Abstract
Background
Adverse outcome pathways (AOPs) link adverse effects in individuals or populations to a molecular initiating event (MIE) that can be quantified using in vitro methods. Practical application of AOPs in chemical-specific risk assessment requires incorporation of knowledge on exposure, along with absorption, distribution, metabolism, and excretion (ADME) properties of chemicals.
Objectives
We developed a conceptual workflow to examine exposure and ADME properties in relation to an MIE. The utility of this workflow was evaluated using a previously established AOP, acetylcholinesterase (AChE) inhibition.
Methods
Thirty chemicals found to inhibit human AChE in the ToxCast™ assay were examined with respect to their exposure, absorption potential, and ability to cross the blood–brain barrier (BBB). Structures of active chemicals were compared against structures of 1,029 inactive chemicals to detect possible parent compounds that might have active metabolites.
Results
Application of the workflow screened 10 “low-priority” chemicals of 30 active chemicals. Fifty-two of the 1,029 inactive chemicals exhibited a similarity threshold of ≥ 75% with their nearest active neighbors. Of these 52 compounds, 30 were excluded due to poor absorption or distribution. The remaining 22 compounds may inhibit AChE in vivo either directly or as a result of metabolic activation.
Conclusions
The incorporation of exposure and ADME properties into the conceptual workflow eliminated 10 “low-priority” chemicals that may otherwise have undergone additional, resource-consuming analyses. Our workflow also increased confidence in interpretation of in vitro results by identifying possible “false negatives.”
Citation
Phillips MB, Leonard JA, Grulke CM, Chang DT, Edwards SW, Brooks R, Goldsmith MR, El-Masri H, Tan YM. 2016. A workflow to investigate exposure and pharmacokinetic influences on high-throughput in vitro chemical screening based on adverse outcome pathways. Environ Health Perspect 124:53–60; http://dx.doi.org/10.1289/ehp.1409450
Introduction
The adverse outcome pathway (AOP) is a conceptual framework originally developed with the goal of utilizing pathways-based data to support ecotoxicology research and risk assessment (Ankley et al. 2010). Researchers in a variety of disciplines have since used AOPs to describe impacts of a chemical on molecular targets and biochemical pathways in a sequential manner (Lapenna et al. 2012; Vinken et al. 2013; Watanabe et al. 2011). The AOP framework begins with a molecular initiating event (MIE), which is defined as the interaction between a xenobiotic and a specific biomolecule (Ankley et al. 2010), such as inhibition of an enzyme due to competitive binding of a chemical in the active site (Russom et al. 2014). The MIE is followed by a progression of a defined series of key events (KEs) that are measurable through in vitro or in vivo assays, necessary for the development of the toxicological outcome, and connected by key event relationships (KERs). These KEs and KERs then lead to an apical outcome that is relevant to regulatory purposes (Villeneuve et al. 2014). Such outcomes may be survival, development, and reproduction at the population level in ecotoxicology; or disease and organ dysfunction in human individuals.
The power of the AOP framework arises from the knowledge that multiple chemicals can act through common biochemical pathways. Because there are tens of thousands of chemicals in commerce (Egeghy et al. 2012; U.S. EPA 2014b), starting from these common pathways provides a more rapid and cost-effective alternative for hazard screening compared with chemical-by-chemical approaches. Rather than relying on traditional toxicity tests conducted for individual chemicals (e.g., costly assays administered one at a time in animals), the AOP framework can support the use of high-throughput in vitro assays to quickly measure the activity of numerous chemicals with respect to a given molecular target. The AOP itself is chemical independent to allow for a general interpretation of results based on common modes of action and biological pathways. Practical application of AOPs in chemical-based risk assessment, however, will require extrapolation of an in vitro concentration expected to trigger an MIE to an in vivo biologically effective target tissue dose, which can then be used to estimate a regulatory-relevant external dose (i.e., using reverse toxicokinetics). This extrapolation cannot be made without considering exposure, as well as the absorption, distribution, metabolism, and excretion (ADME) properties of a chemical (Groh et al. 2015). The most active chemical in an in vitro assay may not induce in vivo toxicity if concentrations necessary to trigger an MIE are unlikely to be attained due to limited exposure or ADME-mediated processes.
To augment the application of an AOP framework in chemical risk assessment, we developed a workflow to incorporate exposure and ADME considerations for refining outcomes from in vitro assays designed based on an MIE. We evaluated the utility of this workflow using in vitro assay results from the ToxCast™ data set for a previously established AOP, acetylcholinesterase (AChE) inhibition (Russom et al. 2014). First, the identities of the active chemicals in the human AChE inhibition assay were obtained from the ToxCast™ data set (U.S. EPA 2012a). Next, the likelihood of these active chemicals to trigger an MIE in the brain was determined by sequentially considering their exposure potential, absorption potential, and ability to cross the blood–brain barrier (BBB) to bind to brain AChE. In addition, structural similarities of active chemicals were compared against structures of inactive chemicals using molecular fingerprint models to detect possible nonactive parents that might become biologically active after undergoing metabolism. This case study demonstrates the ongoing need for a more holistic approach that encompasses various considerations for improving toxicity predictions based on in vitro measurements and for expanding the AOP framework to improve its utility in chemical-specific risk assessment.
Methods
Conceptual structure of the exposure–ADME workflow. The exposure–ADME workflow incorporates exposure and ADME considerations for linking chemical exposure with AOP activation through the MIE. The main utility of this workflow is to refine in vitro results, which can then be used to predict in vivo MIEs that would trigger an AOP. This workflow begins with the selection of an AOP of interest, such as one listed in the AOP Wiki (https://aopkb.org/aopwiki/index.php/AOP_List). Next, active chemicals identified in a specific in vitro assay are examined as parent compounds (Figure 1) or metabolites (Figure 2). Given that these are “known” metabolites, it is assumed that a) they would be generated in the human body after exposure to their parent compounds, and b) the identity of their parent compounds is known. If the metabolite tests positive in vitro, its parent’s exposure and absorption potentials are examined, along with its own capability of reaching the molecular target (Figure 2). If the active metabolite can also be found in the environment, its own exposure potential and ADME-related properties are also examined (Figure 1).
Figure 1.
