Abstract
Objective
Elevated inflammatory cytokine levels have been implicated in the pathogenesis of non3 healing chronic venous insufficiency (CVI) ulcers. The goal of this study was to determine the protein levels of a wide range of inflammatory cytokines in untreated CVI ulcer tissue before and after 4 weeks of high strength compression therapy. These levels were compared to cytokines present in healthy tissue.
Methods
Thirty limbs with untreated CVI and leg ulceration received therapy for 4 weeks with sustained high compression bandaging at an ambulatory wound center. Biopsies were obtained from healthy and ulcerated tissue before and after therapy. A multiplexed protein assay was used to measure multiple cytokines in a single sample. Patients were designated as rapid or delayed healers based on ulcer surface area change.
Results
The majority of pro-inflammatory cytokine protein levels were elevated in ulcer tissue compared to healthy tissue, and compression therapy significantly reduced these cytokines. TGF-β1 was up-regulated in ulcer tissue following compression therapy. Rapid healing ulcers had significantly higher levels of IL-1α, IL-1β, IFN-γ, IL-12p40 and GM-CSF before compression therapy, and IL-1 Ra after therapy. IFN-γ levels significantly decreased following therapy in the rapidly healing patients.
Conclusion
CVI ulcer healing is associated with a pro-inflammatory environment prior to treatment that reflects metabolically active peri-wound tissue that has the potential to heal. Treatment with compression therapy results in healing that is coupled with reduced pro-inflammatory cytokine levels and higher levels of the anti-inflammatory cytokine IL-1 Ra.
Clinical Relevance
This data suggests that cytokines may provide targets in which topical therapeutic inhibition or promotion at appropriate time points in the healing process may provide novel therapeutic approaches to the healing of CVI ulcers.
Keywords: Inflammation, cytokine, venous leg ulcer, compression therapy, multiplex analysis
INTRODUCTION
In the United States, chronic venous insufficiency (CVI) is the most common etiology of chronic non-healing lower extremity ulcers, estimated to be responsible for over 75% of cases. Though rarely a cause of limb amputation, these chronic wounds result in significant morbidity, pain, and are estimated to cause more than 2 million lost work days per year.1 These ulcerations negatively impact a person’s quality of life, especially if delayed healing is experienced.2 The majority of patients with leg ulcers and associated venous insufficiency will experience progressive healing when treated with sustained limb compression of 30 mmHg or greater3–5, but healing typically requires 3–6 months of treatment or longer. Also, a subset of patients experiences delayed or no healing despite receiving medical treatment.6–8
Previous studies have identified up-regulation of various pro-inflammatory cytokines in fluid collected from venous leg ulcers. It has been hypothesized that chronic venous hypertension results in an inflammatory state that is responsible for poor ulcer healing.9, 10 Although compression therapy results in healing of most venous leg ulcers, the mechanism responsible for this effect is not well defined.
Historically, cytokine proteins levels have been quantified separately. Luminex multiplex technology allows for the simultaneous evaluation of multiple cytokines in a single sample. Microspheres, which are dyed beads coated with a specific capture antibody, are added to a sample. The cytokines of interest are captured by the microspheres. Fluorescent tagged detection antibodies are added, and samples are fed through a flow cytometer. A dual laser excites the internal dyes that identify each microsphere particle (cytokine of interest) and reads the quantity of the fluorescent detection antibodies, which are in direct proportion to the bound cytokine. This technology gives the advantages of higher throughput, smaller sample volume, and improved sensitivity. Other researchers have used multiplexed immunoassays to measure cytokines in various bodily fluids.11–13
The main study goal was to quantify both pro- and anti-inflammatory levels in healthy and CVI ulcer tissue before and after sustained compression therapy using a multiplexed assay. The second goal was to identify inflammatory markers related to ulcer healing status. We hypothesized that pro-inflammatory levels would be elevated in venous ulcers with associated low levels of anti-inflammatory cytokine levels compared to healthy tissue. Following compression therapy, ulcer tissue was expected to demonstrate cytokine levels similar to the healthy tissue pattern. Delayed healing ulcers would theoretically have continued elevation of pro-inflammatory levels.
METHODS
Clinical Protocol
Enrolled patients were 18 years of age or greater with onset of lower extremity ulceration and edema (CEAP class 6, primary or secondary etiology) within 6 months of study participation per history, physical exam and referring physician documentation. Only patients that had not received previous compression therapy were included. Abnormal venous function and CEAP criteria were examined using duplex ultrasonography and air plethysmography. Patients that did not meet criteria for venous insufficiency on both studies were excluded. Duplex ultrasonography was performed with patients in a standing position using an automatic inflation/deflation cuff system (Hokanson E20 Rapid Cuff Inflator and AG101 Cuff Inflator Air Source, Issaquah, WA), and CVI was defined as the presence of reflux in the deep or superficial veins of > 0.5 sec.14 An abnormal venous filling index as assessed by air plethysmography was > 2ml/sec.15
Exclusion criteria included an Ankle to Brachial Index (ABI) of less than 0.7, congenital venous insufficiency, previous treatment with any form of high strength compression bandaging, active systemic infection, severe immunocompromised state, dressing allergy, use of an investigational drug, history of vasculitis or poor medical compliance. This protocol was approved by the University of North Carolina at Chapel Hill’s Institutional Review Board and Independent Ethics Committee. Informed consent was obtained from all patients prior to study enrollment.
Wounds were debrided, and a 3-layer or 4-layer compression bandage system (Profore lite or Profore, Smith and Nephew, Hull, United Kingdom) was applied over a polyurethane foam primary dressing. Dressings and compression bandages were changed weekly. Wounds were photographed at the initial and subsequent visits.
Planimetry, which used tracings of the ulcer perimeter, was performed weekly using the Visitrak Digital system (Smith & Nephew) to determine wound surface area changes.16 Previous studies of venous ulcer healing with compression therapy have reported that the percentage change in wound area between baseline and 3 to 4 weeks of treatment is predictive of eventual healing. Kantor and Margolis found that improvement at 4 weeks was predictive of eventual healing and Phillips reported that a reduced wound size of 40% at 3 weeks of treatment was predictive of complete healing at 12 weeks of treatment.17–18 Based on these studies, we chose to treat study patients with weekly compression bandaging for 4 weeks at which time healing progress and repeat tissue analysis was performed. Patients who had at least a 40% reduction in ulcer surface area at 4 weeks were designated as rapid healers and those who healed less than 40% were designated as delayed healers.
Tissue Sample Collection
Prior to biopsy 1% lidocaine was injected for local anesthesia. Full thickness punch biopsies 6mm in diameter and 2–3 mm in depth were obtained from the wound tissue adjacent to intact peri-ulcer skin and the ipsilateral medial thigh (healthy sample) using a trephine before the initiation of therapy. After 4 weeks of sustained compression therapy, the ulcer biopsy was repeated. Following sample collection, the tissue was flash frozen. Samples were kept at −80° C until homogenization was performed. Immunohistochemistry was not performed due to the limited biopsy size.
