Abstract
IMPORTANCE
The role of amyloid in the progression of Alzheimer disease (AD) pathophysiology is of central interest to the design of randomized clinical trials. The presence of amyloid has become a prerequisite for enrollment in several secondary prevention trials for AD, yet the precise effect of elevated amyloid levels on subsequent clinical and biomarker events is less certain.
OBJECTIVE
To explore the effect of elevated amyloid levels on subsequent changes in cognition and biomarkers.
DESIGN, SETTING, AND PARTICIPANTS
A total of 564 cognitively normal individuals (median age, 78 years) from the Mayo Clinic Study of Aging, a population-based longitudinal study in Olmsted County, Minnesota, with serial cognitive data were selected for this study. The data used in this study were collected from January 12, 2006, to January 9, 2014. Individuals included in this study had undergone magnetic resonance imaging, fluorodeoxyglucose positron emission tomography (FDG-PET), and Pittsburgh Compound B (PiB) PET at baseline were not cognitively impaired at baseline and had at least 1 clinical follow-up. A subset of 286 individuals also underwent serial imaging. Elevated amyloid level was defined as a standardized uptake value ratio of greater than 1.5 on PiB PET. Associations with baseline amyloid status and baseline and longitudinal change in clinical and imaging measures were evaluated after adjusting for age and hippocampal volume. APOE4 effects were also evaluated.
MAIN OUTCOMES AND MEASURES
Cognitive measures of memory, language, attention/executive function, visuospatial skills, PiB levels, hippocampal and ventricular volumes, and FDG-PET measures.
RESULTS
At baseline, 179 (31.7%) individuals with elevated amyloid levels had poorer cognition in all domains measured, reduced hippocampal volume, and greater FDG-PET hypometabolism. Elevated amyloid levels at baseline were associated with a greater rate of cognitive decline in all domains (0.04 to 0.09 z score units per year) except language and a greater rate of amyloid accumulation (1.6% per year), hippocampal atrophy (30 mm3 per year), and ventricular enlargement (565 mm3 per year). Elevated amyloid levels were also associated with an increased risk of mild cognitive impairment (hazard ratio, 2.9; 95% CI, 1.7–5.0, and hazard ratio, 1.6; 95% CI, 0.9–2.8, for PiB+ APOE4 carriers and PiB+ noncarriers, respectively, compared with PiB− noncarriers). These associations were largely independent of APOE4.
CONCLUSIONS AND RELEVANCE
In persons selected from a population-based study, elevated amyloid levels at baseline were associated with worse cognition and imaging biomarkers at baseline and with greater clinical decline and neurodegeneration. These results have implications for the design of randomized clinical trials for AD.
Previous models for the putative progression of pathophysiology in Alzheimer disease (AD) assert that the deposition of β-amyloid is an initiating event followed by indexes of neurodegeneration, such as brain glucose hypometabolism and atrophy.1–4 However, recent evidence indicates that the temporal progression of events may need to include alternative scenarios.5,6 Nevertheless, many current models address the importance of amyloid in playing an early role in the neurodegeneration that leads to AD. After these neurodegenerative events have taken place, cognitive changes manifest.1–3With this framework as a foundation, the National Institute on Aging and the Alzheimer Association revised the criteria for the AD spectrum to include a preclinical state in which individuals have no clinical symptoms but may harbor the biologic underpinnings of the AD process.7
Several secondary prevention trials are currently being conducted or planned that operate on the premise that amyloid positivity implies the development of more rapid clinical impairment; although this hypothesis is plausible, the data supporting this approach are inconclusive.8 It is unclear whether amyloid positivity is sufficient to enrich a trial. Some data have been generated concerning cognitive changes among cognitively normal (CN) individuals with and without amyloid,9–11 but relatively little is known about the progression of neurodegenerative markers by amyloid status and virtually none in randomly sampled individuals from the community.12,13
This study includes individuals from the Mayo Clinic Study of Aging (MCSA), a population-based longitudinal study in Olmsted County, Minnesota, to explore the effect of elevated amyloid levels on subsequent changes in cognition and, in a subset of individuals with serial imaging data, changes instructural magnetic resonance imaging (MRI), fluorodeoxyglucose positron emission tomography (FDG-PET), and accumulation of amyloid.
Methods
The MCSA is a population-based study in Olmsted County, Minnesota, in which individuals are examined approximately every 15 months.14 The data used in this study were collected from January 12, 2006, to January 9, 2014. All Olmsted County residents who were aged 70 to 89 years on October 1, 2004, were identified using the Rochester Epidemiology Project medical records linkage system. Since then, we have reenumerated and resampled the population a number of times to maintain an active cohort of approximately 2800 individuals without dementia. The study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards. All participants provided written informed consent.