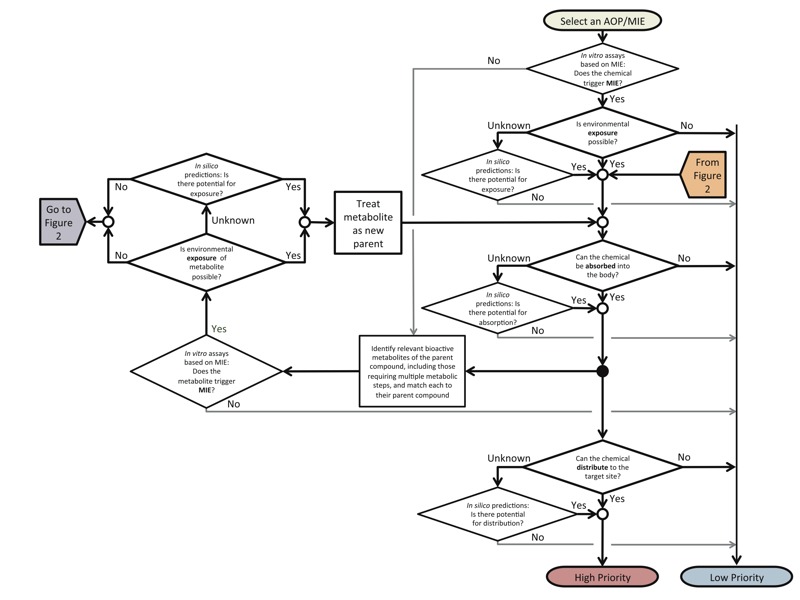
Workflow for including exposure and ADME considerations into the AOP framework. The chemical of interest is a parent compound. Exposure, absorption, distribution, and metabolism are considered for the parent compound, and distribution of a known metabolite of an identified parent compound (described in Figure 2) is considered if the parent exhibits exposure and absorption potential. Each step is evaluated based on available data. When insignificant, the chemical is classified as “low priority.” If any step results in an unknown effect, further research is needed (i.e., high-throughput follow-up studies). “High-priority” chemicals should be further ranked according to relationships among rates of absorption or distribution, activating or detoxifying metabolic processes, and excretion from a biological system. Open circles represent converging steps in the workflow, and solid black circles represent diverging steps.
Figure 2.
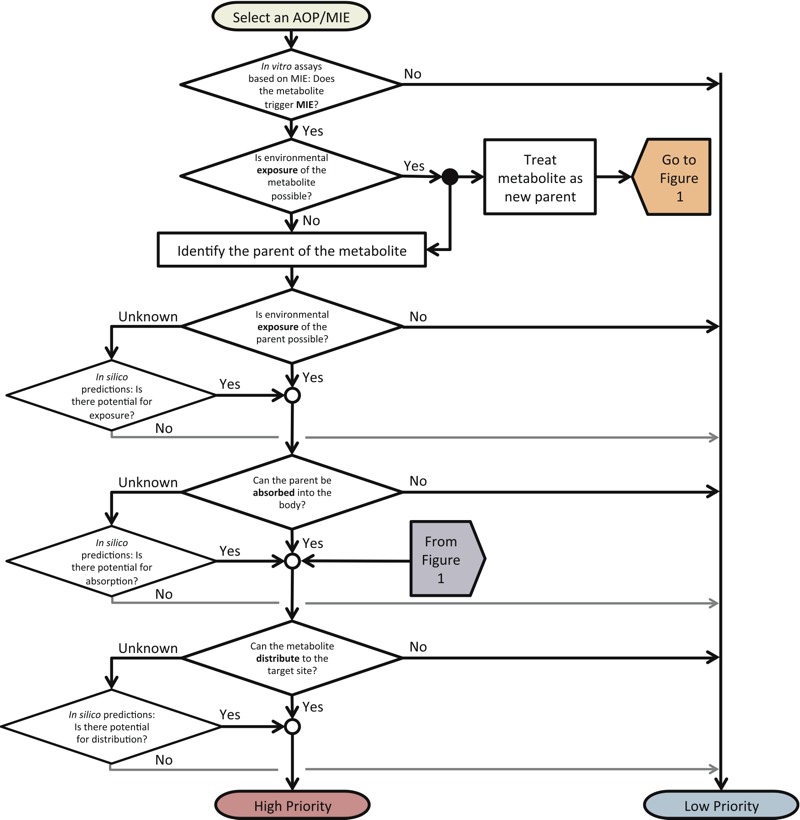
Workflow for including exposure and ADME considerations into the AOP framework. Exposure of the known metabolite is examined, and if exposure is possible the metabolite is then treated similar to a parent compound (described in Figure 1). If exposure of the metabolite is not possible, then its distribution is considered only if its identified parent exhibits exposure and absorption potential. Each step is evaluated based on available data. When insignificant, the chemical is classified as “low priority.” If any step results in an unknown effect, further research is needed (i.e., high-throughput follow-up studies). “High priority” chemicals should be further ranked according to relationships among rates of absorption or distribution, activating or detoxifying metabolic processes, and excretion from a biological system. Open circles represent converging steps in the workflow, and solid black circles represent diverging steps.
Exposure. Each active chemical can be placed into one of three categories based on its exposure potential: widespread exposure, limited exposure (e.g., occupational exposures or patient exposures to specific drugs), or low/no potential of exposure (e.g., drugs that have failed clinical trials). Those chemicals with low/no exposure potential are considered “low priority.” The remaining chemicals are advanced to the next step of the workflow.
Absorption. Next, the physicochemical properties of the chemicals (e.g., lipophilicity or water solubility) are measured to assess absorption potential and bioavailability as related to primary routes of exposure. Many of these properties can be estimated using a combination of generalized molecular-based methods, such as geometric optimization and pharmacophore modeling (Goldsmith et al. 2012). A number of public or commercial platforms can also be used to estimate such properties through specific ADME-related molecular descriptors based on reference two-dimensional (2D) and three-dimensional (3D) chemical structures. Some of these tools and resources include ChemSpider (http://www.chemspider.com), QikProp (http://www.schrodinger.com/QikProp/), RDKit (http://www.rdkit.org), Dragon 6 (Mauri et al. 2006), and Chemistry Development Kit (Steinbeck et al. 2003).
Distribution. The likelihood that a chemical could be sequestered in certain tissues (e.g., fat or bone), bind to plasma proteins, and so on is assessed similarly using physicochemical properties. Chemicals that may be systematically distributed might require evaluation to see if they can access the molecular target. For example, if the target involves the central nervous system, a chemical must cross the BBB before binding. Chemicals not easily absorbed or distributed are considered “low priority.” The remaining chemicals are classified as “high priority,” as are those chemicals for which the data are insufficient to confidently assign “low-priority” status. When exposure, absorption, or distribution potential is uncertain, in silico approaches may be applied to generate estimates for guiding the prioritization process.
Metabolism. When a parent compound tests negative in an in vitro assay, it is still possible that its metabolite could reach a molecular target after the parent is absorbed into the body. Thus, our workflow suggests that known metabolites of inactive parents be subjected to in vitro testing (Figure 1). Predicting the likelihood of a compound being metabolized, as well as predicting the structures of its major metabolites, is challenging (Bertz and Granneman 1997; Shlomi et al. 2008). In the best-case scenario, in vivo testing is used to confirm any predictions, and confirmed metabolites can then be subjected to in vitro toxicity testing (Figure 2). Unfortunately, this scenario requires a substantial investment of time and resources. Alternatively, computational programs [e.g., Meteor Nexus (Lhasa Limited)] or in vitro metabolism assays may be used to predict metabolites based on enzymatic activity. Similarity analyses may then be used to identify which predicted metabolites have structures similar to known active chemicals, giving a preliminary indication that they might interact with the molecular target in a manner sufficient to trigger an MIE.
The previous steps of the workflow primarily address qualitative aspects of exposure and ADME to identify “high-priority” chemicals for additional quantitative analyses. An example of quantitative analysis is generating surrogates for exposure and ADME behaviors based on chemical properties (e.g., predicted biological half-life may be used to extrapolate clearance rate, and a faster clearance rate could be interpreted as lower availability of the chemical to the molecular target). The “high-priority” chemicals, identified from the qualitative evaluation, can then be ranked based on comparisons among measured or predicted intake doses, as well as relative rates of absorption/distribution and metabolism/excretion.
