Highlights
-
•
Bacterial respiratory tract infections have a strong association with worsening or increased severity of symptoms in asthma patients.
-
•
The asthma airway is characterized by an altered microbiota composition, compared with healthy individuals.
-
•
Microbial stimuli and the composition of the airway microbiota can contribute to the shaping of inflammatory responses seen in asthma.
-
•
Approaches to rapidly diagnose or modulate the microbiota associated with the asthmatic airway may represent powerful tools to improve disease prognosis.
Keywords: airway, allergens, asthma, infection, inflammation, microbiota
Abstract
During the past 50 years, the prevalence of asthma has increased and this has coincided with our changing relation with microorganisms. Asthma is a complex disease associated with local tissue inflammation of the airway that is determined by environmental, immunological, and host genetic factors. In a subgroup of sufferers, respiratory infections are associated with the development of chronic disease and more frequent inflammatory exacerbations. Recent studies suggest that these infections are polymicrobial in nature. Furthermore, there is increasing evidence that the recently discovered asthma airway microbiota may play a critical role in pathophysiological processes associated with the disease. Here, we discuss the current data regarding a possible role for infection in chronic asthma with a particular focus on the role bacteria may play. We discuss recent advances that are beginning to elucidate the complex relations between the microbiota and the immune response in asthma patients. We also highlight the clinical implications of these recent findings in regards to the development of novel therapeutic strategies.
The human microbiome and its interaction with the host
Medical and hygiene advances over the past 150 years, such as sanitation measures, vaccines, and antibiotics, have helped arm the fight against infectious microorganisms, curbing infant mortality and greatly increasing life expectancy. However, in recent years, it has become clear that humans have, during the course of their evolution, developed an essential symbiotic relation with microorganisms and this is redefining the way we view infectious disease [1]. It is now understood that we are host to a vast number of bacterial, fungal, viral, and eukaryotic cohabiters termed the microbiota. In the simplest terms, the microbiota can be defined as the microbes associated with a particular environmental niche. Although the term microbiota has been used for decades, clinical and scientific interest in human-associated microbiota is more recent and has been further stimulated by projects like the Human Microbiome Project or sub-projects like the gut microbiome project MetaHIT. These studies utilizing advanced molecular techniques have generated unprecedented insight into the complexity and diversity of microbial communities that exist on and within humans. It is clear that these microbial communities, particularly in the gut, play a key role in the protection from pathogenic organisms and the development and maintenance of immune responses. The study of the role of the gut microbiota in modern diseases such as asthma, obesity, juvenile diabetes, and specific cancers has revealed that perturbations to microbial communities have a profound effect on disease development and treatment response 1, 2, 3.
Technological advances have resulted in the advent of high-resolution molecular investigations into the airway microbiota in pulmonary health and/or disease. Given that the lungs represent one of the largest interfaces between the human host and the external environment, being exposed to >8000 l of inhaled air each day [4], it is unsurprising that a direct connection between aberrant microbial colonization of the airways has been shown to contribute to the development of chronic inflammatory diseases such as chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF) [5].
Until recently, the understanding of the role bacteria play in asthma, another chronic lower airway disease, has lagged behind its counterparts. However, clinicians and researchers have begun to benefit from this deeper understanding to re-evaluate and redirect efforts to understand the relations between asthma and microbial colonization [5]. This has been stimulated by the fact that >300 million people worldwide suffer from this disease, which results in an economic burden of >$80 billion per year in the US alone.
In this review, we discuss recent studies that have shed light on the association of microorganisms with asthma and the potential interactions between the airway microbiota and the immune system. We also discuss how a deeper understanding of the microbiota within the asthmatic airway may provide potential therapeutic avenues.
The asthma airway and associated microbes
Specific microorganisms and asthma disease development
The US Department of Health and Human Services defines asthma as ‘an inflammatory disease of the airways with generally reversible air flow obstruction and airway hyper-responsiveness causing episodic respiratory symptoms’, in which many cells and cellular elements play a role [6]. The chronic inflammation is associated with airway hyper-responsiveness and leads to recurrent episodes of exacerbation marked by wheezing, breathlessness, chest tightness, and coughing [7]. Historically, the contribution of bacteria and other microbes to asthma pathogenesis was investigated using: (i) traditional culture-dependent approaches to sample the airway; (ii) serological testing to identify microbe specific antibodies in the serum; and (iii) by species-targeted PCR (7). The importance of viral infections as a source of asthma exacerbation and chronic inflammation of the airways in children and adults is well demonstrated 8, 9. The potential contribution of fungal infection to asthma is less well understood (Box 1), and in the case of bacterial infections, it is now felt that they can contribute to the development of stable asthma and acute exacerbations [10].
Box 1. Fungus in asthma.
Culturing of fungi from airway samples is challenging due to slow growth, specific media requirements, and the lack of quantitative methods, which is due in part to filamentous morphologies 56, 57, 58. Taken together, this has made it difficult to describe fungal species and their relative burdens in respiratory disease.
Although the fungal microbiota of the asthmatic respiratory tract has not been well characterized to date, there is evidence that specific fungal species can play a part in the clinical progression of disease [59]. Fungi can cause problems in the airways of asthma patients in two ways: by acting as allergens or as pathogens causing infection, with many fungi being able to do both and often concurrently.
It is well documented that the spores of several filamentous species within the genera of Aspergillus, Alternaria, Cladosporius, Penicillium, and Didymella (phylum Ascomycota) may act as allergens and initiate asthma development in atopic individuals. Importantly, fungal infection and exposure have already been linked to several clinical consequences in asthmatics including deterioration of lung function, increased hospital admission, and even mortality. One of the most documented fungal infections observed in asthmatics is allergic bronchopulmonary aspergillosis (ABPA), caused by colonization of the lower respiratory tract with Aspergillus spp. In this situation, the fungus acts as both a source of allergen and as a pathogen [60]. ABPA presents itself by a range of clinical features including asthma exacerbation, recurrent pulmonary infiltrates, elevated total serum IgE, elevated Aspergillus-fumigatus-specific IgE or IgG, central bronchiectasis, eosinophilia, and mucous plug production. Furthermore, ABPA is difficult to predict given that allergens produced by A. fumigatus are diverse in nature and the dormant spores can evade host defense mechanisms until conditions are suitable for germination [60]. Fungi have also been associated with severe asthma termed ‘severe asthma with fungal sensitization’ (SAFS) [61]. SAFS is diagnosed by the presence of severe asthma, fungal sensitization, and the absence of ABPA. Because of the paucity of data and ambiguity in diagnostic criteria, SAFS is currently classed as a diagnosis of exclusion rather than a specific entity.
