Abstract
Metacognition refers to thinking about thinking, and there has been a great deal of interest in how this ability manifests across primates. Based on much of the work to date, a tentative division has been drawn with New World monkeys on one side and Old World monkeys and apes on the other. Specifically, Old World monkeys, apes and humans often show patterns reflecting metacognition, but New World monkeys typically fail to do so, or show less convincing behavioral patterns. However, recent data suggests that this difference may relate to other aspects of some experimental tasks. For example, one possibility is that risk tolerance affects how capuchin monkeys, a New World primate species, tend to perform. Specifically, it has recently been argued that on tasks in which there are two or three options, the ‘risk’ of guessing is tolerable for capuchins since there is a high probability of being correct even if they ‘know they do not know’ or feel something akin to uncertainty. The current study investigated this possibility by manipulating the degree of risk (2-choices versus 6-choices) and found that capuchin monkeys used the uncertainty response more on 6-choice trials than on 2-choice trials. We also found that rate of reward does not appear to underlie these patterns of performance, and propose that the degree of risk is modulating the use of the uncertainty response in capuchin monkeys. Thus, the apparent differences between New and Old world monkeys in metacognition may reflect differences in risk tolerance rather than access to metacognitive states.
Keywords: uncertainty monitoring, metacognition, capuchin monkeys, comparative psychology, risk, probability
Humans are metacognitive. They monitor their knowledge states, assess the strength of their memories, seek information when needed, and can provide assessments of their confidence about most things (Dunlosky & Bjork, 2008; Flavell, 1979; Metcalfe & Kober, 2005; Nelson, 1992; Schwartz, 2008). An increasing number of researchers working in comparative cognition have attempted to look for analogous behaviors in animals (e.g., Basile, Schroeder, Brown, Templer, & Hampton, 2015; Basile, Hampton, Suomi, & Murray, 2009; Beran & Smith, 2011; Beran, Smith, Redford, & Washburn, 2006; Call, 2010; Castro & Wasserman, 2013; Foote & Crystal, 2007, 2012; Fujita, 2009; Hampton, 2001; Iwasaki, Watanabe, & Fujita, 2013; Kornell, Son, & Terrace, 2007; Marsh & MacDonald, 2012a; Morgan, Kornell, Kornblum, & Terrace, 2014; Paukner, Anderson, & Fujita, 2006; Roberts, Feeney, McMillan, MacPherson, & Musolino, 2009; Smith, Beran, Redford, & Washburn, 2006; Smith, Coutinho, Church, & Beran, 2013; Suda-King, 2008; Sutton & Shettleworth, 2008; Templer & Hampton, 2012), and an active debate centers on the question of whether humans are unique in their metacognitive abilities, or whether this characteristic of human psychology is shared with other species (Basile & Hampton, 2014; Beran & Smith, 2014; Carruthers, 2008, 2009, 2014; Crystal, 2014; Crystal & Foote, 2009; Hampton, 2009; Jozefowiez, Staddon, & Cerutti, 2009; Kornell, 2009, 2013, 2014; Le Pelley, 2012; Le Pelley, 2014; Smith, 2009; Smith, Beran, Couchman, & Coutinho, 2008; Smith, Couchman, & Beran, 2014).
Independent of the debate about whether behavioral patterns offered as evidence of metacognition in animals are sufficient or not, there seems to be clear evidence for species differences in many of the tasks that have been designed to look at metacognitive questions. Not all species are created equal when it comes to tests of ‘animal metacognition.’ The great apes have excelled in many such tests, and are perhaps the best candidates for human-like metacognitive abilities (Beran, Smith, & Perdue, 2013; Call, 2010; Call & Carpenter, 2001; Marsh & MacDonald, 2012a, 2012b; Neldner. Collier-Baker, & Nielson, 2015; Suda-King, 2008; Suda-King, Bania, Stromberg, & Subiaul, 2013). But, they are not alone, as some evidence for metacognition has been proposed for rhesus macaques (e.g., Basile et al., 2015; Beran et al., 2006; Hampton, 2001; Kornell et al., 2007; Middlebrooks & Sommer, 2011; Smith et al., 2006, 2010, 2013, Templer & Hampton, 2012), lion-tailed macaques (Marsh, 2014), rats (Foote & Crystal, 2007; Kirk, McMillan, & Roberts, 2014), a dolphin (Smith et al., 1995), and crows (Goto & Watanabe, 2012). Although there are some positive results for certain tests given to pigeons (e.g., Adams & Santi, 2011; Castro & Wasserman, 2013; Iwasaki et al., 2013), other reports suggest that pigeons may not have access to the same signals of difficulty that macaques can access (e.g., Sutton & Shettleworth, 2008). Pigeons (Roberts et al., 2009; Roberts, McMillan, Musolino, & Cole, 2012) also appear to have greater difficulty seeking needed information relative to macaque monkeys (Beran & Smith, 2011; Hampton, Zivin, & Murray, 2004). Likewise, some additional tests with rats call into question whether metacognitive interpretations are appropriate (Foote & Crystal, 2012).
Capuchin monkeys, a New World primate species, offer perplexing patterns of responding in many metacognition tests. They sometimes fail to search appropriately by either looking for food when they have not seen where it is hidden, or reaching directly when they have seen the hiding location (Basile et al., 2009; Paukner et al., 2006), a test that is passed by chimpanzees (Call, 2010; Call & Carpenter, 2001) and rhesus macaques (Hampton et al., 2004). Even though they sometimes do seek information more often when they need to in order to be successful, not all capuchin monkeys show this pattern, and they also fail to use inferences as a means of obtaining information in this kind of test (Vinings & Marsh, 2015). Some capuchin monkeys also fail to assess their own memory strength and do not seem to avoid taking memory tests they are likely to fail (Fujita, 2009) whereas macaques generally do appropriately avoid difficult memory tests (Hampton, 2001). In a computer-based information-seeking task they also lagged behind macaques in efficiently seeking the necessary information needed for correct performance (Beran & Smith, 2011). Most relevant to the present study is that capuchin monkeys rarely or never chose to use an uncertainty response when given a psychophysical discrimination task (Beran, Smith, Coutinho, Couchman, and Boomer, 2009), even when they made many errors on the primary discrimination. Again, this result is in contrast to the adaptive and flexible use of uncertainty responses by some macaques (e.g., Smith et al., 2006, 2010, 2013) in similar tests.
Most recently, a direct comparison of macaque monkeys and capuchin monkeys on a size discrimination task showed that macaques adaptively and frequently made use of the uncertainty response to decline particularly difficult trials, whereas capuchin monkeys either failed to do so, or used the uncertainty response at relatively low levels even on the hardest trials (Beran, Perdue, & Smith, 2014). However, they did use that response, suggesting that they may be sensitive to signals of difficulty and able to respond to those signals. The hypothesis of that study was that macaques and capuchins might have different thresholds for shifting from risk-taking to risk-aversion responses. Specifically, a 50% chance level of being rewarded (as is typically the case in other tasks; e.g., Beran et al., 2009) might be an acceptable level of ‘risk’ for a capuchin monkey even when the discrimination it is trying to make is very difficult. In Beran et al. (2014), however, the chance level of responding was much lower (16%), and in that case, capuchins were more inclined to make uncertainty responses, though at levels much lower than macaques. Beran et al. (2014) concluded that capuchin monkeys may not generate macaque-like (and human-like) patterns on standard uncertainty monitoring tasks with 50% chance levels because they are more tolerant of risk than macaques, not because they lack a metacognitive capacity.