ToxCast™ background and AChE assay results. ToxCast™ is a multiyear effort led by the U.S. Environmental Protection Agency (EPA) to test thousands of chemicals in hundreds of assays, including enzyme inhibition assays (U.S. EPA 2014a). To date, > 2,000 chemicals have been tested in > 700 in vitro assays covering approximately 300 signaling pathways (U.S. EPA 2014a). Chemicals considered within ToxCast™ include, but are not limited to, additives, pesticides and antimicrobial agents, plasticizers, and pharmaceuticals that are in various stages of clinical testing or have been introduced into the commercial market. A full inventory of the chemicals used in the ToxCast™ program, of which the 1,059 considered in this study is a subset, is available online (http://www.epa.gov/ncct/dsstox/sdf_toxcst.html), with more chemicals to be added in the future. Detailed information regarding analytical quality control of procured ToxCast™ chemicals is provided in Supplemental Material, “Chemical quality control.”
The Novascreen acetylcholinesterase (AChE) analysis in ToxCast™ consists of an in vitro cell-free biochemical assay that detects the inhibition of human-derived AChE enzyme, as determined colorimetrically by enzyme reporter activity using the substrate acetylcholine and a positive control of physostigmine (U.S. EPA 2014a). Additional details of the assay procedures can be found in Supplemental Material, “Chemical assays.” Thirty of the 1,059 chemicals tested in the AChE inhibition assay were found to be active (3%).
Prioritization of active chemicals in AChE inhibition assay. The inhibition of AChE by parent compounds and known metabolites was considered to comprise the MIE (the first step in Figures 1 and 2). Literature collected from PubMed, PubChem, Web of Science, and technical documents was used to categorize exposure potential of the 30 active chemicals (Table 1). Data collected included primary route of exposure, chemical use category and history, prevalence of usage across the general population, and documented adverse health effects. Chemicals with low/no exposure potential were designated “low priority.”
Table 1.
Inhibition activity (in decreasing order), exposure probability, and ADME properties of thirty compounds extracted from ToxCast™ data set identified as acetylcholinesterase inhibitors.
| Compound | AC50 (μM) | Exposurea | Absorptionb | Distributionc | Priority | Metabolismd | Source |
|---|---|---|---|---|---|---|---|
| Chlorpyrifos oxon | 0.149 | 1 | Yes | Yes | High | + | Eaton et al. 2008; Smegal 2000 |
| PharmaGSID_47259 | 0.287 | 4 | NA | NA | Low | – | U.S. EPA 2010 |
| Carbofuran | 0.416 | 3 | Yes | Yes | High | ± | Hussain et al. 1990; U.S. EPA 2008 |
| Anthralin | 0.512 | 2 | Yes | No | Low | – | McGill et al. 2005 |
| Naled | 1.01 | 1 | Yes | Yes | High | – | Duprey et al. 2008; U.S. EPA 2006 |
| Carbosulfan | 1.21 | 1 | Limited | Yes | High | ± | Abass et al. 2010 |
| Raloxifene hydrochloride | 1.85 | 2 | Limited | No | Low | – | Kosaka et al. 2011 |
| 1-Benzylquinolinium chloride | 2.48 | 2 | Yes | Yes | High | U | U.S. EPA 2012b |
| Besonprodil | 3.49 | 3 | Yes | Yes | High | – | Ouattara et al. 2009 |
| Bendiocarb | 4.09 | 3 | Yes | Yes | High | – | Berman et al. 2011, 2012 |
| SB236057A | 4.63 | 4 | NA | NA | Low | – | Roberts et al. 2001 |
| GW473178E | 4.79 | 4 | NA | NA | Low | – | U.S. EPA 2010 |
| SSR241586 | 4.86 | 3 | Limited | Yes | High | – | Métro et al. 2011 |
| SSR69071 | 5.05 | 4 | NA | NA | Low | – | Kapui et al. 2003 |
| Mevinphos | 5.11 | 3 | Yes | Yes | High | ± | Cochran et al. 1996; U.S. EPA 1994 |
| Azamethiphos | 6.6 | 2 | Yes | Yes | High | ± | EMEA 1999 |
| Oxamyl | 7.4 | 2 | Yes | Yes | High | – | EXTOXNET 1993; Schilmann et al. 2010 |
| Gentian violet | 7.65 | 1 | Yes | Yes | High | + | TOXNET 2013 |
| Toluene-2,4-diisocyanate | 8.78 | 2 | Yes | Yes | High | ± | U.S. EPA 2013 |
| Didecyldimethylammonium chloride | 12.1 | 1 | Limited | Yes | High | – | Dejobert et al. 1997; Houtappel et al. 2008 |
| Propoxur | 12.7 | 1 | Yes | Yes | High | ± | Ostrea et al. 2014 |
| Methomyl | 13.9 | 3 | Yes | Yes | High | – | EXTOXNET 1996; Van Scoy et al. 2013 |
| Pentamidine isethionate | 16.8 | 2 | Limited | No | Low | NA | Beach et al. 1999; Montgomery et al. 1990 |
| bis(2-Ethylhexyl) decandioate | 17 | 4 | NA | NA | Low | = | NIOSH 1983 |
| SR125047 | 17.6 | 4 | NA | NA | Low | – | Kohlhaas et al. 2006 |
| PharmaGSID_48172 | 18.3 | 4 | NA | NA | Low | – | U.S. EPA 2010 |
| Dodecylbenzenesulfonic acid | 19.3 | 1 | Limited | Yes | High | ± | TOXNET 2002 |
| SSR150106 | 20.9 | 3 | Yes | Yes | High | + | R & D Focus Drug News 2007 |
| Mercuric chloride | 23.1 | 2 | Yes | Yes | High | + | Bernhoft 2012; Boscolo et al. 2009 |
| Bronopol | 23.3 | 1 | Yes | Yes | High | ± | Cui et al. 2011; Travassos et al. 2011 |
| Abbreviations: AC50, concentration of chemical necessary to reduce maximum activity of the AChE enzyme by 50%; ADME, absorption, distribution, metabolism, and excretion; BBB, blood–brain barrier; NA, not applicable. aExposure conditions are as follows: 1, widespread exposure to public; 2, occupational-only or special cases of exposure; 3, unknown exposure; 4, low likelihood of exposure (no further analysis). bAbsorption considers whether chemicals are free of violations (“Yes”) of the “Rule of 5” code (Lipinski et al. 1997); properties that violate these rules include large size and molecular weight, high number of rotatable bonds, and an excessive number of hydrogen bond donors or hydrogen bond acceptors. cDistribution considers whether chemicals can cross (“Yes”) the BBB. dMetabolism is considered to be transformation to an active metabolite (+), detoxification (–), possibility of activation or detoxification (±), metabolite with same toxicity as parent (=), parent excreted with no metabolism (NA), or unknown (U). | |||||||
When comprehensive review resulted in greater confidence that a chemical (or the parent of a tested metabolite) would exhibit widespread or limited exposure, its potential for absorption into the body was queried using the ADMET Predictor™ (Simulations Plus Inc.). These chemicals’ 2D simplified molecular-input line-entry system (SMILES) structures were entered into the ADMET Predictor™ to estimate their physicochemical properties, such as water solubility, octanol-water partition coefficient (log Kow), plasma protein binding, pKa, and skin permeability. Chemicals with negligible absorption were designated as “low priority.”