Protein Sample Preparation
Tissue was weighed and homogenized using a Tissue-Tearor (Biospec Products, Inc., Bartlesville, Oklahoma, United States of America) homogenizer in a buffer of Dulbecco’s-Phosphate Buffered Saline (Sigma, St. Louis, Missouri, USA) and an EDTA-free protease inhibitor cocktail (Cat.# P8340 Sigma). 0.5 ml of buffer was added for each 30 mg of tissue sample. The homogenate was centrifuged (15,000g× 10 min. at 4° C), and the supernatant total protein concentration was determined using a bicinchoninic protein assay kit (Pierce, Rockford, Illinois, USA).19 Cytokine protein levels were normalized to total protein amount.
Protein Assays
Previous studies have validated the use of a multiplex assay for simultaneous measurement of multiple cytokines in various bodily fluids and cell culture.20–22 The preceding protein quantification methods were validated for use with tissue homogenate using both spike and recovery and dilution curve analysis by our group, and similar results have been published.23 Assays (n=30 ulcer tissue, n=23 healthy tissue) were performed in duplicate. Protein levels of 22 cytokines were determined simultaneously in a single sample using the Luminex xMAP system and a commercial assay kit (Millipore, Billerica, Massachusetts, USA). The manufacturer’s instructions were followed, and samples were diluted with the kit’s calibrator diluent to a total protein concentration of 250 g/ml. Samples were read by a Luminex 100 dual laser apparatus (Luminex Corp., Austin, Texas, USA) with StarStation v2.0 software (Applied Cytometry Systems, Sacramento, California, USA).
TGF-β1 protein levels were measured using ELISA kits per the manufacturer’s (R&D Systems, Minneapolis, Minnesota, USA) protocol, as TGF-β1 was not available as a part of the multiplexed panel. The ELISA requires a larger tissue sample volume compared to the multiplex analysis; thus, not all biopsies were studied due to tissue sample size limitations (n=10 ulcer tissue, n=7 healthy tissue). Results were read on a MicroQuant plate reader with KCJunior v1.41 software (both Biotek Instruments, Winooski, Vermont, USA).
Statistical Analysis
Statistical comparisons were made using SAS v9.1 (SAS Institute Inc., Cary, North Carolina, USA). Statistical significance was set at P < 0.05, and measurements are presented as means ± standard deviations. One way analysis of variance (ANOVA) for repeated measures was used to detect differences between healthy tissue and before therapy measures as well as between before and after therapy measures. These models take into account that multiple responses are coming from the same participant. Because Wilks' Lambda, Pillai's Trace, Hotelling-Lawley Trace, and Roy's Greatest Root were all in lock-step agreement throughout (due to two groups in the ANOVA), the F statistic is reported. Comparisons between cytokine values in rapid versus delayed healers were performed using both parametric and non parametric methods. Subjects were divided into these groups based on whether or not the ulcer surface area change was more or less than 40% at 4 weeks of treatment. Modified t-tests comparing the means of cytokine levels in these two groups were performed. The modification was utilized because the comparisons were not made between two homogenous groups. To confirm significance, nonparametric tests were conducted using Wilcoxon’s rank sum test.
RESULTS
Patient Demographics
Thirty-three patients with 34 limbs affected with new untreated ulcers were enrolled in the study. Two were screen failures due to a lack of venous insufficiency on duplex studies and one was found to have an ABI < 0.7. One patient was unable to comply with compression treatment leaving 30 limb ulcers for analysis. Patient demographics and co-morbidities are listed in Table I. CEAP criteria for venous insufficiency etiology, anatomy and pathophysiology are provided in Table II. The average initial ulcer size was 21.5 ± 26.8 cm2. After 4 weeks of treatment, 2 ulcers were completely healed, and 3 ulcers had enlarged. Half of the ulcers healed at least 40% with 4 weeks of treatment, and the average ulcer size decreased by 54%.
Table I.
Patient demographics.
| Number | Percentage | |
|---|---|---|
| Mean Age | 57.9 ± 16.4 yrs | |
| Females | 20 | 68.9 |
| Hypertension | 9 | 31 |
| ABI less than 0.9 | 1 | 3.4 |
| History of DVT | 17 | 58.6 |
| Diabetes | 7 | 24.1 |
| Hypercoagulable State # | 2 | 6.9 |
| Cardiac Disease * | 7 | 24.1 |
Per patient report/documentation. Not routinely measured by our group in CVI ulcer patients.
CHF, CAD, Atherosclerosis, MI, Dysrhythmia
Table II.
CEAP criteria.
| Etiology | ||
| Primary | 8 | 27% |
| Secondary | 22 | 73% |
| Anatomy | ||
| Deep venous reflux | 12 | 40% |
| Superficial venous reflux | 4 | 13% |
| Deep and superficial reflux | 14 | 47% |
| Pathophysiology | ||
| Reflux only | 21 | 70% |
| Reflux and obstruction | 9 | 30% |
Cytokine protein levels
Twenty two cytokines were measured using a multiplex assay. Six of the 22 cytokines (IL-2, -5, -7, -12p70, -15, and -17) had either undetectable tissue measurements or no statistical significance when comparing healthy and ulcer tissue before and after compression therapy. The significant cytokines measured using the Luminex xMAP system and TGF-β1 are reported in Table III.
Table III.
Cytokine means ± standard deviations (levels expressed as pg/ug protein).