Additional evidence that chance levels of responding affect degree of metacognition in animals comes from Marsh and MacDonald (2012b) who reported that orangutans were more likely to seek information about where food was hidden when there were more choices and the likelihood of guessing correctly was lower compared to when there were only two choices and guessing would be successful half of the time. Vinings and Marsh (2015) also reported that capuchin monkeys responded differently in terms of information-seeking responses when the number of response options varied. In that case, capuchin monkeys went from showing near-ceiling levels of information-seeking responses where there were more choices to more appropriate information-seeking responses when there were only two choices (i.e., they sought information more when they had not seen where the item was hidden compared to when they had). Vinings and Marsh suggested that a potential explanation for this effect of the number of choices was that a smaller number of choices decreased the cognitive load in that type of task relative to larger numbers of choices.
In the present study, we manipulated the degree of risk for capuchin monkeys performing the same size discrimination task. Many of the same monkeys from the Beran et al. (2014) study again participated, although we also added some new monkeys. We conducted this study solely with capuchin monkeys because we already know that rhesus monkeys can and will use an uncertainty response even with a high chance level. Our question is whether manipulating that chance level within the same subjects will impact the use of an uncertainty response, and so capuchin monkeys are the appropriate species to assess this question.
We gave each monkey alternating blocks of trials. In some blocks, they had to select the larger of two squares on a computer screen. In others, they had to select the largest of six squares. In both cases, trial to trial difficulty was varied in terms of how similar in size the squares were. Thus, we could assess whether capuchin monkeys would frequently (and appropriately) use an uncertainty response when chance levels of responding were low but not use the same response when chance levels were higher. This result, if it occurred, would indicate that this species modulates its use of an uncertainty response to decline hard trials not solely on the basis of trial difficulty but also on the basis of the relative risk in making a response. The data from Beran et al. (2014) suggested this might be true, but the present approach directly compares each monkey’s performance in each risk condition, controlling the kind of stimuli and response options available.
Experiment 1
Methods
Participants
We tested nine adult capuchin monkeys (Cebus apella; 5 males and 4 females, ages 6 to 24 years). All monkeys had been trained previously to use a joystick to control a cursor on a computer screen (see Evans, Beran, Chan, Klein, & Menzel, 2008). They had all participated in numerous previous computerized experiments (e.g., Beran, 2008; Beran, Evans, Klein, & Einstein, 2012; Beran & Parrish, 2012), including participation by most of these animals in previous tests of metacognition (e.g., Beran & Smith, 2011; Beran et al., 2009, 2014). The monkeys had continuous access to water and worked for fruit flavored food pellets. They also received a daily diet of fruits and vegetables. They were not food deprived for the purposes of this experiment. The experiments were conducted with approval of the Georgia State University Institutional Animal Care and Use Committee and followed all federal guidelines.
Apparatus
The monkeys were tested using the Language Research Center’s Computerized Test System which consists of a personal computer, digital joystick, color monitor, and pellet dispenser (Evans et al., 2008; Richardson et al., 1990). Monkeys manipulated the joystick with their hands to produce isomorphic movements of a small cursor on the computer screen. Contacting stimuli with the cursor sometimes resulted in the delivery of 45-mg banana-flavored chow pellets (Bio-Serv, Frenchtown, NJ) via a pellet dispenser that was connected to the computer through a digitial I/O board (PDISO8A; Keithley Instruments, Cleveland, OH). The task program was written in Visual Basic 6.0.
Design and procedure
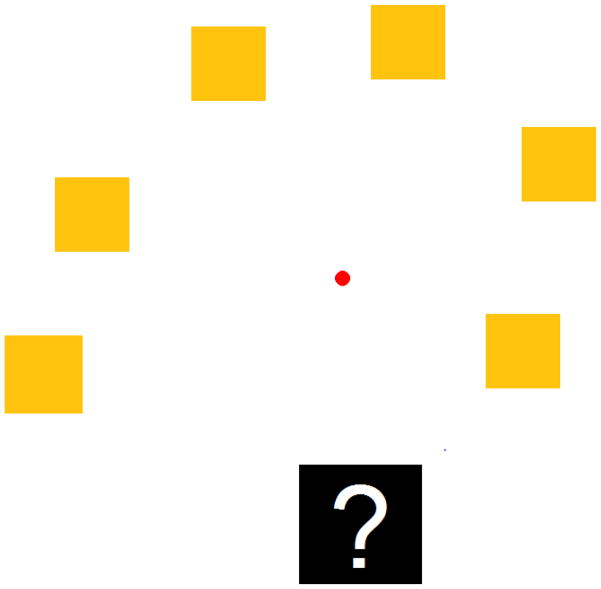
On each trial, monkeys saw either two or six squares on the computer screen, presented in one of six designated locations on the screen (Figure 1). These squares were presented in a rough semicircle from bottom left upward and then back down to the bottom right part of the screen. All squares were presented in widths and heights measured in twips in Visual Basic 6.0, and each twip is 1/567 cm. Monkeys made a selection among the squares by contacting one of those squares with the joystick-controlled cursor. If the monkey chose the largest square on the screen, a melodic chime sounded and the monkey received a single food pellet. Incorrect selections led to a buzz tone, no food was given, and then a 30 second time-out period began during which the screen remained blank. A new trial was then presented after a 1 s inter-trial interval. The locations of the squares on the screen were randomly determined on each trial from one of the six possible locations.
Figure 1.
A size-discrimination trial illustrated. The squares were presented in an orange color, and the largest had to be selected for food reward. Here, the largest square is to the bottom left. The ? is the uncertainty response. The small circle in the center of the screen is the cursor controlled by the monkey using a joystick. It was red in color.
The size of the largest square on each trial was chosen randomly to be one of 13 sizes, ranging from 1000 twips (approximately 67 pixels) per side to 2200 twips (approximately 147 pixels) per side, in 100 step increments (1000, 1100, …, 2200). All foil (incorrect) squares were the same size as each other on a given trial. They differed from the largest square across 20 trial-difficulty levels with the smallest difference consisting of only 25 twips per side (Level 1) and the largest consisting of 500 twips per side (Level 20). This provided a variety of difficulty levels for the discrimination.
On all trials, a question mark appeared at the bottom center of the screen. This was the uncertainty response (UR), and its selection operated to clear the screen and end the trial. The 1 s ITI then occurred before presentation of the next trial. The UR operated solely as a means of not making the primary discrimination to a given trial - no food pellet, timeout, or auditory feedback was given when the UR was selected, and the next trial was not guaranteed to be easier or harder than the present trial.
The session duration was approximately four hours, and monkeys determined the pace of working and the number of trials completed by resting and working when they chose. Monkeys completed variable numbers of test sessions weekly, depending on their participation in other (unrelated) experimental tasks, and whether they chose to work on a given day. Participation was voluntary. Typically they worked on the task for two to four sessions per week.
Phase 1
In this phase, each monkey completed ten blocks of 1,500 trials per block. For the first block, there were two squares presented on each trial as choice options. The second block presented six squares as choice options, and Block 3 through Block 10 continued this alternation in the number of choice options. When monkeys completed a block of trials, the program ended, although on some occasions the program might be started again later in the same session with a new number of squares presented.
At the end of the first two blocks (1,500 trials with two choices and 1,500 trials with six choices) none of the monkeys had ever used the UR. This was despite the previous experiences of some of them in the Beran et al. (2014) study. Thus, we had to institute ‘forced’ trials in which the monkeys had to choose the UR and experience its function. This occurred through the normal presentation of a trial, but the cursor only would move in the direction of the UR, and so the monkey was forced to make that response rather than to choose one of the squares. These forced trials occurred with a probability of .15, and they were randomly determined in terms of when they were presented. Thus, forced trials could occur on any trial difficulty level. In this way, monkeys were not trained when they should use the UR in terms of optimal responding, but instead only learned what it did when selected. These forced trials were used for Block 3 through Block 10.