Next, BBB permeability (i.e., distribution to the molecular target) was queried using the ADMET Predictor™ for the remainder of the chemicals (those not considered “low priority”), as well as inactive chemicals structurally similar to the original 30 active chemicals. Molecular structures of these chemicals were washed of extraneous salts, had protonation states rebalanced, had explicit hydrogen atoms augmented, and had their energy states minimized through conversion into a 3D conformation using Molecular Operating Environment (MOE) software (Chemical Computing Group) before being entered as a predictive data set in the ADMET Predictor™. The chemical space of SMILES structures of washed and energy-minimized chemicals was compared to that of the ADMET Predictor™ S+BBB filter (a binary classifier of “high” or “low” permeability collected from 1,942 chemicals from multiple sources and with a classification concordance value of 93%). Chemicals deemed unable to cross the BBB were designated “low priority.” Chemicals with widespread or limited exposure, possible absorption into the body, and potential to reach brain AChE were designated as “high priority” candidates, which can be further ranked in the future based on their relative rates of absorption/distribution and metabolism/excretion.
Similarity analysis. We identified inactive chemicals falling within a related functional or structural class as active chemicals, along with parent compounds of known metabolites that exhibited a positive response in the AChE inhibition assay, by evaluating their structural similarities to such active chemicals. Similarity tests were conducted through use of MOE, in which molecular fingerprints were selected based on the presence or absence of one of the 166 public MDL Information Systems’ structural Molecular Access System (MACCS) keys (Willett et al. 1998). The fingerprint of each of the 30 active chemicals was used to identify the nearest neighbor inactive chemical using a Tanimoto similarity threshold of 75%, which is considered to be an appropriate cut-off value for fingerprint searches (Rahman et al. 2009). Briefly, the Tanimoto similarity threshold coefficient is the ratio of the number of bit-key characteristics common to both sample sets (the size of the intersection) divided by the number of bit-key characteristics found in either or both sets (the size of the union), and thus explains the similarity and diversity of the sample sets (Baldi and Nasr 2010).
Results
The ToxCast™ human AChE assay had 30 active chemicals. Following the steps in our workflow, seven active chemicals were assigned as “low priority” due to a low likelihood of exposure to the general population or workers (Table 1). A majority of these “low-priority” chemicals were pharmaceuticals that had failed in clinical trials. Eight chemicals had a low likelihood of exposure to the general population, but might be of concern to workers who regularly come into contact with them or to individuals with special medical conditions that would require their use. Another 8 chemicals were considered as presenting a high exposure potential to the general public. The exposure potential of the 7 remaining chemicals was unknown. These chemicals included pesticides for which manufacture or distribution have been cancelled but may still be present in the environment or that may have derivatives that are still in use, as well as pharmaceuticals that may be cleared for public use after the later stages of clinical safety trials.
Most of the 23 chemicals with high/limited exposure potential were predicted to have significant oral absorption, followed by inhalation. Only anthralin and bendiocarb are expected to have greater dermal than oral absorption. Six chemicals were predicted to have barriers to absorption as a result of physicochemical properties such as excessive charge, high molecular weight, or a high degree of lipophilicity (Table 1). Two of these 6 chemicals—raloxifene hydrochloride and pentamidine isethionate—were also predicted to have low potential for BBB penetration. The third chemical with predicted inability to cross the BBB was anthralin (Table 1). The remaining 4 chemicals with limited absorption were retained as “high priority” chemicals but can be assigned a lower ranking in future quantitative analysis. Application of our workflow resulted in a total of 10 “low-priority” chemicals (7 due to low exposure potential and 3 due to low BBB permeability), leaving approximately 67% to be further analyzed.
Metabolism was shown to affect a chemical’s activity in several ways. It was an activating step for some chemicals such as the organophosphate (OP) pesticide chlorpyrifos (Table 1), which is metabolized to the most active chemical in the AChE assay, chlorpyrifos oxon. For other chemicals, metabolism was a detoxifying step (Table 1), as was the case for the OP pesticide naled, which is metabolized to the less potent chemical dichlorvos before being further metabolized and excreted from the body.
Fifty-two of the 1,029 inactive chemicals exhibited a similarity threshold > 75% with their nearest active neighbors. Twenty-nine chemicals were structurally similar to bis(2-ethylhexyl) decanedioate (a “low-priority” chemical), which has very limited absorption through the primary exposure routes of skin and lungs and little to no adverse toxicity upon incidental oral ingestion (NIOSH 1983). Although individuals in the general public may be exposed by using products containing this chemical, it would not be absorbed through the skin (Clayton et al. 1994). Thus, these 29 chemicals that are structurally similar to bis(2-ethylhexyl) decanedioate were also designated as “low priority.” Zamifenacin (an M3 selective muscarinic antagonist) demonstrated 76% similarity with the poorly absorbed and distributed “low-priority” chemical raloxifene hydrochloride, so it was also given a “low-priority” status, leaving 22 chemicals remaining on the list of “possible false negatives” (see Supplemental Material, Table S1, for similarity scores for these 22 chemicals). The elimination of 30 chemicals using our prescreening approach allowed focus on the more relevant “false negatives.”
Examples of these “false negatives” of interest included chlorpyrifos, which showed 91% similarity with its oxon metabolite; dichlorvos, which showed 83% similarity with its parent naled; and aldicarb, which showed 88% similarity with the carbamate methomyl (see Supplemental Material, Table S1). At least two of these inactive chemicals—chlorpyrifos and chlorpyrifos methyl—were parents of active metabolites likely to inhibit AChE. In addition, at least four of these inactive chemicals—aldicarb, trichlorfon, dichlorvos, and phosalone—were themselves known as moderate to weak AChE inhibitors in vivo.
Discussion
In an AOP framework, known adverse outcomes may be linked via KEs to an upstream MIE. Knowledge of an MIE can be used to design high-throughput in vitro assays to screen for chemicals able to trigger that MIE. However, exposure potential and ADME properties that may influence a chemical’s ability to reach a molecular target in vivo are rarely considered beyond in vitro outcomes. In the present study we attempted to address this issue through the development of a conceptual workflow that provides general guidance for considering exposure and ADME when interpreting in vitro results. The utility of this workflow was demonstrated using the active chemicals from the human AChE inhibition assay in the ToxCast™ data set. Ten of 30 chemicals were designated as “low priority” from our analyses: 7 had very low exposure potential, and 3 were unlikely to cross the BBB. Those remaining were designated as “high priority” and can be subjected to future ranking based on quantitative considerations of ADME.
