Highlights
-
•
We address the cytoplasmic mRNA decay processes that determine the mRNAs half-life.
-
•
We briefly describe the major, evolutionary conserved, ageing pathways and mechanisms.
-
•
We summarize critical findings that link mRNA turnover and ageing modulators.
Keywords: mRNA decay, Ageing, P-body, Stress granule, Protein synthesis
Abstract
Messenger RNA (mRNA) turnover that determines the lifetime of cytoplasmic mRNAs is a means to control gene expression under both normal and stress conditions, whereas its impact on ageing and age-related disorders has just become evident. Gene expression control is achieved at the level of the mRNA clearance as well as mRNA stability and accessibility to other molecules. All these processes are regulated by cis-acting motifs and trans-acting factors that determine the rates of translation and degradation of transcripts. Specific messenger RNA granules that harbor the mRNA decay machinery or various factors, involved in translational repression and transient storage of mRNAs, are also part of the mRNA fate regulation. Their assembly and function can be modulated to promote stress resistance to adverse conditions and over time affect the ageing process and the lifespan of the organism. Here, we provide insights into the complex relationships of ageing modulators and mRNA turnover mechanisms.
1. Introduction
As ageing advances, a progressive decline in the ability of cells to preserve a functional proteome occurs, leading to widespread protein aggregation over time (Koga et al., 2011). Also, defects in cellular protein quality control mechanisms may constitute a common basis for the abnormal accumulation of proteins in age-dependent neurodegenerative disorders (Vilchez et al., 2014). In a physiological context, several levels of protein quality control exist, starting from the regulation of transcription and translation to the maintenance of proteome through clearance and repair mechanisms. Post-transcriptional control of gene expression at the level of messenger RNA (mRNA) translation and degradation is fundamental for determining protein levels, given that mRNA half-life ranges from a few minutes to many hours. Post-transcriptional mechanisms can regulate not only the amount but also the time and place of mRNAs that are translated into new proteins (Sonenberg and Hinnebusch, 2009, Spriggs et al., 2010). This regulation is vital for an organism to develop, grow and survive under both physiological and stress conditions. Furthermore, it has been shown across species that major signaling pathways that control the translation process can promote longevity and prevent age-related diseases. In this frame, cellular mechanisms and factors that direct cytoplasmic mRNAs between the translational and decay machineries or guide them to RNA granules for storage may have a great impact on the process of ageing and the development of diseases. However, current knowledge of their significance in the modulation of the ageing process and its consequences is limited. In this review, we summarize recent advances in understanding the relationship between mRNA decay and ageing and discuss the impact of key ageing modulators on mRNA turnover and vice versa. In particular, we first address the cytoplasmic mRNA decay processes that determine the half-life of normal and aberrant transcripts focusing on the latest discoveries in the field. Next, we briefly describe the major ageing pathways and mechanisms that affect lifespan in many species. Finally, we present critical findings that link mRNA decay and ageing modulators, supporting a functional interplay between them. Emphasis is given on cytoplasmic RNA granules and their factors that control mRNA turnover under normal and stress conditions, discussing their possible implications in the modulation of ageing.
2. Cytoplasmic mRNA turnover
Degradation of cytoplasmic RNAs is an essential cellular process, serving as a key regulator of gene expression at the post-transcriptional level, under various conditions of growth. Proper regulation of the mRNA decay process is critical for eukaryotic cells to maintain their homeostasis, not only by degrading transcripts that are no longer needed or aberrant transcripts that could lead to the production of toxic proteins, but also by stabilizing or destabilizing specific mRNAs, thus allowing a rapid fine-tuning of gene expression under changeable conditions. The fate of mRNAs in the cytoplasm is determined by a common cellular strategy: packaging of mRNAs with proteins into messenger ribonucleoprotein complexes (mRNPs) that regulate mRNA turnover.