| Healthy Tissue | Ulcer Before Therapy | Ulcer After Therapy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| IL-1α | 23 | 2.59 | 3.00 | 30 | 0.89 | 1.72 | 30 | 0.28 | 0.24 | |
| IL-1β | 23 | 0 | 0 | 30 | 0.17 | 0.27 | 30 | 0.03 | 0.04 | |
| IL-1Ra* | 23 | 172.87 | 414.19 | 30 | 16.38 | 13.27 | 30 | 20.13 | 20.1 | |
| IL-4 | 23 | 0.01 | 0.02 | 30 | 0.03 | 0.04 | 30 | 0.02 | 0.03 | |
| IL-6 | 23 | 0 | 0.01 | 30 | 1.27 | 1.7 | 30 | 0.62 | 0.8 | |
| IL-8 | 23 | 0.03 | 0.03 | 30 | 15.18 | 21.89 | 30 | 3.80 | 4.78 | |
| IL-10* | 23 | 0.01 | 0.02 | 30 | 0.04 | 0.05 | 30 | 0.04 | 0.06 | |
| IL-12p40 | 23 | 0.14 | 0.27 | 30 | 1.65 | 1.68 | 30 | 0.85 | 0.78 | |
| IL-13 | 23 | 0.02 | 0.02 | 30 | 0.05 | 0.04 | 30 | 0.04 | 0.03 | |
| G-CSF | 23 | 0 | 0.02 | 30 | 0.27 | 0.28 | 30 | 0.14 | 0.16 | |
| GM-CSF | 23 | 0 | 0.01 | 30 | 0.07 | 0.07 | 30 | 0.02 | 0.04 | |
| MCP-1 | 23 | 0.11 | 0.18 | 30 | 1.03 | 0.55 | 30 | 0.94 | 0.58 | |
| IFN-γ | 23 | 0.01 | 0.02 | 30 | 0.27 | 0.27 | 30 | 0.14 | 0.16 | |
| TNF-α | 23 | 0 | 0 | 30 | 0.02 | 0.03 | 30 | 0.01 | 0.01 | |
| MIP-1α | 23 | 0.05 | 0.07 | 30 | 0.54 | 1.02 | 30 | 0.24 | 0.15 | |
| MIP-1β | 23 | 0.12 | 0.07 | 30 | 0.32 | 0.5 | 30 | 0.19 | 0.11 | |
| TGF-β1* | 7 | 0.10 | 0.04 | 10 | 0.24 | 0.07 | 10 | 0.34 | 0.12 | |
Anti-inflammatory.
Cytokines in healthy tissue compared to ulcer tissue before compression
Table IV lists the cytokines demonstrating a significant difference between concentrations in healthy tissue compared to ulcer tissue prior to compression therapy. All of these proteins demonstrated significantly higher concentrations in ulcer tissue than in healthy tissue with the exception of IL-1α.
Table IV.
Cytokines demonstrating significant differences between healthy tissue and ulcer tissue before compression therapy (levels expressed as pg/ug protein). All cytokines, except TGF-β1, n=23. TGF-β1 n=7.
| Healthy | Before Therapy | |||||
|---|---|---|---|---|---|---|
| Cytokines | Mean | (SE) | Mean | (SE) | F-Statistic | P-Value |
| IL-1α | 2.59 | (0.61) | 0.58 | (0.16) | 10.15 | 0.004 |
| IL-1β | 0.004 | (0.001) | 0.13 | (0.05) | 7.86 | 0.01 |
| IL-4 | 0.005 | (0.003) | 0.02 | (0.01) | 4.56 | 0.044 |
| IL-6 | 0.004 | (0.002) | 0.99 | (0.16) | 39.54 | 0 |
| IL-8 | 0.03 | (0.01) | 16.57 | (4.87) | 11.53 | 0.002 |
| IL-10* | 0.013 | (0.004) | 0.04 | (0.01) | 8.30 | 0.008 |
| IL-12p40 | 0.14 | (0.05) | 1.75 | (0.37) | 17.35 | 0 |
| IL-13 | 0.02 | (0.01) | 0.04 | (0.01) | 5.95 | 0.023 |
| G-CSF | 0.005 | (0.003) | 0.22 | (0.04) | 25.98 | 0 |
| GM-CSF | 0.003 | (0.001) | 0.07 | (0.01) | 22.38 | 0 |
| MCP-1 | 0.11 | (0.04) | 0.94 | (0.09) | 80.06 | 0 |