Clinical Evaluations
At each visit, a study coordinator collected information regarding medical and family history and also interviewed a study partner about the individual to complete a modified Clinical Dementia Rating.15A physician then performed a medical history review, mental status examination, and neurologic examination. A comprehensive neuropsychological evaluation was performed that consisted of 9 tests from 4 cognitive domains: memory (Wechsler Memory Scale–Revised Logical Memory II [delayed recall], Wechsler Memory Scale–Revised Visual Reproductions II [delayed recall], and the Auditory Verbal Learning Test [delayed recall]), attention-executive function (Trail Making Test Part B and Digit Symbol Substitution from the Wechsler Adult Intelligent Scale–Revised), language (Boston Naming Test and category fluency scores), and visuospatial skills (Block Design and Picture Completion tests from the Wechsler Adult Intelligent Scale–Revised).
Cognitive Impairment Determination
For the current study, we required individuals to be CN at baseline. To inform this determination, the raw scores from each test were transformed into age-adjusted scores using independent normative data from the Mayo’s Older Americans Normative Studies.16 Domain scores were obtained by averaging the adjusted scaled scores within each domain and then scaled to allow for comparisons across domains. Individuals with domain scores at least 1 SD below the age-specific mean in the general population were considered for possible mild cognitive impairment (MCI). However, we emphasize that no algorithm was used to assign the diagnosis of MCI; rather, the study coordinator, neuropsychologist, and examining physician collectively assigned a diagnosis of MCI according to published criteria,17which included the following: (1) cognitive concern by the individual, informant, or physician; (2) impairment in one or more cognitive domains; (3) essentially normal functional activities; and (4) absence of dementia (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition). As indicated above, individuals who were cognitively impaired at baseline were excluded. However, individuals who developed MCI or dementia during follow-up were not.
Continuous Measures of Cognitive Performance for Longitudinal Analysis
The age-normed scores discussed above were used only for clinical classifications. The continuous measures of cognitive performance used in the longitudinal analysis were not age normed. Instead, longitudinal global-and domain-specific z scores were calculated by first converting individual test scores to z scores. Domain-specific z scores were created by averaging the test-specific z scores, and a global z score was created by averaging the domain-specific z scores. The reference mean (SD) for all z scores was obtained from the 2004 MCSA CN participants’ baseline performance.
Imaging Methods
A 3-dimensional, magnetization-prepared, rapid acquisition gradient-echo sequence was used to perform MRI at 3T. Images were corrected for distortion due to gradient nonlinearity and for bias field. Hippocampal volume was measured with Free-Surfer software, version 5.3, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). Total intracranial volume (TIV) was measured using an in-house method. ATIV-adjusted hippocampal volume (HVa) measure was defined by calculating the residual from a linear regression of hippocampal volume vs TIV among 133 CN individuals aged 30 to 59 years.18 This adjusted measure was used as a covariate in the models. Ventricular volume was measured with the boundary shift integral technique.19
Carbon 11–labeled Pittsburgh Compound B (PiB) PET that consisted of four 5-minute dynamic frames was performed 40 to 60 minutes after injection.20 One hour after PiB PET, FDG-PET was performed. Individuals were injected with fluorodeoxyglucose 18–labeled FDG and imaged after 30 to 38 minutes for an 8-minute image acquisition that consisted of four 2-minute dynamic frames.
Quantitative image analysis for both PiB and FDG was performed using our in-house, fully automated image processing pipeline.20 A global, cortical, PiB PET standardized uptake value ratio (SUVR) was obtained by calculating the median uptake in the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, posterior cingulate, and precuneus regions for each study participant and dividing this result by the median uptake in the cerebellar gray matter. An FDG-PET SUVR was obtained in a similar manner. We used angular gyrus, posterior cingulate, and inferior temporal cortical regions to construct an AD-signature meta region of interest, normalized to pons and vermis uptake.21
Inclusion Criteria
All MCSA participants were invited to undergo imaging. Participants included in this study had received MRI, FDG-PET, and PiB PET; were CN at the time of the first imaging visit; and had at least 1 clinical follow-up. A subset of individuals also had received serial imaging and were used in modeling changes in imaging measures over time.
Statistical Analysis
The new criteria for MCI due to AD require the classification of biomarker and cognitive test results as normal or abnormal.22 Individuals were classified as having a normal PiB PET result (PiB−) or abnormal PiB PET result (PiB+) at baseline. We defined PiB+ as an SUVR greater than 1.5, a level at which individuals have clearly elevated amyloid levels on PET and that distinguishes between borderline cases, which is an approach consistent with the goals of typical clinical trials.
