Summary
Bortezomib is active in mantle cell lymphoma (MCL), with approval in upfront and relapsed settings. Given inevitable recurrence following induction chemoimmunotherapy, maintenance approaches are a rational strategy to improve clinical outcomes. We conducted a phase II study to evaluate the safety and efficacy of six cycles of R-CHOP plus bortezomib (1.3 mg/m2 days 1 and 4 of 21 day cycles) followed by bortezomib maintenance (1.3 mg/m2 days 1, 4, 8, and 11 every 3 months for 2 years). Sixty-five eligible patients were enrolled. The treatment was well-tolerated and toxicities were mainly hematologic. The rate of grade ≥3 peripheral neuropathy was low (5%). With a median follow-up of 6.8 years, 2-year progression-free survival (PFS) was 62%, and 2-year overall survival (OS) was 85%. At 5 years, PFS was 28% and OS was 66%. MIPI scores were significantly associated with 2-year PFS, but did not predict long-term (≥5-year) PFS. Baseline Ki-67 index was significantly associated with survival. Combination R-CHOP with bortezomib followed by maintenance bortezomib appears to improve outcomes compared historically with R-CHOP alone, with prolonged remissions in a subset of patients. These results suggest that inclusion of bortezomib with induction chemotherapy and/or maintenance is promising in MCL and warrants further exploration.
Keywords: lymphoma, chemotherapy, mantle cell lymphoma, maintenance therapy, proteasome inhibition
Introduction
Mantle cell lymphoma (MCL) is a distinct subtype of B cell non-Hodgkin lymphoma characterised by the CCND1/IGH (t[11;14]) translocation leading to overexpression of cyclin D1. Although the survival of patients with MCL has improved over the last decade, much of this benefit is associated with selected populations of younger patients receiving intensive therapy (Delarue et al., 2013; Geisler et al., 2008; Hermine et al., 2010). Unfortunately, aggressive therapies are not feasible for many patients with MCL, and in population-based studies, where the median age at diagnosis is 70 or older, the median overall survival (OS) remains 3–5 years (Abrahamsson et al., 2014; Leux et al., 2014; van de Schans et al., 2010). The regimen of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is a standard induction therapy for MCL that is associated with high response rates, but remissions are not durable, with a median progression-free survival (PFS) of 14 to 22 months (Howard et al., 2002; Lenz et al., 2005; Robak et al., 2015; Rummel et al., 2013). Thus, improved therapies are clearly needed, especially for the growing population of patients with MCL not eligible for intensive regimens (Aschebrook-Kilfoy et al., 2013).
Bortezomib is a low-molecular weight compound that inhibits the protease activity of the 26S proteasome, leading to important regulatory changes in many intracellular proteins relevant to MCL biology, including inhibition of NF-κB activity (Pham et al., 2003), increased levels of p21 and p27, which regulate cyclin/cdk activity, resulting in cell cycle arrest (Bogner et al., 2003), and cleavage of the anti-apoptotic protein Bcl-2 via caspase 3 activation, leading to apoptosis (Bogner et al., 2006). The clinical activity of bortezomib in MCL has been demonstrated in several phase II trials, in which bortezomib as a single agent produced response rates ranging from 30–50% in patients with relapsed disease (Belch et al., 2007; Fisher et al., 2006; Gerecitano et al., 2009; Goy et al., 2009; Goy et al., 2005; O'Connor et al., 2005; Strauss et al., 2006), and a phase III trial recently showed a progression-free survival (PFS) benefit for bortezomib combined with frontline chemotherapy (Robak et al., 2015). Based on these studies, it is approved in the U.S. and Europe for the treatment of MCL in both the relapsed and upfront settings. The anti-CD20 antibody rituximab has been shown to be synergistic in vitro with bortezomib (Wang et al., 2008). Additionally, anthracyclines induce NF-κB in malignant cells, thus potentially increasing cellular dependency on NF-κB for survival and sensitising cells to proteasome inhibition (Guzman et al., 2002). Combining bortezomib with R-CHOP is therefore a promising strategy to improve the frequency and quality of remissions with induction therapy for MCL.
The pattern of continuous relapse despite initial response that is characteristic of MCL has led to the hypothesis that maintenance therapy may be beneficial in prolonging remissions. A randomised phase III intergroup trial comparing two different maintenance approaches in elderly patients with MCL showed that rituximab maintenance after CHOP-based induction improved both progression-free and overall-survival compared to interferon maintenance (Kluin-Nelemans et al., 2012); thus, several recent or ongoing trials have incorporated a maintenance component to therapy. Given the single-agent activity of bortezomib and relatively low toxicity, maintenance therapy with this drug may be an effective approach to improve PFS duration.
S0601 was therefore designed with the goal of testing the feasibility and efficacy of adding bortezomib to an R-CHOP backbone, and prolonging PFS by incorporating a bortezomib maintenance strategy for patients with newly diagnosed MCL. The objective of this study was to estimate the 2 year PFS, with the goal of improving the rate and duration of remissions for patients with MCL without serious additive toxicity.
Patients and Methods
Study registration
This phase II multicenter trial was initiated in August 2006 and closed to accrual in June 2008. The protocol was approved by the Institutional Review Board at each site, and written consent was obtained from all patients prior to enrollment. The study was conducted in accordance with the Declaration of Helsinki and was registered with ClinicalTrials.gov prior to enrolling patients (ClinicalTrials.gov Identifier: NCT00376961).
Eligibility
Patients with biopsy-proven, previously untreated mantle cell lymphoma were eligible if they were 18 years of age or older, had bi-dimensionally measurable stage III, IV, or bulky (≥10 cm) stage II disease, and a Zubrod performance status of 0–2. Patients were excluded if they received any prior therapy, were HIV-positive, had CNS involvement, had grade ≥2 peripheral neuropathy, were pregnant or nursing, or had a prior malignancy (except non-melanoma skin cancer, in situ cervical cancer, other stage I/II cancers in complete remission, or other cancers in remission ≥5 years). Investigators were counseled to adhere to good medical practice guidelines with regard to organ function, hematopoiesis, and hepatitis B virus screening, but results of these tests did not determine eligibility. All patients submitted written informed consent in accordance with institutional and federal guidelines.
Pathology Review
Representative paraffin-embedded sections from the original pre-treatment diagnostic biopsies were requested for central pathology review, which was required to determine eligibility. Diagnoses were established according to the World Health Organisation criteria (Jaffe et al., 2001). The characteristic morphologic features of MCL along with supporting documentation of co-expression of CD19 or CD20 and cyclin D1 protein, and/or the t(11;14)(q13;q32) translocation demonstrated by cytogenetics or fluorescent in situ hybridisation (FISH) were required for inclusion.
Baseline Studies
Baseline evaluation included a history and physical examination, radiographic imaging (computerised tomography of the chest, abdomen, and pelvis and fluorodeoxyglucose positron emission tomography [FDG-PET]), routine laboratory studies, bone marrow evaluation, and an echocardiogram.
