the SH2B2 gene via alternative mRNA splicing. SH2B2α contains a DD, PH, and SH2 domain, which are encoded by nine exons. In contrast, SH2B2β contains only a DD and PH domain, which are encoded by five exons. Exons 1–4 of SH2B2α and SH2B2β are identical. Exon 5 of SH2B2β contains both exons 5 and 6 of SH2B2α plus intronic DNA sequences between exons 5 and 6 and between exons 6 and 7 (Fig. 1C). The insertion of the additional DNA sequences between exons 5 and 6 of SH2B2α causes a shift in reading frame, resulting in SH2B2β-specific C-terminal 32 amino acids (Fig. 1A).
SH2B2β bound to both SH2B1 and SH2B2α as demonstrated by GST fusion protein pull-down and coimmunoprecipitation assays. Because the DD domain of SH2B2α mediates SH2B2α homodimerization and SH2B2α heterodimerization with SH2B1 (2, 38), the DD domain of SH2B2β, which is identical with the DD domain of SH2B2α, may mediate the interaction of SH2B2β with SH2B1 or SH2B2α.
The SH2 domain of SH2B1 or SH2B2α binds to phosphorylated tyrosine(s) in JAK2 or the insulin receptor, resulting in the enhancement of JAK2 activation or insulin signaling, respectively (3, 5, 21, 22, 24–26). SH2 domain defective SH2B1 or SH2B2α acts as dominant-negative mutants to inhibit JAK2 activation and insulin signaling (2, 21). Because SH2B2β lacks the entire SH2 domain, it may function as an endogenous inhibitor for SH2B1 and/or SH2B2α. Consistent with these ideas, we demonstrated that SH2B2β markedly inhibited SH2B1-promoted JAK2 activation and JAK2-mediated tyrosine phosphorylation of IRS1. Moreover, SH2B2β inhibited GH-stimulated JAK2 phosphorylation. SH2B2β significantly attenuated both SH2B1- and SH2B2α-promoted tyrosine phosphorylation of IRS1 in response to insulin. SH2B2β may inhibit JAK2 activation and insulin signaling by directly binding to SH2B1 and/or SH2B2α via its DD domain, thus sequestering SH2B1 and/or SH2B2α.
SH2B1 is a key positive regulator of leptin and insulin sensitivity in vivo as revealed by severe leptin resistance, insulin resistance, obesity, and type 2 diabetes in SH2B1-deficient mice (5, 6). SH2B2β is expressed in multiple tissues, including targets of insulin, GH, and leptin (e.g. the brain, adipose tissue, and skeletal muscle). Therefore, SH2B2β may function as an endogenous-negative regulator of insulin, GH, and/or leptin sensitivity by antagonizing SH2B1 action. SH2B2α also positively regulates insulin signaling and JAK2 activation in cultured cells; surprisingly, deletion of the SH2B2 gene results in enhanced insulin sensitivity and cytokine action (36, 39). Because SH2B2β is also deleted in SH2B2−/− knockout mice, SH2B2β deficiency may contribute to the phenotypes observed in SH2B2−/− knockout mice.
In summary, we identified SH2B2β as a novel isoform of SH2B2. SH2B2β binds to both SH2B1 and SH2B2α, thereby inhibiting both SH2B1- and SH2B2α-promoted cellular responses, including JAK2 activation and insulin signaling. SH2B2β may function as an endogenous inhibitor of SH2B1 and/or SH2B2α, contributing to negative regulation of cellular responses to insulin and/or cytokines that require JAK2 for cell signaling.
Acknowledgments
We thank Dr. Lin Jiang for his technical assistance, Dr. Satoshi Takaki (University of Tokyo) for providing SH2B2 knockout mice, and Dr. Christin Carter-Su (University of Michigan) for providing γ2A cells.
This work was supported by Career Development Award (7-03-CD-11) from the American Diabetes Association and RO1 DK 065122 and RO1 DK073601 from National Institutes of Health (NIH) (all to L.R.). This work used the cores supported by the Michigan Diabetes Research and Training Center (funded by NIH 5P60 DK20572), University of Michigan's Cancer Center (funded by National Institutes of Health 5 P30 CA46592).
Abbreviations
- BAT
Brown adipose tissue
- DD
dimerization domain
- GHR
GH receptor
- GST
glutathione S-transferase
- IRS
insulin receptor substrate
- JAK2
Janus kinase-2
- αJAK2
anti-JAK2
- αMyc
anti-Myc
- PH
pleckstrin homology
- αpJAK2
α-phospho-JAK2
- αPY
anti-phospho-tyrosine
- SH2
Src homology 2
- STAT
signal transducer and activator of transcription
Footnotes
Disclosure Statement: All authors have nothing to declare.