2.1. Typical mRNA decay pathways
An ordinary mature mRNA consists of the spliced coding region, 3’ and 5’ untranslated regions (UTRs) that contain regulatory sequences important for stability and translation, as well as two extra regulatory elements that play a central role in splicing, polyadenylation, nuclear export, translation and degradation of the transcript: a cap structure at their 5’ end and a stretch of adenine residues (poly(A)-tail) at their 3’ end. The 5’-cap consists of methylated or trimethylated guanine and is added shortly after the initiation of transcription, while the poly(A)-tail is added during transcriptional termination of all transcripts (with the exception of histone mRNA). Both structures protect the mRNA from degradation and promote interactions that facilitate translation initiation in the cytoplasm, through ‘circularization’ of the mRNA molecules (Preiss and Hentze, 2003). The length of poly(A) tail provides a first level of translational control (Weill et al., 2012), with long tails having a stabilizing effect in the bulk of mRNAs, whereas poly(A)-tail shortening (deadenylation) generally initiates the degradation of mRNAs (Fig. 1).
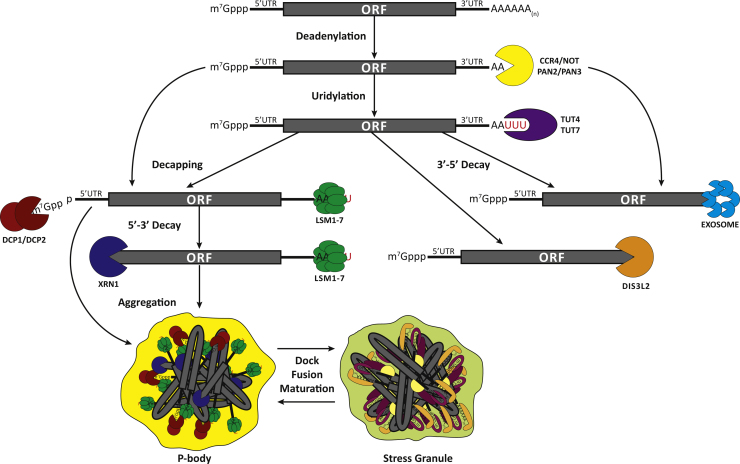
Fig. 1.
General mRNA decay pathways. Bulk mRNA degradation is initiated with the shortening of the poly(A) tail by the combined action of CCR4/NOT and PAN2/PAN3 complexes, optionally followed by the addition of a short uridine tract at the 3’ end. Subsequently, mRNAs can be degraded by two mechanisms: 3’-5’ decay mediated by the exosome (with the aid of 3’-5’ exoribonuclease DIS3L2, in the case of uridylated transcripts), or removal of the 5’ cap by the DCP1/DCP2 decapping complex and 5’-3’ decay mediated by XRN1 exonuclease. Translationally inactive and degradable transcripts can assemble in conjunction with their protein-binding partners into cytoplasmic RNA granules termed P-bodies (PBs). PBs are often found to dock, fuse or mature to stress granules, a different type of RNA granules, which contain mRNAs stalled in the process of translation initiation.
Deadenylation is mainly executed by the combined action of PAN2/PAN3 and CCR4/NOT deadenylation complexes (Wahle and Winkler, 2013) and, in most cases, is the rate limiting step of cytoplasmic mRNA decay, but is still reversible. Following deadenylation, the mRNA can be degraded in the 3’→5’ direction through the action of the cytoplasmic RNA exosome, a multisubunit complex containing the DIS3 exoribonuclease. Most often though, deadenylation is followed by the removal of the 5’-cap by the decapping enzyme DCP2, exposing the 5’ end mono-phosphorylated RNA to exoribonucleolytic degradation by the cytoplasmic 5’→3’ exoribonuclease XRN1 (Garneau et al., 2007). DCP1 is an essential cofactor of DCP2 in budding yeast Saccharomyces cerevisiae, as it interacts directly with, and introduces conformational changes in DCP2, stimulating its activity. The DCP1/DCP2 decapping complex is conserved in higher eukaryotes but additional factors are needed for their interaction (Jonas and Izaurralde, 2013). Also the DCP1/DCP2 complex communicates with a variety of enhancers or activators that act in diverse ways to stimulate decapping activity (Arribas-Layton et al., 2013). These enhancers may serve as a scaffold for complex assembly or stimulate decapping activity indirectly by repressing translation initiation.