Protocol Treatment
Patients were treated with six cycles of rituximab (375 mg/m2 IV on day 1) plus standard CHOP chemotherapy (Press et al., 2003) (VR-CHOP) with the addition of bortezomib 1.3 mg/m2 on days 1 and 4 of every 21 day cycle, consistent with a previously established tolerated dose of bortezomib with R-CHOP in a phase I/II trial (Furman et al., 2010). Day 1 rituximab was withheld if the circulating absolute lymphocyte count (i.e. leukemic mantle cells) was > 5000 cells/microliter to minimise the risk of tumor lysis and cytokine release syndrome. Patients achieving at least stable disease after induction were eligible for maintenance therapy, consisting of bortezomib 1.3 mg/m2 IV days 1, 4, 8, and 11 every 3 months for 8 cycles (one cycle was defined as 3 months for the maintenance phase). Standard dose reductions were used for hematologic, hepatic, and renal toxicity. Bortezomib was dose-reduced to 1.0 mg/m2 (dose level -1) and 0.7 mg/m2 (dose level -2) for peripheral neuropathy or grade ≥3 non-hematologic toxicities. Myeloid and erythroid growth factors were permitted at the discretion of the treating physician. Patients were removed early from the protocol treatment for progressive disease, unacceptable toxicity, delay of treatment for more than three weeks, or patient preference.
Assessment of Clinical Responses and Toxicity
Data were centrally reviewed, and clinical responses (partial remission [PR], complete remission [CR], or unconfirmed CR [CRu]) were coded according to International Workshop NHL criteria (Cheson et al., 1999). Remission status was assessed 3–6 weeks after the 6th cycle of induction to determine eligibility for maintenance therapy, and subsequent restaging was performed every 6 months for 2 years. At each time point a patient history and physical examination, blood counts, and diagnostic CT scans were performed. An FDG-PET scan was required only for the first restaging after induction. National Cancer Institute Common toxicity criteria (version 3.0) were used to grade toxicities (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf).
Baseline tumor tissue biological correlates analysis
For studies of SOX11 expression, specimens were formalin-fixed paraffin-embedded tissues from diagnostic tumor biopsy specimens as either tissue microarrays (TMA) or unstained tissue slides. TMAs and slides were then stained with antibodies to SOX11 (MRQ-58; mouse monoclonal antibody, Cell Marque Corporation, Rocklin, CA) followed by PowerVision Homo-Mouse Poly HRP IHC Detection System (Leica Biosystems, Buffalo Grove, IL); For quantification of Ki-67, specimens were tissue slides which were stained with Ki-67 (Dako; diluted to 1:100) followed by Dako Flex HRP kit with a low pH retrieval with hematoxylin counterstain. To assess the fraction of Ki-67 positive cells, regions for analysis were selected per the European MCL Network consensus guidelines (Klapper et al., 2009) with the following necessary exceptions: extranodal specimens were included, TMAs were used in addition to biopsies, for several very small specimens the regions were selected non-randomly to ensure that they were not overlapping, and the number of total tumor cells evaluated exceeded 200 in all specimens. The association between the fraction of Ki-67 positive cells and PFS or OS was assessed using a Cox regression statistical analysis.
Statistical Analysis
The primary endpoint of this phase II single arm study was to estimate progression-free survival (PFS). We planned to accrue 60 eligible patients, which gives sufficient power to estimate the 2-year PFS rate to within ±13% (95% CI). Given the historical 2-year PFS rate of 30% of R-CHOP alone in this patient population at the time the study was designed, we considered a 2-year PFS estimate of ≥42% to warrant further investigation of this therapy. Sixty patients would also provide 85% power at a 0.05 level (one-sided) to detect an improvement in CR/CRu rate from the historical rate of 48% with R-CHOP alone to 65%. Any adverse event occurring with at least 5% probability would be likely seen at least once (95% chance), and toxicity rates would be estimated to within ±13%. Toxic effects were coded using the NCI's CTCAE, v3.0 and PFS was defined as the time from registration to the first observation of progressive disease or death due to any cause. Patients who were alive and progression-free at the time of final data analysis were censored at the last assessment. OS and PFS were estimated according to the Kaplan-Meier method (Kaplan and Meier, 1958) and all patients were included in these analyses regardless of whether they received maintenance therapy. Analyses of survival differences by prognostic factors were carried out using Cox regression (Cox, 1972). For univariate analyses, the factors evaluated included age, marrow involvement, bulky disease, extranodal involvement, hemoglobin, LDH, serum beta-2 microglobulin, MIPI risk group, performance status, gender, spleen involvement, stage, and presence of B symptoms. For multivariate analysis, stepwise selection and best subset selection methods were used for model selection. The above factors were included in the model selection, with the exception of factors used to calculate MIPI score (age, performance status, LDH, and WBC). The final model was selected based on minimum AIC from the subset selection. Four factors (bulky disease, spleen involvement, MIPI risk group, and serum beta-2 microglobulin) were included in the final model. Data as of March 30, 2015 were included in the analysis.
For the immunohistochemical analyses, the reviewing pathologists (WRB and OP) and technologists were blinded to all outcome data.
Results
Patient Characteristics
Between November 30, 2006 and May 30, 2008, 68 patients were registered. Central pathology review was performed on all patients. Three patients were determined to be ineligible due to incorrect histologic diagnosis; thus, 65 patients were included in the final analysis and were evaluable for both toxicity and efficacy. Baseline clinical characteristics are detailed in Table 1. The median age of enrolled patients was 61 years (range, 36 to 85), and 80% were male. All patients had advanced disease except one patient who had bulky stage II. The distribution of MIPI scores was 45% low risk, 43% intermediate risk, and 12% high risk.
Table 1.