| IFN-γ | 0.009 | (0.004) | 0.26 | (0.06) | 18.91 | 0 |
| TNF-α | 0.004 | (0.001) | 0.01 | (0.00) | 15.02 | 0.001 |
| MIP-1α | 0.05 | (0.01) | 0.36 | (0.06) | 23.08 | 0 |
| MIP-1β | 0.12 | (0.01) | 0.22 | (0.03) | 7.70 | 0.011 |
| TGF-β1* | 0.10 | (0.01) | 0.26 | (0.02) | 22.55 | 0 |
Anti-inflammatory.
Cytokines in ulcer tissue before compression compared to after compression
Table V lists the cytokines demonstrating a significant difference between concentrations in ulcer tissue before compared to after 4 weeks of compression therapy. All of the listed proteins demonstrated significantly higher concentrations in ulcer tissue before compression therapy with the exception of TGF-β1 which increased significantly in ulcer tissue after compression for 4 weeks.
Table V.
Cytokines demonstrating significant differences in ulcer tissue before compression compared to ulcer tissue after 4 weeks of compression therapy (pg/ug protein). All cytokines, except TGF-β1, n=30. TGF-β1 n=10.
| Before Therapy | After Therapy | |||||
|---|---|---|---|---|---|---|
| Cytokines | Mean | (SE) | Mean | (SE) | F-Statistic | P-Value |
| IL-1α | 0.89 | (0.31) | 0.28 | (0.04) | 4.40 | 0.045 |
| IL-1β | 0.17 | (0.05) | 0.03 | (0.01) | 8.54 | 0.007 |
| IL-6 | 1.27 | (0.31) | 0.62 | (0.15) | 6.94 | 0.013 |
| IL-8 | 15.18 | (4) | 3.80 | (0.87) | 7.06 | 0.013 |
| IL-12p40 | 1.65 | (0.31) | 0.85 | (0.14) | 6.58 | 0.016 |
| G-CSF | 0.27 | (0.05) | 0.14 | (0.03) | 4.63 | 0.04 |
| GM-CSF | 0.07 | (0.01) | 0.02 | (0.01) | 12.41 | 0.001 |
| IFN-γ | 0.27 | (0.05) | 0.14 | (0.03) | 8.94 | 0.006 |
| TNF-α | 0.02 | (0.01) | 0.01 | (0) | 4.18 | 0.05 |
| TGF-β1 | 0.24 | (0.02) | 0.34 | (0.04) | 5.14 | 0.031 |
Association of cytokines and ulcer healing status
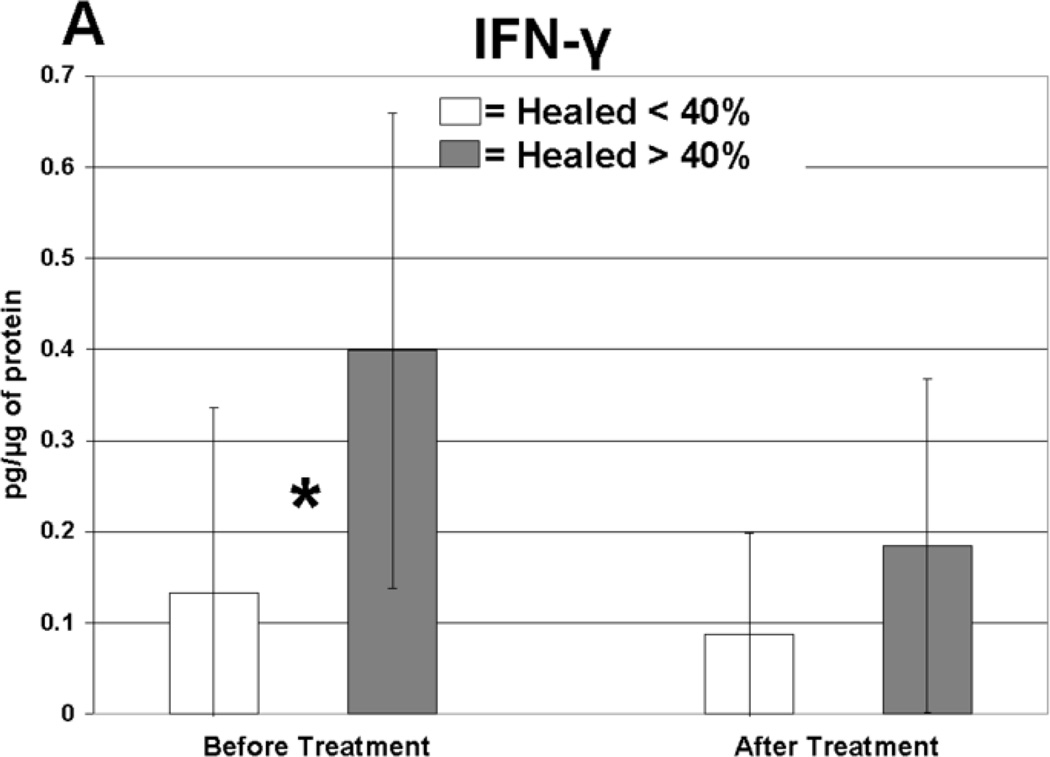
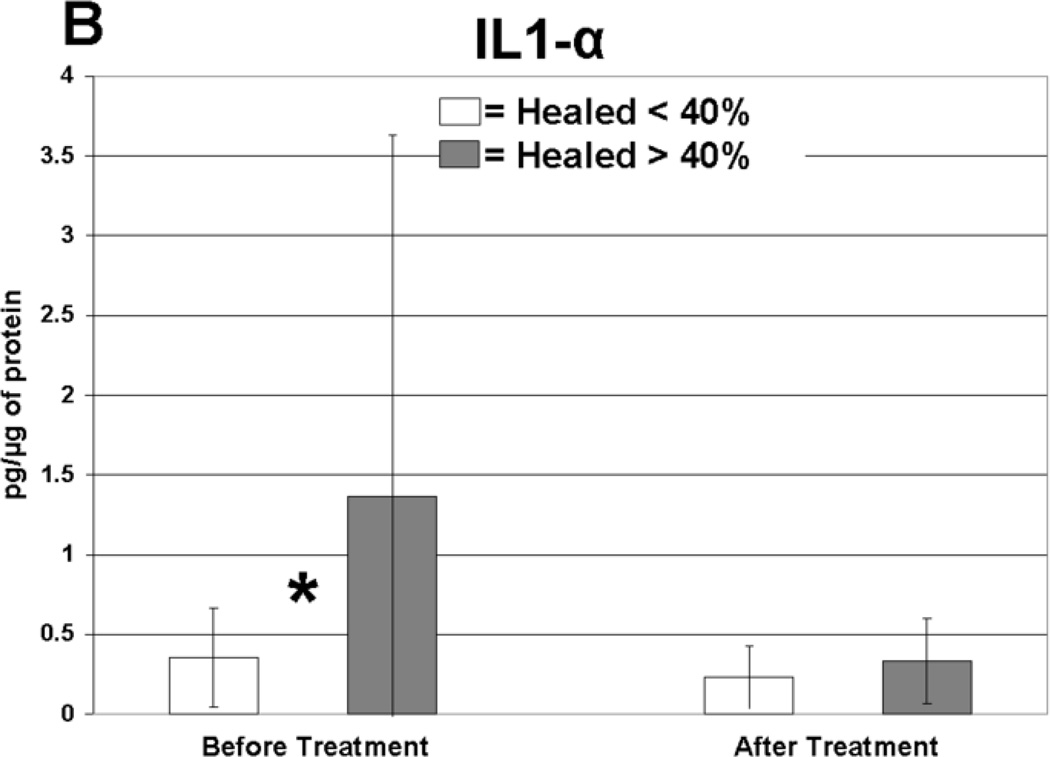
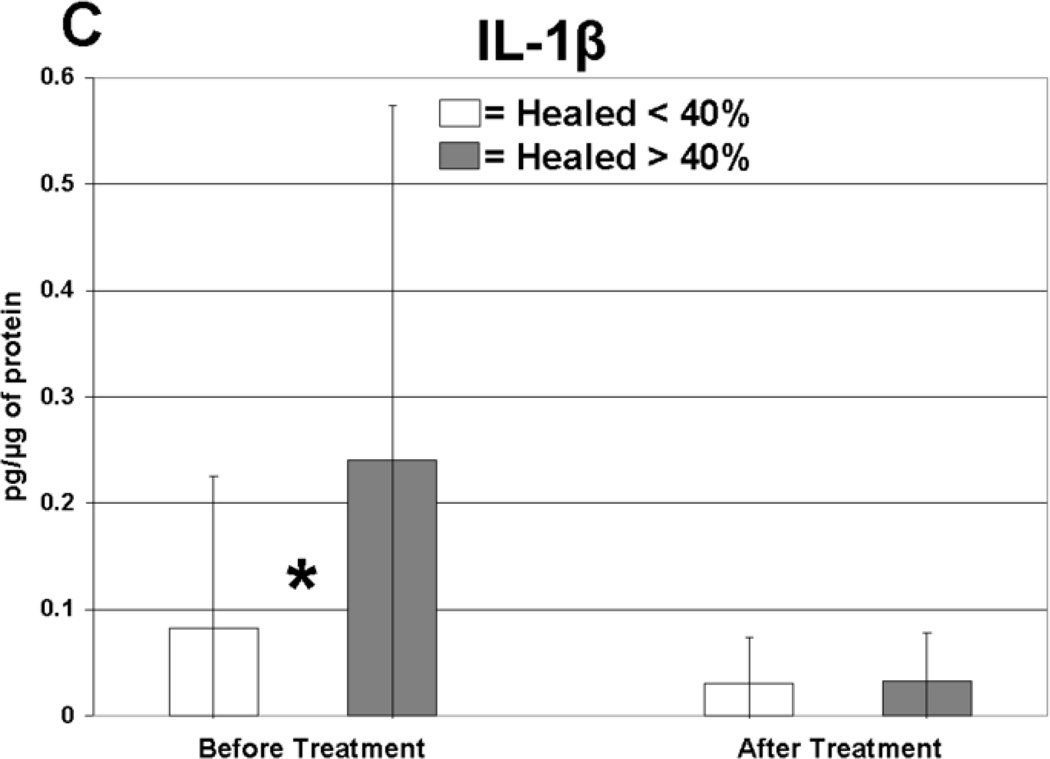
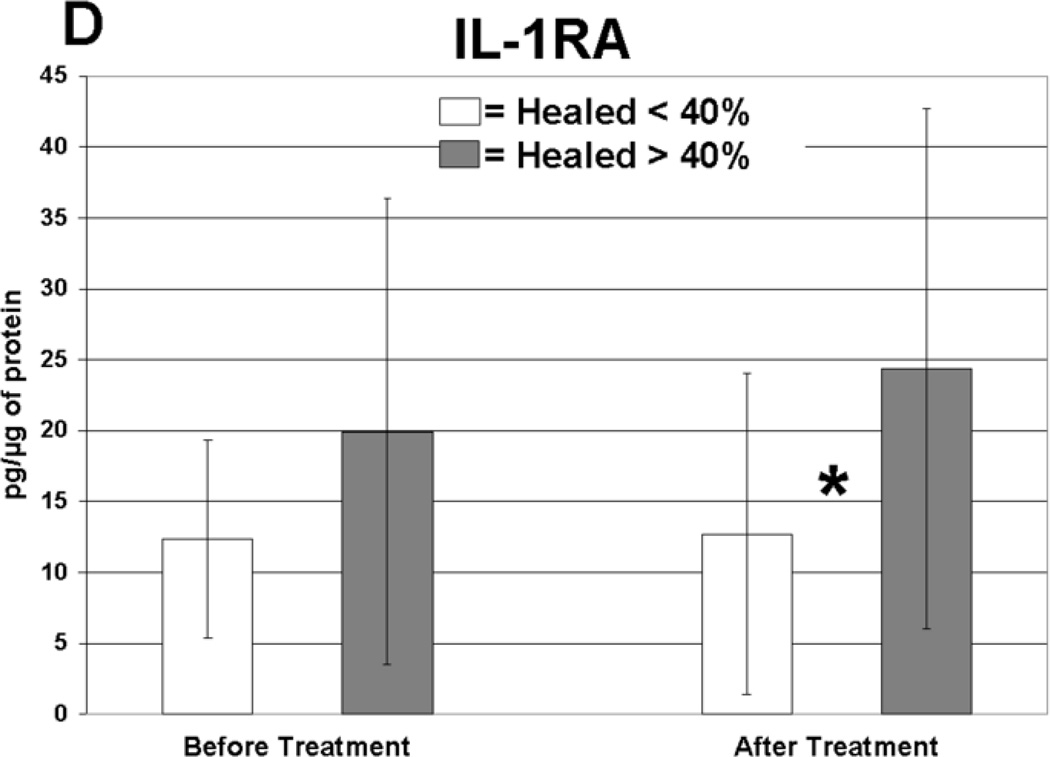
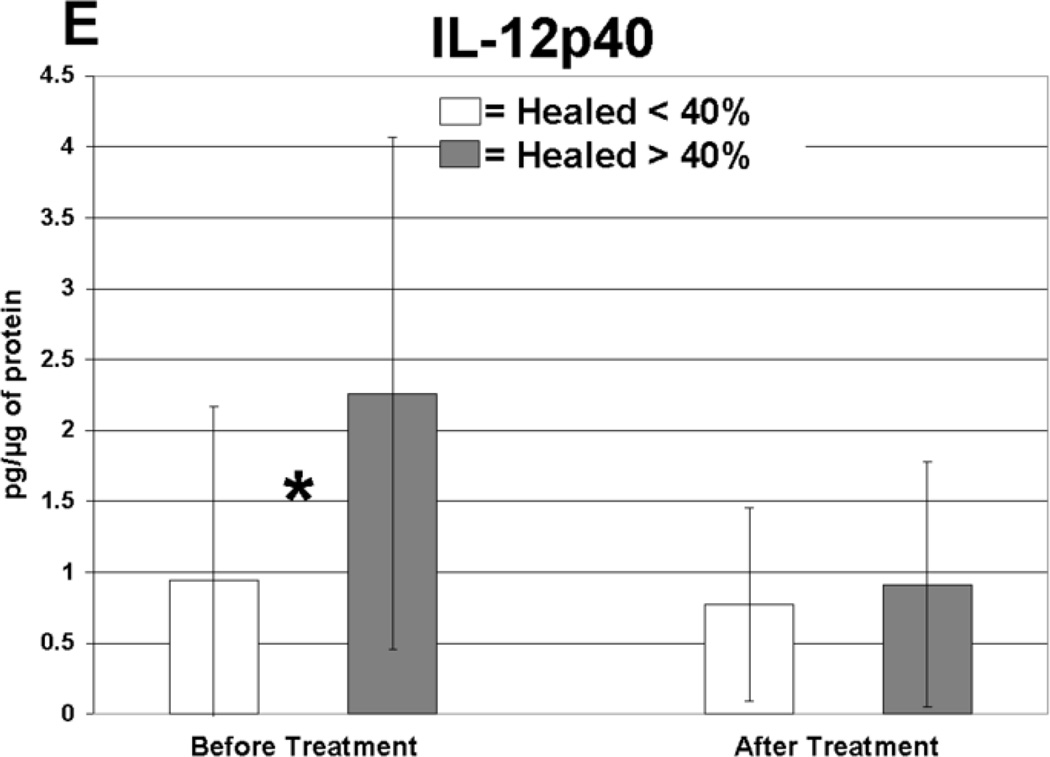
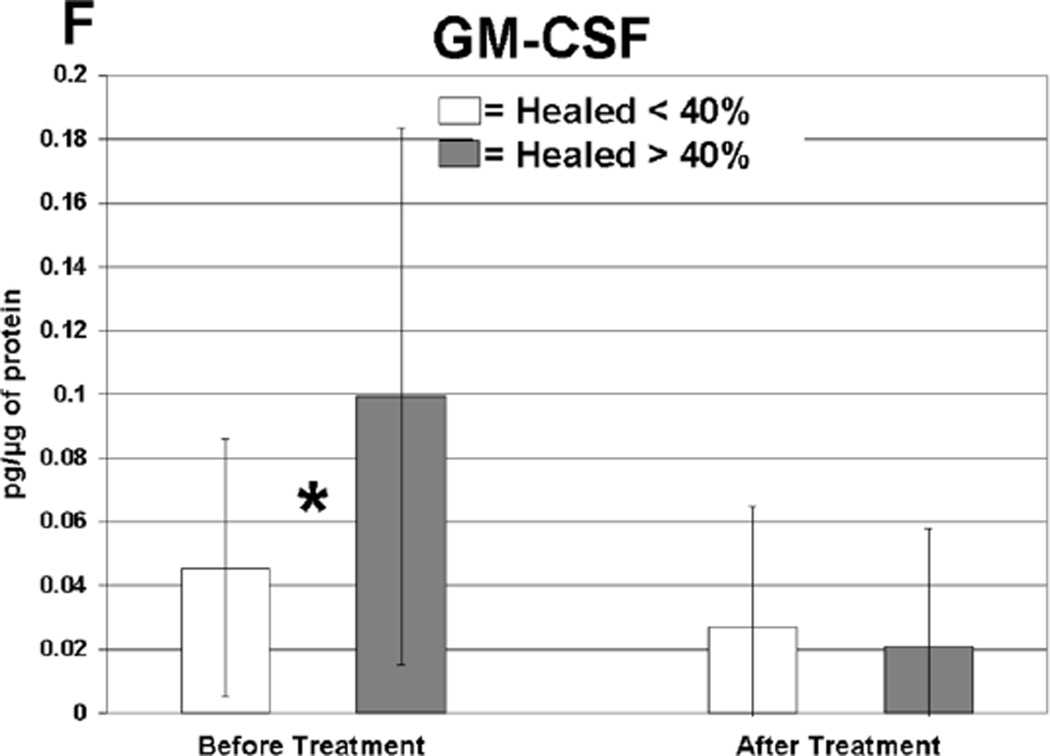
Cytokine levels in ulcer tissue were studied to determine whether they were able to predict rapid (40% or greater reduction in ulcer surface area) or delayed (less than 40% reduction in ulcer surface area) wound healing after 4 weeks of treatment. Table VI summarizes the findings. Prior to compression therapy, ulcers with higher levels of IL-1α, IL-1β, IFN-γ, IL-12p40 and GM-CSF were more likely to be rapid healers. After 4 weeks of compression, ulcers with higher levels of IL-1 Ra were more likely to be rapid healers. Total change in IFN-γ levels was also predictive of healing. Protein levels before and after compression for the cytokines demonstrating significant associations with healing are illustrated in Figure 1. The relationship between TGF-β1 levels and ulcer healing could not be determined due to an insufficient number of measurements.