In addition to DCP2, a second mammalian mRNA decapping enzyme, termed NUDT16, with no obvious ortholog in S. cerevisiae, Caenorhabditis elegans or Drosophila melanogaster, has been found to regulate the stability of a subset of mRNAs, at least in some tissues (Song et al., 2010). Further data suggest that both DCP2 and NUDT16 decapping enzymes could be differentially utilized for specific cellular mRNA decay processes (Li et al., 2011). Likewise it was shown that the scavenger decapping enzyme, DCPS in mammals or DCS1 in yeast, can regulate mRNA stability in a transcript-selective manner, acting in conjunction with or as co-factor of the 5’→3’ exoribonuclease XRN1 (Zhou et al., 2015). DCPS/DCS1 is known to hydrolyze in vitro the residual cap structure from 3’ end decay (m7GpppN), but its role in the degradation of the product generated by 5’→3’ decay (m7GDP) pathway was unclear (Milac et al., 2014). Recent data revealed the existence of a complex process for the elimination of all free cap structures, arising as a consequence of both 5’→3’ and 3’→5’ mRNA decay pathway and involves DCPS/DCS1 as well as a new scavenger decapping enzyme, named APH1 in yeast or FHIT in mammals (Taverniti and Seraphin, 2015).
The two directions described above, 5’→3’ and 3’→5’, of cytoplasmic mRNA degradation represent the major decay mechanisms, at least in S. cerevisiae, and are both dependent on deadenylation. Recently, an alternative to these mRNA decay pathways has been identified in fission yeast Schizosaccharomyces pombe and human cells (Lubas et al., 2013, Malecki et al., 2013, Rissland and Norbury, 2009). It involves the uridylation of adenylated or deadenylated mRNAs, that is the addition of a short stretch of uridyl residues -often only one or two- at their 3’ end by terminal uridylyl transferases (TUT4/7) and subsequent decay by multiple ways; decapping and 5’→3’ degradation by XRN1 exoribonuclease, 3’→5’ degradation by exosome or, degradation by a novel 3’→5’ exoribonuclease, named DIS3L2, which functions independently of the exosome. Uridylation was known to be a critical step for the degradation of non-polyadenylated histone mRNAs in mammals, but the above studies suggest that addition of a uridine (U)-tract at the 3’ end, of both coding and non-coding RNAs, is a broad phenomenon determining RNAs half-life (Lim et al., 2014).
2.2. Specialized mRNA decay pathways
Eukaryotic cells have also evolved specific pathways for the recognition and degradation of aberrant transcripts in a deadenylation-independent manner. Their major surveillance pathway is the nonsense mediated decay (NMD) that serves in the elimination of mRNA molecules harboring a premature translation stop codon (PTC) (Behm-Ansmant et al., 2006), albeit specific natural mRNAs can be also regulated by NMD in many organisms (Peccarelli and Kebaara, 2014). The recognition of a PTC during translation leads to translational repression, ribosome release/recycling and accelerated mRNA decay by either endonucleolytic cleavage or recruitment of decapping enzymes, the 5’→3’ exoribonuclease XRN1 and various exosome components, promoting degradation of the targeted mRNA in both directions (Kervestin and Jacobson, 2012). A second surveillance mechanism, the no-go decay (NGD), is activated when translational elongation is stalled due to a wide range of events, including strong secondary structures or contiguous rare codons. NGD leads to endonucleolytic cleavage and subsequent degradation of the 5’ and 3’ products (Doma and Parker, 2006). Finally, the non-stop decay pathway (NSD) is triggered when ribosomes fail to encounter an in-frame stop codon during translation, reaching the poly(A)-tail of the transcript. Stalled ribosomes at the 3’ end of mRNAs lead to the recruitment of the SKI complex, causing rapid degradation by the exosome, while a contribution of the 5’→3’ degradation machinery is possible (van Hoof et al., 2002). An additional specific mRNA decay mechanism is the regulated IRE1-dependent decay (RIDD) that targets mRNAs localized to the ER membrane to maintain ER homeostasis under stress (Hollien et al., 2009) or in certain physiological conditions (Coelho and Domingos, 2014).