Because not all individuals underwent serial imaging, we compared baseline characteristics of those with vs without serial imaging using Wilcoxon rank sum and χ2 tests. The PiB+ individuals were compared with the PiB− individuals overall and in the serial imaging subset using Wilcoxon rank sum and χ2 tests. The subset of individuals without serial imaging are compared with those with serial imaging in Table 1, but longitudinal analyses that involved only this group were not performed. Linear regression was used to assess differences in baseline cognitive and imaging measures by PiB status after adjusting for age, sex, educational level, HVa, number of times the cognitive tests had been administered, and TIV where appropriate. Linear mixed-effects models with random subject specific intercepts and slopes were used to assess associations between PiB status at baseline and the longitudinal cognitive and imaging end points. All models included PiB status, time in years from baseline, and their interaction. To isolate the PiB effects and address possible confounding, we adjusted for age, sex, educational level, and number of times the cognitive tests had been administered by the baseline visit. Hippocampal and ventricular volume models were additionally adjusted for TIV. All models, except the hippocampal volume models, were also adjusted for HVa as a measure of neurodegeneration. Age and HVa interactions with time were included to allow rates of cognitive decline to vary by age and HVa. PiB and FDG models used log transformations of the response. Thus, coefficient estimates are interpreted as the approximate annual percent change in SUVR. In further modeling, we tested for interactions between APOE4 and PiB status. All individuals were included in the cognitive end point models, whereas the imaging end point models only included individuals with serial imaging data.
Table 1.
Baseline Characteristics of the Study Participants
| Characteristic | All (N = 564) |
Subset | P Value | |
|---|---|---|---|---|
| With Serial Imaging (n = 286) |
Without Serial Imaging (n = 278) |
|||
| Age, median (IQR), y | 78 (75 to 83) | 78 (75 to 82) | 79 (74 to 83) | .21 |
| Male, No. (%) | 313 (55.5) | 178 (62.2) | 135 (48.6) | .001 |
| APOE4 carrier, No. (%) | 139 (24.7) | 80 (28.0) | 59 (21.3) | .07 |
| Educational level, median (IQR), y | 14 (12 to 16) | 14 (12 to 16) | 14 (12 to 16) | .41 |
| Short Test of Mental Status score, median (IQR) | 35 (33 to 37) | 35 (33 to 37) | 35 (34 to 37) | .12 |
| Cognitive z scores, median (IQR) | ||||
| Global | 0.59 (−0.10 to 1.21) | 0.48 (−0.15 to 1.13) | 0.66 (−0.04 to 1.32) | .05 |
| Memory | 0.53 (−0.26 to 1.32) | 0.42 (−0.38 to 1.16) | 0.64 (−0.09 to 1.43) | .01 |
| Language | 0.38 (−0.19 to 0.96) | 0.33 (−0.32 to 0.88) | 0.45 (−0.11 to 1.07) | .08 |
| Attention | 0.46 (−0.25 to 1.06) | 0.42 (−0.27 to 0.92) | 0.53 (−0.24 to 1.27) | .03 |
| Visuospatial | 0.50 (−0.15 to 1.11) | 0.48 (−0.17 to 1.09) | 0.52 (−0.12 to 1.15) | .77 |
| PiB SUVR, median (IQR) | 1.38 (1.31 to 1.63) | 1.38 (1.31 to 1.61) | 1.38 (1.32 to 1.67) | .62 |
| PiB+ (SUVR >1.5), No. (%) | 179 (31.7) | 86 (30.1) | 93 (33.5) | .39 |
| FDG SUVR, median (IQR) | 1.40 (1.30 to 1.50) | 1.40 (1.30 to 1.50) | 1.41 (1.30 to 1.50) | .51 |
| Hippocampal volume, median (IQR), cm3 | 7.1 (6.5 to 7.6) | 7.1 (6.5 to 7.6) | 7.1 (6.5 to 7.7) | .42 |
| No. of visits, No. (%)a | ||||
| 2 | 139 (24.6) | 226 (79.0) | 104 (37.4) | … |
| 3 | 275 (48.8) | 57 (19.9) | 143 (51.4) | … |
| 4 | 126 (22.3) | 3 (1.0) | 24 (8.6) | … |
| ≥5 | 24 (4.3) | 0 | 7 (2.5) | … |
| Follow-up time, median (range), ya | 2.7 (1.1 to 7.7) | 2.2 (1.0 to 6.4) | 2.6 (1.1 to 7.7) | … |
Abbreviations: FDG, fluorodeoxyglucose; IQR, interquartile range; PiB, Pittsburgh Compound B; SUVR, standardized uptake value ratio. Ellipses indicate data not applicable.
The number of follow-up visits and follow-up time reported in the All and Subset Without Serial Imaging columns were based on visits with clinical (cognitive) data. The number of follow-up visits and follow-up time reported in the Subset With Serial Imaging column were based on visits with imaging (magnetic resonance imaging and positron emission tomography) data. Some serial imaging participants may have had longer follow-up due to additional visits with only clinical data.
We fit a Cox proportional hazards regression model to assess the association between PiB status and progression to MCI or dementia. Age was used as the time scale using the start-stop or period-at-risk formulation of the Cox proportional hazards model. The model included PiB status,APOE4 carrier status, and their interaction and was adjusted for educational level and HVa. The model was stratified by sex to allow for differing baseline hazards for men vs women. Time of MCI or dementia onset was defined as the midpoint between the last CN assessment and the first assessment with MCI or dementia. Individuals who did not progress during the available follow-up were censored at their last assessment. We also performed a sensitivity analysis using a parametric Weibull model to directly account for interval censoring and obtained results that were similar to the Cox proportional hazards regression model results.