Table VI.
Cytokines displaying significantly different protein levels in rapid (healed > 40%) compared to delayed (healed < 40%) healers (pg/ug protein).
| Healed > 40% | Healed < 40% | |||||
|---|---|---|---|---|---|---|
| Cytokines | Mean | SD | Mean | SD | Compression | P-Value |
| IL-1α | 1.43 | 2.3 | 0.35 | 0.30 | Before | 0.02 |
| IL-1β | 0.26 | 0.34 | 0.08 | 0.14 | Before | 0.03 |
| IFN-γ | 0.42 | 0.25 | 0.12 | 0.20 | Before | 0.001 |
| IL-12p40 | 2.24 | 1.8 | 0.93 | 1.2 | Before | 0.01 |
| GM-CSF | 0.11 | 0.08 | 0.04 | 0.04 | Before | 0.02 |
| IL-1 Ra* | 25.3 | 18.6 | 15 | 13.5 | After | 0.02 |
Anti-inflammatory.
Fig 1.
Levels of cytokine in ulcer tissue before and after 4 weeks of compression therapy for ulcers that healed > 40% compared to ulcers that healed < 40% at 4 weeks. A: Interferon-gamma (IFN-γ), B: Interleukin-1α (IL–1α), C: Interleukin-1β (IL-1β), D: Interleukin-1 receptor antagonist (IL-1RA), E: Interleukin-12p40 (IL-12p40), F: Granulocyte macrophage colony stimulating factor (GM-CSF). All levels are expressed in picograms of cytokine per microgram of protein in the tissue biopsy sample. * = statistically significant with P < .05.
DISCUSSION
This study reports for the first time, to our know ledge, the relationships between cytokine protein levels before and after compression therapy in venous leg ulcers and healthy tissue. A multiplexed assay provided simultaneous measurements of cytokines in a single tissue homogenate sample.
Although the pathogenesis of venous insufficiency leading to ulceration is likely multi-factorial, our results indicate that specific inflammatory mediators are associated with time- and pattern-dependent healing progression. It appears from our data that the presence of a robust pro-inflammatory environment is likely required in the early stages of wound repair. Several authors have argued that the initial inflammatory response to injury likely programs the end of inflammation through ‘pro-resolution signaling networks’, including lipid mediator classes.24, 25 Specific to wound healing, the importance of the initial inflammatory phase has been noted in several studies, as a paucity of pro-inflammatory cells in a newly created wound bed results in delayed healing.26–28 Hubner et al showed a strong and early induction of pro-inflammatory cytokines following cutaneous injury. This induction