Baseline characteristics of eligible patients (N = 65)
| Characteristic | N | % |
|---|---|---|
| Age | ||
| Median, y (range) | 61 (36–85) | |
| Sex | ||
| Female | 13 | 20 |
| Race | ||
| White | 61 | 94 |
| Black | 2 | 3 |
| Asian | 1 | 2 |
| Pacific Islander | 1 | 2 |
| B symptoms | ||
| Present | 18 | 28 |
| LDH | ||
| Elevated | 24 | 37 |
| Bulky disease (≥10 cm) | ||
| Present | 10 | 15 |
| Marrow involvement | ||
| Present | 51 | 78 |
| Disease stage | ||
| Bulky II | 1 | 2 |
| III | 10 | 15 |
| IV | 54 | 83 |
| Performance status | ||
| 0 | 39 | 60 |
| 1 | 24 | 37 |
| 2 | 2 | 3 |
| MIPI risk score | ||
| Low | 29 | 45 |
| Intermediate | 28 | 43 |
| High | 8 | 12 |
| Blastoid histology | ||
| No | 53 | 81 |
| Yes | 4 | 6 |
| Not assessed† | 8 | 12 |
| SOX11 expression | ||
| Yes | 45 | 69 |
| No | 3 | 5 |
| Not assessed† | 17 | 26 |
| Ki-67 index | ||
| ≤ 10% | 23 | 35 |
| > 10% | 15 | 23 |
| Not assessed† | 27 | 42 |
Tissue was not available to assess blastoid morphology in 8 patients. Reasons for not assessing Ki-67 or SOX11 included: patients not consenting for correlative studies (n = 7), no tissue available or insufficient specimen (n= 20 for Ki-67, n = 10 for SOX11)
Treatment
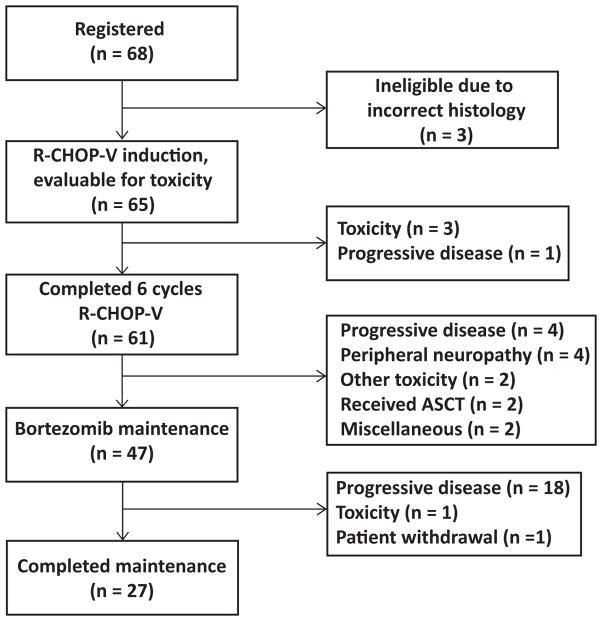
Sixty-one patients (94%) completed six cycles of VR-CHOP, and 27 patients (42%) completed the entire 2 years of maintenance bortezomib (Fig 1). Reasons for early termination included: progressive disease (1 patient during induction, 4 between induction and maintenance, and 18 during maintenance), toxicity (n = 3 during induction, due to fatigue and pneumonitis; n = 6 between induction and maintenance including 4 with peripheral neuropathy; and 1 during maintenance due to fatigue), withdrawal to pursue autologous stem cell transplantation (n = 2), or other reasons (n = 3). The median time on maintenance was 20.7 months (range 14 days – 24.8 months), with progressive disease being the most common reason for not completing the planned maintenance course.
Figure 1.
CONSORT diagram of patients enrolled in S0601. VR-CHOP: bortezomib, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; ASCT: autologous stem cell transplantation
During induction therapy, the mean dose intensity (total dose delivered/expected total dose) was 93% for bortezomib, 96% for doxorubicin, 96% for cyclophosphamide, and 96% for vincristine if based on capped dose of 2 mg, or 69% if calculated based on full 1.4 mg/m2 dose. Bortezomib doses were reduced or omitted in 12 of 65 patients (18%), and one additional patient was dosed at 1 mg/m2 for all 6 cycles contrary to protocol. Vincristine doses were reduced or omitted in 7 patients (11%). During maintenance, one patient did not receive any protocol maintenance therapy due to disease progression after registering to the maintenance step. Bortezomib doses were reduced or omitted during maintenance in 16 of 46 patients (35%), although these 16 patients still completed a median of 6 of the planned 8 cycles of maintenance, and overall maintenance dose intensity was fairly well preserved (91%), excluding cycles not given due to coming off study for disease progression, and excluding one patient who withdrew from the study due to insurance issues.
Safety
During induction therapy, grade 3–4 neutropenia and thrombocytopenia occurred in 52% and 17% of the patients, respectively. Twelve patients (17%) experienced febrile neutropenia. Details of the most significant toxicities are shown in Table 2. One treatment-related death occurred during the study in a patient who developed complete heart block in the setting of pneumonia, hypotension, and acute respiratory distress syndrome. The most common grade 1–2 non-hematologic toxicities during induction were fatigue (69%), alopecia (58%), sensory neuropathy (57%), nausea (51%), musculoskeletal pain (49%), and constipation (48%).
Table 2.
Adverse events at least possibly related to study treatment. All Grade 4 or 5 adverse events, as well as common (≥5% of patients) Grade 3 events are included.
| Associated with VR-CHOP n (%) of eligible patients (total n = 65) | Associated with bortezomib maintenance n (%) of eligible patients (total n = 47) | |||||
|---|---|---|---|---|---|---|
| Adverse Event (grade ≥3) | Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 |
| Hematologic/infection | ||||||
| Neutropenia | 9 (14) | 25 (38) | - | - | - | - |
| Febrile neutropenia | 11 (17) | 1 (2) | - | - | - | - |
| Thrombocytopenia | 5 (8) | 6 (9) | - | - | - | - |
| Anemia | 3 (5) | - | - | - | - | - |
| Lymphopenia | 11 (17) | 4 (6) | - | 1 (2) | 1 (2) | - |
| Leukopenia | 16 (25) | 10 (15) | - | - | - | - |
| Lung infection | 4 (6) | - | - | 1 (2) | - | - |
| Cardiovascular | - | - | 1 (2) | - | - | - |
| Diarrhea | 3 (5) | - | - | - | - | - |
| Fatigue | 4 (6) | 2 (3) | - | 1 (2) | - | - |
| Hyperglycemia | 3 (5) | - | - | - | - | - |
| Hyperuricemia | - | 1 (2) | - | - | - | - |
| Sensory neuropathy | 3 (5) | - | - | 1 (2) | - | - |
Maintenance therapy was well tolerated, with almost all toxicities limited to grade 1 or 2. Sensory peripheral neuropathy was experienced during induction as grade 1 in 48% of patients, grade 2 in 9%, and grade 3 in 3%. Four patients (6%) experienced motor neuropathy during induction, two as grade 2 and two as grade 3. During maintenance bortezomib, sensory neuropathy occurred as grade 1, 2, and 3 in 36%, 34%, and 2% of patients, and two patients (4%) experienced grade 1 motor neuropathy.
Clinical Responses
Of the 65 patients assessed for response following induction therapy with VR-CHOP, 52 (80%) achieved an objective response to therapy, including partial response (PR; n = 23) or a confirmed (CR; n = 20) or unconfirmed (CRu; n = 9) complete response based upon prespecified criteria (Cheson et al., 1999). Nine additional patients (14%) did not have adequate assessments to determine response but were included in the denominator as nonresponders. Following maintenance therapy, 7 partial responders to induction therapy plus one patient with inadequate assessment after induction converted to a CR/CRu, and one patient with stable disease converted to a PR, for an overall best response rate of 83% (57% CR/CRu and 26% PR) during the study. Median duration of response was 27.7 months, and median duration of CR/CRu was 30.3 months.