Results
Table 1 provides the baseline characteristics of all 564 CN individuals and contrasts those with (n = 286) vs without (n = 278) serial imaging. Compared with individuals with no serial imaging, individuals with serial imaging were more likely to be male and APOE4 carriers. They also tended to have lower memory, attention, language, and global z scores at baseline, although these differences were quite modest.
Baseline Data
Table 2 provides the differences in individuals who were PiB+ and PiB− at baseline. In general, the PiB+ individuals were older, were more likely to test positive for APOE4, and had lower scores on global cognitive function and memory and language tests compared with the PiB− individuals. The PiB+ individuals tended to have lower scores on attention and visuospatial tests as well, but these differences were not statistically significant. The PiB+ individuals had lower FDG ratios and smaller hippocampal volumes relative to the PiB− individuals. Similar patterns were seen among the subset of individuals with serial imaging studies.
Table 2.
Baseline Characteristics of Study Participants by PiB Status
| All | Subset With Serial Imaging | |||||
|---|---|---|---|---|---|---|
| Characteristic | PiB− (n = 385) |
PiB+ (n = 179) |
P Valuea | PiB− (n = 200) |
PiB+ (n = 86) |
P Valuea |
| Age, median (IQR), y | 77 (74 to 82) | 80 (76 to 83) | <0.001 | 77 (74 to 82) | 79 (76 to 82) | .01 |
| Male, No. (%) | 210 (54.5) | 103 (57.5) | .51 | 121 (60.5) | 57 (66.3) | .36 |
| APOE4 carrier, No. (%) | 66 (17.1) | 73 (41.0) | <.001 | 39 (19.5) | 41 (47.7) | <.001 |
| Educational level, median (IQR), y | 14 (12 to 16) | 14 (12 to 16) | .61 | 14 (12 to 16) | 14 (12 to 16) | .81 |
| Short Test of Mental Status score, median (IQR) | 35 (34 to 37) | 35 (33 to 36) | .18 | 35 (33 to 37) | 34 (33 to 36) | .28 |
| Cognitive z scores, median (IQR) | ||||||
| Global | 0.69 (0.03 to 1.33) | 0.34 (−0.34 to 1.01) | .001 | 0.66 (0.00 to 1.17) | 0.28 (−0.41 to 0.83) | .006 |
| Memory | 0.63 (−0.08 to 1.43) | 0.31 (−0.56 to 1.12) | <.001 | 0.53 (−0.22 to 1.23) | 0.07 (−0.83 to 1.04) | .005 |
| Language | 0.49 (−0.09 to 1.08) | 0.13 (−0.43 to 0.72) | .001 | 0.41 (−0.16 to 0.97) | 0.08 (−0.58 to 0.64) | .03 |
| Attention | 0.61 (−0.14 to 1.11) | 0.14 (−0.40 to 0.91) | .07 | 0.57 (−0.17 to 0.94) | 0.12 (−0.47 to 0.75) | .02 |
| Visuospatial | 0.55 (−0.09 to 1.17) | 0.32 (−0.35 to 0.95) | .07 | 0.50 (−0.13 to 1.09) | 0.44 (−0.35 to 1.04) | .33 |
| SUVR, median (IQR) | ||||||
| PiB | 1.33 (1.29 to 1.38) | 1.86 (1.67 to 2.14) | … | 1.33 (1.29 to 1.39) | 1.93 (1.68 to 2.21) | … |
| FDG | 1.42 (1.32 to 1.51) | 1.37 (1.27 to 1.45) | .002 | 1.42 (1.32 to 1.51) | 1.37 (1.25 to 1.43) | .001 |
| Hippocampal volume, cm3 | 7.2 (6.6 to 7.7) | 7.0 (6.3 to 7.5) | .02 | 7.1 (6.6 to 7.6) | 6.9 (6.3 to 7.5) | .19 |
Abbreviations: FDG, fluorodeoxyglucose; IQR, interquartile range; PiB, Pittsburgh Compound B; SUVR, standardized uptake value ratio; TIV, total intracranial volume. Ellipses indicate data not applicable.
P values for cognitive and imaging measures are from regression models adjusted for age, sex, educational level, TIV-adjusted hippocampal volume, number of times the cognitive tests had been administered, and TIV where appropriate.
Longitudinal Changes
Figure 1 shows the estimated mean annual change in cognitive function by PiB. The annual cognitive decline was greater for PiB+ individuals compared with PiB− individuals on all cognitive measures except language, after adjusting for age, sex, educational level, HVa, and number of times the cognitive tests had been administered. On average, the PiB+ individuals were declining approximately 0.1 z score units per year faster than PiB− individuals on the global measure and in the memory and attention domains. The difference was approximately half this amount in the language and visuospatial domains. On average, PiB+ individuals were declining approximately 0.2 points per year faster on the Short Test of Mental Status compared to PiB− individuals. Of note, these differences in rates of cognitive decline between PiB+ and PiB− individuals were of similar magnitude to the differences in rates of cognitive decline between an 80-year-old vs a 70-year-old person.
Figure 1. Cognition and Amyloid Status.