Recent studies have suggested the possible benefit of antifungal therapy in the treatment of asthmatics, with clear improvements seen in lung function, even when fungal species have not been cultured or detected from airway secretions [56]. Although little is known of the airway fungal community in the pathogenesis of asthma, these observations suggest that rigorous study should be undertaken. This is even more important given recent studies highlighting the complexity of fungal communities found in the oral cavity of healthy individuals [59], the lower airways of CF, and in COPD patients 56, 57, 58, 62 using pan-fungal primer amplification followed by pyrosequencing. These landmark studies provide the initial standard for studying the fungal microbiota along the respiratory tract. Taken together, it is clear that future examination of the fungal microbiota along the respiratory tract in relation to asthma inflammation and phenotypes could be of great interest. Further studies will be required to characterize the impact that fungal colonization has on the bacterial communities associated with the asthma airway and the potential cross-kingdom interactions that may occur.
Despite the lack of definitive evidence, many controlled clinical studies have demonstrated an association between chronic stable asthma and bacteria 11, 12, 13, as infected subjects were found to have elevated markers of inflammation, increased severity of obstruction identified by FEV1 (forced expiratory volume in one second), higher daytime symptom score, and required high doses of inhaled corticosteroids in comparison with noninfected controls. A strong connection between acute exacerbations of asthma and infection with Chlamydia pneumoniae and/or Mycoplasma pneumoniae has also been reported [14], however, there is insufficient data to allow for definite conclusions about the role of such bacteria in late asthma development [15].
Evidence is also available suggesting that exposure to Staphylococcus aureus and/or its enterotoxins function as an environmental risk factor for the development and severity of asthma [16]. The locally or systemically released enterotoxins show superantigen activity and may provoke eosinophilic stimulation leading to deterioration of upper and lower respiratory tract atopic diseases [16]. Specific antibodies against S. aureus enterotoxins are more likely to be found in patients with asthma [16].
Other respiratory bacteria such as Haemophilus influenzae, Moraxella catarrhalis and Streptococcus pneumoniae have been shown to cause severe persistent wheeze in children [17]. It was also found that neonates colonized in the pharyngeal region are under increased risk for recurrent wheeze and asthma within the first 5 years of life [17]. Particularly evident is the association of these pathogens with a subset of stable asthma, known as neutrophilic asthma, where inflammation is primarily mediated by neutrophils and less by eosinophils. H. influenzae was isolated from the airways of patients with neutrophilic asthma, and infection-induced inflammation [17].
Overall, this work has shown that acute respiratory infections by specific bacteria trigger asthma exacerbations through colonization or infection of the airway. However, it is still unclear and much argued whether these single bacterial infections are true causative agents of asthma exacerbation or act as markers for an increased risk of asthma onset [5]. Furthermore, it is not clear what role changes to airway architecture as a result of infection-related damage may impinge upon the future development of airway disease and the susceptibility to future infection. The interpretation of these observations has become even more complex in light of the revelation that the airways, that were once assumed to be sterile, have a diversity of microorganisms associated with them in asthmatic patients.
Microbiota associated with the asthmatic airways
As discussed above, the role of microbial infection in asthma pathogenesis remains unclear. However, the developments in defining the human microbiota have demonstrated that the composition of bacterial communities colonizing mucosal surfaces, rather than simply the presence of individual species, can be important in defining states of health or disease. Researchers have been utilizing new high-resolution microbial profiling methods to describe the microbial composition of the asthmatic lung (Box 2). Results from these studies in the past 5 years indicate that the composition of the bacterial microbiota detected from the airways of asthmatic patients differs in comparison to those of healthy subjects, although there are variations in the details of these findings 5, 12, 18, likely reflecting the heterogeneity across asthmatic populations.
Box 2. Characterizing the airway microbiota.
Culture-dependent approaches have proved useful clinical tools in the detection and characterization of specific microbes present in the respiratory tract, which are responsible for infection [63]. Despite limitations, these methods have been crucial for studying the function of community members. However, the selectivity of culture media and the fastidious nature of many microbes have resulted in many important microbiota members being overlooked. In recent years, application of culture-independent methods such as microarray hybridization and DNA sequencing technologies has given greater detail and information regarding airway-associated microbial communities [50]. These approaches as well as determining identity and abundance of the microbiota present can provide the researcher with information concerning richness, evenness, and dominance. These measures can in some cases be clinically edifying.
Studying the microbiota of the airways is considered far more problematic than the gut due to the lower burden of biological material and the risk of contamination from the upper airway during sampling. Therefore, it is proposed that lower and upper respiratory samples are obtained from each patient. Several materials that are collected routinely by scientists from the respiratory tract include sputum, bronchial brushings and BAL. Sputum is obtained by induced coughing in subjects that results in the expectoration of mucus from the lower airways. Sputum is a useful material because it contains immune system components as well as host and bacterial products, including RNA, which can be used to infer the functional properties of communities. Bronchial brushings are obtained by passing a protected bronchoscopy cytology brush down the endotracheal tube until resistance is felt. Resistance indicates that the brush has reached the end of the trachea; at this point the brush is extended beyond the tip of the bronchoscope and past the end of the protective sheath and moved back and forth on the surface of the airway mucosa to collect the sample. The brush is then retracted into the sheath before being retracted through the bronchoscope to minimize contamination of the lower airway sample. BAL involves a bronchoscope being passed through the endotracheal tube and into the bronchus. A saline solution is then delivered through the bronchoscope and aspirated back up into a container.
Recently, Rogers and colleagues proposed several criteria that should be considered when determining the microbiota of the airway [49]. These included the following. (i) Respiratory material collection can be done in several ways for microbiota studies. Regardless of the material collected, it is important to consider the ease, safety, reproducibility, and the potential for contamination. (ii) Sample heterogeneity is also an important consideration if the samples are collected spatially and temporally. This depends on the research question being addressed. (iii) Nucleic acid extraction becomes paramount once sample material has been collected. Several studies have examined the importance of deploying stringent extraction procedures. Most have illustrated the ease at which distortion can creep into microbiota profiles due to poor, inconsistent or less than rigorous nucleic acid extraction. (iv) Methods for generating microbiota profiles are commonly carried out using generation of 16S rRNA clone libraries, terminal RFLP analysis, microarray hybridization, 16S rRNA phylogenetic microarray analysis, or deep sequencing of 16S rRNA amplicon pools. Although the choice of method deployed typically depends on the researchers budget and time required, ideally, this choice should be driven by the primary hypothesis to be addressed.(v) Analyzing the resulting data in an informed and relevant manner is imperative. Although there are numerous methods for data analysis available, the processing should take into account the potential for spurious signals generated by the profiling method, the potential for contamination during the processing of the samples, and the potential of imperfect taxa identification.
These recently highlighted criteria for assessment of airway microbiota are rather time-consuming and the research community has called for greater standardization and documentation of microbiota profiling methods, particularly regarding work aimed at gaining clinical insight into respiratory disease.