The value added by considering exposure and ADME properties of a chemical to refine high-throughput in vitro results can be illustrated using the top five active chemicals in the ToxCast™ assay. PharmaGSID_47259 is a failed pharmaceutical for which little information is available to the public. Because of a high likelihood of no exposure potential, it was designated as “low priority.” Anthralin is a topical medication approved for treating psoriasis; it is sequestered in the mitochondria of keratinocytes, where it triggers apoptosis and promotes the growth of new skin tissue (Sehgal et al. 2014). Due to its lipophilicity and sequestration in dermal tissues, anthralin is unlikely to enter the systemic circulation to a significant extent and is also predicted to have low BBB permeability, resulting in it being a “low-priority” chemical. The other three chemicals—carbofuran, naled, and chlorpyrifos oxon—are known AChE inhibitors. Carbofuran is a metabolite of carbosulfan, another highly reactive compound, and is itself metabolized to the equally toxic form 3-hydroxycarbofuran through hydroxylation, or to the less toxic moiety 3-ketocarbofuran through oxidation (Gupta 1994). Naled is metabolized to dichlorvos or to the nontoxic chemicals dimethyl phosphate and bromodichloroacetaldehyde (Roberts and Hutson 1999). The most interesting case, however, involves chlorpyrifos oxon.
Chlorpyrifos oxon is a chief metabolite of chlorpyrifos after enzymatic activity occurs within the body, and humans are exposed to trace amounts of oxon directly as chlorpyrifos is environmentally degraded (Mackay et al. 2014). It is well-established that AChE inhibition is the AOP arising from chlorpyrifos exposure (U.S. EPA 2011). Chlorpyrifos itself is not an AChE inhibitor, as shown in both in vivo and in vitro studies (Chambers and Carr 1993). From an in vivo perspective, chlorpyrifos can be considered a “false negative” as a result of its inability to demonstrate reactivity in the AChE in vitro assay, but its metabolite exhibits potent activity. This chemical highlights the critical need to consider ADME, especially metabolism, in establishing a more holistic interpretation of high-throughput in vitro results based on AOPs. In our study, structural similarities of inactive and active chemicals were compared in order to detect “true in vitro negatives, but false in vivo negatives,” such as chlorpyrifos, that might become biologically active when accounting for metabolism.
The carbamate pesticide aldicarb is a known AChE inhibitor in vivo (Wyld et al. 1992) but is considered to be inactive in the AChE inhibition assay. From our analysis, it was identified as a possible false negative because it was structurally similar to methomyl (88%). Although aldicarb was banned in the United States in 2010, distribution from the manufacturing company is not expected to be completely eliminated until 2017 (Cone 2010), suggesting that exposure to the population remains possible until then. Two other inactive chemicals identified in similarity analysis were the OPs trichlorphon (86% similar to naled) and phosalone (81% similar to azamethiphos). Trichlorphon and phosalone are considered moderate to weak AChE inhibitors in vivo and represent other examples of “possible false negatives.”
In a prior analysis of 309 ToxCast™ chemicals, 14 were considered AChE inhibitors in both rat and human in vitro assays (Knudsen et al. 2011). Eight of these chemicals were included in our current “high-priority” list, and the 6 remaining chemicals were very weak human AChE inhibitors, 2 of which, aldicarb and dichlorvos, were identified as possible “false negatives” from our similarity analysis at a threshold of 75%. If this threshold was further decreased to 60%, an additional 3 chemicals would be selected, leaving only malaoxon (56% similarity with mevinphos) unidentified from our analysis. The comparison of our results with those of a previously published study demonstrates the usefulness of similarity analysis in detecting possible “false negatives” from in vitro data. A logical next step to enhance the utility of our workflow would be to compare these possible “in vivo false negatives” with in silico predictions [e.g., quantitative structure–activity relationship (QSAR) or protein-docking models] that are built to identify initiation of MIEs by chemical classes rather than by individual chemicals. In vitro assays and in silico models are complementary approaches able to incorporate high-throughput analyses into AOP frameworks.
There is often some difficulty in the extrapolation of in vitro and in silico results to in vivo observations due to a variety of factors. Sometimes, in silico predictions contradict in vitro results, and further evaluation of the appropriateness of either approach is necessary. A possibility exists that the fundamental assumptions in either or both approaches are inadequate. For example, the use of 2D descriptors alone in predictive in silico QSAR models may lack the required specificity to account for protein–ligand interactions observed in homochiral protein environments (Chang et al. 2012; Vedani and Smiesko 2009). Domain of applicability issues may also arise for chemicals that fall outside the chemical descriptor space that was used to build QSAR models (Dragos et al. 2009; Jaworska et al. 2005; Stanforth et al. 2007; Tan et al. 2012). Identification of factors responsible for interassay variability is necessary to also avoid interpretation errors of in vitro results (Beresford et al. 2000; LeBlanc et al. 2011). Human error or, more often, promiscuous chemicals that can bind or interfere with assay reagents and targets may lead to incorrect conclusions derived from in vitro results. Such problems were discovered by Baell and Walters (2014) when applying in vitro testing to drug development, and similar problems are likely to exist with environmental chemicals as well. For example, pan-assay interference compounds (PAINS) can show signs of activity in assays due to redox cycling, degradation, or other nonspecific processes that lead to a signal, even when not truly binding to a molecular target’s active site (Baell and Walters 2014). In the present study, the SMILES strings of the 20 high priority chemicals were entered into the open-source BioActivity Data Associative Promiscuity Pattern Learning Engine (BADAPPLE plugin) (UNM 2014) and into MOE to evaluate the promiscuity of these chemicals. Both programs yielded high promiscuity scores for gentian violet, likely due to interference of the dye with assay absorption spectra (Baell and Holloway 2010), as well as for the fracking agent 1-benzylquinolinium. Although 1-benzylquinolinium may be toxic, it is unlikely to specifically inhibit AChE. Recognition of promiscuous compounds allowed for further reduction in our list of “high-priority” chemicals.
Finally, when both in silico and in vitro methods suggest the same outcome, consideration of ADME-mediated behaviors of chemicals continues to be important, because it is difficult for either approach to depict the complexity of biological processes. Much like the concept of prodrugs (Rautio et al. 2008), an inactive parent may become active through metabolic processes, or a compound considered active may not reach its molecular target as a result of limited absorption or rapid clearance. Therefore, our workflow can be used to examine outcomes predicted by in silico and in vitro approaches.
Excretion, although a critical component of ADME, was addressed only briefly in the present study because we investigated only qualitative aspects of ADME in detail. Qualitative consideration of excretion alone does not sufficiently predict the ability of a chemical to reach its molecular target. Rather, it is the rate of excretion compared with the rate of absorption that will determine whether a chemical can bind with its molecular target at a concentration sufficient to trigger an MIE. Thus, rate of excretion, along with rates of metabolism, absorption, and distribution will be addressed in a future work to illustrate the quantitative aspects of our workflow.
Conclusions
The importance of incorporating exposure and ADME properties in refining results of high-throughput in vitro assays designed based on an MIE was demonstrated through our developed workflow. Twenty of 30 possible active chemicals identified in a human AChE inhibition assay were prioritized for future quantitative testing. Similarity analysis allowed 22 inactive chemicals from the in vitro assay to be identified as possible “false negatives.” Some of these chemicals are either parents of potential active metabolites or weak AChE inhibitors in vivo. Our workflow improves the reliability of in vitro testing by identifying false negatives (e.g., inactive parents of active metabolites) and reduces cost and time by screening out false positives (e.g., active chemicals with no exposure potential) that may otherwise have undergone unnecessary analyses.
Supplemental Material
Acknowledgments
We thank D. Villeneuve, D. Lyons, and R. Tornero-Velez for their review and comments.