Estimated mean annual change in cognitive measures from linear mixed-effects regression models by Pittsburgh Compound B (PiB) status. Error bars indicate 95% CIs.
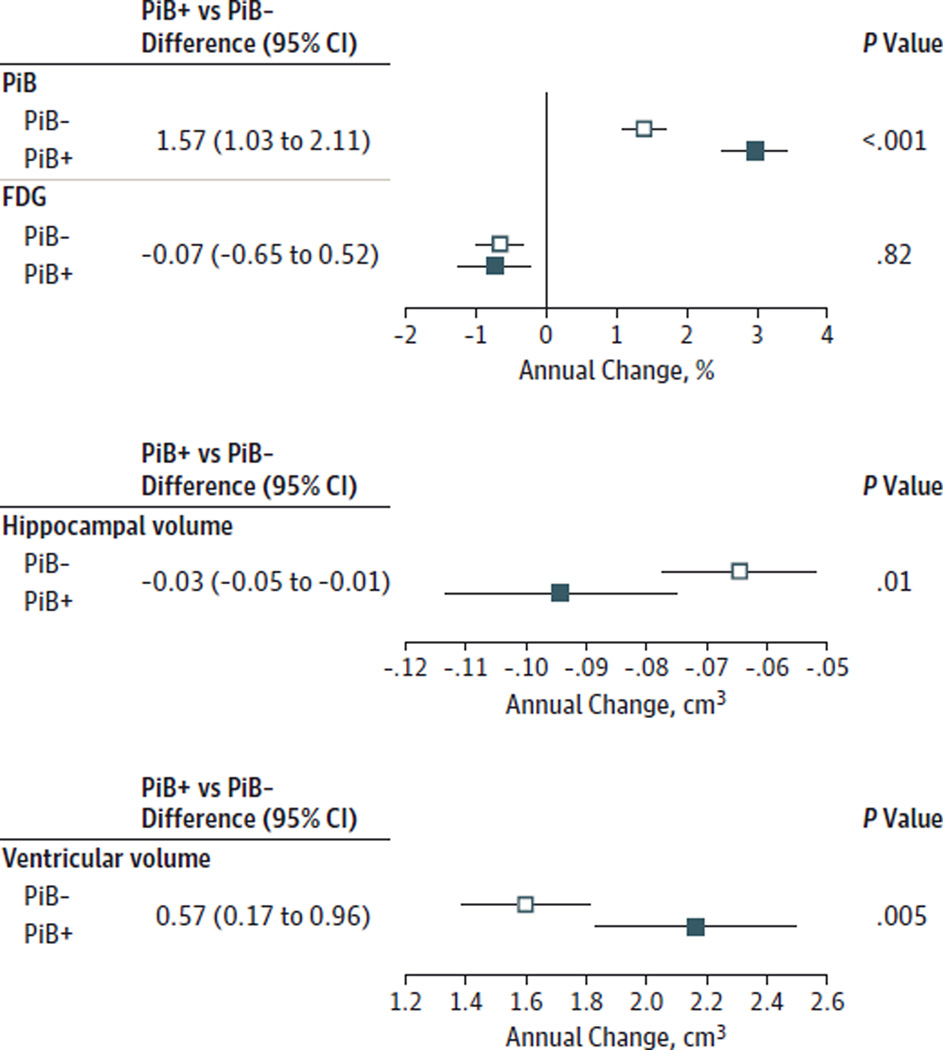
Figure 2 shows the estimated mean annual percent change in the PiB and FDG ratios, annual change in hippocampal volumes, and annual change in ventricular volumes by PiB status. The PiB+ individuals had a higher annual rate of PiB accumulation (by 1.6 percentage points), greater annual increase in ventricular volumes (by 565 mm3), and greater annual decrease in hippocampal volumes (by 30 mm3) compared with the PiB− individuals. Change in FDG was not significantly associated with PiB. Age was not independently associated with change in PiB (P = .82), hippocampal volume (P = .37), or ventricular volume (P = .09), but older age was associated with greater declines in FDG (P = .03). eFigure 1 in the Supplement complements the summaries in Figure 1 and Figure 2 by showing the fitted regression lines for PiB+ and PiB− individuals.
Figure 2. Annual Imaging Changes by Amyloid Status.
Estimated mean annual percent change or annual change in imaging measures from linear mixed-effects regression models by Pittsburgh Compound B (PiB) status. Error bars indicate 95% CIs. FDG indicates fluorodeoxyglucose.