One of the landmark studies that utilized 16S rRNA clone libraries to examine the respiratory samples from adults and children with asthma found the lung bacterial community to contain members of the Proteobacteria phylum, in particular, Haemophilus spp., Neisseria spp., and Moraxella spp. Although the resolution of this approach is lower than many newer profiling methods, it did find that these strains were more commonly found in asthmatics than the healthy controls tested [19]. A subsequent study of bronchial brushings from 75 patients with mild to moderate asthma, using a higher-resolution 16S rRNA phylogenetic microarray platform to assay the airway microbiome, found 100 bacterial taxa that were strongly correlated to airway hyper-responsiveness [11]. This study like others found that members of the phylum Proteobacteria were significantly enriched in asthmatics and also included families of potential pathogenic bacteria such as Haemophilus and Streptococcus spp. More recently, an examination of induced sputum samples from treatment-resistant severe asthmatics during a non-exacerbation phase of their disease using lower-resolution terminal restriction fragment length polymorphism (T-RFLP) analysis revealed again that patient airway colonization was predominantly with Haemophilus spp., Streptococcus spp., or M. catarrhalis [20].
Studies involving larger numbers of asthmatic patients have observed relations between the clinical outcome and airway microbiota diversity [11]. One of the most comprehensive studies, which examined the microbiota profile of 65 adults who suffered from suboptimally controlled asthma showed positive correlation between the abundance of specific airway microbiome members (Comamonadaceae, Sphingomonadaceae, and Oxalobacteraceae) and the degree of airway hyperactivity. Although correlation is not causality, it suggests that the presence of specific microbial species in the lower airway is associated with the degree of bronchial hyper-responsiveness, a key feature of asthma [11]. More recently, in patients with severe asthma, relations have been reported between airway microbiota and body mass fluctuations [11], as well as neutrophils and interleukin (IL)-8 accumulation in sputum [18]. These relations may influence the type or degree of airway inflammation seen in certain patients and may contribute to the complexity in defining phenotypes within asthma.
Although all these studies describe extensive diversity in the airways of examined asthmatics, it is important to note that little consideration was given to the impact that patient treatments such as inhaled corticosteroids, bronchodilators, and antibiotic treatments had on these results. The first studies addressing this gap in our knowledge was carried out using 16S rRNA profiling of DNA extracted from bronchoalveolar lavage (BAL) fluid to compare the airway microbiota of corticosteroid-resistant and corticosteroid-sensitive asthmatics [21]. Although, this work failed to highlight changes in the microbial community that were consistently associated with steroid-resistant asthma, the work did go on to define the influence of Haemophilus parainfluenzae on the expression of corticosteroid-regulated genes of BAL macrophages in an in vitro co-culture model. It concluded that co-culture with H. parainfluenzae as opposed to the commensal genus Prevotella resulted in inhibition of the steroid response. Another longitudinal study was conducted to measure the impact of the antibiotic azithromycin on the airway microbiota of five adult patients with asthma [22]. The result was the reduction in the representation of the Haemophilus, Pseudomonas, and Staphylococcus genera within the community, which coincided, with establishment of Anaerococcus as the most dominant members of the community.
Despite limitations of small sample sizes, potential biases in sampling methods, and unanswered questions regarding treatment influence, the overall findings between most studies are generally consistent and provide further evidence that airway microbiota diversity associated with asthma is likely due to the disease itself (Figure 1). The other possibility is that the microbiome drives disease and/or features of the disease such as steroid non-responsiveness. Most importantly, now that these communities are being understood in greater detail, there is a pressing need to explore the mechanistic link between microbial communality and asthma development/severity, with research into the interaction between the microbiota and mucosal immunity being vitally important.
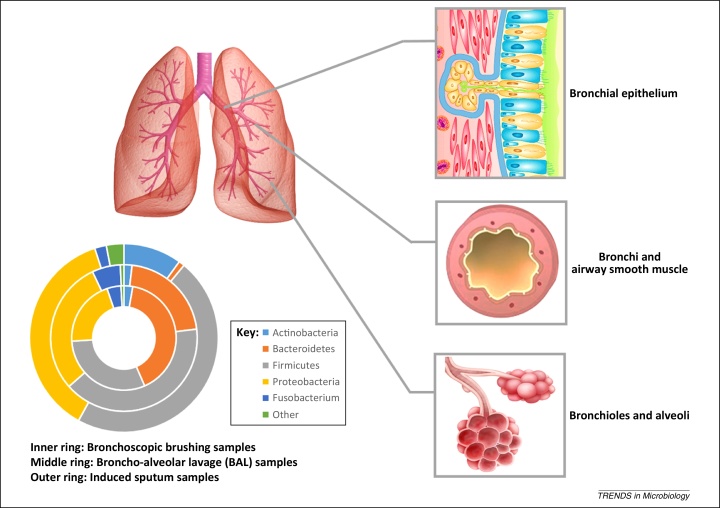
Figure 1.
Microorganisms involved in asthma airway colonization. A cross-section of the human lower respiratory tract is depicted, showing sites of infection for different microorganisms and the effects that they have on airway function. The phylogenetic ring depicts the percentage abundance of bacterial phyla identified in various biological samples taken from the airways of asthma patients (Information used to compile figure was reported in 12, 19. Inner ring (bronchoscopic brushing samples); middle ring (BAL samples), and outer ring (induced sputum samples). Abbreviation: BAL, bronchoalveolar lavage.
The potential contribution of airway microbiota to asthma subtypes and the immune response
One of the classic features of asthma is the presence of specific immune cells in the airway. Simpson and colleagues [23] have used this to describe four inflammatory subtypes of asthma based on the immune cell profile of sputum taken from patients (Table 1). These subtypes include eosinophilic (eosinophils >3%), neutrophilic (neutrophils >61%), mixed granulocytic (increased eosinophils and neutrophils), and paucigranulocytic asthma (normal levels of both of these specific immune cell types). Numerous researchers and clinicians have used these asthma subtypes for classification. Recent work has begun to elucidate the association between lung microbiota function and immune response in eosinophilic and neutrophilic asthma subtypes that we describe below (Figure 2).
Table 1.
Asthma subtypes and molecular characteristics
| Asthma subtypes | Molecular pathology |
|---|---|
| Eosinophilic | Eosinophils >3% in sputum taken from the airway Can be associated with high or low Th2 Stimulation of IL-5 and other various cytokines Associated with steroid responsiveness Thickening of the basement membrane |
| Neutrophilic | Neutrophils >61% in sputum taken from the airway Can be associated with high Th17 Stimulation of IL-8 Associated with steroid non-responsiveness Normal basement membrane thickness |
| Mixed-granulocytic | Increased eosinophils and neutrophils, cytokine stimulation |
|
Paucigranulocytic |
Normal levels of eosinophils and neutrophils, cytokine stimulation |
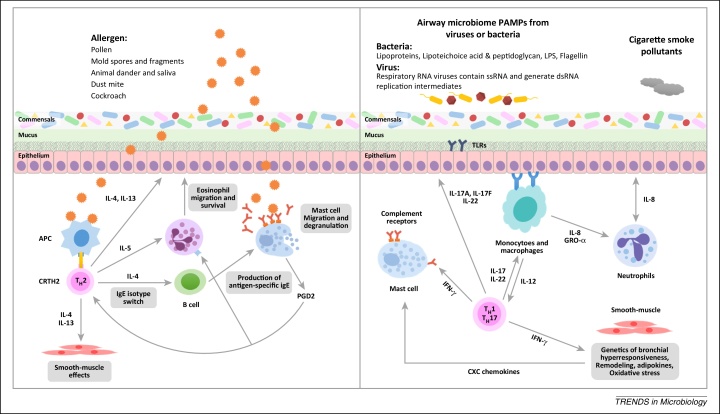
Figure 2.