References
- 1.Rui L, Mathews LS, Hotta K, Gustafson TA, Carter-Su C. Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol Cell Biol. 1997;17:6633–6644. doi: 10.1128/mcb.17.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishi M, Werner ED, Oh BC, Frantz JD, Dhe-Paganon S, Hansen L, Lee J, Shoelson SE. Kinase activation through dimerization by human SH2-B. Mol Cell Biol. 2005;25:2607–2621. doi: 10.1128/MCB.25.7.2607-2621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelms K, O'Neill TJ, Li S, Hubbard SR, Gustafson TA, Paul WE. Alternative splicing, gene localization, and binding of SH2-B to the insulin receptor kinase domain. Mamm Genome. 1999;10:1160–1167. doi: 10.1007/s003359901183. [DOI] [PubMed] [Google Scholar]
- 4.Yousaf N, Deng Y, Kang Y, Riedel H. Four PSM/SH2-B alternative splice variants and their differential roles in mitogenesis. J Biol Chem. 2001;276:40940–40948. doi: 10.1074/jbc.M104191200. [DOI] [PubMed] [Google Scholar]
- 5.Duan C, Yang H, White MF, Rui L. Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol Cell Biol. 2004;24:7435–7443. doi: 10.1128/MCB.24.17.7435-7443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab. 2005;2:95–104. doi: 10.1016/j.cmet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Ohtsuka S, Takaki S, Iseki M, Miyoshi K, Nakagata N, Kataoka Y, Yoshida N, Takatsu K, Yoshimura A. SH2-B is required for both male and female reproduction. Mol Cell Biol. 2002;22:3066–3077. doi: 10.1128/MCB.22.9.3066-3077.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank SJ. Growth hormone signalling and its regulation: preventing too much of a good thing. Growth Horm IGF Res. 2001;11:201–212. doi: 10.1054/ghir.2001.0237. [DOI] [PubMed] [Google Scholar]
- 9.Lanes R. Metabolic abnormalities in growth hormone deficiency. Pediatr Endocrinol Rev. 2004;2:209–215. [PubMed] [Google Scholar]
- 10.Carter-Su C, Smit LS. Signaling via JAK tyrosine kinases: growth hormone receptor as a model system. Recent Prog Horm Res. 1998;53:61–82. discussion 82–83. [PubMed] [Google Scholar]
- 11.Frank SJ, Gilliland G, Kraft AS, Arnold CS. Interaction of the growth hormone receptor cytoplasmic domain with the JAK2 tyrosine kinase. Endocrinology. 1994;135:2228–2239. doi: 10.1210/endo.135.5.7956946. [DOI] [PubMed] [Google Scholar]
- 12.Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993;74:237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- 13.Yi W, Kim SO, Jiang J, Park SH, Kraft AS, Waxman DJ, Frank SJ. Growth hormone receptor cytoplasmic domain differentially promotes tyrosine phosphorylation of signal transducers and activators of transcription 5b and 3 by activated JAK2 kinase. Mol Endocrinol. 1996;10:1425–1443. doi: 10.1210/mend.10.11.8923468. [DOI] [PubMed] [Google Scholar]
- 14.Liang L, Jiang J, Frank SJ. Insulin receptor substrate-1-mediated enhancement of growth hormone-induced mitogen-activated protein kinase activation. Endocrinology. 2000;141:3328–3336. doi: 10.1210/endo.141.9.7673. [DOI] [PubMed] [Google Scholar]
- 15.Smit LS, Vanderkuur JA, Stimage A, Han Y, Luo G, Yu-Lee LY, Schwartz J, Carter-Su C. Growth hormone-induced tyrosyl phosphorylation and deoxyribonucleic acid binding activity of Stat5A and Stat5B. Endocrinology. 1997;138:3426–3434. doi: 10.1210/endo.138.8.5332. [DOI] [PubMed] [Google Scholar]
- 16.Argetsinger LS, Hsu GW, Myers MG, Jr, Billestrup N, White MF, Carter-Su C. Growth hormone, interferon-γ, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1. J Biol Chem. 1995;270:14685–14692. doi: 10.1074/jbc.270.24.14685. [DOI] [PubMed] [Google Scholar]
- 17.Argetsinger LS, Norstedt G, Billestrup N, White MF, Carter-Su C. Growth hormone, interferon-γ, and leukemia inhibitory factor utilize insulin receptor substrate-2 in intracellular signaling. J Biol Chem. 1996;271:29415–29421. doi: 10.1074/jbc.271.46.29415. [DOI] [PubMed] [Google Scholar]
- 18.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 19.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 20.Kellerer M, Koch M, Metzinger E, Mushack J, Capp E, Haring HU. Leptin activates PI-3 kinase in C2C12 myotubes via janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia. 1997;40:1358–1362. doi: 10.1007/s001250050832. [DOI] [PubMed] [Google Scholar]