Phase 2
Given the successes of some of the monkeys (see Results), we gave all monkeys four more iterations of 2-choice and 6-choice conditions (again, with 1,500 trials per iteration). Now, we removed the forced trials so that the monkeys never had to select the UR. All other aspects of the experiment were the same as in Phase 1.
Results
Phase 1
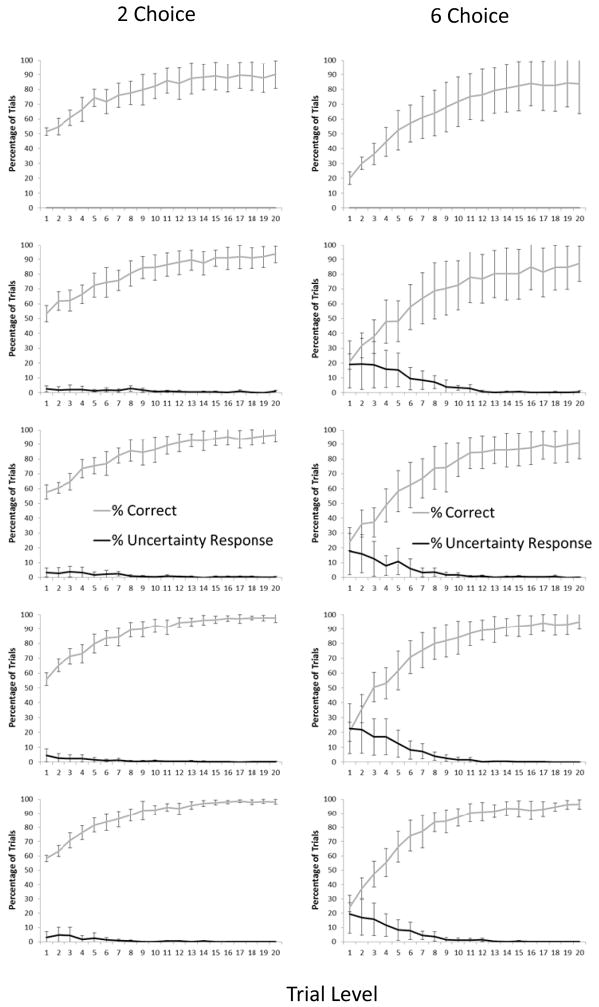
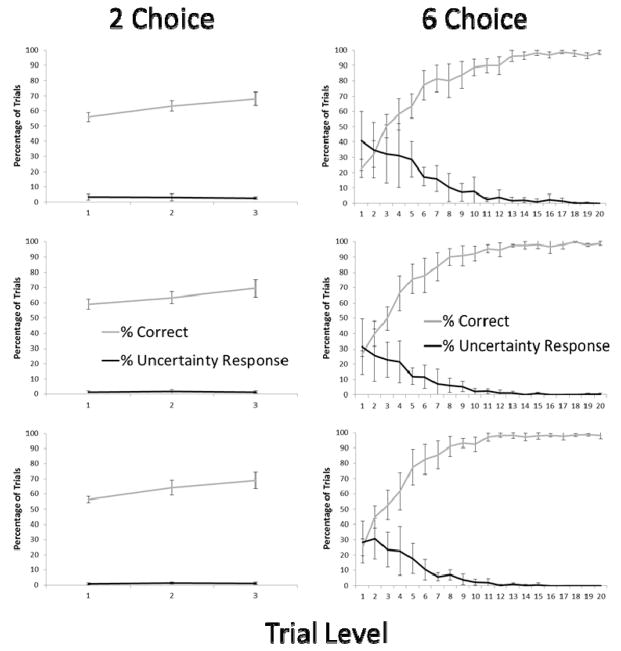
Figure 2 presents the percentage of trials correct and the percentage of trials in which the UR was voluntarily selected at each difficulty level for each of the five alternating blocks of trials with the 2-choice and the 6-choice conditions. As noted, for the first alternating block of trials the monkeys never used the UR, and so there was no difference between the 2-choice and 6-choice options. All remaining figures and analyses show the proportion of UR responses with the forced trials excluded. As shown by the remaining graphs in Figure 2, there was a consistent increase in the use of the UR in the 6-choice condition relative to the 2-choice condition. Group-level analyses were conducted for the final alternation (the last block of 2-choice trials and last block of 6-choice trials) using repeated measures analysis of variance, with Level and Set Size as within-subject factors.
Figure 2.
Monkeys’ performance in Exp. 1 as a function of trial level and condition (2-choice – left column, 6-choice – right column) for each of the five alternating blocks of trials (earliest at top and latest at bottom). Error bars show 95% confidence intervals.
First, we examined performance when monkeys chose to attempt the discrimination trial by choosing one of the squares rather than the UR. There was a significant interaction between Level and Condition, F (19, 152) = 23.24, p < .001, np2 = .74. This reflected a stronger effect of level on performance in the 6-choice condition.
Next, we examined monkeys’ use of the UR to decline trials. There was a significant interaction of Level and Condition, F (19, 152) = 5.78, p < .001, np2 = .42. This indicated that the UR was used more extensively for some levels (the harder ones) in the 6-choice condition than in the 2-choice condition. However, to confirm whether the effect of level occurred in both conditions, we examined the correlation between level and percentage of trials choosing the UR. A significant negative correlation would indicate that the UR was selected more often as a function of increasing objective trial difficulty. For the 6-item condition, there was a significant negative correlation, r (18) = −.87, p < .001. For the 2-item condition, there also was a significant negative correlation, r (18) = −.81, p < .001.
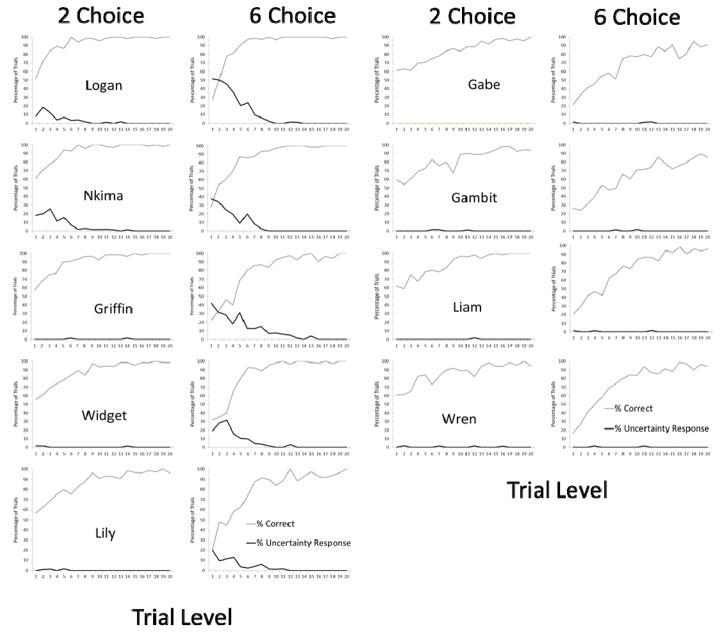
The graphs in Figure 2 show group performance, but it is important also to document the individual differences in monkey’s use (or not) of the UR as a function of choice number and level. We again relied on the final alternating block of trials to document these differences, although the patterns reflected here held for earlier iterations as well for each monkey. These data are presented graphically in Figure 3. It is easy to see from the figure that the monkeys fell into one of two categories – those that rarely, if ever, used the UR in either condition (Gabe, Gambit, Liam, Wren) and those that used the UR, and did so much more often in the 6-choice condition (Griffin, Lily, Logan, Nkima, Widget).