Progression-free and overall survival
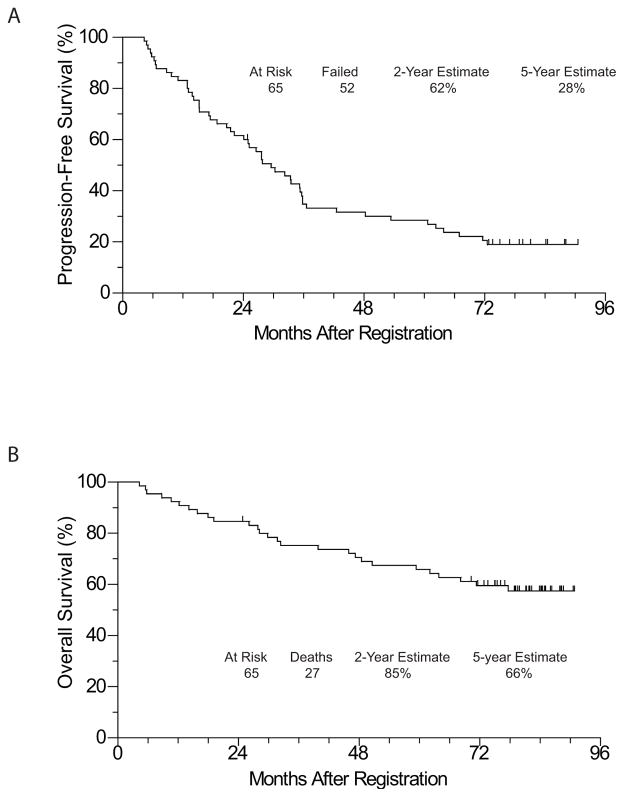
The median follow up of the patients still alive is 6.8 years. To date, 52 patients have either manifested progressive disease or died, with a median PFS of 29.5 months. The Kaplan-Meier estimate of 2-year PFS is 62% (95% CI: 48.6 – 72.1%), and the estimate of 5-year PFS is 28% (95% CI: 18.0 – 39.7%). Twenty-seven patients died, with a median OS not yet reached. The Kaplan-Meier estimate of 2-year OS is 85% (95% CI: 73.3 – 91.4%) and 5-year OS of 66% (95% CI: 52.9 – 76.0%). Survival curves of the entire cohort are shown in Fig 2.
Figure 2.
(A) PFS and (B) OS of the entire cohort
Prognostic Factor Analysis
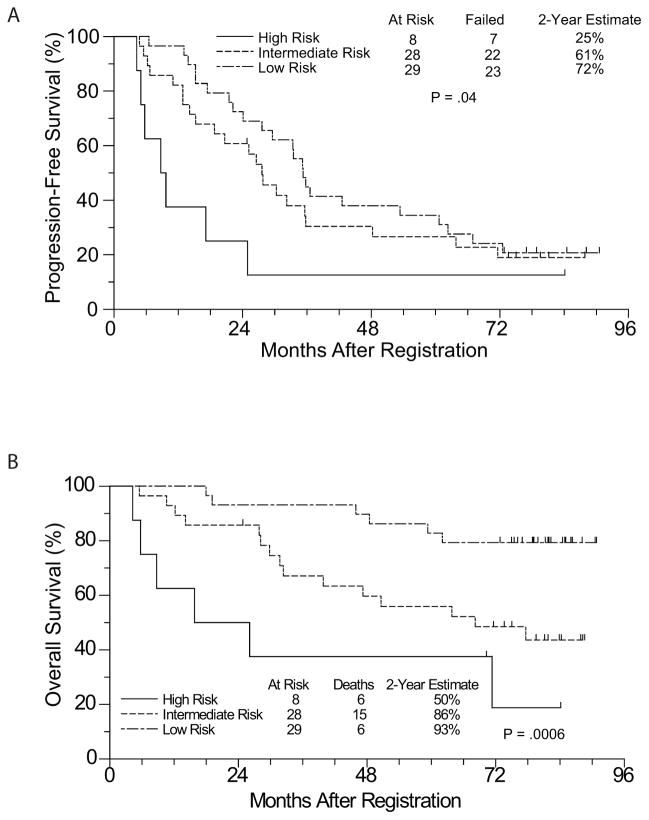
We conducted univariate and multivariate Cox regression analyses to identify baseline clinical characteristics associated with PFS and OS. In the univariate analysis (Table 3), bulky disease, MIPI risk group, and performance score > 0 were significantly associated with inferior PFS and OS, and spleen involvement was significantly associated with inferior PFS. The MIPI was not defined at the time this study was initiated, but we reviewed the database for all 65 eligible patients to assign MIPI scores retroactively according to published criteria (Hoster et al., 2008). In a multivariate analysis (excluding performance score since it is included in the calculation of the MIPI score), bulky disease remained statistically significantly associated with both PFS and OS, whereas splenic involvement was associated with PFS but not OS. High-risk MIPI score was associated with a worse OS and a trend towards worse PFS. Beta-2 microglobulin was also significantly associated with OS (Table 4).
Table 3.
Univariate Cox regression analysis of baseline factors for PFS and OS in the 65 eligible patients
| Factor | Progression-Free Survival HR (95% CI) | Overall Survival HR (95% CI) |
|---|---|---|
| Age > 60 | 0.61 (0.35–1.06) P=.08 |
1.65 (0.74–3.68) P=.22 |
| Bone marrow Involvement | 1.12 (0.59–2.13) P=.74 |
1.06 (0.43–2.63) P=.90 |
| Bulky disease | 2.30 (1.12–4.75) P=.0240 |
3.66 (1.53–8.75) P=.0035 |
| Extranodal involvement | 0.69 (0.34–1.37) P=.29 |
0.58 (0.24–1.45) P=.25 |
| Hemoglobin ≤median (13.6 g/dl) | 1.29 (0.74–2.22) P=.37 |
1.52 (0.69–3.32) P=.30 |
| Elevated LDH | 1.68 (0.96–2.93) P=.07 |
1.86 (0.87–3.96) P=.11 |
| Beta-2 microglobulin > median (2.55 mg/L) | 1.31 (0.75–2.28) P=.34 |
0.89 (0.42–1.89) P=.76 |
| High risk MIPI | 2.60 (1.16–5.81) P=.0201 |
3.63 (1.46–9.04) P=.0057 |
| Performance status 1 or 2 | 2.07 (1.19–3.60) P=.0096 |
3.38 (1.56–7.32) P=.0020 |
| Male sex | 1.09 (0.53–2.24) P=.82 |
0.74 (0.30–1.84) P=.52 |
| Spleen involvement | 2.07 (1.19–3.61) P=.0103 |
1.46 (0.68–3.11) P=.33 |
| Stage IV | 0.75 (0.37–1.49) P=.41 |
0.45 (0.19–1.06) P=.07 |
| B symptoms | 1.42 (0.79–2.56) P=.25 |
1.37 (0.61–3.05) P=.44 |
Table 4.