Bacterial and viral infections of the airways activate immune and structural cells, promoting inflammation and influencing responses to other pathogens, allergens and pollution. The schematic depicts potential triggers and innate immune response of eosinophilic (Th2 dependent) and neutrophlic (non-Th2 dependent) asthma. Left panel: Environmental allergens such as pollen and mold spores can trigger Th2 asthma. Th2 immune processes begin with the development of Th2 cells and their production of the cytokines IL-4, IL-5, and IL-13. These cytokines stimulate allergic and eosinophilic inflammation as well as epithelial and smooth-muscle changes that contribute to asthma pathobiology. Right panel: Cigarette smoke, pollutants and the PAMPs from airway microbes including LPS from bacteria or ssRNA from respiratory viruses can potentially trigger non-Th2 asthma. There is a range of factors that can contribute to the development of non-Th2 asthma. These factors include infection-related elements, Th1 and Th17 immunity, non-Th2 associated smooth-muscle changes and the development of neutrophlic inflammation. Abbreviations: APC, antigen-presenting cell; CRTH2, chemoattractant receptor-homologous molecule expressed on Th2 cells; dsRNA, double-stranded RNA; PGD2, prostaglandin D2. IFN, interferon; GRO, growth-regulated oncogene; IL, interleukin; LPS, lipopolysaccharide; PAMP, pathogen associated molecular pattern; ssRNA, single-stranded RNA; Th, T helper; TLR, Toll-like receptor.
Eosinophilic asthma
Eosinophil accumulation in the airway (eosinophilia) is associated with thickening of the basement membrane and is often responsive to corticosteroid treatment [24]. This eosinophilia is most commonly associated with allergic or atopic asthma and accounts for the majority of disease. The generally held view for allergic asthma is that it is driven by sensitization to an allergen that is followed by subsequent challenge. The result of this multifactorial event is the activation of T cells and the conversion to a T helper (Th)2 type immune response. Th2-associated cytokines IL-4, IL-5, IL-9, and IL-13 promote the presence of eosinophils in the airway and in turn lead to an increase in IgE levels in the blood. Additionally, IgE levels are known to be elevated in the respiratory tract of eosinophilic asthmatics in response to antigens from many common airway microbial colonizers [25]. IgE binds to IgE receptors on the surface of mast cells and becomes crosslinked upon binding of allergens resulting in mast cell degranulation. The result of mast cell degranulation is the release of preformed mediators of inflammation such as histamine, tumor necrosis factor (TNF)-α, IL-13, and IL-4. Ultimately, this results in enhanced vascular permeability and the recruitment of inflammatory cells to the airway. The link between Th2 responses and allergic inflammation was initially established in mice and subsequently Th2 cytokines were then detected in asthmatic patients. Transcripts of IL-4, IL-5, and IL-13 in patient sputum can be detected and quantified by PCR and can be used as biomarkers of a ‘Th2-high’ response [26]. Gene expression microarrays demonstrated that monitoring of CLCA1, periostin, and serpinB2 expression could be used as markers to estimate and monitor a Th2 response [27].
Recent studies have begun to challenge the long held view that allergic reactions represent unintentional immune responses [28]. Instead it is proposed that allergic host defenses have evolved as protection against harmful substances such as irritants, noxious chemicals, and foreign toxins. The extent to which microbial derived products, toxins, or components present in the airway contribute to allergic responses remains a largely unexplored but exciting question. This suggests that the ability to cause tissue damage may be a common feature of substances that trigger appropriate allergic responses regardless of their source [28]. Little is known about the contribution of the airway microbiota to such mechanisms but clues are emerging that highlight the need for further investigation. For example, it is known that group 2 innate lymphoid cells (ILC2s) can be activated directly by microbes acting on Toll-like receptors (TLRs) 29, 30. Recent work in mice has led to the discovery that ILC2s can release Th2-type cytokines in response to epithelial damage and this may be a potential mechanism by which damage caused by members of the airway microbiota could elicit an asthmatic response 31, 32, 33.
It has also been shown that low levels of microbe-derived lipopolysaccharide (LPS) sensed through TLR4 can enhance the Th2 response [34]; responses are context-dependent and LPS also induces Th1 responses. This again indicates that the airway microbiota has the potential to modulate the allergic response where different manifestations may be heavily dependent on the environmental context. Work with neonatal mice showed that manipulating the lung microbiota composition provided a potential protective role in allergic responses [35]. The work demonstrated that immediately after birth the airway mucosa was primed for a strong response to allergen challenge. However, 2 weeks postpartum the bacterial load in the lungs increased and there was a change in composition from Gammaproteobacteria and Firmicute dominance to the establishment of Bacteroidetes. This shift in community was associated with the inducement of the Helios T regulatory cell subset and decreased aeroallergen responsiveness. Future work should further elucidate the interaction between specific microbes and components of the immune system contributing to allergy.
Neutrophilic asthma
Neutrophils are one of the earliest immune cells recruited to sites of damage and infection of the airway. Many lung diseases such as COPD and CF are associated with neutrophilic inflammation 36, 37. Asthma is no exception with ∼30% of patients suffering from neutrophilic asthma. This is characterized by substantial and sustained increase in airway neutrophils (neutrophilia), which is linked to a poor response to inhaled corticosteroid treatment.
Although eosinophilic asthma has been connected with the Th2 response, neutrophilic asthma is believed to be a non-Th2 immune response [38]. It is recognized that this potent innate immune response can be stimulated by Gram-negative and Gram-positive bacteria and their products, including endotoxins, with cell wall and outer membrane components all potentially acting as pathogen-associated molecular patterns (PAMPs), which are recognized by TLRs, G protein-coupled receptors (GPCRs), CD14, and collectins. Activation of TLRs leads to inflammatory cascades that result in production of the proinflammatory cytokines IL-8 [also known as chemokine CXC ligand (CXCL)8], IL-1, and TNF-α, generating a shift toward a Th1 and Th17 response, extensive neutrophil recruitment, and a change in inflammatory cell differential profile. Consistent with this, there is evidence in treatment-resistant severe asthma of increased TNF-α gene and protein expression within the airways compared to that in mild asthma [39]. Some members of the GPCR family transduce signals through cytoplasmic G proteins to cytoskeletal proteins controlling the movement of leukocytes. The GPCRs FPR1 and FPR2 (human formyl peptide receptor 1 and 2) as well as GPCRs 41 and 43 (GPR41 and GPR43) are all expressed by neutrophils and can detect PAMPs. Activation of these receptors results in the recruitment of neutrophils from the bloodstream to sites of infection, activation of the oxidative burst and IL-8 release [40].