Figure 3.
Performances of each monkey in the final alternating block of trials in Exp 1. For each monkey, there are two graphs. The one with the monkey name is performance in the 2-choice condition and the graph to the right of that is performance in the 6-choice condition. The monkeys at left showed varying degrees of uncertainty responding in the 6-choice condition whereas monkeys at left did not.
Phase 2
Figure 4 presents the two alternating blocks of trials with the 2-choice and the 6-choice conditions when there were no longer any forced UR trials. The same analyses were conducted for the final alternating block of trials, with the same result as Phase 1. There was a significant interaction of Level and Condition, F(19, 152) = 14.06, p < .001, np2 = .64. The effect of level on performance was stronger in the 6-choice condition.
Figure 4.
Performance by the monkeys in the two alternating blocks of trials (first alternating block is the top row, second alternating block is the bottom row) with the 2-choice and the 6-choice conditions when there were no longer any forced UR trials (Phase 2, Experiment 1). Error bars show 95% confidence intervals.
Next, we examined monkeys’ use of the UR to decline trials. There was a significant interaction of Level and Condition, F(19, 152) = 5.45, p < .001, np2 = .41. This indicates that the UR was used more extensively for some levels (the harder ones) in the 6-choice condition than in the 2-choice condition. Again, to confirm whether the effect of level occurred in both conditions, we examined the correlation between level and percentage of trials choosing the UR. For the 6-item condition, there was a significant negative correlation, r(18) = −.84, p < .001. For the 2-item condition, there also was a significant negative correlation, r(18) = −.85, p < .001.
The individual differences seen in Phase 1 were almost perfectly recaptured in Phase 2. The same monkeys gave the same pattern of either no use of the UR, or greater use of the UR in the 6-choices condition compared to the 2-choices condition. The only exception to this was Liam, who showed some use of the UR in the 2-choice condition, but not in the 6-choice condition.
We examined an additional aspect of these data. We compared the last alternating block of trials in Phase 2 on the basis of a comparison metric of food reward delivery for the five monkeys that used the UR more in the 6-choice condition compared to the 2-choice condition. We calculated this metric in the following way. Each incorrect response added 30 seconds to the total temporal measure. Each correct response or UR added 1 second to this measure. This summed valued represented the total time engaging in the task. The total number of pellets earned in the task for correct responses then was divided by this number to produce a pellets-per-second score for time engaged with the task, where a larger value indicated a more profitable reward rate for working. This metric indicated that, for four of the five monkeys, the 2-choice condition was always more profitable than the 6-choice condition (Table 1). The only exception was Logan, who earned slightly more pellets per second in the 6-choice condition than in the 2-choice condition.
Table 1.
Reward rates for various conditions, shown as the number of pellets per second working on the task.
| Experiment 1 Phase 2 |
Experiment 2 | Experiment 3 | |||
|---|---|---|---|---|---|
| 2-choice | 6-choice | 2-choice | 6-choice | 2-choice | |
| Griffin | .27 | .17 | .05 | .13 | .09 |
| Lily | .22 | .16 | .04 | .12 | .08 |
| Logan | .35 | .39 | .06 | .30 | .12 |
| Nkima | .32 | .23 | .07 | .22 | .11 |
| Widget | .25 | .17 | .05 | .17 | .10 |
Discussion
Three results emerged from Experiment 1. First, as in Beran et al. (2014), capuchin monkeys as a group used the UR statistically more often when faced with six choice options than faced with 2 choice options, and they used it on the difficulty levels with the greatest risk of making a classification error. The difference between 2-choice performance and 6-choice performance suggests that contexts with lower levels of reward for chance responding tend to facilitate greater use of the UR by this species. Second, this result, although true at the group level, again must be qualified, as in Beran et al. (2014), by the recognition that some monkeys rarely or never used the UR in either condition. Thus, use of the UR remains a rather fragile phenomenon in this species, although it does occur.
The third result is most critical, because it may shed some light on why capuchin monkeys perform as they do in varying risk contexts with different chance levels. We calculated a measure of reward that took into account pellets earned and time directly engaged in the task. We found that for four of the five monkeys, the rate of reward procurement was actually better in the condition in which they rarely used the UR compared to the condition where they used the UR more frequently. When chance was 50%, the monkeys may have opted not to use the UR because their rate of reward was sufficiently high. However, when six choices were available, and chance levels of responding were much lower, the monkeys may have been motivated to use the UR more often in an attempt to raise the general rate of reinforcement. Even with their use of the UR, however, four of five monkeys did not manage to equal their reward rate in the 2-choice condition without using the UR.
This last result has important implications for understanding how capuchin monkey might view the UR, and what psychological states might preface its use (or disuse) in these kinds of tasks. We have argued (e.g., Beran et al., 2009, 2014; Beran & Smith, 2011) that capuchin monkeys may lack the same degree of metacognitive monitoring abilities that rhesus monkeys and apes possess, perhaps because of selective pressure among some Old World primates for better cognitive control and monitoring of internal states of perception, memory, and knowledge. Alternatively, capuchin monkeys may simply be less risk averse than rhesus monkeys, preferring to make primary responses when chance levels are high enough to still provide a relatively fair rate of reward overall. The present results cannot distinguish between these two possibilities. In Experiment 1, capuchin monkeys were more successful in the 2-choice condition compared to the 6-choice condition in terms of pellets earned for the same unit of time, even though they rarely used the UR to avoid errors.
In Experiment 2, we changed the task to illuminate whether capuchin monkeys use the UR as a tool for indicating uncertainty or as a means of maintaining acceptable rates of reward. The crucial step we took was to manipulate the reward landscape so that now the 2-choice condition as performed by the capuchins (with little or no use of the UR) had an equal or lower reward rate relative to the 6-choice condition. If the monkeys approached the UR as a tool for maintaining acceptable rates of reward they would incorporate that response into the 2-choice condition. But, if monkeys’ interest in the UR extends beyond its reward-maximization properties, toward fending off uncertainty or the necessity for making choices with low chance levels of being correct, they would still favor the UR in the 6-option condition, and our equalization of the reinforcement landscape would make little difference.
Experiment 2
Methods
Participants and Apparatus
We tested the five monkeys that showed higher use of the UR in the 6-choice condition in Experiment 1 (Griffin, Lily, Logan, Nkima, and Widget). The apparatus was identical to that in Experiment 1.
Design and procedure
Each monkey completed six blocks of 1,500 trials per block. Again, the number of choices alternated across these blocks, starting with six choices in Block 1, two choices in Block 2, and so on. Now, however, we modified the 2-choice version of the task. Only Levels 1 through 3 were presented, and so the discrimination was much more difficult for the monkeys as these were the hardest trial levels.