Multivariate Cox regression analysis of baseline factors for PFS and OS in the 65 eligible patients
| Factor | Progression-Free Survival HR (95% CI) | Overall Survival HR (95% CI) |
|---|---|---|
| Bulky disease | 3.16 (1.41–7.09) P=.0045 |
6.62 (2.45–17.90) P=.0002 |
| Spleen involvement | 2.23 (1.21–4.08) P=.0097 |
1.48 (0.65–3.39) P=.35 |
| High risk MIPI | 2.38 (0.96–5.89) P = 0.0604 |
7.35 (2.24–24.05) P = 0.0010 |
| Serum beta-2 microglobulin > median (2.55) | 1.33 (0.70–2.55) P=.39 |
2.94 (1.12–7.71) P=.0289 |
Given the relatively high number of patients with long-term disease-free survival compared with historical rates with R-CHOP alone, we attempted to define baseline clinical characteristics predictive of long-term remissions by comparing patients with 5 or more years of PFS with those with a PFS of less than 5 years. In this univariate logistic regression analysis, only absence of splenic involvement was significantly associated with a PFS of ≥5 years (odds ratio 3.9, P = 0.02). MIPI score was not significantly associated with 5 year PFS, with the distribution of MIPI risk categories amongst patients with long-term PFS being similar to the baseline distribution.
Biological correlates
Baseline tumor characteristics were assessed by evaluating for blastoid morphology and immunohistochemical analysis for Ki-67 and SOX11. Of 48 eligible patients who consented to correlative studies, 23 had a Ki-67 score of 0–10%, 9 had a score of 11–30%, and 6 had a score of >30%, with a median score of 5.5%, somewhat lower than that reported in other studies (15–20%) (Geisler et al., 2008; Hoster et al., 2014; Tiemann et al., 2005) potentially due to technical differences. Ten patients who could not have Ki-67 scored were excluded from the analysis. Tumor samples from 45 patients (94%) expressed SOX11, with any staining scored as positive, and 3 patients (6%) had no SOX11 expression, comparable to the rates reported in other studies (Nordstrom et al., 2014; Nygren et al., 2012). Of 57 patients with available tissue, four (7%) had blastoid variant morphology, also consistent with expected rates (Tiemann et al., 2005).
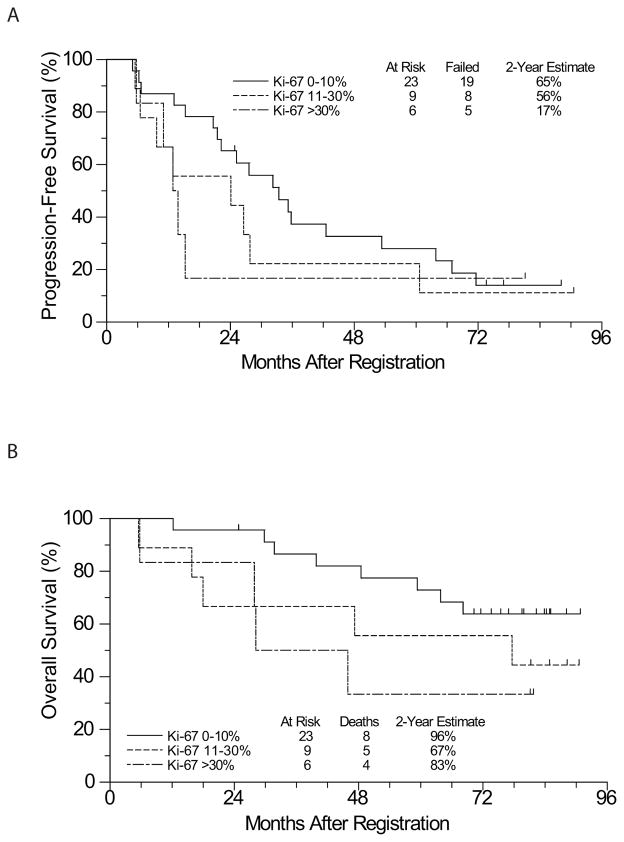
A Cox regression analysis was performed to assess for an association between continuous Ki- 67 score and PFS and OS. Given that Ki-67 scores were skewed towards lower scores, the analysis was performed using a log[Ki-67] score to normalise the distribution. There was a significant association between higher Ki-67 score and mortality (HR 1.47, P = 0.018), although the association with PFS was not significant (HR 1.2, P = 0.07). Kaplan-Meier estimates of PFS and OS stratified by Ki-67 score are shown (Fig 4). An analysis of the association of SOX11 and blastoid morphology with clinical outcome was not possible due to the small numbers of patients (only 3 SOX11-negative patients and 4 patients with blastoid morphology).
Figure 4.
(A) PFS and (B) OS by Ki-67 proliferation index from baseline tumor samples
Discussion
This multicenter study has demonstrated the safety and efficacy of administering combination R-CHOP plus bortezomib induction followed by bortezomib maintenance in patients with previously untreated MCL. The observed 2-year PFS of 62% exceeded our pre-defined threshold (2-year PFS of 42%) for further evaluation, despite this being an older patient population with the majority of patients having intermediate or high risk MIPI scores. Several prospective trials of R-CHOP have reported the median PFS for previously untreated MCL to be 14–22 months (Howard et al., 2002; Lenz et al., 2005; Robak et al., 2015; Rummel et al., 2013). While the current study was not randomised, the median PFS of 30 months in a comparable population of patients (based on similar median age, gender ratio, performance status, and number of patients with elevated LDH, B symptoms, and stage IV disease) suggests that the addition of bortezomib to R-CHOP improves clinical outcomes. These findings support the results recently reported from the phase III LYM-3002 trial in which newly diagnosed patients with MCL randomised to VR-CAP (R-CHOP plus bortezomib, omitting vincristine) had a superior PFS compared to patients who received R-CHOP (median 24.7 vs. 14.4 months) (Robak et al., 2015).
The long follow-up in this study allowed us to assess the frequency of long-term remissions, and we found that nearly one-third of patients remained progression-free at 5 years, a rate significantly higher than has been reported with R-CHOP alone. Perhaps somewhat surprisingly, prolonged responders could not be identified simply on the basis of a low MIPI score, suggesting a potential biological underpinning for this observation that warrants future study. It is also worth noting the durable overall survival in this study, with median OS not yet reached despite nearly 7 years of follow-up. This attests to the progress in therapy for relapsed/refractory in MCL in general and also specifically the ability to salvage relapsed patients following this regimen.
The Ki-67 proliferation index is a well-established prognostic factor for MCL, and consistent with prior studies (Goy et al., 2010; Tiemann et al., 2005), we found that a higher Ki-67 was significantly associated with an inferior overall survival. The lack of separation of the PFS curves at late timepoints (> 5 years) suggests that this regimen may overcome the poor prognosis of a high Ki-67 index in a subset of patients, and that other biologic features may be contributing to outcome.
VR-CHOP with bortezomib maintenance was generally well-tolerated, with toxicities being primarily hematologic, occurring during induction, and comparable to those historically seen with R-CHOP alone (Flinn et al., 2014; Howard et al., 2002; Lenz et al., 2005; Rummel et al., 2013). There was little apparent additive toxicity from inclusion of bortezomib, with the exception of increased rates of peripheral neuropathy. This required some dose reductions of bortezomib, but dose intensity was nevertheless fairly well preserved. It is likely that the rate of neurotoxicity could be reduced by subcutaneous administration of bortezomib, or potentially substitution of newer proteasome inhibitors. Additionally, omission of vincristine may also be helpful in this regard, though it is possible that this could have a small negative impact on efficacy. Interestingly, the rate of grade 3–4 thrombocytopenia was significantly lower (17%) with VR- CHOP than with the rate reported with VR-CAP (57%), suggesting that the lower dose (2.6 mg/m2/cycle vs 5.2 mg/m2/cycle in VR-CAP) and duration (ending day 4 vs day 11) of bortezomib used in our study is important in preserving thrombopoiesis, consistent with the known effects of proteasome inhibition on platelet production (Lonial et al., 2005; Shi et al., 2014).
