Lipoprotein-associated phospholipase A2 (Lp-PLA2) produces the proinflammatory mediators lysophosphatidylcholine and oxidized free fatty acids through hydrolysis of oxidized phospholipids carried on low-density lipoproteins in atherosclerotic plaques. Although increased Lp-PLA2 activity has been associated with higher risks of occlusive vascular diseases, recent phase III trials of the Lp-PLA2 inhibitor darapladib, which reduces Lp-PLA2 activity by ∼60%, failed to establish a protective role of darapladib for prevention of major vascular events in patients with stable coronary heart disease (CHD) or acute coronary syndrome 1, 2.
A V279F loss-of-function variant in the PLA2G7 gene encoding Lp-PLA2, resulting in 50% lower activity for each copy of the variant, is common in East Asians (3) and allows an unbiased assessment of the causal effects of lifelong lower Lp-PLA2 activity, analogous to randomized trials of Lp-PLA2 inhibition (4). We genotyped PLA2G7 V279F (rs76863441) in 91,428 individuals randomly selected from the China Kadoorie Biobank prospective cohort of 0.5M participants recruited in 2004 to 2008 from 10 regions of China (5). Follow-up for International Statistical Classification of Diseases and Related Health Problems 10th Revision–coded incident events continued to January 1, 2014, through linkage with morbidity and mortality registries and a nationwide health insurance system. The pre-specified primary endpoint was major vascular events (MVE) (vascular death, myocardial infarction, stroke), with >90% power to detect a 20% lower relative risk at p < 0.01. As a context, the trials were powered to detect 15% risk reductions, and differences in lifelong exposure associated with genetic variants would be expected to have greater effects than intervening for a few years in later life (4). Secondary endpoints, for which power was lower, included major coronary events (CHD death, myocardial infarction), major occlusive events (CHD death, myocardial infarction, ischemic stroke), and stroke subtypes.
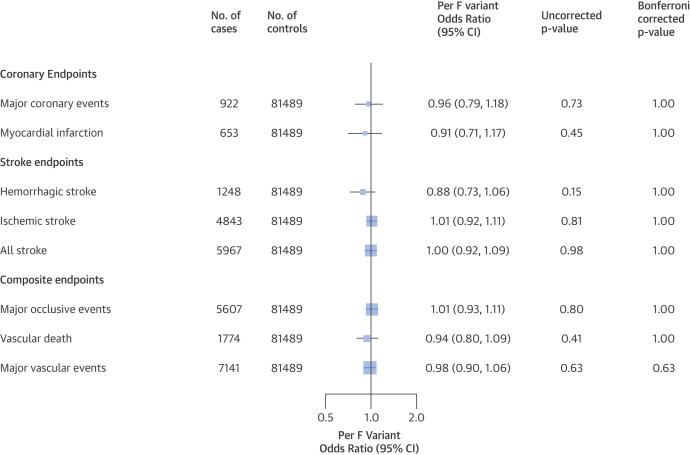
The mean baseline age of participants was 51 years and 40% were men. History of CHD was reported by 3%, stroke or transient ischemic attack by 2%, diabetes by 6%, and hypertension by 12% of participants. Antihypertensive or statin use was reported by 5% and 0.2% of participants, respectively. The frequency of PLA2G7 V279F was 5% overall, but varied from 3% to 7% by region (pheterogeneity <0.0001) and 10.6% of participants (n = 9,691) had at least 1 loss-of-function variant. There were no differences in blood pressure, adiposity, or other baseline characteristics by genotype. There was no significant association of PLA2G7 V279F with MVE, either overall (odds ratio: 0.98; 95% confidence interval: 0.90 to 1.06; p = 0.63) as assessed among 7,141 cases and 81,489 controls who had no prior history of vascular disease, or in specific population subgroups defined by sex, 10-year age group, region, or smoking status. Nor was PLA2G7 V279F associated with any of the components of MVE (Figure 1).
Figure 1.
Association of PLA2G7 V279F With Vascular Diseases
Odds ratio and 95% confidence interval (CI) per lipoprotein-associated phospholipase A2–lowering F variant, adjusted for sex, region, age, and relatedness. Bonferroni-corrected p values are based on 1 (primary) and 7 (secondary) endpoint tests.
This is the largest single study to investigate the associations of PLA2G7 V279F with risk of vascular diseases, and the results exclude any protective effect on MVE >10% in this general population. These findings are consistent with a previous meta-analysis of PLA2G7 V279F involving 3,611 East Asian CHD cases (3), and with the recent findings from randomized trials of Lp-PLA2 inhibition 1, 2. In contrast with the trials, MVE in this study predominantly involved stroke, reflecting the differences in cardiovascular disease rates between East Asian and European populations.
In conclusion, genetically determined lower Lp-PLA2 activity appeared to have no substantial causal effects on major vascular diseases, consistent with findings from randomized trials of Lp-PLA2–lowering therapy. These findings indicate that it may be valuable to use functional genetic variants in large-scale prospective studies with follow-up of a range of health outcomes to help predict any beneficial and adverse effects of novel therapeutic strategies (as well as to identify any potential alternative indications) prior to undertaking costly clinical trials.
Footnotes
Please note: The study was partly funded by a grant from GlaxoSmithKline, who collaborated in developing the study design, analysis plan, results interpretation, and reporting. The study was also partly funded by UK Wellcome Trust, Medical Research Council, British Heart Foundation, Kadoorie Charitable Foundation Hong Kong, Chinese National Natural Science Foundation, and Merck. All data were analyzed independently at the Clinical Trial Service Unit & Epidemiological Studies Unit, University of Oxford (United Kingdom). Drs. Waterworth, Johnson, Yeo, Chissoe, and Cardon are full-time employees of GlaxoSmithKline and own company stock. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.White H.D., Held C., Stewart R. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370:1702–1711. doi: 10.1056/NEJMoa1315878. [DOI] [PubMed] [Google Scholar]
- 2.O'Donoghue M.L., Braunwald E., White H.D. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA. 2014;312:1006–1015. doi: 10.1001/jama.2014.11061. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q., Hao Y., Mo X. PLA2G7 gene polymorphisms and coronary heart disease risk: a meta-analysis. Thromb Res. 2010;126:498–503. doi: 10.1016/j.thromres.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Evans D.M., Davey Smith G. Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annu Rev Genomics Hum Genet. 2015;16:327–350. doi: 10.1146/annurev-genom-090314-050016. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z., Chen J., Collins R. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40:1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]