Footnotes
M.B.P. and J.A.L. were funded through the Oak Ridge Institute for Science and Education Research Participation Program at the U.S. EPA.
The U.S. EPA provided administrative review and approved this paper for publication. The views expressed in this paper are those of the authors and do not necessarily reflect the views of the U.S. EPA.
M.-R.G. and D.T.C. are employed by the Chemical Computing Group Inc., the publisher of the Molecular Operating Environment (MOE) software. The other authors declare they have no actual or potential competing financial interests.
References
- Abass K, Reponen P, Mattila S, Pelkonen O. Metabolism of carbosulfan II. Human interindividual variability in its in vitro hepatic biotransformation and the identification of the cytochrome p450 isoforms involved. Chem Biol Interact. 2010;185:163–173. doi: 10.1016/j.cbi.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- Baell J, Walters MA. Chemistry: chemical con artists foil drug discovery. Nature. 2014;513:481–483. doi: 10.1038/513481a. [DOI] [PubMed] [Google Scholar]
- Baldi P, Nasr R. When is chemical similarity significant? The statistical distribution of chemical similarity scores and its extreme values. J Chem Inf Model. 2010;50:1205–1222. doi: 10.1021/ci100010v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach JR, Campbell M, Andrews DJ. Exposure of health care workers to pentamidine isethionate. Occup Med (Lond) 1999;49:243–245. doi: 10.1093/occmed/49.4.243. [DOI] [PubMed] [Google Scholar]
- Beresford N, Routledge EJ, Harris CA, Sumpter JP. Issues arising when interpreting results from an in vitro assay for estrogenic activity. Toxicol Appl Pharmacol. 2000;162:22–33. doi: 10.1006/taap.1999.8817. [DOI] [PubMed] [Google Scholar]
- Berman T, Amitai Y, Almog S, Richter ED. Human biomonitoring in Israel: past, present, future. Int J Hyg Environ Health. 2012;215:138–141. doi: 10.1016/j.ijheh.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Berman T, Hochner-Celnikier D, Barr DB, Needham LL, Amitai Y, Wormser U, et al. Pesticide exposure among pregnant women in Jerusalem, Israel: results of a pilot study. Environ Int. 2011;37:198–203. doi: 10.1016/j.envint.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Bernhoft RA.2012Mercury toxicity and treatment: a review of the literature. J Environ Public Health 2012460508; doi: 10.1155/2012/460508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertz RJ, Granneman GR. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet. 1997;32:210–258. doi: 10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- Boscolo M, Antonucci S, Volpe AR, Carmignani M, Di Gioacchino M. Acute mercury intoxication and use of chelating agents. J Biol Regul Homeost Agents. 2009;23:217–223. [PubMed] [Google Scholar]
- Chambers JE, Carr RL. Inhibition patterns of brain acetylcholinesterase and hepatic and plasma aliesterases following exposures to three phosphorothionate insecticides and their oxons in rats. Fundam Appl Toxicol. 1993;21:111–119. doi: 10.1006/faat.1993.1079. [DOI] [PubMed] [Google Scholar]
- Chang DT, Goldsmith MR, Tornero-Velez R, Tan YM, Grulke CM, Chen LJ, et al. In: Parameters for Pesticide QSAR and PBPK/PD Models for Human Risk Assessment (Knaak JB, Timchalk C, Tornero-Velez R, eds) New York: Oxford University Press, 245–269; 2012. In silico strategies for modeling stereoselective metabolism of pyrethroids. [Google Scholar]
- Clayton GD, Clayton FE, Patty FA, eds. 1994. Patty’s Industrial Hygiene and Toxicology. 4th ed. New York:John Wiley & Sons Inc. [Google Scholar]
- Cochran RC, Formoli TA, Silva MH, Kellner TP, Lewis CM, Pfeifer KF. Risks from occupational and dietary exposure to mevinphos. Rev Environ Contam Toxicol. 1996;146:1–24. doi: 10.1007/978-1-4613-8478-6_1. [DOI] [PubMed] [Google Scholar]
- Cone M. Insecticide To Be Banned—Three Decades after Tainted Melons Sickened 2,000 People. Environmental Health News. 2010 Available: http://www.environmentalhealthnews.org/ehs/news/aldicarb-phaseout [accessed 27 October 2014]
- Cui N, Zhang X, Xie Q, Wang S, Chen J, Huang L, et al. Toxicity profile of labile preservative bronopol in water: the role of more persistent and toxic transformation products. Environ Pollut. 2011;159:609–615. doi: 10.1016/j.envpol.2010.09.036. [DOI] [PubMed] [Google Scholar]
- Dejobert Y, Martin P, Piette F, Thomas P, Bergoend H. Contact dermatitis from didecyldimethylammonium chloride and bis-(aminopropyl)-lauryl amine in a detergent-disinfectant used in hospital. Contact Dermatitis. 1997;37:95–96. doi: 10.1111/j.1600-0536.1997.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Dragos H, Gilles M, Alexandre V. Predicting the predictability: a unified approach to the applicability domain problem of QSAR models. J Chem Inf Model. 2009;49:1762–1776. doi: 10.1021/ci9000579. [DOI] [PubMed] [Google Scholar]
- Duprey Z, Rivers S, Luber G, Becker A, Blackmore C, Barr D, et al. Community aerial mosquito control and naled exposure. J Am Mosq Control Assoc. 2008;24:42–46. doi: 10.2987/5559.1. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol. 2008;38(suppl 2):1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Egeghy PP, Judson R, Gangwal S, Mosher S, Smith D, Vail J, et al. The exposure data landscape for manufactured chemicals. Sci Total Environ. 2012;414:159–166. doi: 10.1016/j.scitotenv.2011.10.046. [DOI] [PubMed] [Google Scholar]
- EMEA (European Agency for the Evaluation of Medical Products). London, UK: EMEA; 1999. Committee for Medicinal Veterinary Products. Azamethiphos. Summary Report (1). EMEA/MRL//001/95-FINAL. Available: http://www.emea.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500010778.pdf [accessed 30 April 2015] [Google Scholar]
- EXTOXNET (Extension Toxicology Network). Pesticide Information Profile: Oxamyl. 1993 Available: http://pmep.cce.cornell.edu/profiles/extoxnet/metiram-propoxur/oxamyl-ext.html [accessed 3 October 2014]
- EXTOXNET (Extension Toxicology Network). Pesticide Information Profile: Methomyl. 1996 Available: http://extoxnet.orst.edu/pips/methomyl.htm [accessed 4 October 2014]
- Goldsmith MR, Peterson SD, Chang DT, Transue TR, Tornero-Velez R, Tan YM, et al. Informing mechanistic toxicology with computational molecular models. Methods Mol Biol. 2012;929:139–165. doi: 10.1007/978-1-62703-050-2_7. [DOI] [PubMed] [Google Scholar]