An intriguing hypothesis that is gaining notoriety is that sustained microbial colonization of the airway could promote the neutrophilia observed in asthma. Studies lend strength to this argument by showing that S. aureus, M. catarrhalis, and H. influenzae are detected in sputum from neutrophilic and stable severe asthmatics 13, 41. Furthermore, strong associations have been revealed between increased bacterial load and higher concentrations of airway neutrophils and the neutrophil chemoattractant IL-8. Also linked to neutrophilic asthmatics is the resistance to corticosteroid treatment. Although only a small number of studies have been carried out, it appears that patients resistant to corticosteroid treatment are predominantly colonized by the same communities of bacteria as neutrophilic asthmatics are. Work investigating the influence of H. parainfluenzae on the expression of corticosteroid-regulated genes of BAL macrophages in an in vitro co-culture model concluded that the presence of H. parainfluenzae resulted in inhibition of the steroid response as opposed to the presence of commensal members of the genus Prevotella [21].
A recent study examining bronchial biopsies implicated IL-17 in causing substantial neutrophil recruitment in the airways suggesting that the neutrophilic asthmatic response is more complex than believed [42]. IL-17 drives Th17 responses, which provide protection from infection at mucosal surfaces. Further work provides evidence that the Th17 lineage of T cells has a role in controlling pulmonary bacterial infections and have already been implicated in those caused by Klebsiella pneumoniae infection [43]. Furthermore, Th17 cytokines are associated with moderate and severe asthma with IL-17A, IL-17F, and IL-22, functioning to increase smooth muscle mass, mucus production, and the indirect recruitment of neutrophils to the airways by inducing the release of the chemokines CXCL1 and IL-8 from epithelial cells [44]. It should be noted that recent work looking at bronchial biopsies failed to find an association with IL-17A/IL-17F and neutrophilia [42]. Interestingly, the cytokine profile and presence of microbial products at a site of infection can determine the lifespan of neutrophils. Activation of pattern recognition receptors (PRRs) on the surface of neutrophils such as TLR 1, 2, 4, 5, 6, 8, and 9 have been shown to enhance neutrophil survival where nuclear factor (NF)-κB and mitogen-activated protein kinase (MAPK) signaling is important [45]. The pattern recognition function of neutrophils may represent an important pathway directly linking the detection of lung microbiota members and neutrophil survival in the airway.
Although investigation into the microbial contributions to these various subtypes of asthma are in the initial stages, they provide confidence that research should be undertaken to link microbial burden and community composition with specific immune responses. The categorization of asthmatics based on the immune cell profile of the sputum is an invaluable tool that should be utilized to elucidate the functional properties of the lung microbiota. However, there are many questions regarding the stability of these inflammatory phenotypes over time, especially in children with asthma, and more longitudinal studies are required. This variability may be partly explained by variations in environmental exposure over time. Other complementary approaches are required to understand these complex immunopathologies. A recent study looking at differential expression of host genes found a six-gene signature that could be used as a biomarker to distinguish different inflammatory subtypes [39]. In the future, these clinically measurable outputs should be combined with sputum metabolomics data, patient genetics, and patient information (including history of infection) to better define asthma endotypes, as outlined in a recent review [46], and the contribution of the lung microbiota to each of these. Ultimately, this will lead to the replacement of asthma as an umbrella term for a range of inter-related but distinct conditions [38].
Asthma treatment in the light of improved understanding of the associated airway microbiota
Many research groups have begun to devise and test methods to exploit and convert this newfound knowledge of the microbiota of the respiratory tract into improved or even new treatment strategies for asthma and other inflammation-based diseases. Below, we discuss some of the approaches that have been proposed in potentially improving treatment of asthma.
Predication of onset of asthma exacerbation
The asthma severity correlates with microbiota dysfunction and inflammation in the airways 47, 48. Acute periods of exacerbation are one of the most important causes of morbidity in asthma sufferers. Consequently, the control of asthma has depended on the management of inflammation. The precise triggers of exacerbation are complex but it has become clear that the current techniques to measure dysfunction and inflammation in asthma patients (history, physical examination, and spirometry) are somewhat insensitive [49]. One of the key objectives of asthma clinicians now is to improve the ability to accurately monitor airway inflammation through noninvasive means. Although several inflammatory-marker-based methods have been developed, such factors are typically only elevated during the inflammatory/exacerbation process, providing limited periods where treatment intervention will be useful. Advances in our understanding of microbiota in asthma and the rapid diagnostic tools that are being developed in tandem now allow the examination of these microbial communities, with the likelihood of identifying potential microbial signatures on which rapid diagnostic assays can be based. To date, there is little work carried out examining if microbial signatures can predict the onset of asthma exacerbation [50]. However, studies that have mapped the bacterial community structure during periods of stability and exacerbation in the airways of CF patients suggest this approach may be feasible. For example, a recent study has identified several orders of anaerobic bacteria that are more predominant in CF patient airways undergoing exacerbation [50]. Although it is unlikely that the microorganisms colonizing the CF airway are the sole contributors to exacerbation, further longitudinal studies could establish if the changes identified preceded the onset of exacerbation as measured by clinical parameters. The identification and deployment of microbial signatures in asthma diagnosis is less advanced than other diseases but at this point longitudinally collected sample sets need to be investigated for their potential.
Immune manipulation using prebiotics, probiotics, and other microbiological supplements
There is increasing evidence to suggest that the intestinal microbiota, particularly during early infancy, plays a critical role in regulating immune responses [51]. Several studies have shown that modulations of the resident gut commensal microbiota can shape the global immune response of the host, reducing sensitization and inflammation [51]. Several recent studies have illustrated that microbiota manipulation via oral prebiotics, probiotics, and other supplementation promotes healthy gut microbiota and reduces overt airway immune responses in asthma patients 52, 53. Studies examining the impact of prebiotics on the immunogenicity of asthma are summarized in Table 2. Although data from these tests are not altogether convincing, they do give strength to the possibility of promoting a healthy microbiota in asthmatics, which may have potential health benefits. These approaches show some merit in treatment of other inflammation-based diseases such as obesity and juvenile diabetes but specific work on asthmatics is still required to surmount the scientific, health, and regulatory concerns.
Table 2.