Recently reported randomised trials have demonstrated a benefit for maintenance rituximab following R-CHOP or autologous stem cell transplantation (Kluin-Nelemans et al., 2012; Le Gouill et al., 2014), supporting a potential role for maintenance strategies in MCL. In the absence of a control arm, our data cannot determine the contribution of maintenance bortezomib to prolonging PFS and/ or OS relative to induction alone. Interim results of a small randomised study did not indicate a benefit of bortezomib maintenance after DA-EPOCH-R plus bortezomib (Dunleavy et al., 2012). While direct comparisons across studies is not feasible, the observation of a slightly longer median PFS, as well as a higher 5-year PFS rate, in our study as compared to the very similar VR-CAP regimen which lacked a maintenance component is provocative, suggesting that prolonged proteasome inhibition may be beneficial, at least in a subset of patients.
Since the initiation of this study in 2006 it has become clear that R-CHOP may not be the optimal induction regimen for MCL, and other therapies such as R-bendamustine (Flinn et al., 2014; Rummel et al., 2013), or, in younger patients, high-dose cytarabine-containing regimens (Delarue et al., 2013; Geisler et al., 2008; Hermine et al., 2010; Romaguera et al., 2005) are more promising and may serve as superior platforms for combination therapy with proteasome inhibitors (Chang et al., 2014; Friedberg et al., 2011). Based in part on our findings, an ongoing randomised U.S. Intergroup study (E1411) is evaluating the role of adding bortezomib to R-bendamustine induction.
In summary, our results support the feasibility and efficacy of including bortezomib as part of induction and maintenance on an R-CHOP backbone for the treatment of newly diagnosed MCL. The high overall survival rate is noteworthy in this elderly population with more than 50% of patients with intermediate or high risk MIPI scores. Therapy for MCL is a rapidly evolving field, with many exciting new agents being tested in clinical trials, and it remains to be determined how best to combine bortezomib or newer proteasome inhibitors with other therapies. Despite the many active treatments for MCL, it is likely that proteasome inhibition will continue to play a role given the distinct mechanism of action and expected lack of cross-resistance with other therapies.
Figure 3.
(A) PFS and (B) OS by MIPI score
Acknowledgments
The authors thank the patients and their families, as well as all of the SWOG member institutions without whom this study would not have been possible. This investigation was supported in part by the following PHS/DHHS grant numbers awarded by the National Cancer Institute (NCI), National Clinical Trials Network (NCTN): CA180888, CA180819, CA180834, CA180818; by the NCI Community Oncology Research Program (NCORP): CA189830, CA189858, CA189808, CA189860, CA189957, CA189872, CA189953, CA189954, CA189804; NIH/NCI: CA11083, CA35119; and in part by Millennium Pharmaceuticals, Inc. B.G.T. is a Damon Runyon-Pfizer Clinical Investigator.
Footnotes
Author Contributions
S.H.B, J.W.F, R.I.F. designed the study; B.G.T., H.L., S.H.B, R.I.F, W.R.B., L.M.R., J.D.F., M.A.D., D.F.M., O.P., S.M.S, M.L., and J.W.F. performed research; H.L. and M.L. performed statistical analyses; B.G.T., H.L., S.M.S., M.L., and J.W.F. analyzed and interpreted the data; B.G.T. wrote the manuscript, and all authors reviewed the draft manuscript and approved the final version for submission.
ClinicalTrials.gov Identifier: NCT00376961
Authors’ Disclosures of Potential Conflicts of Interest
B.G.T. has received research funding from Roche/Genentech. R.I.F. is a consultant for Johnson & Johnson and MorphoSys AG.
References
- Abrahamsson A, Albertsson-Lindblad A, Brown PN, Baumgartner-Wennerholm S, Pedersen LM, D'Amore F, Nilsson-Ehle H, Jensen P, Pedersen M, Geisler CH, & Jerkeman M. Real world data on primary treatment for mantle cell lymphoma: a Nordic Lymphoma Group observational study. Blood. 2014;124:1288–1295. doi: 10.1182/blood-2014-03-559930. [DOI] [PubMed] [Google Scholar]
- Aschebrook-Kilfoy B, Caces DB, Ollberding NJ, Smith SM, Chiu BC. An upward trend in the age-specific incidence patterns for mantle cell lymphoma in the USA. Leukemia & Lymphoma. 2013;54:1677–1683. doi: 10.3109/10428194.2012.760041. [DOI] [PubMed] [Google Scholar]
- Belch A, Kouroukis CT, Crump M, Sehn L, Gascoyne RD, Klasa R, Powers J, Wright J, Eisenhauer EA. A phase II study of bortezomib in mantle cell lymphoma: the National Cancer Institute of Canada Clinical Trials Group trial IND.150. Annals of Oncology. 2007;18:116–121. doi: 10.1093/annonc/mdl316. [DOI] [PubMed] [Google Scholar]
- Bogner C, Peschel C, Decker T. Targeting the proteasome in mantle cell lymphoma: a promising therapeutic approach. Leukemia & Lymphoma. 2006;47:195–205. doi: 10.1080/10428190500144490. [DOI] [PubMed] [Google Scholar]
- Bogner C, Ringshausen I, Schneller F, Fend F, Quintanilla-Martinez L, Hacker G, Goetze K, Oostendorp R, Peschel C, Decker T. Inhibition of the proteasome induces cell cycle arrest and apoptosis in mantle cell lymphoma cells. British Journal of Haematology. 2003;122:260–268. doi: 10.1046/j.1365-2141.2003.04438.x. [DOI] [PubMed] [Google Scholar]
- Chang JE, Li H, Smith MR, Gascoyne RD, Paietta EM, Yang DT, Advani RH, Horning SJ, Kahl BS. Phase 2 study of VcR-CVAD with maintenance rituximab for untreated mantle cell lymphoma: an Eastern Cooperative Oncology Group study (E1405) Blood. 2014;123:1665–1673. doi: 10.1182/blood-2013-08-523845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. Journal of Clinical Oncology. 1999;17:2454–2460. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables (with discussion) Journal of the Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- Delarue R, Haioun C, Ribrag V, Brice P, Delmer A, Tilly H, Salles G, Van Hoof A, Casasnovas O, Brousse N, Lefrere F, Hermine O. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d'Etude des Lymphomes de l'Adulte. Blood. 2013;121:48–53. doi: 10.1182/blood-2011-09-370320. [DOI] [PubMed] [Google Scholar]