- 21.Rui L, Carter-Su C. Identification of SH2-bβ as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc Natl Acad Sci USA. 1999;96:7172–7177. doi: 10.1073/pnas.96.13.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurzer JH, Argetsinger LS, Zhou YJ, Kouadio JL, O'Shea JJ, Carter-Su C. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-Bβ. Mol Cell Biol. 2004;24:4557–4570. doi: 10.1128/MCB.24.10.4557-4570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan C, Li M, Rui L. SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J Biol Chem. 2004;279:43684–43691. doi: 10.1074/jbc.M408495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotani K, Wilden P, Pillay TS. SH2-Bα is an insulin-receptor adapter protein and substrate that interacts with the activation loop of the insulin-receptor kinase. Biochem J. 1998;335(Pt 1):103–109. doi: 10.1042/bj3350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riedel H, Wang J, Hansen H, Yousaf N. PSM, an insulin-dependent, pro-rich, PH, SH2 domain containing partner of the insulin receptor. J Biochem (Tokyo) 1997;122:1105–1113. doi: 10.1093/oxfordjournals.jbchem.a021868. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed Z, Pillay TS. Adapter protein with a pleckstrin homology (PH) and an Src homology 2 (SH2) domain (APS) and SH2-B enhance insulin-receptor autophosphorylation, extracellular-signal-regulated kinase and phosphoinositide 3-kinase-dependent signalling. Biochem J. 2003;371:405–412. doi: 10.1042/BJ20021589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien KB, O'Shea JJ, Carter-Su C. SH2-B family members differentially regulate JAK family tyrosine kinases. J Biol Chem. 2002;277:8673–8681. doi: 10.1074/jbc.M109165200. [DOI] [PubMed] [Google Scholar]
- 28.Hu J, Liu J, Ghirlando R, Saltiel AR, Hubbard SR. Structural basis for recruitment of the adaptor protein APS to the activated insulin receptor. Mol Cell. 2003;12:1379–1389. doi: 10.1016/s1097-2765(03)00487-8. [DOI] [PubMed] [Google Scholar]
- 29.Moodie SA, Alleman-Sposeto J, Gustafson TA. Identification of the APS protein as a novel insulin receptor substrate. J Biol Chem. 1999;274:11186–11193. doi: 10.1074/jbc.274.16.11186. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed Z, Smith BJ, Pillay TS. The APS adapter protein couples the insulin receptor to the phosphorylation of c-Cbl and facilitates ligand-stimulated ubiquitination of the insulin receptor. FEBS Lett. 2000;475:31–34. doi: 10.1016/s0014-5793(00)01621-5. [DOI] [PubMed] [Google Scholar]
- 31.Ahn MY, Katsanakis KD, Bheda F, Pillay TS. Primary and essential role of the adaptor protein APS for recruitment of both c-Cbl and its associated protein CAP in insulin signaling. J Biol Chem. 2004;279:21526–21532. doi: 10.1074/jbc.M307740200. [DOI] [PubMed] [Google Scholar]
- 32.Hu J, Hubbard SR. Structural characterization of a novel Cbl phosphotyrosine recognition motif in the APS family of adapter proteins. J Biol Chem. 2005;280:18943–18949. doi: 10.1074/jbc.M414157200. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, DeYoung SM, Hwang JB, O'Leary EE, Saltiel AR. The roles of Cbl-b and c-Cbl in insulin-stimulated glucose transport. J Biol Chem. 2003;278:36754–36762. doi: 10.1074/jbc.M300664200. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Kimura A, Baumann CA, Saltiel AR. APS facilitates c-Cbl tyrosine phosphorylation and GLUT4 translocation in response to insulin in 3T3–L1 adipocytes. Mol Cell Biol. 2002;22:3599–3609. doi: 10.1128/MCB.22.11.3599-3609.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li M, Ren D, Iseki M, Takaki S, Rui L. Differential role of SH2-B and APS in regulating energy and glucose homeostasis. Endocrinology. 2006;147:2163–2170. doi: 10.1210/en.2005-1313. [DOI] [PubMed] [Google Scholar]
- 36.Minami A, Iseki M, Kishi K, Wang M, Ogura M, Furukawa N, Hayashi S, Yamada M, Obata T, Takeshita Y, Nakaya Y, Bando Y, Izumi K, Moodie SA, Kajiura F, Matsumoto M, Takatsu K, Takaki S, Ebina Y. Increased insulin sensitivity and hypoinsulinemia in APS knockout mice. Diabetes. 2003;52:2657–2665. doi: 10.2337/diabetes.52.11.2657. [DOI] [PubMed] [Google Scholar]
- 37.Qian X, Ginty DD. SH2-B and APS are multimeric adapters that augment TrkA signaling. Mol Cell Biol. 2001;21:1613–1620. doi: 10.1128/MCB.21.5.1613-1620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhe-Paganon S, Werner ED, Nishi M, Hansen L, Chi Y-I, Shoelson SE. A phenylalanine zipper mediates APS dimerization. Nat Struct Mol Biol. 2004;11:968–974. doi: 10.1038/nsmb829. [DOI] [PubMed] [Google Scholar]
- 39.Iseki M, Kubo C, Kwon SM, Yamaguchi A, Kataoka Y, Yoshida N, Takatsu K, Takaki S. Increased numbers of B-1 cells and enhanced responses against TI-2 antigen in mice lacking APS, an adaptor molecule containing PH and SH2 domains. Mol Cell Biol. 2004;24:2243–2250. doi: 10.1128/MCB.24.6.2243-2250.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]