was significantly reduced in healing impaired glucocorticoid treated mice.29 Consistent with multiple other studies, our data also indicates that the quantity of pro-inflammatory cytokines is significantly diminished in the tissue of rapid healers. We believe that CVI ulcer delayed healers have wounds that cannot mount an appropriate initial inflammatory response; thus, the prolonged cellular effects of inflammation are not propagated.
In comparison to healthy tissue samples, inflammatory cytokines, in addition to TGF-β1, were significantly elevated in ulcer tissue before multilayer compression therapy. The exception was IL-1α, which had lower levels in untreated CVI ulcer tissue than in healthy tissue. IL-1 is a pleiotropic cytokine, and previous studies have reported that IL-1α is present in lower levels in acute wound fluid when compared to fluid from chronic wounds.30
In our study, treatment of CVI ulcers with 4 weeks of compression therapy resulted in a decrease in the levels of the majority of the pro-inflammatory ulcer cytokines. Trengove et al reported that the pro-inflammatory cytokines IL-1, IL-6 and TNF-α have significantly higher levels in the fluid of non-healing wounds. In their study, in vitro fibroblast proliferative response was stunted when this non-healing wound fluid was added to cultures; thus, healing may be impaired by specific inflammatory mediators.31
Several of the proteins studied in this project have known anti-inflammatory properties, including TGF-β1, IL-10 and IL-1 Ra. TGF-β1 levels in ulcer tissue were significantly increased after 4 weeks of sustained compression therapy. TGF-β1 has been reported to regulate dermal tissue fibrosis, although the addition of TGF-β1 to venous ulcer fibroblasts has not been shown to induce proliferation.32, 33 Likewise, the potent anti-inflammatory cytokine IL-10 had higher levels following compression which approached statistical significance (P=0.076).
Rapidly healing wounds were associated with higher levels of pro-inflammatory cytokines in the ulcer tissue at initial presentation before compression treatment. Five pro-inflammatory cytokines displayed this significant finding, including IL-1α, IL-1β, IFN-γ, IL-12p40 and GM-CSF. Elevated expression of these cytokines may indicate that a wound has mounted and maintained an appropriate level of defensive mechanisms and is prepared for future coordinated cellular repair. Our data indicates that although delayed healing CVI ulcers contain inflammatory proteins, these cytokines were present at significantly lower levels, perhaps due to increased cellular senescence.