Results and Discussion
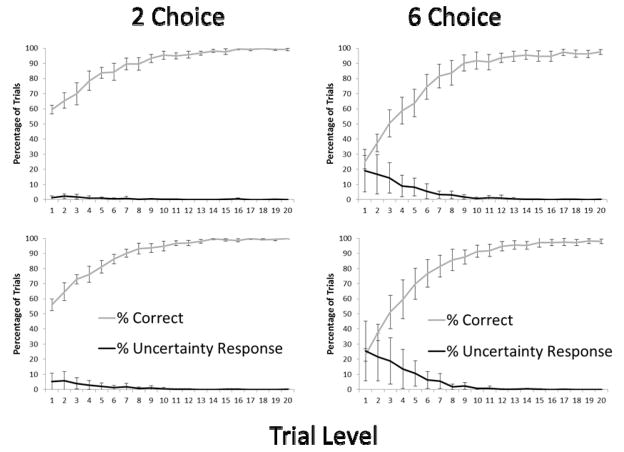
Figure 5 presents the overall performance patterns and patterns of UR use for each condition as a function of level (1–20 for the 6-choice condition and 1–3 for the 2-choice condition). The use of the UR was nearly nonexistent for the 2-choice condition but occurred at much higher levels for the 6-choice condition. For the final alternation of trials blocks we compared performance at Level 1, Level 2, and Level 3 in the two conditions using two-tailed, paired samples t-tests to confirm this statistically. For each level there were significantly more UR responses in the 6-choice condition: Level 1 t(4) = 4.00, p = .02; Level 2 t(4) = 4.30, p = .012; Level 3 t(4) = 3.89, p = .02.
Figure 5.
The performance patterns and patterns of UR use for each condition In Experiment 2 as a function of level (1–20 for the 6-choice condition and 1–3 for the 2-choice condition). Error bars show 95% confidence intervals.
In addition, this manipulation effectively reversed the rewards per second outcome in the two conditions (Table 1). Now, monkeys earned more pellets per second in the 6-choice condition than the 2-choice condition. If these monkeys were sensitive to this ongoing rate of reward and were attempting to hold that rate steady, they should have increased their use of the UR for the hard trials in the 2-choice condition. That they did not again indicates that capuchin monkeys are not inclined to integrate the UR into a response pattern when the chance level of responding correctly is high.
However, there is an additional consideration. Because only the three most difficult levels were presented in the 2-choice condition, it was true that the UR did not offer the same kind of ‘escape’ that it offered in the 6-choice condition. Even if monkeys chose that response, they were likely to see another nearly as difficult trial (as shown in the light grey lines in the figure), and perhaps this led to their disuse of that response. If the UR served no functional value, it was not a fair assessment of the hypothesis that capuchin monkeys rely on the rate of reward to drive responding independent of whether they also can monitor states of certainty and uncertainty. Experiment 3 addressed this limitation of Experiment 2.
Experiment 3
Methods
Participants and Apparatus
We tested the same five monkeys as in Experiment 2. The apparatus was identical to that in Experiment 1.
Design and procedure
Each monkey completed one block of 3,000 trials. There were always two choices on the screen. On half of the trials, the level was chosen randomly from the range of 1 to 3 (the three hardest levels), and on the other half of the trials, level was chosen randomly from the range 1 to 15, with these levels being the same as those used in Experiment 1. The difference was a larger proportion of trials from the objectively most difficult levels. In this way, the task now offered a much greater variety of trial levels than in Experiment 2, and many more objectively easier trials that could follow after the use of the UR.
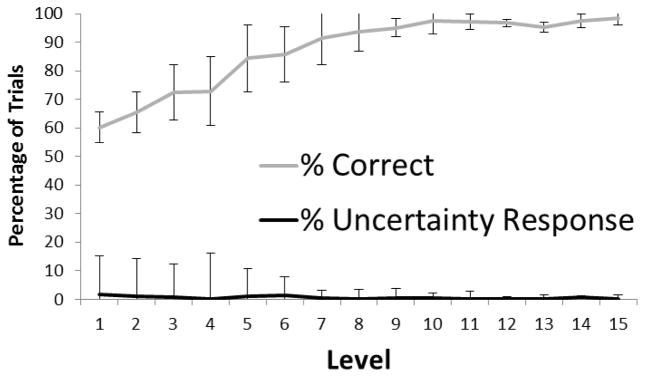
Results and Discussion
Figure 6 presents the overall performance pattern and pattern of UR use. The use of the UR remained very low in this variation of the 2-choice condition. This manipulation doubled or nearly doubled the rewards per second measure for each monkey compared to 2-choice condition in Experiment 2 but still kept it below the rewards per second for the 6-choice condition in Experiment 2 (Table 1) and the 2-choice condition in Experiment 1. The monkeys earned more pellets per second engaged in the task than in Experiment 2 in the 2-choice condition, and had they used the UR appropriately on the trials for which they were at greatest risk for error, they could have earned even more pellets. If the monkeys’ use of the UR depended on keeping the rewards per trial consistent across conditions, they should have used the UR more in the 2-choice condition, but they did not.
Figure 6.
The overall performance pattern and pattern of UR use in Experiment 3. Error bars show 95% confidence intervals.
General Discussion
Beran et al. (2009) demonstrated that capuchin monkeys, unlike rhesus monkeys, were unable or unwilling to integrate the UR into their behavioral response patterns in a psychophysical test, even when task difficulty and error rates were very high. Beran et al. (2014) demonstrated that some of the same monkeys, when given a task in which the chance level of responding correctly was much lower than the typical 50%, did integrate the use of the UR to some degree. The present study extended that report by directly manipulating, in some cases from one test session to the next, the objective degree of risk for guessing during a psychophysical discrimination. The objective degree of risk for making a primary response (choosing a square) modulated the use of the uncertainty response in capuchin monkeys, in two ways. As expected, whenever the uncertainty response was consistently used by a monkey in a condition, it was used more often for the objectively most difficult trials. Second, when the probability of being rewarded, by chance, was higher (50%), capuchin monkeys rarely or never used the uncertainty response, even for difficult trials when they were performing near this chance level. When the probability of being rewarded, by chance, was lower (16%), some capuchin monkeys began to use the uncertainty response, and they consistently used it when the likelihood of making an error was highest. However, even in this situation, not all capuchin monkeys used the uncertainty response – some continued to make only primary responses no matter the degree of risking errors by doing so. These individual differences are consistent with those reported in previous work with capuchins that made use of uncertainty (Beran et al., 2009) and information-seeking responses (Basile et al., 2009).
Experiment 2 and Experiment 3 were designed to assess whether this increased use of the uncertainty response was actually modulated by the increased risk or whether reward rate differences could account for the results. In Experiment 1, capuchin monkeys had differing rates of rewards per time spent in the 2-choice and 6-choice conditions, and even without any use of the uncertainty response the 2-choice condition was more profitable. However, in Experiment 2, we reversed this reward rate relation, without evoking any change in the frequency of uncertainty response use in the two conditions. If capuchin monkeys used the uncertainty response to maintain a consistent level of reward within a task condition, they should have increased their use in the 2-choice condition. They should have done this because there was a very large reduction in the reward rate in this condition relative to the 6-choice condition in Experiment 1 and Experiment 2. However, they did not. Experiment 3 confirmed that this was not the result of our having made all of the 2-choice trials difficult. Even when there were plenty of objectively easier trials, capuchin monkeys often made erroneous responses to the hardest trial levels in the 2-choice condition.
This suggests that capuchin monkeys are risk tolerant when chance levels of correct responding are sufficiently high, and that even when overall error rates are increased, and overall task difficulty is increased, they still make primary responses rather than incorporating an unrewarded uncertainty response. Rhesus monkeys, however, do incorporate the uncertainty response into their overall behavioral repertoire in these 2-choice circumstances, and do so in an effective and flexible manner (Beran et al., 2006, 2009, 2014; Smith et al., 2006, 2010, 2014). These results with capuchin monkeys nicely match those found in orangutans in an information-seeking paradigm with hidden food items, where orangutans were more likely to seek information rather than choose without looking when chance levels of responding were low compared to when they were higher (Marsh and MacDonald, 2012b).