Therefore, mRNA decay can be triggered in a transcript-specific manner through the interaction of mRNAs with specific RNA-binding proteins (RBPs). Examples include the UPF proteins that are the key regulators of the NMD pathway or the PUF proteins that recognize specific cis-acting elements in 3’ UTR of mRNAs and promote translation repression and/or deadenylation, decapping and degradation across species (Miller and Olivas, 2010). In mammalian cells, a common cis element that confers instability to an mRNA is the AU-rich element (ARE), identified in the 3’ UTR of 5–8% of human mRNAs, encoding for proteins of diverse functions (Bakheet et al., 2006). Several ARE-binding proteins (ABPs) have been identified, including the HuR, AUF1, BRF, TIA-1 and TTP, which regulate mRNA stability and translation through their interaction with AREs. Some of the ABPs stimulate rapid degradation of mRNAs, via both directions, while others can stabilize their target mRNAs preventing their decay, e.g. when confronted with stress (von Roretz et al., 2010). They have important physiological and pathological functions, as they are implicated in tightly regulated processes, like immune response and inflammation, cell cycle and carcinogenesis. Similar to AREs, but far less studied, is the family of GU-rich elements (GREs), found in the 3’ UTR of many human mRNAs that are involved in processes like cell growth, migration and apoptosis, conferring transcript instability (Vlasova and Bohjanen, 2008). Another type of trans-acting factors that recognize specific cis elements on the target mRNA, the non-coding miRNAs, regulates the steady-state transcript levels of a large number of genes in animals and plants by promoting silencing and/or degradation (discussed below). Of note, miRNAs can interact with ABPs, which are able to promote or antagonize their function and in turn, miRNAs may modulate the expression of ABPs themselves (Fabian and Sonenberg, 2012, Pascale and Govoni, 2012).
2.3. RNA granules regulating mRNA turnover
In eukaryotic cells, components of the 5’→3’ decay have been found to colocalize and exert their function in distinct cytoplasmic foci termed processing bodies (PBs) or P-bodies (Fig. 1), initially referred to as GW bodies in mammalian cells (Eystathioy et al., 2003, Sheth and Parker, 2003). PBs have been characterized as cytoplasmic sites of mRNA decay and storage of translationally repressed mRNAs, as they harbor non-translating mRNAs and several proteins implicated in the translational silencing and degradation of transcripts (Cougot et al., 2004, Teixeira et al., 2005). Both CCR4-NOT and PAN2-PAN3 deadenylation complexes, the decapping complex and the 5’→3’ exoribonuclease XRN1 are localized to PBs, but not exosome subunits or the scavenger decapping enzyme DCS1 (Brengues et al., 2005, Cougot et al., 2004, Teixeira and Parker, 2007). The newly identified 3’→5’ exoribonuclease DIS3L2, in fission yeast, localizes in cytoplasmic foci which are docked to PBs (Malecki et al., 2013). Moreover, PBs have been found to contain degradation intermediates, components of aberrant mRNA surveillance pathways, as well as proteins involved in ARE- or miRNA-mediated degradation of transcripts, suggesting that they are active sites of mRNA decay and not just storage places of the decay machinery (Kulkarni et al., 2010). In S. cerevisiae, PBs also function in the elimination of mature but aberrant 18S rRNAs, which are subjected to a cytoplasmic quality control mechanism named nonfunctional RNA decay (NRD), the latter being mechanistically related to no-go mRNA decay (Cole et al., 2009).