- Dunleavy K, Grant C, Hessler J, Miller BW, Steinberg SM, Pittaluga S, Roschewski M, Jaffe ES, Wiestner A, Wilson WH. Bortezomib + DA-EPOCH-R Induction Therapy Followed by Maintenance Bortezomib Versus Observation in Newly Diagnosed Mantle Cell Lymphoma. Blood (ASH Annual Meeting Abstracts) 2012;120:Abstract 3672. [Google Scholar]
- Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, Epner E, Krishnan A, Leonard JP, Lonial S, Stadtmauer EA, O'Connor OA, Shi H, Boral AL, Goy A. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. Journal of Clinical Oncology. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- Flinn IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, Hertzberg M, Kwan YL, Simpson D, Craig M, Kolibaba K, Issa S, Clementi R, Hallman DM, Munteanu M, Chen L, Burke JM. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123:2944–2952. doi: 10.1182/blood-2013-11-531327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg JW, Vose JM, Kelly JL, Young F, Bernstein SH, Peterson D, Rich L, Blumel S, Proia NK, Liesveld J, Fisher RI, Armitage JO, Grant S, Leonard JP. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood. 2011;117:2807–2812. doi: 10.1182/blood-2010-11-314708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman RR, Martin P, Ruan J, Cheung YK, Vose JM, LaCasce AS, Elstrom R, Coleman M, Leonard JP. Phase 1 trial of bortezomib plus R-CHOP in previously untreated patients with aggressive non-Hodgkin lymphoma. Cancer. 2010;116:5432–5439. doi: 10.1002/cncr.25509. [DOI] [PubMed] [Google Scholar]
- Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, Eriksson M, Nordstrom M, Kimby E, Boesen AM, Kuittinen O, Lauritzsen GF, Nilsson-Ehle H, Ralfkiaer E, Akerman M, Ehinger M, Sundstrom C, Langholm R, Delabie J, Karjalainen-Lindsberg ML, Brown P, Elonen E. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecitano J, Portlock C, Moskowitz C, Hamlin P, Straus D, Zelenetz AD, Zhang Z, Dumitrescu O, Sarasohn D, Lin D, Pappanicholaou J, Cortelli BM, Neylon E, Hamelers R, Wright J, O'Connor OA. Phase 2 study of weekly bortezomib in mantle cell and follicular lymphoma. British Journal of Haematology. 2009;146:652–655. doi: 10.1111/j.1365-2141.2009.07775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goy A, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, Epner E, Krishnan A, Leonard JP, Lonial S, Nasta S, O'Connor OA, Shi H, Boral AL, Fisher RI. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Annals of Oncology. 2009;20:520–525. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goy A, Bernstein SH, McDonald A, Pickard MD, Shi H, Fleming MD, Bryant B, Trepicchio W, Fisher RI, Boral AL, Mulligan G. Potential biomarkers of bortezomib activity in mantle cell lymphoma from the phase 2 PINNACLE trial. Leukemia & Lymphoma. 2010;51:1269–1277. doi: 10.3109/10428194.2010.483302. [DOI] [PubMed] [Google Scholar]
- Goy A, Younes A, McLaughlin P, Pro B, Romaguera JE, Hagemeister F, Fayad L, Dang NH, Samaniego F, Wang M, Broglio K, Samuels B, Gilles F, Sarris AH, Hart S, Trehu E, Schenkein D, Cabanillas F, Rodriguez AM. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. Journal of Clinical Oncology. 2005;23:667–675. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- Guzman ML, Swiderski CF, Howard DS, Grimes BA, Rossi RM, Szilvassy SJ, Jordan CT. Preferential induction of apoptosis for primary human leukemic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermine O, Hoster E, Walewski J, Ribrag V, Brousse N, Thieblemont C, Bouabdallah R, Stilgenbauer S, Feugier P, Forstpointner R, Haioun C, Kneba M, Hänel M, Casasnovas R-O, Finke J, Hallek M, Wandt H, Bosly A, Klapper W, Gisselbrecht C, Coiffier B, Hiddemann W, Unterhalt M, Dreyling MH. Alternating Courses of 3x CHOP and 3x DHAP Plus Rituximab Followed by a High Dose ARA-C Containing Myeloablative Regimen and Autologous Stem Cell Transplantation (ASCT) Is Superior to 6 Courses CHOP Plus Rituximab Followed by Myeloablative Radiochemotherapy and ASCT In Mantle Cell Lymphoma: Results of the MCL Younger Trial of the European Mantle Cell Lymphoma Network (MCL net) Blood (ASH Annual Meeting Abstracts) 2010;116:Abstract #110. [Google Scholar]
- Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, Pfreundschuh M, Reiser M, Metzner B, Einsele H, Peter N, Jung W, Wormann B, Ludwig WD, Duhrsen U, Eimermacher H, Wandt H, Hasford J, Hiddemann W, Unterhalt M. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- Hoster E, Klapper W, Hermine O, Kluin-Nelemans HC, Walewski J, van Hoof A, Trneny M, Geisler CH, Di Raimondo F, Szymczyk M, Stilgenbauer S, Thieblemont C, Hallek M, Forstpointner R, Pott C, Ribrag V, Doorduijn J, Hiddemann W, Dreyling MH, Unterhalt M. Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. Journal of Clinical Oncology. 2014;32:1338–1346. doi: 10.1200/JCO.2013.52.2466. [DOI] [PubMed] [Google Scholar]
- Howard OM, Gribben JG, Neuberg DS, Grossbard M, Poor C, Janicek MJ, Shipp MA. Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survival. Journal of Clinical Oncology. 2002;20:1288–1294. doi: 10.1200/JCO.2002.20.5.1288. [DOI] [PubMed] [Google Scholar]
- Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- Klapper W, Hoster E, Determann O, Oschlies I, van der Laak J, Berger F, Bernd HW, Cabecadas J, Campo E, Cogliatti S, Hansmann ML, Kluin PM, Kodet R, Krivolapov YA, Loddenkemper C, Stein H, Moller P, Barth TE, Muller-Hermelink K, Rosenwald A, Ott G, Pileri S, Ralfkiaer E, Rymkiewicz G, van Krieken JH, Wacker HH, Unterhalt M, Hiddemann W, Dreyling M. Ki-67 as a prognostic marker in mantle cell lymphoma-consensus guidelines of the pathology panel of the European MCL Network. Journal of Hematopathology. 2009;2:103–111. doi: 10.1007/s12308-009-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluin-Nelemans HC, Hoster E, Hermine O, Walewski J, Trneny M, Geisler CH, Stilgenbauer S, Thieblemont C, Vehling-Kaiser U, Doorduijn JK, Coiffier B, Forstpointner R, Tilly H, Kanz L, Feugier P, Szymczyk M, Hallek M, Kremers S, Lepeu G, Sanhes L, Zijlstra JM, Bouabdallah R, Lugtenburg PJ, Macro M, Pfreundschuh M, Prochazka V, Di Raimondo F, Ribrag V, Uppenkamp M, Andre M, Klapper W, Hiddemann W, Unterhalt M, Dreyling MH. Treatment of older patients with mantle-cell lymphoma. New England Journal of Medicine. 2012;367:520–531. doi: 10.1056/NEJMoa1200920. [DOI] [PubMed] [Google Scholar]