If capuchin monkeys are more tolerant of risk than macaques, this might help explain why they generate limited or lower uncertainty responding compared to rhesus macaques. Specifically, a capuchin monkey making a response that involved a difficult discrimination might indeed feel that the choice is a difficult one. It also could be sensitive to the chances of guessing correctly, and be tolerant of that level of risk, and that tolerance might not be as high for rhesus macaques. Although the species difference might be related to differential sensitivity to uncertainty, it is plausible that instead differential tolerance of risk is playing a critical role. There appear to be such species differences among primates with regard to risk tolerance, even in as closely related species as chimpanzee and bonobos (e.g., Heilbronner et al., 2008). However, experiments that have directly assessed risk preference in rhesus monkeys and capuchin monkeys in the same task have not been conducted. What we do know is that, when tested in isolation, rhesus monkeys are more risk tolerant than risk averse (e.g., Xu & Kralik, 2014). This also seems true for capuchin monkeys, again tested in isolation. De Petrillo, Ventricelli, Ponsi, and Addessi (2015) reported that capuchin monkeys tended to prefer a ‘risky’ option compared to a ‘safe’ one in terms of probability of reward, although they also closely attended to the overall probabilities of receiving reward and decreased their risk-tolerance when the reward levels for taking risks were decreased relative to safe option. What remains to be determined is what the relative degree of risk tolerance in that species is when compared directly to rhesus monkeys. Such data, in relation to those reported in the present paper, would be highly informative.
We predict that, given an identical test, capuchin monkeys would make more risky choices than rhesus monkeys. This might occur no matter whether both species showed a general risk tolerance or risk aversion. An alternative hypothesis is that perhaps capuchin monkeys and rhesus monkeys would be equally risk tolerant on tasks they are equally good at, but capuchin monkey metacognition is weaker and so monitoring uncertainty and generating behavioral responses to reflect that uncertainty requires greater cognitive effort for them. If this is the case they might be willing to tolerate more risk before attempting to monitor uncertainty. For rhesus monkeys, it might be easier to monitor uncertainty. Thus, they might be more willing to use the information from that monitoring in less risky situations because the risk/effort trade-off is in favor of that use.
There are a number of ideas about when and why species might show greater risk tolerance. For example, reward contexts such as the size or amount of reward or the length of delay between response and reward delivery can play a role in risk tolerance in species ranging from pigeons to humans (e.g., Lagorio & Hackenberg, 2010; Ludvig, Madan, Pisklak, & Spetch, 2014). More broadly, a number of ecological perspectives support the idea that capuchin monkeys may be more risk tolerant than macaques. One idea is that individuals who are at a higher risk of starvation may be more likely to take risks in foraging behavior, and therefore, low body weight species (that are at a greater risk for starvation) would be more risk tolerant (see Caraco, 1981; Kacelnik & Bateson, 1996; Stephens, 1981). Capuchins have smaller-bodies than macaques. Another idea is that risk preferences reflect the environments in which species evolved and reflect the feeding ecologies of species (De Petrillo et al., 2015; Gigerenzer & Todd 1999; Heilbronner et al., 2008). From this perspective, capuchin monkeys should be relatively more risk tolerant than macaques because they show highly flexible and opportunistic foraging behavior by exploiting a variety of food sources including some that are unpredictable (such as insect nests), they use tools to extract food, and they live in a variety of forest environments (see Fragaszy, Visalberghi, & Fedigan, 2004). This variability in environmental conditions and food sources leads to the prediction that greater risk tolerance would be beneficial for capuchin monkeys because taking chances on unfamiliar or unpredictable sources, or innovating in terms of tool use, may be essential to obtaining food. On the other hand, rhesus macaques are omnivores that live in a wide variety of habitats and tend to exploit readily available food sources rather than specialize in certain types of foods. This reliance on readily available food sources might not benefit as much from risk tolerance and instead favor more risk aversion relative to capuchin monkeys.
As we have noted in our earlier work (Beran et al., 2009, 2014), there are individual differences in use of the uncertainty response in capuchin monkeys (and also in rhesus monkeys) and in other metacognitive responses (e.g., Basile et al., 2009). Beran et al. (2009) found that one monkey (Logan) eventually came to use an uncertainty response, but only when there was a long penalty for responding incorrectly (a 90 s timeout, which is triple what these monkeys typically experience in all of their other computerized testing), and only when many trials were presented at his perceptual threshold for making a dense-sparse discrimination. That result, reframed in the context discussed here, suggests that uncertainty responding by Logan occurred only when the penalty and overall performance levels were strongly aligned to make the task difficult even though he still had a 50% chance of responding correctly. Other monkeys also faced that stiff test, and they suffered greatly in terms of their performance, but none incorporated the uncertainty response. Beran et al. (2014) showed that a direct comparison of rhesus monkeys to capuchin monkeys on the same discrimination task suggested that the two species approached the impact of chance levels of responding rather differently with regard to their use of the uncertainty response. All macaques adopted and effectively used the uncertainty response when chance was very low (in comparison to other tasks these monkey perform) whereas only some capuchin monkeys did, and even then at much lower levels than macaques. More work is needed not only to better understand the apparent species differences in this area, but also the individual differences that are observed. Such approaches could focus on the role of differing risk thresholds or perhaps other cognitive capacities such as working memory capacity in predicting differences in behavior responses that might reflect metacognitive abilities.
As noted, it seems that a productive next step in this comparative assessment of uncertainty monitoring by rhesus monkeys and capuchin monkeys is to more directly address issues such as risk sensitivity, awareness of differing reward probabilities, and other aspects of choice environments that lend themselves to an accurate (or inaccurate) understanding of what ‘one’s chances are.’ This also should include assessing the effects of variations in things such as reward quality and quantity which have been reported to affect information seeking responses in other primate species (e.g., Call, 2010; Marsh & MacDonald, 2012b). Perhaps rhesus monkeys and capuchin monkeys are different in some of these regards, and those differences may impact their propensity for learning about, and then adaptively using, uncertainty responses. Without those data, we must remain agnostic as to whether capuchin monkeys lack the degree of metacognitive monitoring that some other primates show. Instead, their monitoring capacities may be modulated because of a stronger inclination to accept risk independently of any experienced uncertainty as they make primary responses. A broader database on risk tolerance, probabilistic choice behavior, and uncertainty monitoring within the species compared in the animal metacognition literature may generate new insights into why differences in uncertainty monitoring occur between species.
Acknowledgments
This research was supported by NIH grants HD061455 and HD060563 and by NSF grant BCS0956993.