APOE4 and PiB
eFigure 2 and eFigure 3 in the Supplement show rates of change in cognitive and imaging measures for PiB+ and PiB− individuals stratified byAPOE4 carrier status. In general, rates for PiB+ APOE4 carriers and noncarriers were similar, with both groups worsening more rapidly than their PiB− counterparts. Although APOE4 carrier status was generally associated with change in cognition when ignoring PiB status, after adjusting for PiB status, APOE4 was not independently associated with cognitive declines. However, we found APOE4 modified the rate of change for the PiB and hippocampal volume estimates. The rate of PiB accumulation was lower among PiB− APOE4 noncarriers compared with PiB− APOE4 carriers (P = .001), PiB+ APOE4 noncarriers (P < .001), and PiB+ APOE4 carriers (P < .001).We also found that hippocampal volume was declining less quickly in the PiB− APOE4 carrier group compared with PiB− APOE4 noncarriers (P= .02), PiB+ APOE4 noncarriers (P = .008), and PiB+ APOE4 carriers (P < .001). eFigure 4 in the Supplement complements the summaries in eFigure 2 and eFigure 3 in the Supplement by showing the fitted regression lines for PiB+ and PiB− individuals by APOE4 status.
Progression to MCI or Dementia
During a median of 2.5 (interquartile range, 1.3–2.9) years of follow-up, 88 individuals progressed clinically to MCI or dementia (n = 84 to MCI and n = 4 to dementia). Baseline characteristics in individuals who developed MCI or dementia and in individuals who remained CN are given in the eTable in the Supplement. The hazard ratios (HRs) from the Cox proportional hazards regression models are summarized in Figure 3 for the 4 groups defined by PiB and APOE4 carrier status, with APOE4 noncarriers who were PiB− serving as the reference. After controlling for sex, educational level, and HVa, PiB+ individuals had a greater risk for progression to MCI or dementia, with the greatest risk in the PiB+ APOE4 carriers (HR, 2.9; 95% CI, 1.7–5.0) and a still-elevated risk among PiB+ APOE4 noncarriers (HR, 1.6; 95% CI, 0.9–2.8). This association between PiB and progression to MCI or dementia is consistent with the greater change in continuous measures seen among PiB+ individuals. However, an APOE4-PiB interaction was seen in the Cox proportional hazards regression model (P = .03), whereas this effect modification was not observed for the continuous cognitive outcomes.
Figure 3. Progression to Mild Cognitive Impairment.
Hazard ratios from a Cox proportional hazards regression model for progression from cognitively normal to mild cognitive impairment using age as the time scale. The model includes educational level in years, total intracranial volume–adjusted hippocampal volume (HVa), and the interaction between APOE4 and Pittsburgh Compound B (PiB) status. Error bars indicate 95% CIs.
Discussion
These data, using individuals selected from a population based cohort of CN people, shed light on the role of amyloid deposition as detected by PET. At baseline, CN individuals with clearly elevated levels of amyloid performed worse on most cognitive measures and measures of neurodegeneration even after accounting for potential confounders, including age, sex, educational level, and hippocampal volume (when applicable). The literature on this topic has been variable, with some studies finding no significant associations with amyloid and cognition cross-sectionally and others finding a significant effect.10,11,23–27 Several of these studies were performed on relatively small populations, and the source of the participants was variable. Our data represent one of the first characterizations of individuals drawn from a population-based sample.
Over time, PiB+ individuals had greater declines in global- and domain-specific cognitive measures and higher rates of amyloid accumulation, hippocampal atrophy, and ventricular expansion compared with PiB− individuals. Given that we adjusted for key confounders, such as age, sex, educational level, and hippocampal volume (when applicable), we conclude that PiB is independently associated with cognitive decline, hippocampal atrophy, and ventricular expansion. Although differences between the PiB+ and PiB− individuals in terms of annual cognitive change were modest, the effect size generally corresponded to that of a 10-year difference in age. The PiB+ individuals were also more likely to progress clinically to MCI or dementia. As such, if these changes persisted on an annual basis, the effect would be quite noticeable.
The Australian Imaging, Biomarkers and Lifestyle (AIBL) study11 has also reported associations with amyloid and cognitive decline. Among 320 healthy older individuals, amyloid positive individuals (n = 76) had a greater decline in verbal and visual episodic memory during 36 months of follow-up compared with amyloid-negative individuals. However, unlike our current study, the AIBL study did not find differences in cognitive decline in nonmemory domains by amyloid status.
The literature is mixed in regard to the association between amyloid and APOE genotype in predicting cognitive decline. The AIBL study was enriched for APOE4 carriers and found similar rates of cognitive decline in amyloid-positive APOE4 carriers and noncarriers. Lim et al,28 who specifically examined amyloid and APOE effects, suggested that amyloid effects on cognitive decline were greater than APOE effects. However, a subsequent AIBL report29 that focused on 84amyloid-positive individuals found faster rates of decline on memory for the APOE4 carriers. In a recent report30 that combined data from 3 observational convenience cohorts (n = 490), CN individuals who were both amyloid positive and APOE4 carriers had a greater decline in Logical Memory Delayed Recall and Mini-Mental State Examination scores compared with all other groups defined by amyloid and APOE4. Of interest, the PiB− individuals and PiB+ noncarriers had a slight improvement during the median follow-up of 1.5 years.