Studies since 2011 showing the varied impact of prebiotics, probiotics, and other supplements on the immunogenicity of asthma or associated symptoms from a range of clinical trials, epidemiological studies, and murine models
| Approach | Objective | Delivery | Outcome | Refs |
|---|---|---|---|---|
| Prebiotics | ||||
| Bacteria oligosaccharides | Examination of infants in the first six months of life without clinical evidence of allergy, both with and without risk factors for allergic disease and food allergy. | Metadata assessing a collection of delivery methods. | Evidence suggested that the prebiotic supplement added to infant feeds potentially prevents eczema. It was unclear whether it may have an effect on asthma. | [64] |
| Various nutrients & Mediterranean diet | A total of 1428 subjects across 21 cohorts. | Metadata assessing a collection of delivery methods. | The evidence is supportive of vitamins A, D, and E; zinc; fruits and vegetables; and a Mediterranean diet contributing to the prevention of asthma. | [65] |
| Mediterranean diet | A general population of children assessed in studies up to May 2012. | Metadata assessing a collection of delivery methods | Mediterranean diet tended to be associated with lower occurrence of the asthma symptoms. | [66] |
| Antioxidant | 2442 8-year-old children from the Swedish birth cohort study BAMSE. | Oral | Magnesium intake seems to have a protective effect on childhood asthma. | [67] |
| Cord blood vitamin D | 257 children from the Copenhagen prospective studies on asthma in childhood (COPSAC2000) at-risk mother-child cohort. | Oral | No association between cord blood 25(OH)-vitamin D level and changes in lung function, sensitization, rhinitis or eczema were observed. | [68] |
| Cord blood vitamin D | 158 children at age 3 years. | Oral | Prenatal vitamin D supplementation in late pregnancy had a modest effect on cord blood vitamin D level. This study found that cord blood vitamin D level was not associated with decreased wheezing in offspring at age three years. | [69] |
| Fish oil | 420 infants considered high atopic risk. | Oral | Postnatal fish oil supplementation improved infant n-3 status but did not prevent childhood allergic disease including asthma. | [70] |
| Probiotics | ||||
| Single strain or mixture of bacterial strains – mainlyLactobacillus rhamnosusGG (LGG) | 4031 subjects in 20 cohorts | Metadata assessing a collection of delivery methods | Early probiotic administration reduces the risk of atopic sensitization, but it does not reduce the risk of developing asthma. | [71] |
| Lactobacillus rhamnosusGG (LGG) | Murine model of allergic asthma. | Oral | LGG had an anti-inflammatory effect on OVA-induced airway inflammation. | [72] |
| Single strain or mixture of bacterial strains – mainlyLactobacillus rhamnosusGG (LGG) | 4866 children. | Metadata assessing a collection of delivery methods | No evidence to support a protective association between perinatal use of probiotics and doctor diagnosed asthma or childhood wheeze. | [73] |
| Strains ofLactobacillusorBifidobacteriumwere mainly used | 995 participants involving all age groups. | Metadata assessing a collection of delivery methods | Due to the high degree of heterogeneity in the data assess few solid outcomes can be ascertained. | [74] |
| Lactobacillus reuteriATCC 55730 | 232 families with allergic disease, of whom 184 completed a 7-yr follow-up. | Metadata assessing a collection of delivery methods | The effect of L. reuteri on sensitization and IgE-associated eczema in infancy did not lead to a lower prevalence of respiratory allergic disease in school age. Thus, the effect of L. reuteri on the immune system appeared to be transient. | [75] |
| Lactobacillus paracaseisspparacaseiF19 | 171 children that completed the intervention, 121 were assessed at age 8–9 | Oral | No long-term effect of LF19 on any diagnosed allergic disease, airway inflammation or IgE sensitization. | [76] |
| Microbial products | ||||
| Lipopeptide | Murine model of asthma. | Intraperitoneal | Protection against sensitization and airway inflammation was observed. | [77] |
| Heat-killedBifidobacterium breveC50 andStreptococcus thermophilus065 (HKBBST) | Infants at high risk of atopy. | Oral | Decreased the incidence of potentially allergic AE (PAAE)s. | 75, 78 |
Tailored antimicrobial or vaccine therapy of specific microbiota community members
As discussed earlier, airway infections by specific microbiota or bacteria may induce, augment, or potentially even modify the predominant type of airway inflammation seen in asthma. It has been proposed that more focused antibiotic or vaccine therapies based on comprehensive characterization of the microbiota present in the airway could inform the use of antimicrobials that target specific microbiota in asthmatics 49, 54. Conversely, microbiome-targeted treatment approaches could be interpreted from the perspective of how to promote a more functionally balanced airway microbiome, such that pathogenic microbiota are not able to exert dominant effects. Thus, an understanding of microbiota that are negatively associated with features of asthma could be useful toward developing approaches to promote these microbiota, or specific functions they express that counteract detrimental inflammatory processes. These approaches have been studied and highlighted more in the gut microbiome literature but suggest that the approach may be feasible in the treatment of asthma [55].
Although still in their infancy, these potential new approaches for the treatment of asthma, used in combination with other therapeutic measures including vaccines and steroid treatment, could improve, alleviate, and treat asthma symptoms. Many obstacles exist to the use of these approaches but key among these is the complex heterogeneity of asthma as a disease and the variation in microbiome composition observed across this patient population. As discussed above, this heterogeneity of asthma reflects different underlying clinical characteristics, biology, and genetics, which can all make different contributions to shaping microbial diversity found in the asthmatic airway. Therefore, it is becoming paramount that the phenotypes or subtypes of asthma are more clearly defined to determine specifically when the microbiota plays an important role in asthma. As touched on above, a selection of schemes have been proposed to classify asthma phenotypes. It will become important that a stringent and more feasible classification approach is adopted in order to enable more focused development of microbiota driven treatment in asthma.
Concluding remarks
It is now clear that high-resolution microbiota sequencing efforts and translational models of lung disease are providing new insight into the roles of microorganisms in asthma pathogenesis and exacerbation. Bacteria clearly have roles to play in the disease progress and clinical outcome of asthma but also appear, in certain cases, to play protective functions. There is also vast experimental and clinical evidence that asthma can be viewed as a chronic inflammatory disease that can be initiated and modulated by the airway microbiota. These interactions with the host immune system can involve commensal bacteria as well as pathogens that most likely mediate a range of molecular mechanisms. It may be that increases in asthma prevalence worldwide are due in part to a changing relation with microbes in the lower respiratory tract. More research is needed to provide better mechanistic insights into interactions between the airway microbiota and host immune response, as well as address several outstanding questions in the field (Box 3). Understanding the molecular basis and biological effect the airway microbiota has on the host immune response may lead to the discovery of microbial or immunological targets that are amenable to manipulation for the development of new treatments.
Box 3. Outstanding questions.
-
•
To what extent does an altered airway microbiota effect the development or worsening of asthma symptoms?
-
•
Do standard treatments, such as steroids, antibiotics or inhaled medication, contribute to the shaping of the asthma airway microbiota and does this have implications for disease symptoms?
-
•
Many microorganisms (virus, bacteria, and fungi) can coexist in the respiratory tract of an asthmatic patient. If interplay occurs between these organisms, what impact does it have on shaping the inflammatory response and the response to treatment?
-
•
How best can we classify phenotypes of asthma to determine precisely what role the microbiota plays in the development of disease and patient prognosis?
-
•
Many challenges apply to modeling asthma disease. However, with new translational models of asthmatic lung disease, can models of microbiota interactions with the host be developed?
-
•
Are changes in microbiota composition predictive of asthma exacerbation or respiratory tract infection? Will this provide a platform for preventative treatment using targeted interference strategies and antimicrobial therapy?