Importantly, the rapid healing ulcers not only had very high pre-compression levels of IFN-γ but also had a significant reduction in this cytokine following compression therapy. IFN-γ is a glycoprotein with numerous immunological functions. IFN-γ has been shown to suppress numerous genes responsible for the cell cycle, DNA replication and RNA metabolism of keratinocytes; thus, it is thought to increase host defense against viruses by denying access into cells in which to replicate.34 Konur has shown that IFN-γ is the key mediator in keratinocyte apoptosis.35 IFN-γ is also a positive regulator for the production of TNF-α from keratinocytes, and it acts synergistically with IL-1α to produce TNF-α.36 Administration of recombinant murine IFN-γ to mice with surgically created incisions caused significant impairment in wound healing.37 Likewise, multiple sclerosis patients receiving injections of interferon have experienced skin inflammation and skin breakdown.38, 39 Data suggests that IFN-γ expression is important during the inflammatory phase of acute wound healing.
We believe that up-regulation of pro-inflammatory cytokines at baseline may be a marker for healthier peri-wound tissue that has better potential to heal. However, it is similarly important for up-regulated cytokine levels to be controlled to allow resumption of healing. We agree with the hypothesis previously proposed by Simka that an anti-IFN-γ agent may have a significant clinical impact on the treatment of CVI ulceration, and anti-interferon therapy is already used to treat a variety of diseases.40
Rapid healers also expressed a significantly higher level of IL-1 Ra in ulcer tissue compared to delayed healers after compression therapy. This suggests that up-regulation of anti-inflammatory cytokines is important during treatment to support the healing process. Mice homozygous for a null mutation in the gene encoding IL-1 Ra develop lethal arterial inflammation.41 An imbalance between IL-1 Ra and IL-1 also predisposes patients to inflammatory arthritis and arteritis forming the basis for the treatment of resistant cases of rheumatoid arthritis with IL-1 Ra (Anakinra; Amgen, Thousand Oaks, CA).42
This study has significant limitations that must be considered during interpretation of the data. With a study population of 30 limbs, subset analysis is not possible for patient demographics and risk factors and some relevant associations between cytokine levels and outcomes may be missed due to a lack of power. Also, when studying a panel of 22 predictors it is possible that any positive finding may be related to chance. Therefore, we believe that further studies in human tissue in this population and in populations with other types of non-healing ulcers are required to strengthen the associations outlined in this manuscript.
CONCLUSION
The results of this study indicate tha t CVI ulcers have high levels of inflammatory proteins prior to compression therapy. Importantly, rapidly healing ulcers likely need an elevated level of pro-inflammatory cytokines, including IL-1α, IL-1β, IFN-γ, IL-12p40 and GM-CSF within the ulcer bed before initiating compression therapy. Compression therapy is associated with a reduction in the pro-inflammatory ulcer environment, especially INF-γ, and promotes anti-inflammatory proteins. This data suggests that cytokines may provide targets in which inhibition or promotion at appropriate time points in the healing process may provide novel therapeutic approaches to CVI ulcer healing.
ACKNOWLEDGEMENTS
Technical help: Lisa Rothlein, BS and Quintin Anderson
Contributions: Paul Riesenman, MD and Dhavalkumar Patel, MD, PhD
Statistical assistance: Riten Mitra, BS and Pranab Sen, PhD
Financial Material Support:
The BSN-Jobst Research Fellowship in Venous and Lymphatic Disease
NIH/National Institute of General Medicine Science, Grant #: 5 T32GM00845013
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 20th Annual Meeting of the American Venous Forum Charleston, SC, February 21, 2008
REFERENCES
- 1.McGuckin M, Waterman R, Brooks J, Cherry G, Porten L, Hurley S, et al. Validation of venous leg ulcer guidelines in the United States and United Kingdom. American journal of surgery. 2002;183:132–137. doi: 10.1016/s0002-9610(01)00856-x. [DOI] [PubMed] [Google Scholar]
- 2.Franks PJ, Moffatt CJ. Health related quality of life in patients with venous ulceration: use of the Nottingham health profile. Qual Life Res. 2001;10:693–700. doi: 10.1023/a:1013825924765. [DOI] [PubMed] [Google Scholar]
- 3.Cullum N, Nelson EA, Fletcher AW, Sheldon TA. Compression for venous leg ulcers. Cochrane database of systematic reviews (Online) 2001 doi: 10.1002/14651858.CD000265. CD000265. [DOI] [PubMed] [Google Scholar]
- 4.Kunimoto BT. Management and prevention of venous leg ulcers: a literature-guided approach. Ostomy/wound management. 2001;47:36–42. 4–9. [PubMed] [Google Scholar]
- 5.Nelson EA, Bell-Syer SE, Cullum NA. Compression for preventing recurrence of venous ulcers. Cochrane database of systematic reviews (Online) 2000 doi: 10.1002/14651858.CD002303. CD002303. [DOI] [PubMed] [Google Scholar]
- 6.Marston WA, Carlin RE, Passman MA, Farber MA, Keagy BA. Healing rates and cost efficacy of outpatient compression treatment for leg ulcers associated with venous insufficiency. J Vasc Surg. 1999;30:491–498. doi: 10.1016/s0741-5214(99)70076-5. [DOI] [PubMed] [Google Scholar]
- 7.Erickson CA, Lanza DJ, Karp DL, Edwards JW, Seabrook GR, Cambria RA, et al. Healing of venous ulcers in an ambulatory care program: the roles of chronic venous insufficiency and patient compliance. J Vasc Surg. 1995;22:629–636. doi: 10.1016/s0741-5214(95)70051-x. [DOI] [PubMed] [Google Scholar]
- 8.Mayberry JC, Moneta GL, Taylor LM, Jr, Porter JM. Fifteen-year results of ambulatory compression therapy for chronic venous ulcers. Surgery. 1991;109:575–581. [PubMed] [Google Scholar]
- 9.Wlaschek M, Scharffetter-Kochanek K. Oxidative stress in chronic venous leg ulcers. Wound Repair Regen. 2005;13:452–461. doi: 10.1111/j.1067-1927.2005.00065.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen WY, Rogers AA. Recent insights into the causes of chronic leg ulceration in venous diseases and implications on other types of chronic wounds. Wound Repair Regen. 2007;15:434–449. doi: 10.1111/j.1524-475X.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 11.Prabhakar U, Eirikis E, Davis HM. Simultaneous quantification of proinflammatory cytokines in human plasma using the LabMAP assay. Journal of immunological methods. 2002;260:207–218. doi: 10.1016/s0022-1759(01)00543-9. [DOI] [PubMed] [Google Scholar]