In our data while ignoring PiB altogether, APOE4 carrier status was generally associated with greater changes in the continuous cognitive measures over time. With both PiB status and APOE4 as predictors, PiB was predictive but APOE4 was not predictive of decline. Therefore, the effect of APOE4 is largely mediated by amyloid positivity. We also found that PiB+ APOE4 carriers and noncarriers had similar rates of longitudinal cognitive decline. Although our data cannot be used to rule out faster declines among PiB+ APOE4 carriers vs noncarriers, noncarriers with high levels of amyloid are also likely to experience cognitive declines.
We observed evidence of an increased hazard for amyloid-positive APOE4 carriers and noncarriers alike. Still, the association of PiB and progression to MCI or dementia was appreciably stronger in the APOE4 carriers. The AIBL investigators have also reported that PiB+ CN individuals were more likely to progress clinically to MCI or dementia during 36 months of follow-up (odds ratio,4.8;95% CI, 1.9–12).31 The increased odds held for noncarriers and carriers, although the latter group was at higher risk.
One potentially conflicting result from our study was that PiB+ APOE4 carriers progressed clinically to MCI or dementia at a higher rate than that of noncarriers even though their rates of cognitive change were similar. This finding can largely be explained by differences at baseline. As shown in eFigure 4 in the Supplement, by having worse baseline performance but similar rates of change, the PiB+ APOE4 carriers tended to have lower cognition than the PiB+ noncarriers throughout all follow-up and thus were at an increased risk of MCI compared with PiB+ noncarriers.
Conclusions
Our data reveal that the presence of elevated levels of amyloid is associated with cognitive and neurodegenerative marker changes over time. The evidence of neurodegenerative abnormalities depicted by MRI and FDG-PET at baseline imply that these changes had begun, but do not speak to the temporal ordering of the changes. Recent data indicate that individuals who are amyloid negative but neurodegeneration positive can also have an AD signature.32–34
The implications of these results for the design of randomized clinical trials are evident. Individuals with clearly elevated levels of amyloid appear to be predisposed to progress clinically and on measures of neurodegeneration and can serve as a source of enrichment for clinical trials, and additional evidence of neurodegeneration may not be necessary.
Supplementary Material
Acknowledgments
Dr Petersen reported serving on a data monitoring committee for Pfizer Inc and Janssen Alzheimer Immunotherapy; working as a consultant for Merck Inc, Roche Inc, Biogen Inc, Eli Lily and Company, and Genentech Inc; receiving royalties from publishing Mild Cognitive Impairment (Oxford University Press, 2003), and receiving research support from grants P50 AG016574 (principal investigator), U01 AG006786 (principal investigator), U01 AG024904 (coinvestigator), and R01 AG011378 (coinvestigator) from the National Institutes of Health. Dr Rocca reported receiving research support from grants R01 AG034676 (principal coinvestigator), U01 AG006786 (coinvestigator), and P50 AG044170 (codirector) from the National Institutes of Health. Dr Roberts reported receiving research support from grant U01 AG006786 (coinvestigator) from the National Institutes of Health, AbbVie Health Economics and Outcome Research, and the Walter S. and Lucienne Driskill Foundation. Dr Mielke reported receiving funding from the Alzheimer’s Drug Discovery Foundation, the Driskill Foundation, the Michael J. Fox Foundation, and the National Institutes of Health. Dr Lowe reported working as a consultant for Bayer Schering Pharma and Piramal Imaging and receiving research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, the National Institutes of Health (National Institute on Aging and National Cancer Institute), the Elsie and Marvin Dekelboum Family Foundation, the MN Partnership for Biotechnology and Medical Genomics, and the Leukemia & Lymphoma Society. Dr Knopman reported serving as an Associate Editor for Neurology, serving on a data safety monitoring board for Lilly Pharmaceuticals, working as an investigator in a clinical trial sponsored by Janssen Pharmaceuticals, and receiving research support from grants R01 AG011378 (coinvestigator), P50 AG016574 (coinvestigator), U01 AG006786 (coinvestigator), AG029550 (coinvestigator), AG032306 (coinvestigator), and U01 HL096917 (coinvestigator) from the National Institutes of Health. Dr Pankratz reported receiving funding from grants R01 AG040042, U01 AG006786, P50 AG016574, and R01 AG032990 from the National Institutes of Health. Dr Jack reported providing consulting services for Janssen Research & Development LLC and receiving research funding from the grants R01 AG011378, U01 HL096917, U01 AG024904, RO1 AG041851, R01 AG037551, R01 AG043392, and U01 AG006786 from the National Institutes of Health and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation.
Funding/Support: This study was supported by grant U01 AG006786 from the National Institute on Aging (Dr Petersen), grants R01 AG011378 and RO1 AG041581 from the National Institutes of Health (Dr Jack), and the Mayo Foundation.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Petersen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Petersen, Wiste, Weigand.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Wiste, Weigand, Pankratz.
Obtained funding: Petersen, Roberts, Lowe, Jack.
Administrative, technical, or material support: Petersen, Roberts, Lowe, Jack.
Study supervision: Petersen, Lowe, Jack.
Conflict of Interest Disclosures:
No other disclosures were reported.