Contributor Information
Michael J. Beran, Georgia State University
Bonnie M. Perdue, Agnes Scott College
Barbara A. Church, University at Buffalo, The State University of New York
J. David Smith, University at Buffalo, The State University of New York.
References
- Adams A, Santi A. Pigeons exhibit higher accuracy for chosen memory tests than for forced memory tests in duration matching-to-sample. Learning and Behavior. 2011;39:1–11. doi: 10.1007/s13420-010-0001-7. [DOI] [PubMed] [Google Scholar]
- Basile BM, Hampton RR. Metacognition as discrimination: Commentary on Smith et al. (2014) Journal of Comparative Psychology. 2014;128:135–137. doi: 10.1037/a0034412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Hampton RR, Suomi SJ, Murray EA. An assessment of memory awareness in tufted capuchin monkeys (Cebus apella) Animal Cognition. 2009;12:169–180. doi: 10.1007/s10071-008-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Schroeder GR, Brown EK, Templer V, Hampton RR. Evaluation of seven hypotheses for metamemory performance in rhesus monkeys. Journal of Experimental Psychology: General. 2015;144:85–102. doi: 10.1037/xge0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ. Capuchin monkeys (Cebus apella) succeed in a test of quantity conservation. Animal Cognition. 2008;11:109–116. doi: 10.1007/s10071-007-0094-3. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA, Klein ED, Einstein GO. Rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella) remember future responses in a computerized task. Journal of Experimental Psychology: Animal Behavior Processes. 2012;38:233–243. doi: 10.1037/a0027796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Parrish AE. Sequential responding and planning in capuchin monkeys (Cebus apella) Animal Cognition. 2012;15:1085–1094. doi: 10.1007/s10071-012-0532-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Perdue BM, Smith JD. What are my chances? Closing the gap in uncertainty monitoring between rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella) Journal of Experimental Psychology: Animal Learning and Cognition. 2014;40:303–316. doi: 10.1037/xan0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD. Information seeking by rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella) Cognition. 2011;120:90–105. doi: 10.1016/j.cognition.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD. The uncertainty response in animal metacognition researchers. Journal of Comparative Psychology. 2014;128:155–159. doi: 10.1037/a0036564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD, Coutinho MVC, Couchman JJ, Boomer J. The psychological organization of “uncertainty” responses and “middle” responses: A dissociation in capuchin monkeys (Cebus apella) Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:371–381. doi: 10.1037/a0014626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD, Perdue BM. Language-trained chimpanzees name what they have seen, but look first at what they have not seen. Psychological Science. 2013;24:660–666. doi: 10.1177/0956797612458936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD, Redford JS, Washburn DA. Rhesus macaques (Macaca mulatta) monitor uncertainty during numerosity judgments. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:111–119. doi: 10.1037/0097-7403.32.2.111. [DOI] [PubMed] [Google Scholar]
- Call J. Do apes know that they could be wrong? Animal Cognition. 2010;13:689–700. doi: 10.1007/s10071-010-0317-x. [DOI] [PubMed] [Google Scholar]
- Call J, Carpenter M. Do apes and children know what they have seen? Animal Cognition. 2001;4:207–220. [Google Scholar]
- Caraco T. Energy budgets, risk and foraging preferences in dark-eyed juncos (Junco hyemalis) Behavioral Ecology & Sociobiology. 1981;8:213–217. [Google Scholar]
- Carruthers P. Meta-cognition in animals: a skeptical look. Mind and Language. 2008;23:58–89. [Google Scholar]
- Carruthers P. How we know our own minds: The relationship between mindreading and metacognition. Behavioral and Brain Sciences. 2009;32:121–182. doi: 10.1017/S0140525X09000545. [DOI] [PubMed] [Google Scholar]
- Carruthers P. Two concepts of metacognition. Journal of Comparative Psychology. 2014;128:138–139. doi: 10.1037/a0033877. [DOI] [PubMed] [Google Scholar]
- Castro L, Wasserman EA. Information-seeking behavior: Exploring metacognitive control in pigeons. Animal Cognition. 2013;16:241–254. doi: 10.1007/s10071-012-0569-8. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Where is the skepticism in animal metacognition? Journal of Comparative Psychology. 2014;128:152–154. doi: 10.1037/a0034427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal JD, Foote AL. Metacognition in animals: Trends and challenges. Comparative Cognition and Behavior Reviews. 2009;4:54–55. doi: 10.3819/ccbr.2009.40005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrillo F, Ventricelli M, Ponsi G, Addessi E. Do tufted capuchin monkeys play the odds? Flexible risk preferences in Sapajus spp. Animal Cognition. 2015;18:119–130. doi: 10.1007/s10071-014-0783-7. [DOI] [PubMed] [Google Scholar]
- Dunlosky J, Bjork RA, editors. Handbook of memory and metamemory. New York: Psychology Press; 2008. [Google Scholar]
- Evans TA, Beran MJ, Chan B, Klein ED, Menzel CR. An efficient computerized testing method for the capuchin monkey (Cebus apella): Adaptation of the LRC-CTS to a socially housed nonhuman primate species. Behavior Research Methods. 2008;40:590–596. doi: 10.3758/brm.40.2.590. [DOI] [PubMed] [Google Scholar]
- Flavell JH. Metacognition and cognitive monitoring: A new area of cognitive- developmental inquiry. American Psychologist. 1979;34:906–911. [Google Scholar]
- Foote AL, Crystal JD. Metacognition in the rat. Current Biology. 2007;17:551–555. doi: 10.1016/j.cub.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote AL, Crystal JD. “Play it again”: A new method for testing metacognition in animals. Animal Cognition. 2012;15:187–199. doi: 10.1007/s10071-011-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragaszy DM, Visalberghi E, Fedigan LM. The complete capuchin: The biology of genus Cebus. Cambridge University Press; Cambridge: 2004. [Google Scholar]
- Fujita K. Metamemory in tufted capuchin monkeys (Cebus apella) Animal Cognition. 2009;12:575–585. doi: 10.1007/s10071-009-0217-0. [DOI] [PubMed] [Google Scholar]
- Gigerenzer G, Todd PM. Simple heuristics that make us smart. Oxford University Press; Oxford: 1999. [Google Scholar]
- Goto K, Watanabe S. Large-billed crows (Corvus macrorhynchos) have retrospective but not prospective metamemory. Animal Cognition. 2012;15:27–35. doi: 10.1007/s10071-011-0428-z. [DOI] [PubMed] [Google Scholar]
- Hampton RR. Rhesus monkeys know when they remember. Proceedings of the National Academy of Sciences. 2001;98:5359–5362. doi: 10.1073/pnas.071600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR. Multiple demonstrations of metacognition in nonhumans: Converging evidence or multiple mechanisms? Comparative Cognition and Behavior Reviews. 2009;4:17–28. doi: 10.3819/ccbr.2009.40002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR, Zivin A, Murray EA. Rhesus monkeys (Macaca mulatta) discriminate between knowing and not knowing and collect information as needed before acting. Animal Cognition. 2004;7:239–246. doi: 10.1007/s10071-004-0215-1. [DOI] [PubMed] [Google Scholar]
- Heilbronner SR, Rosati AG, Stevens JR, Hare B, Hauser MD. A fruit in the hand or two in the bush? Divergent risk preferences in chimpanzees and bonobos. Biology Letters. 2008;4:246–9. doi: 10.1098/rsbl.2008.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Watanabe S, Fujita K. Do pigeons (Columba livia) seek information when they have insufficient knowledge? Animal Cognition. 2013;16:211–221. doi: 10.1007/s10071-012-0566-y. [DOI] [PubMed] [Google Scholar]
- Jozefowiez J, Staddon JER, Cerutti DT. Metacognition in animals: How do we know that they know? Comparative Cognition and Behavior Reviews. 2009;4:29–39. [Google Scholar]