- 12.Radstake TR, Roelofs MF, Jenniskens YM, Oppers-Walgreen B, van Riel PL, Barrera P, et al. Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis and rheumatism. 2004;50:3856–3865. doi: 10.1002/art.20678. [DOI] [PubMed] [Google Scholar]
- 13.Natelson BH, Weaver SA, Tseng CL, Ottenweller JE. Spinal fluid abnormalities in patients with chronic fatigue syndrome. Clinical and diagnostic laboratory immunology. 2005;12:52–55. doi: 10.1128/CDLI.12.1.52-55.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labropoulos N, Tiongson J, Pryor L, Tassiopoulos AK, Kang SS, Ashraf Mansour M, et al. Definition of venous reflux in lower-extremity veins. J Vasc Surg. 2003;38:793–798. doi: 10.1016/s0741-5214(03)00424-5. [DOI] [PubMed] [Google Scholar]
- 15.Criado E, Farber MA, Marston WA, Daniel PF, Burnham CB, Keagy BA. The role of air plethysmography in the diagnosis of chronic venous insufficiency. J Vasc Surg. 1998;27:660–670. doi: 10.1016/s0741-5214(98)70231-9. [DOI] [PubMed] [Google Scholar]
- 16.Gorin DR, Cordts PR, LaMorte WW, Manzoian JO. The influence of wound geometry on the measurement of wound healing rates in clinical trials. J Vasc Surg. 1996;23:524–528. doi: 10.1016/s0741-5214(96)80021-8. [DOI] [PubMed] [Google Scholar]
- 17.Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. The British journal of dermatology. 2000;142:960–964. doi: 10.1046/j.1365-2133.2000.03478.x. [DOI] [PubMed] [Google Scholar]
- 18.Phillips TJ, Machado F, Trout R, Porter J, Olin J, Falanga V. Prognostic indicators in venous ulcers. Journal of the American Academy of Dermatology. 2000;43:627–630. doi: 10.1067/mjd.2000.107496. [DOI] [PubMed] [Google Scholar]
- 19.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Analytical biochemistry. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 20.Carson RT, Vignali DA. Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. Journal of immunological methods. 1999;227:41–52. doi: 10.1016/s0022-1759(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 21.dupont NC, Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. Journal of reproductive immunology. 2005;66:175–191. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray CA, Bowsher RR, Smith WC, De vanarayan V, Willey MB, Brandt JT, et al. Development, validation, and implementation of a multiplex immunoassay for the simultaneous determination of five cytokines in human serum. Journal of pharmaceutical and biomedical analysis. 2005;36:1037–1044. doi: 10.1016/j.jpba.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair Regen. 2008 doi: 10.1111/j.1524-475X.2008.00415.x. In print. [DOI] [PubMed] [Google Scholar]
- 24.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nature immunology. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 25.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 26.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. The American journal of pathology. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 27.Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. The American journal of pathology. 1990;136:1235–1246. [PMC free article] [PubMed] [Google Scholar]
- 28.Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, et al. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. The American journal of pathology. 2000;157:237–247. doi: 10.1016/S0002-9440(10)64534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996;8:548–556. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- 30.Barone EJ, Yager DR, Pozez AL, Olutoye OO, Crossland MC, Diegelmann RF, et al. Interleukin-1alpha and collagenase activity are elevated in chronic wounds. Plastic and reconstructive surgery. 1998;102:1023–1027. discussion 8–9. [PubMed] [Google Scholar]
- 31.Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8:13–25. doi: 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 32.Pappas PJ, You R, Rameshwar P, Gorti R, DeFouw DO, Phillips CK, et al. Dermal tissue fibrosis in patients with chronic venous insufficiency is associated with increased transforming growth factor-beta1 gene expression and protein production. J Vasc Surg. 1999;30:1129–1145. doi: 10.1016/s0741-5214(99)70054-6. [DOI] [PubMed] [Google Scholar]
- 33.Lal BK, Saito S, Pappas PJ, Padberg FT, Jr, Cerveira JJ, Hobson RW, 2nd, et al. Altered proliferative responses of dermal fibroblasts to TGF-beta1 may contribute to chronic venous stasis ulcer. J Vasc Surg. 2003;37:1285–1293. doi: 10.1016/s0741-5214(02)75295-6. [DOI] [PubMed] [Google Scholar]
- 34.Banno T, Adachi M, Mukkamala L, Blumenberg M. Unique keratinocyte-specific effects of interferon-gamma that protect skin from viruses, identified using transcriptional profiling. Antiviral therapy. 2003;8:541–554. [PubMed] [Google Scholar]
- 35.Konur A, Schulz U, Eissner G, Andreesen R, Holler E. Interferon (IFN)-gamma is a main mediator of keratinocyte (HaCaT) apoptosis and contributes to autocrine IFN-gamma and tumour necrosis factor-alpha production. The British journal of dermatology. 2005;152:1134–1142. doi: 10.1111/j.1365-2133.2005.06508.x. [DOI] [PubMed] [Google Scholar]
- 36.Matsuura K, Otsuka F, Fujisawa H. Effects of interferons on tumour necrosis factor alpha production from human keratinocytes. Cytokine. 1998;10:500–505. doi: 10.1006/cyto.1997.0326. [DOI] [PubMed] [Google Scholar]
- 37.Miles RH, Paxton TP, Zacheis D, Dries DJ, Gamelli RL. Systemic administration of interferon-gamma impairs wound healing. The Journal of surgical research. 1994;56:288–294. doi: 10.1006/jsre.1994.1045. [DOI] [PubMed] [Google Scholar]
- 38.Buttmann M, Goebeler M, Toksoy A, Schmid S, Graf W, Berberich-Siebelt F, et al. Subcutaneous interferon-beta injections in patients with multiple sclerosis initiate inflammatory skin reactions by local chemokine induction. Journal of neuroimmunology. 2005;168:175–182. doi: 10.1016/j.jneuroim.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Ozden MG, Erel A, Erdem O, Oztas MO. Dermal fibrosis and cutaneous necrosis after recombinant interferon-beta1a injection in a multiple sclerosis patient. J Eur Acad Dermatol Venereol. 2005;19:112–113. doi: 10.1111/j.1468-3083.2004.01086.x. [DOI] [PubMed] [Google Scholar]
- 40.Simka M. A potential role of interferon-gamma in the pathogenesis of venous leg ulcers. Medical hypotheses. 2006;67:639–644. doi: 10.1016/j.mehy.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 41.Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. The Journal of experimental medicine. 2000;191:303–312. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furst DE. Anakinra: review of recombinant human interleukin-I receptor antagonist in the treatment of rheumatoid arthritis. Clinical therapeutics. 2004;26:1960–1975. doi: 10.1016/j.clinthera.2004.12.019. [DOI] [PubMed] [Google Scholar]