REFERENCES
- 1.Mormino EC, Kluth JT, Madison CM, et al. Alzheimer’s Disease Neuroimaging Initiative. Episodic memory loss is related to hippocampal-mediated β-amyloid deposition in elderly subjects. Brain. 2009;132(pt 5):1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Alzheimer’s Disease Neuroimaging Initiative. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132(pt 5):1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461(7266):916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack CR, Jr, Holtzman DM. Biomarker modeling of Alzheimer’s disease. Neuron. 2013;80(6):1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack CR, Jr, Wiste HJ, Weigand SD, et al. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology. 2013;81(20):1732–1740. doi: 10.1212/01.wnl.0000435556.21319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6(228):228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chételat G, Villemagne VL, Pike KE, et al. Australian Imaging Biomarkers and Lifestyle Study of ageing (AIBL) Research Group. Independent contribution of temporal β-amyloid deposition to memory decline in the pre-dementia phase of Alzheimer’s disease. Brain. 2011;134(pt 3):798–807. doi: 10.1093/brain/awq383. [DOI] [PubMed] [Google Scholar]
- 10.Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80(14):1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim YY, Maruff P, Pietrzak RH, et al. AIBL Research Group. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain. 2014;137(pt 1):221–231. doi: 10.1093/brain/awt286. [DOI] [PubMed] [Google Scholar]
- 12.Chételat G, Villemagne VL, Villain N, et al. AIBL Research Group. Accelerated cortical atrophy in cognitively normal elderly with high β-amyloid deposition. Neurology. 2012;78(7):477–484. doi: 10.1212/WNL.0b013e318246d67a. [DOI] [PubMed] [Google Scholar]
- 13.Schott JM, Bartlett JW, Fox NC, Barnes J Alzheimer’s Disease Neuroimaging Initiative Investigators. Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Aβ1-42. Ann Neurol. 2010;68(6):825–834. doi: 10.1002/ana.22315. [DOI] [PubMed] [Google Scholar]
- 14.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 16.Ivnik RJ, Malec JF, Smith GE, et al. Mayo’s Older Americans Normative Studies: WAIS-R, WMS-R, and AVLT norms for ages 56 through 97. Clin Neuropsychol. 1992;6:1–104. [Google Scholar]
- 17.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 18.Jack CR, Jr, Wiste HJ, Knopman DS, et al. Rates of β-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82(18):1605–1612. doi: 10.1212/WNL.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans Med Imaging. 1997;16(5):623–629. doi: 10.1109/42.640753. [DOI] [PubMed] [Google Scholar]
- 20.Jack CR, Jr, Wiste HJ, Vemuri P, et al. Alzheimer’s Disease Neuroimaging Initiative. Brain β-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133(11):3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landau SM, Harvey D, Madison CM, et al. Alzheimer’s Disease Neuroimaging Initiative. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32(7):1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doraiswamy PM, Sperling RA, Johnson K, et al. AV45-A11 Study Group. Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Mol Psychiatry. 2014;19(9):1044–1051. doi: 10.1038/mp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chételat G, La Joie R, Villain N, et al. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. Neuroimage Clin. 2013;2:356–365. doi: 10.1016/j.nicl.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villemagne VL, Burnham S, Bourgeat P, et al. Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 26.Kantarci K, Lowe V, Przybelski SA, et al. APOE modifies the association between Aβ load and cognition in cognitively normal older adults. Neurology. 2012;78(4):232–240. doi: 10.1212/WNL.0b013e31824365ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66(12):1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim YY, Ellis KA, Pietrzak RH, et al. AIBL Research Group. Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology. 2012;79(16):1645–1652. doi: 10.1212/WNL.0b013e31826e9ae6. [DOI] [PubMed] [Google Scholar]
- 29.Pietrzak RH, Lim YY, Ames D, et al. Australian Imaging, Biomarkers and Lifestyle (AIBL) Research Group. Trajectories of memory decline in preclinical Alzheimer’s disease: results from the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing. Neurobiol Aging. 2015;36(3):1231–1238. doi: 10.1016/j.neurobiolaging.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Mormino EC, Betensky RA, Hedden T, et al. Alzheimer’s Disease Neuroimaging Initiative; Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing; Harvard Aging Brain Study. Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82(20):1760–1767. doi: 10.1212/WNL.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe CC, Bourgeat P, Ellis KA, et al. Predicting Alzheimer disease with β-amyloid imaging: results from the Australian Imaging, Biomarkers, and Lifestyle Study of Ageing. Ann Neurol. 2013;74(6):905–913. doi: 10.1002/ana.24040. [DOI] [PubMed] [Google Scholar]
- 32.Petersen RC, Aisen P, Boeve BF, et al. Mild cognitive impairment due to Alzheimer disease in the community. Ann Neurol. 2013;74(2):199–208. doi: 10.1002/ana.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to NIA-AA crtiteria for preclinical Alzheimer’s disease. Ann Neurol. 2012;71(6):765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wirth M, Villeneuve S, Haase CM, et al. Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol. 2013;70(12):1512–1519. doi: 10.1001/jamaneurol.2013.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.