-
•
Can biotherapeutic strategies such as the use of prebiotics or probiotics be developed to counteract microbiota dysbiosis in asthmatic patients and could this have a knock-on effect on care?
Acknowledgments
We thank Susan Lynch, Yvonne McCarthy and the Ryan Laboratory for their helpful comments and critical reading of the manuscript. The work of the authors has been supported in part by grants awarded by the Wellcome Trust (WT100204AIA senior fellowship grant to R.P.R.) and the European Union's Seventh Framework Programme (Grant No. 603038 to R.P.R.). We apologize to the colleagues whose work could not be cited due to space constraints.
References
- 1.Kuczynski J. Experimental and analytical tools for studying the human microbiome. Nat. Rev. Genet. 2012;13:47–58. doi: 10.1038/nrg3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinross J.M. Gut microbiome-host interactions in health and disease. Genome Med. 2011;3:1–11. doi: 10.1186/gm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravel J. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U.S.A. 2011;108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopf M. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 2015;16:36–44. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y.J., Boushey H.A. The microbiome and asthma. Ann. Am. Thorac. Soc. 2014;1:S48–S51. doi: 10.1513/AnnalsATS.201306-187MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dougherty, D. et al. (2007) National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Bethesda (MD): National Heart, Lung, and Blood Institute (US)
- 7.Clark K.L. The asthma accession standard: a survival analysis of military recruits, 1995 to 1997. Mil. Med. 2000;165:852–854. [PubMed] [Google Scholar]
- 8.Gern J.E. Rhinovirus and the initiation of asthma. Curr. Opin. Allergy Clin. Immunol. 2009;9:73–78. doi: 10.1097/ACI.0b013e32831f8f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papadopoulos N.G., Kalobatsou A. Respiratory viruses in childhood asthma. Curr. Opin. Allergy Clin. Immunol. 2007;7:91–95. doi: 10.1097/ACI.0b013e328013d501. [DOI] [PubMed] [Google Scholar]
- 10.Singh A.M., Busse W.W. Asthma exacerbations 2: aetiology. Thorax. 2006;61:809–816. doi: 10.1136/thx.2005.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y.J. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J. Allergy Clin. Immunol. 2011;127:372–689. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marri P.R. Asthma-associated differences in microbial composition of induced sputum. J. Allergy Clin. Immunol. 2013;131:346–351. doi: 10.1016/j.jaci.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green B.J. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS ONE. 2014;9:e100645. doi: 10.1371/journal.pone.0100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guilbert T.W., Denlinger L.C. Role of infection in the development and exacerbation of asthma. Expert Rev. Resp. Med. 2010;4:71–83. doi: 10.1586/ers.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choroszy-Krol I. Infections caused by Chlamydophila pneumoniae. Adv. Clin. Exp. Med. 2014;23:123–126. doi: 10.17219/acem/37035. [DOI] [PubMed] [Google Scholar]
- 16.Redinbo M.R. The microbiota, chemical symbiosis, and human disease. J. Mol. Biol. 2014;426:3877–3891. doi: 10.1016/j.jmb.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korppi M. Bacterial infections and pediatric asthma. Immunol. Allergy Clin. North Am. 2010;30:565–571. doi: 10.1016/j.iac.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y.J. The respiratory microbiome and innate immunity in asthma. Curr. Opin. Pulm. Med. 2015;21:27–32. doi: 10.1097/MCP.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilty M. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han M.K. Significance of the microbiome in obstructive lung disease. Thorax. 2012;67:456–463. doi: 10.1136/thoraxjnl-2011-201183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goleva E. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am. J. Respir. Crit. Care Med. 2013;188:1193–1201. doi: 10.1164/rccm.201304-0775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong E.H.C. The role of macrolides in asthma: current evidence and future directions. Lancet Respir. Med. 2014;2:657–670. doi: 10.1016/S2213-2600(14)70107-9. [DOI] [PubMed] [Google Scholar]
- 23.Simpson J.L. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 24.Berry M. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–1049. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel K.K. Infectious Chlamydia pneumoniae is associated with elevated interleukin-8 and airway neutrophilia in children with refractory asthma. Pediatr. Infect. Dis. J. 2010;29:1093–1098. doi: 10.1097/inf.0b013e3181eaebdc. [DOI] [PubMed] [Google Scholar]
- 26.Peters M.C. Measures of gene expression in sputum cells can identify T(H)2-high and T(H)2-low subtypes of asthma. J. Allergy Clin. Immunol. 2014;133:388–394. doi: 10.1016/j.jaci.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodruff P.G. Genome-wide profiling identifies elpithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc. Natl. Acad. Sci. U.S.A. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palm N.W. Allergic host defenses. Nature. 2012;484:38–41. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansel T.T. Microbes and mucosal immune responses in asthma. Lancet. 2013;381:861–873. doi: 10.1016/S0140-6736(12)62202-8. [DOI] [PubMed] [Google Scholar]
- 30.Philip N.H., Artis D. New friendships and old feuds: relationships between innate lymphoid cells and microbial communities. Immunol. Cell Biol. 2013;91:225–231. doi: 10.1038/icb.2013.2. [DOI] [PubMed] [Google Scholar]
- 31.Morris A. Airway obstruction is increased in pneumocystis-colonized human immunodeficiency virus-infected outpatients. J. Clin. Microbiol. 2009;47:3773–3776. doi: 10.1128/JCM.01712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neill D.R. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1369. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price A.E. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. U.S.A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenbarth S.C. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gollwitzer E.S. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med. 2014;20:642–647. doi: 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
- 36.Cohen-Cymberknoh M. Airway inflammation in cystic fibrosis: molecular mechanisms and clinical implications. Thorax. 2013;68:1157–1162. doi: 10.1136/thoraxjnl-2013-203204. [DOI] [PubMed] [Google Scholar]
- 37.Hoenderdos K., Condliffe A. The neutrophil in chronic obstructive pulmonary disease too little, too late or too Much, too soon? Am. J. Respir. Cell Mol. Biol. 2013;48:531–539. doi: 10.1165/rcmb.2012-0492TR. [DOI] [PubMed] [Google Scholar]