- Groh KJ, Carvalho RN, Chipman JK, Denslow ND, Halder M, Murphy CA, et al. Development and application of the adverse outcome pathway framework for understanding and predicting chronic toxicity: I. Challenges and research needs in ecotoxicology. Chemosphere. 2015;120:764–777. doi: 10.1016/j.chemosphere.2014.09.068. [DOI] [PubMed] [Google Scholar]
- Gupta RC. Carbofuran toxicity. J Toxicol Environ Health. 1994;43:383–418. doi: 10.1080/15287399409531931. [DOI] [PubMed] [Google Scholar]
- Hussain M, Yoshida K, Atiemo M, Johnston D. Occupational exposure of grain farmers to carbofuran. Arch Environ Contam Toxicol. 1990;19:197–204. doi: 10.1007/BF01056087. [DOI] [PubMed] [Google Scholar]
- Houtappel M, Bruijnzeel-Koomen CA, Röckmann H. Immediate-type allergy by occupational exposure to didecyl dimethyl ammonium chloride. Contact Dermatitis. 2008;59:116–117. doi: 10.1111/j.1600-0536.2008.01336.x. [DOI] [PubMed] [Google Scholar]
- Jaworska J, Nikolova-Jeliazkova N, Aldenberg T. QSAR applicabilty domain estimation by projection of the training set descriptor space: a review. Altern Lab Anim. 2005;33:445–459. doi: 10.1177/026119290503300508. [DOI] [PubMed] [Google Scholar]
- Kapui Z, Varga M, Urban-Szabo K, Mikus E, Szabo T, Szeredi J, et al. Biochemical and pharmacological characterization of 2-(9-(2-piperidinoethoxy)-4-oxo-4H-pyrido[1,2-a]pyrimidin-2-yloxymethyl)-4-(1-methylethyl)-6-methoxy-1,2-benzisothiazol-3(2H)-one-1,1-dioxide (SSR69071), a novel, orally active elastase inhibitor. J Pharmacol Exp Ther. 2003;305:451–459. doi: 10.1124/jpet.102.044263. [DOI] [PubMed] [Google Scholar]
- Knudsen TB, Houck KA, Sipes NS, Singh AV, Judson RS, Martin MT, et al. Activity profiles of 309 ToxCast™ chemicals evaluated across 292 biochemical targets. Toxicology. 2011;282:1–15. doi: 10.1016/j.tox.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Kohlhaas KL, Rueter LE, Bitner RS. Alpha7 Neuronal Nicotinic Receptor Ligand and Antipsychotic Compositions. U.S. Patent Application Publication. US2006/0211686 A1. 2006 Available: http://patentimages.storage.googleapis.com/pdfs/US20060211686.pdf [accessed 30 April 2015]
- Kosaka K, Sakai N, Endo Y, Fukuhara Y, Tsuda-Tsukimoto M, Ohtsuka T, et al. Impact of intestinal glucuronidation on the pharmacokinetics of raloxifene. Drug Metab Dispos. 2011;39:1495–1502. doi: 10.1124/dmd.111.040030. [DOI] [PubMed] [Google Scholar]
- Lapenna S, Gabbert S, Worth A. Training needs for toxicity testing in the 21st century: a survey-informed analysis. Altern Lab Anim. 2012;40:313–320. doi: 10.1177/026119291204000604. [DOI] [PubMed] [Google Scholar]
- LeBlanc GA, Kullman SW, Norris DO, Baldwin WS, Kloas W, Greally JM. 2011. Draft Detailed Review Paper. State of the Science on Novel in Vitro and in Vivo Screening and Testing Methods and Endpoints for Evaluating Endocrine Disruptors. Research Triangle Park, NC:RTI International. Available: http://www.oecd.org/chemicalsafety/testing/49002244.pdf [accessed 30 April 2015] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Mackay D, Giesy JP, Solomon KR. Fate in the environment and long-range atmospheric transport of the organophosphorus insecticide, chlorpyrifos and its oxon. Rev Environ Contam Toxicol. 2014;231:35–76. doi: 10.1007/978-3-319-03865-0_3. [DOI] [PubMed] [Google Scholar]
- Mauri A, Consonni V, Pavan M, Todeschini R. DRAGON software: an easy approach to molecular descriptor calculations. Match-Commun Math Comput Chem. 2006;56:237–248. [Google Scholar]
- McGill A, Frank A, Emmett N, Turnbull DM, Birch-Machin MA, Reynolds NJ. The anti-psoriatic drug anthralin accumulates in keratinocyte mitochondria, dissipates mitochondrial membrane potential, and induces apoptosis through a pathway dependent on respiratory competent mitochondria. FASEB J. 2005;19:1012–1014. doi: 10.1096/fj.04-2664fje. [DOI] [PubMed] [Google Scholar]
- Métro TX, Cochi A, Gomez Pardo D, Cossy J. Asymmetric synthesis of an antagonist of neurokinin receptors: SSR 241586. J Org Chem. 2011;76:2594–2602. doi: 10.1021/jo102471r. [DOI] [PubMed] [Google Scholar]
- Montgomery AB, Corkery KJ, Brunette ER, Leoung GS, Waskin H, Debs RJ. Occupational exposure to aerosolized pentamidine. Chest. 1990;98:386–388. doi: 10.1378/chest.98.2.386. [DOI] [PubMed] [Google Scholar]
- NIOSH (National Institute for Occupational Safety and Health). NOES. National Occupational Exposure Survey Conducted from 1981 to 1983. 1983 Available: http://www.cdc.gov/noes/ [accessed 24 October 2014]
- Ostrea EM, Jr, Villanueva-Uy E, Bielawski D, Birn S, Janisse JJ. Trends in long term exposure to propoxur and pyrethroids in young children in the Philippines. Environ Res. 2014;131:13–16. doi: 10.1016/j.envres.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Ouattara B, Belkhir S, Morissette M, Dridi M, Samadi P, Grégoire L, et al. Implication of NMDA receptors in the antidyskinetic activity of cabergoline, CI-1041, and Ro 61-8048 in MPTP monkeys with levodopa-induced dyskinesias. J Mol Neurosi. 2009;38:128–142. doi: 10.1007/s12031-008-9137-8. [DOI] [PubMed] [Google Scholar]
- R & D Focus Drug News. SSR 150106 Sanofi-Aventis Phase Change II, Europe(inflammation). 2007 Available: http://business.highbeam.com/436989/article-1G1-173125154/ssr-150106-sanofiaventis-phase-change-ii-europe [accessed 4 October 2014]
- Rahman SA, Bashton M, Holliday GL, Schrader R, Thornton JM.2009Small molecule subgraph detector (SMSD) toolkit. J Cheminform 112; doi: 10.1186/1758-2946-1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Järvinen T, et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- Roberts C, Watson J, Price GW, Middlemiss DN. SB-236057-A: a selective 5-HT1B receptor inverse agonist. CNS Drug Rev. 2001;7:433–444. doi: 10.1111/j.1527-3458.2001.tb00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T, Hutson D. Cambridge, UK: Royal Society of Chemistry Publishing, 79–103; 1999. Macrocyclic insecticides. In: Metabolic Pathways of Agrochemicals: Part 2: Insecticides and Fungicides (Roberts TR, Hutson HD, Jewess PJ, Lee PW, Nicholls PH, Plimmer JR, eds) [Google Scholar]