- Kacelnik A, Bateson M. Risky theories: The effects of variance on foraging decisions. American Journal of Zoology. 1996;36:402–434. [Google Scholar]
- Kirk CR, McMillan N, Roberts WA. Rats respond for information: Metacognition in a rodent? Journal of Experimental Psychology: Animal Learning and Cognition. 2014;40:249–259. doi: 10.1037/xan0000018. [DOI] [PubMed] [Google Scholar]
- Kornell N. Metacognition in humans and animals. Current Directions in Psychological Science. 2009;18:11–15. [Google Scholar]
- Kornell N. Where is the “meta” in animal metacognition? Journal of Comparative Psychology. 2013 doi: 10.1037/a0033444. [DOI] [PubMed] [Google Scholar]
- Kornell N, Son LK, Terrace HS. Transfer of metacognitive skills and hint seeking in monkeys. Psychological Science. 2007;18:64–71. doi: 10.1111/j.1467-9280.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- Kornell N. Where is the “meta” in animal metacognition? Journal of Comparative Psychology. 2014;128:143–149. doi: 10.1037/a0033444. [DOI] [PubMed] [Google Scholar]
- Lagorio CH, Hackenberg TD. Risky choice in pigeons and humans: a cross-species comparison. Journal of the Experimental Analysis of Behavior. 2010;93:27–44. doi: 10.1901/jeab.2010.93-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pelley ME. Metacognitive monkeys or associative animals? Simple reinforcement learning explains uncertainty in nonhuman animals. Journal of Experimental Psychology Learning, Memory, and Cognition. 2012;38:686–708. doi: 10.1037/a0026478. [DOI] [PubMed] [Google Scholar]
- Le Pelley ME. Primate polemic: Commentary on Smith, Couchman and Beran (2013) Journal of Comparative Psychology. 2014;128:132–134. doi: 10.1037/a0034227. [DOI] [PubMed] [Google Scholar]
- Ludvig EA, Madan CR, Pisklak JM, Spetch ML. Reward context determines risky choice in pigeons and humans. Biology Letters. 2014;10:20140451. doi: 10.1098/rsbl.2014.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh HL. Metacognitive-like information seeking in lion-tailed macaques: A generalized search response after all? Animal Cognition. 2014;17:1313–1328. doi: 10.1007/s10071-014-0767-7. [DOI] [PubMed] [Google Scholar]
- Marsh HL, MacDonald SE. Information seeking by orangutans: A generalized search strategy? Animal Cognition. 2012a;15:293–304. doi: 10.1007/s10071-011-0453-y. [DOI] [PubMed] [Google Scholar]
- Marsh HL, MacDonald SE. Orangutans (Pongo abelii) “play the odds”: Information-seeking strategies in relation to cost, risk, and benefit. Journal of Comparative Psychology. 2012b;126:263–278. doi: 10.1037/a0025906. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Kober H. Self-reflective consciousness and the projectable self. In: Terrace HS, Metcalfe J, editors. The missing link in cognition: Origins of self-reflective consciousness. New York, NY: Oxford University Press; 2005. [Google Scholar]
- Middlebrooks PG, Sommer MA. Metacognition in monkeys during an oculomotor task. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2011;37:327–337. doi: 10.1037/a0021611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan G, Kornell N, Kornblum T, Terrace HS. Retrospective and prospective metacognitive judgments in rhesus macaques (Macaca mulatta) Animal Cognition. 2014;17:249–257. doi: 10.1007/s10071-013-0657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neldner K, Collier-Baker E, Nielsen M. Chimpanzees (Pan troglodytes) and human children (Homo sapiens) know when they are ignorant about the location of food. Animal Cognition. 2015;18:683–699. doi: 10.1007/s10071-015-0836-6. [DOI] [PubMed] [Google Scholar]
- Nelson TO, editor. Metacognition: Core readings. Toronto: Allyn and Bacon; 1992. [Google Scholar]
- Paukner A, Anderson JR, Fujita K. Redundant food searches by capuchin monkeys (Cebus apella): A failure of metacognition? Animal Cognition. 2006;9:110–117. doi: 10.1007/s10071-005-0007-2. [DOI] [PubMed] [Google Scholar]
- Richardson WK, Washburn DA, Hopkins WD, Savage-Rumbaugh ES, Rumbaugh DM. The NASA/LRC Computerized Test System. Behavior Research Methods, Instruments, and Computers. 1990;22:127–131. doi: 10.3758/bf03203132. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Feeney MC, McMillan N, MacPherson K, Musolino E, Petter M. Do pigeons (Columba livia) study for a test? Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:129–142. doi: 10.1037/a0013722. [DOI] [PubMed] [Google Scholar]
- Roberts WA, McMillan N, Musolino E, Cole MW. Information seeking in animals: Metacognition? Comparative Cognition and Behavior Reviews. 2012;7:85–109. [Google Scholar]
- Schwartz BL. Working memory load differentially affects tip-of-the-tongue states and feeling-of-knowing judgments. Memory & Cognition. 2008;36:9–19. doi: 10.3758/mc.36.1.9. [DOI] [PubMed] [Google Scholar]
- Smith JD. The study of animal metacognition. Trends in Cognitive Sciences. 2009;13:389–396. doi: 10.1016/j.tics.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Smith JD, Beran MJ, Couchman JJ, Coutinho MVC. The comparative study of metacognition: Sharper paradigms, safer inferences. Psychonomic Bulletin and Review. 2008;15:679–691. doi: 10.3758/pbr.15.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Beran MJ, Redford JS, Washburn DA. Dissociating uncertainty responses and reinforcement signals in the comparative study of uncertainty monitoring. Journal of Experimental Psychology: General. 2006;135:282–297. doi: 10.1037/0096-3445.135.2.282. [DOI] [PubMed] [Google Scholar]
- Smith JD, Couchman JJ, Beran MJ. Animal metacognition: A tale of two comparative psychologies. Journal of Comparative Psychology. 2014;128:115–131. doi: 10.1037/a0033105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Coutinho MVC, Church BA, Beran MJ. Executive-attentional uncertainty responses by rhesus macaques (Macaca mulatta) Journal of Experimental Psychology: General. 2013;142:458–475. doi: 10.1037/a0029601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Redford JS, Beran MJ, Washburn DA. Rhesus monkeys (Macaca mulatta) adaptively monitor uncertainty while multi-tasking. Animal Cognition. 2010;13:93–101. doi: 10.1007/s10071-009-0249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Schull J, Strote J, McGee K, Egnor R, Erb L. The uncertain response in the bottlenosed dolphin (Tursiops truncatus) Journal of Experimental Psychology: General. 1995;124:391–408. doi: 10.1037//0096-3445.124.4.391. [DOI] [PubMed] [Google Scholar]
- Stephens DW. The logic of risk-sensitive foraging preferences. Animal Behaviour. 1981;29:628–629. [Google Scholar]
- Suda-King C. Do orangutans (Pongo pygmaeus) know when they do not remember? Animal Cognition. 2008;11:21–42. doi: 10.1007/s10071-007-0082-7. [DOI] [PubMed] [Google Scholar]
- Suda-King C, Bania AE, Stromberg EE, Subiaul F. Gorillas’ use of the escape response in object choice memory tests. Animal Cognition. 2013;16:65–84. doi: 10.1007/s10071-012-0551-5. [DOI] [PubMed] [Google Scholar]
- Sutton JE, Shettleworth SJ. Memory without awareness: Pigeons do not show metamemory in delayed matching to sample. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34:266–282. doi: 10.1037/0097-7403.34.2.266. [DOI] [PubMed] [Google Scholar]
- Templer VL, Hampton RR. Rhesus monkeys (Macaca mulatta) show robust evidence for memory awareness across multiple generalization tests. Animal Cognition. 2012;15:409–419. doi: 10.1007/s10071-011-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vining AQ, Marsh HL. Information seeking in capuchins (Cebus apella): A rudimentary form of metacognition? Animal Cognition. 2015;18:667–681. doi: 10.1007/s10071-015-0835-7. [DOI] [PubMed] [Google Scholar]
- Xu ER, Kralik JD. Risky business: Rhesus monkeys exhibit persistent preferences for risky options. Frontiers in Psychology. 2014;5:258. doi: 10.3389/fpsyg.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]