- 38.Ray A. Emerging molecular phenotypes of asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;308:L130–L140. doi: 10.1152/ajplung.00070.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baines K.J. Transcriptional phenotypes of asthma defined by gene expression profiling of induced sputum samples. J. Allergy Clin. Immunol. 2011;127:153–160. doi: 10.1016/j.jaci.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Bloes D.A. Enemy attraction: bacterial agonists for leukocyte chemotaxis receptors. Nat. Rev. Microbiol. 2015;13:95–104. doi: 10.1038/nrmicro3390. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Q. Bacteria in sputum of stable severe asthma and increased airway wall thickness. Respir. Res. 2012;13:35. doi: 10.1186/1465-9921-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doe C. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest. 2010;138:1140–1147. doi: 10.1378/chest.09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aujla S.J. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newcomb D.C., Peebles R.S. Th17-mediated inflammation in asthma. Curr. Opin. Immunol. 2013;25:755–760. doi: 10.1016/j.coi.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas C.J., Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol. 2013;34:317–328. doi: 10.1016/j.it.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Lotvall J. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J. Allergy Clin. Immunol. 2011;127:355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 47.Dulek D.E., Peebles R.S. Bacteria and asthma: more there than we thought. Expert Rev. Respir. Med. 2011;5:329–332. doi: 10.1586/ers.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Y.J., Lynch S.V. The emerging relationship between the airway microbiota and chronic respiratory disease: clinical implications. Expert Rev. Respir. Med. 2011;5:809–821. doi: 10.1586/ers.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers G.B. Respiratory microbiota: addressing clinical questions, informing clinical practice. Thorax. 2015;70:74–81. doi: 10.1136/thoraxjnl-2014-205826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Short F.L. Polybacterial human disease: the ills of social networking. Trends Microbiol. 2014;22:508–516. doi: 10.1016/j.tim.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagalingam N.A. Probiotic strategies for treatment of respiratory diseases. Trends Microbiol. 2013;21:485–492. doi: 10.1016/j.tim.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds C. Natural killer T cells in bronchial biopsies from human allergen challenge model of allergic asthma. J. Allergy Clin. Immunol. 2009;124:860–862. doi: 10.1016/j.jaci.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 53.Sakamoto Y. Chronic intestinal nematode infection induces Stat6-independent interleukin-5 production and causes eosinophilic inflammatory responses in mice. Immunology. 2004;112:615–623. doi: 10.1046/j.1365-2567.2004.01909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogers G.B. Enhancing the utility of existing antibiotics by targeting bacterial behaviour? Br. J. Pharmacol. 2012;165:845–857. doi: 10.1111/j.1476-5381.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arrieta M.C. The intestinal microbiome in early life: health and disease. Front. Immunol. 2014;5:427. doi: 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueller F-MC, Seidler M. Characteristics of pathogenic fungi and antifungal therapy in cystic fibrosis. Expert. Rev. Anti. Infect. Ther. 2010;8:957–964. doi: 10.1586/eri.10.72. [DOI] [PubMed] [Google Scholar]
- 57.Norris K.A., Morris A. Pneumocystis infection and the pathogenesis of chronic obstructive pulmonary disease. Immunol. Rev. 2011;50:175–180. doi: 10.1007/s12026-011-8218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pasqualotto A.C. The effects of antifungal therapy on severe asthma with fungal sensitization and allergic bronchopulmonary aspergillosis. Respirology. 2009;14:1121–1127. doi: 10.1111/j.1440-1843.2009.01640.x. [DOI] [PubMed] [Google Scholar]
- 59.Ghannoum M.A. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6:e1000713. doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reihill J.A. Effect of Aspergillus fumigatus and Candida albicans on pro-inflammatory response in cystic fibrosis epithelium. J. Cyst. Fibros. 2011;10:401–406. doi: 10.1016/j.jcf.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 61.Parulekar A.D. Antifungals in severe asthma. Cur. Opin. Pulm. Med. 2015;21:48–54. doi: 10.1097/MCP.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 62.Horre R. Detection of hyphomycetes in the upper respiratory tract of patients with cystic fibrosis. Mycoses. 2011;54:514–522. doi: 10.1111/j.1439-0507.2010.01897.x. [DOI] [PubMed] [Google Scholar]
- 63.Beck J.M. The microbiome of the lung. Transl. Res. 2012;160:258–266. doi: 10.1016/j.trsl.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Osborn D.A., Sinn J.K.H. Prebiotics in infants for prevention of allergy. Cochrane Database Syst. Rev. 2013;3:CD006474. doi: 10.1002/14651858.CD006474.pub3. [DOI] [PubMed] [Google Scholar]
- 65.Nurmatov U. Nutrients and foods for the primary prevention of asthma and allergy: Systematic review and meta-analysis. J. Allergy Clin. Immunol. 2011;127:724–733. doi: 10.1016/j.jaci.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Marcos L. Influence of mediterranean diet on asthma in children: a systematic review and meta-analysis. Pediatr. Allergy Immunol. 2013;24:330–338. doi: 10.1111/pai.12071. [DOI] [PubMed] [Google Scholar]
- 67.Rosenlund H. Antioxidant intake and allergic disease in children. Clin. Exp. Allergy. 2012;42:1491–1500. doi: 10.1111/j.1365-2222.2012.04053.x. [DOI] [PubMed] [Google Scholar]
- 68.Li C.B. Cord blood 25(OH)-vitamin D deficiency and childhood asthma, allergy and eczema: the COPSAC2000 birth cohort study. PLoS ONE. 2014;9:e99856. doi: 10.1371/journal.pone.0099856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldring S.T. Prenatal vitamin D supplementation and child respiratory health: a randomised controlled trial. PLoS ONE. 2013;8:e66627. doi: 10.1371/journal.pone.0066627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.D’Vaz N. Postnatal fish oil supplementation in high-risk infants to prevent allergy: randomized controlled trial. Pediatrics. 2012;130:674–682. doi: 10.1542/peds.2011-3104. [DOI] [PubMed] [Google Scholar]
- 71.Elazab N. Probiotic administration in early life, atopy, and asthma: a meta-analysis of clinical trials. Pediatrics. 2013;132:e666. doi: 10.1542/peds.2013-0246. [DOI] [PubMed] [Google Scholar]
- 72.Wuf C-T. Effects of immunomodulatory supplementation with Lactobacillus rhamnosus on airway inflammation in a mouse asthma model. J. Allergy Clin. Immunol. 2014;1:1–11. doi: 10.1016/j.jmii.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Azad M.B. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. BMJ. 2013;347:f6471. doi: 10.1136/bmj.f6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Das R.R. Probiotics as additives on therapy in allergic airway diseases: a systematic review of benefits and risks. Biomed Res. Int. 2013;2013 doi: 10.1155/2013/231979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abrahamsson T.R. No effect of probiotics on respiratory allergies: a seven-year follow-up of a randomized controlled trial in infancy. Pediatr. Allergy Immunol. 2013;24:556–561. doi: 10.1111/pai.12104. [DOI] [PubMed] [Google Scholar]
- 76.West C.E. Probiotics in primary prevention of allergic disease – follow-up at 8-9 years of age. Allergy. 2013;68:1015–1020. doi: 10.1111/all.12191. [DOI] [PubMed] [Google Scholar]
- 77.Stiehm M. A novel synthetic lipopeptide is allergy-protective by the induction of LPS-tolerance. Clin. Exp. Allergy. 2013;43:785–797. doi: 10.1111/cea.12116. [DOI] [PubMed] [Google Scholar]
- 78.Morisset M. A non-hydrolyzed, fermented milk formula reduces digestive and respiratory events in infants at high risk of allergy. Eur. J. Clin. Nutr. 2011;65:175–183. doi: 10.1038/ejcn.2010.250. [DOI] [PubMed] [Google Scholar]