- Le Gouill S, Thieblemont C, Oberic L, Bouabdallah K, Gyan E, Damaj G, Ribrag V, Bologna S, Gressin R, Casasnovas O, Haioun C, Solal-Celigny P, Maisonneuve H, Van Den Neste E, Moreau A, Bene MC, Salles G, Tilly H, Lamy T, Hermine O. Rituximab Maintenance Versus Wait and Watch after Four Courses of R-DHAP Followed By Autologous Stem Cell transplantation in Previously Untreated Young Patients with Mantle Cell Lymphoma: First Interim Analysis of the Phase III Prospective Lyma Trial, a Lysa Study. Blood (ASH Annual Meeting Abstracts) 2014;124:Abstract #146. [Google Scholar]
- Lenz G, Dreyling M, Hoster E, Wormann B, Duhrsen U, Metzner B, Eimermacher H, Neubauer A, Wandt H, Steinhauer H, Martin S, Heidemann E, Aldaoud A, Parwaresch R, Hasford J, Unterhalt M, Hiddemann W. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) Journal of Clinical Oncology. 2005;23:1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- Leux C, Maynadie M, Troussard X, Cabrera Q, Herry A, Le Guyader-Peyrou S, Le Gouill S, Monnereau A. Mantle cell lymphoma epidemiology: a population-based study in France. Annals of Hematology. 2014;93:1327–1333. doi: 10.1007/s00277-014-2049-5. [DOI] [PubMed] [Google Scholar]
- Lonial S, Waller EK, Richardson PG, Jagannath S, Orlowski RZ, Giver CR, Jaye DL, Francis D, Giusti S, Torre C, Barlogie B, Berenson JR, Singhal S, Schenkein DP, Esseltine DL, Anderson J, Xiao H, Heffner LT, Anderson KC. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106:3777–3784. doi: 10.1182/blood-2005-03-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom L, Sernbo S, Eden P, Gronbaek K, Kolstad A, Raty R, Karjalainen ML, Geisler C, Ralfkiaer E, Sundstrom C, Laurell A, Delabie J, Ehinger M, Jerkeman M, Ek S. SOX11 and TP53 add prognostic information to MIPI in a homogenously treated cohort of mantle cell lymphoma--a Nordic Lymphoma Group study. British Journal of Haematology. 2014;166:98–108. doi: 10.1111/bjh.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren L, Baumgartner Wennerholm S, Klimkowska M, Christensson B, Kimby E, Sander B. Prognostic role of SOX11 in a population-based cohort of mantle cell lymphoma. Blood. 2012;119:4215–4223. doi: 10.1182/blood-2011-12-400580. [DOI] [PubMed] [Google Scholar]
- O'Connor OA, Wright J, Moskowitz C, Muzzy J, MacGregor-Cortelli B, Stubblefield M, Straus D, Portlock C, Hamlin P, Choi E, Dumetrescu O, Esseltine D, Trehu E, Adams J, Schenkein D, Zelenetz AD. Phase II Clinical Experience With the Novel Proteasome Inhibitor Bortezomib in Patients With Indolent Non-Hodgkin's Lymphoma and Mantle Cell Lymphoma. Journal of Clinical Oncology. 2005;23:676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Pham LV, Tamayo AT, Yoshimura LC, Lo P, Ford RJ. Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. The Journal of Immunology. 2003;171:88–95. doi: 10.4049/jimmunol.171.1.88. [DOI] [PubMed] [Google Scholar]
- Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, LeBlanc M, Gaynor ER, Rivkin SE, Fisher RI. A phase 2 trial of CHOP chemotherapy followed by tositumomab/iodine I 131 tositumomab for previously untreated follicular non-Hodgkin lymphoma: Southwest Oncology Group Protocol S9911. Blood. 2003;102:1606–1612. doi: 10.1182/blood-2003-01-0287. [DOI] [PubMed] [Google Scholar]
- Robak T, Huang H, Jin J, Zhu J, Liu T, Samoilova O, Pylypenko H, Verhoef G, Siritanaratkul N, Osmanov E, Alexeeva J, Pereira J, Drach J, Mayer J, Hong X, Okamoto R, Pei L, Rooney B, van de Velde H, Cavalli F. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. New England Journal of Medicine. 2015;372:944–953. doi: 10.1056/NEJMoa1412096. [DOI] [PubMed] [Google Scholar]
- Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, McLaughlin P, Younes A, Samaniego F, Goy A, Sarris AH, Dang NH, Wang M, Beasley V, Medeiros LJ, Katz RL, Gagneja H, Samuels BI, Smith TL, Cabanillas FF. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. Journal of Clinical Oncology. 2005;23:7013–7023. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M, Balser C, Kaiser U, Weidmann E, Durk H, Ballo H, Stauch M, Roller F, Barth J, Hoelzer D, Hinke A, Brugger W. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- Shi DS, Smith MC, Campbell RA, Zimmerman PW, Franks ZB, Kraemer BF, Machlus KR, Ling J, Kamba P, Schwertz H, Rowley JW, Miles RR, Liu ZJ, Sola-Visner M, Italiano JE, Jr, Christensen H, Kahr WH, Li DY, Weyrich AS. Proteasome function is required for platelet production. The Journal of Clinical Investigation. 2014;124:3757–3766. doi: 10.1172/JCI75247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss SJ, Maharaj L, Hoare S, Johnson PW, Radford JA, Vinnecombe S, Millard L, Rohatiner A, Boral A, Trehu E, Schenkein D, Balkwill F, Joel SP, Lister TA. Bortezomib therapy in patients with relapsed or refractory lymphoma: potential correlation of in vitro sensitivity and tumor necrosis factor alpha response with clinical activity. Journal of Clinical Oncology. 2006;24:2105–2112. doi: 10.1200/JCO.2005.04.6789. [DOI] [PubMed] [Google Scholar]
- Tiemann M, Schrader C, Klapper W, Dreyling MH, Campo E, Norton A, Berger F, Kluin P, Ott G, Pileri S, Pedrinis E, Feller AC, Merz H, Janssen D, Hansmann ML, Krieken H, Moller P, Stein H, Unterhalt M, Hiddemann W, Parwaresch R. Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): a clinicopathological study from the European MCL Network. British Journal of Haematology. 2005;131:29–38. doi: 10.1111/j.1365-2141.2005.05716.x. [DOI] [PubMed] [Google Scholar]
- van de Schans SA, Janssen-Heijnen ML, Nijziel MR, Steyerberg EW, van Spronsen DJ. Validation, revision and extension of the Mantle Cell Lymphoma International Prognostic Index in a population-based setting. Haematologica. 2010;95:1503–1509. doi: 10.3324/haematol.2009.021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Han XH, Zhang L, Yang J, Qian JF, Shi YK, Kwak LW, Romaguera J, Yi Q. Bortezomib is synergistic with rituximab and cyclophosphamide in inducing apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leukemia. 2008;22:179–185. doi: 10.1038/sj.leu.2404959. [DOI] [PubMed] [Google Scholar]