- Russom CL, LaLone CA, Villeneuve DL, Ankley GT. Development of an adverse outcome pathway for acetylcholinesterase inhibition leading to acute mortality. Environ Toxicol Chem. 2014;33:2157–2169. doi: 10.1002/etc.2662. [DOI] [PubMed] [Google Scholar]
- Schilmann A, Lacasaña M, Blanco-Muñoz J, Aguilar-Garduño C, Salinas-Rodríguez A, Flores-Aldana M, et al. Identifying pesticide use patterns among flower growers to assess occupational exposure to mixtures. Occup Environ Med. 2010;67:323–329. doi: 10.1136/oem.2009.047175. [DOI] [PubMed] [Google Scholar]
- Sehgal VN, Verma P, Khurana A. Anthralin/dithranol in dermatology. Int J Dermatol. 2014;53:e449–e460. doi: 10.1111/j.1365-4632.2012.05611.x. [DOI] [PubMed] [Google Scholar]
- Shlomi T, Cabili MN, Herrgård MJ, Palsson BØ, Ruppin E. Network-based prediction of human tissue-specific metabolism. Nat Biotechnol. 2008;26:1003–1010. doi: 10.1038/nbt.1487. [DOI] [PubMed] [Google Scholar]
- Smegal DC. Washington, DC: U.S. Environmental Protection Agency, Office of Pesticide Programs; 2000. Human Health Risk Assessment: Chlorpyrifos. Available: http://www.epa.gov/scipoly/sap/meetings/2008/september/hed_ra.pdf [accessed 30 April 2015] [Google Scholar]
- Stanforth RW, Kolossov E, Mirkin B. A measure of domain of applicability for QSAR modelling based on intelligent K-means clustering. QSAR Comb Sci. 2007;26:837–844. [Google Scholar]
- Steinbeck C, Han Y, Kuhn S, Horlacher O, Luttmann E, Willighagen E. The Chemistry Development Kit (CDK): an open-source Java library for chemo- and bioinformatics. J Chem Inf Comput Sci. 2003;43:493–500. doi: 10.1021/ci025584y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YM, Sobus J, Chang D, Tornero-Velez R, Goldsmith M, Pleil J, et al. Reconstructing human exposures using biomarkers and other “clues.”. J Toxicol Environ Health B Crit Rev. 2012;15:22–38. doi: 10.1080/10937404.2012.632360. [DOI] [PubMed] [Google Scholar]
- TOXNET (Toxicology Data Network). Dodecyl Benzene Sulfonic Acid. CASRN: 27176-87-0. 2002 Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+6285 [accessed 30 April 2015]
- TOXNET. Gentian Violet. CASRN: 548-62-9. 2013 Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+4366 [accessed 30 April 2015]
- Travassos AR, Claes L, Boey L, Drieghe J, Goossens A. Non-fragrance allergens in specific cosmetic products. Contact Dermatitis. 2011;65:276–285. doi: 10.1111/j.1600-0536.2011.01968.x. [DOI] [PubMed] [Google Scholar]
- UNM (University of New Mexico). Badapple. 2014 Available: http://pasilla.health.unm.edu/tomcat/badapple/badapple [accessed 9 October 2014]
- U.S. EPA (U.S. Environmental Protection Agency). R.E.D. FACTS: Mevinphos. EPA-738-F-94-020; EPA-738-R-94-023. Washington, DC:U.S. EPA. 1994 Available: http://archive.epa.gov/pesticides/reregistration/web/pdf/0250fact.pdf [accessed 9 November 2015]
- U.S. EPA. US Environmental Protection Agency. Office of Pesticide Programs. Reregistration Eligibility Decision for Naled. 2006 Available: http://archive.epa.gov/pesticides/reregistration/web.old/pdf/naled_red.pdf [accessed 9 November 2015]
- U.S. EPA. Carbofuran I.R.E.D. Facts. 2008 Available: http://archive.epa.gov/pesticides/reregistration/web/html/carbofuran_ired_fs.html [accessed 9 November 2015]
- U.S. EPA. Toxicity Forecaster (ToxCast™) Data. 2010 Available: http://www.epa.gov/ncct/toxcast/index.html [accessed 3 May 2015]
- U.S. EPA. Chlorpyrifos: Preliminary Human Health Risk Assessment for Registration Review. EPA/HQ/OPP/2008/0850/0025. Washington, DC:U.S. EPA. 2011 Available: http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2008-0850-0025 [accessed 9 November 2015]
- U.S. EPA. Risk Assessment. Human Health Risk Assessments. 2012a Available: http://www.epa.gov/risk_assessment/health-risk.htm [accessed 9 October 2014]
- U.S. EPA. Study of the Potential Impacts of Hydraulic Fracturing on Drinking Water Resources: Progress Report. EPA 601/R-12/011. Washington, DC:U.S. EPA. 2012b Available: http://www2.epa.gov/sites/production/files/documents/hf-report20121214.pdf [accessed 9 November 2015]
- U.S. EPA. Technology Transfer Network – Air Toxics Web Site. Tolulene. 2013 Available: http://www3.epa.gov/airtoxics/hlthef/toluene.html [accessed 11 November 2015]
- U.S. EPA. Toxicity Forecasting: Advancing the Next Generation of Chemical Safety Evaluation. 2014a Available: http://www2.epa.gov/chemical-research/toxicity-forecasting [accessed 11 November 2014]
- U.S. EPA. TSCA Chemical Substance Inventory Homepage. 2014b Available: http://www.epa.gov/oppt/existingchemicals/pubs/tscainventory/ [accessed 30 April 2015]
- Van Scoy AR, Yue M, Deng X, Tjeerdema RS. Environmental fate and toxicology of methomyl. Rev Environ Contam Toxicol. 2013;222:93–109. doi: 10.1007/978-1-4614-4717-7_3. [DOI] [PubMed] [Google Scholar]
- Vedani A, Smiesko M. In silico toxicology in drug discovery—concepts based on three-dimensional models. Altern Lab Anim. 2009;37:477–496. doi: 10.1177/026119290903700506. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, et al. Adverse outcome pathway development II: best practices. Toxicol Sci. 2014;142:321–330. doi: 10.1093/toxsci/kfu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken M, Landesmann B, Goumenou M, Vinken S, Shah I, Jaeschke H, et al. Development of an adverse outcome pathway from drug-mediated bile salt export pump inhibition to cholestatic liver injury. Toxicol Sci. 2013;136:97–106. doi: 10.1093/toxsci/kft177. [DOI] [PubMed] [Google Scholar]
- Watanabe KH, Andersen ME, Basu N, Carvan MJ, III, Crofton KM, King KA, et al. Defining and modeling known adverse outcome pathways: domoic acid and neuronal signaling as a case study. Environ Toxicol Chem. 2011;30:9–21. doi: 10.1002/etc.373. [DOI] [PubMed] [Google Scholar]
- Willett P, Barnard JM, Downs GM. Chemical similarity searching. J Chem Inf Comp Sci. 1998;38:983–996. [Google Scholar]
- Wyld PJ, Watson CE, Nimmo WS, Watson N. Rhone-Poulenc, Lyon, France. Inveresk Clinical Research Report No. 7786. MRID No. 423730-01. HED Doc. No. 0010459. Washington, DC: U.S. Environmental Protection Agency; 1992. A Safety and Tolerability Study of Aldicarb at Various Dose Levels in Healthy Male and Female Volunteers. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