Nonetheless, mRNA degradation in yeast, Drosophila and human cells seems to begin while transcripts are still associated with ribosome complexes (Antic et al., 2015, Eulalio et al., 2007b, Hu et al., 2010, Hu et al., 2009, Pelechano et al., 2015). Also, under conditions that impair PB formation, all related functions continue to occur in the cytoplasm (Eulalio et al., 2007b, Stalder and Muhlemann, 2009) making obscure the reasons for their assembly; it might be crucial for fine-tuning regulation of mRNAs’ fate, e.g. to strengthen mRNA decay rate and/or specificity, along with keeping silenced mRNAs away from translational machinery. PBs are highly dynamic foci, constantly present in the cytoplasm under normal conditions, but rapidly increased in number and size upon exposure to various forms of stress (Kedersha et al., 2005, Rousakis et al., 2014, Teixeira et al., 2005) (Fig. 2). The rate limiting step in their assembly is the presence of translationally incompetent mRNAs, since a decrease in their abundance by trapping mRNAs into polysomes or inhibiting transcription results to reduced PBs, while an increase in the pool of non-translating mRNAs by blocking their degradation or translation initiation increased PBs in yeast, worms and mammals (Cougot et al., 2004, Sheth and Parker, 2003, Teixeira et al., 2005). The release of an mRNA from the translation machinery seems to be a perquisite for its targeting to PBs, since ribosomal proteins and translation initiation factors are excluded from PBs with the exception of eIF4E. The simultaneous presence of eIF4E-T (an eIF4E-binding protein that inhibits translation) however suggests that eIF4E is associated with translationally repressed, not active, molecules (Andrei et al., 2005, Ferraiuolo et al., 2005). Conversely, not all mRNAs found in PBs are committed to decapping and decay and intact transcripts can exit PBs and return to translation (Bhattacharyya et al., 2006, Brengues et al., 2005).
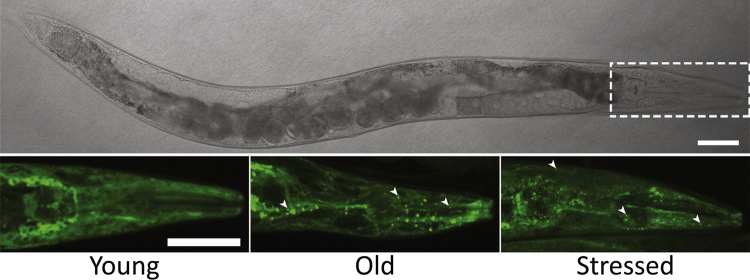
Fig. 2.
Accumulation of P-bodies (PBs) during stress and ageing. PBs are visualized in somatic cells of C. elegans using a translational fusion of DCAP-1 (the worm homologue of DCP1) with GFP. Confocal images of the head region of 1-day (Young) and 7-day adults (old) grown under normal conditions, or of young adults transiently subjected to heat–shock (stressed) are shown. Arrowheads indicate formed PBs. Scale bars = 50 μm.
PBs have frequently been found to physically and functionally interact with a second type of cytoplasmic RNA granules, named stress granules (SGs). These are also dynamic structures containing non-translating mRNAs but they differ from PBs in the aspect that they harbor poly(A)+ mRNAs, translation initiation factors, PABP and 40S ribosomal subunits, but lack enzymes of the mRNA deadenylation and degradation machinery (Kedersha et al., 2005, Kimball et al., 2003). Their composition suggests that SGs are aggregates of mRNPs stalled in translation initiation step, probably formed to protect sequestered mRNAs from degradation until the cell recovers. Indeed, SGs become microscopically visible only under environmental stress, where translation initiation is generally inhibited or as a result of genetic manipulations and addition of drugs that impair translation initiation (Buchan and Parker, 2009). Also, stress-induced SGs stabilize and accumulate labile mRNAs, such as ARE- or NMD-targets, through the recruitment of cognate RNA-binding proteins (von Roretz et al., 2010). However, translational arrest does not always induce the formation of SGs, indicating that proteins and non-translating mRNAs move to SGs under specific conditions (Mokas et al., 2009, Ohn et al., 2008). Despite their differences though, PBs and SGs share many common proteins and mRNA species whilst under certain stress conditions, a dynamic relationship between them has been observed; they can interact, dock for a short period of time, presumably in order to exchange components, fuse or mature from one type to another (Buchan et al., 2008, Decker and Parker, 2012, Kedersha et al., 2005, Wilczynska et al., 2005). The dynamic nature of PBs and SGs probably reflects their capacity to respond quickly and transiently to various cellular signals. Global analysis of yeast mRNPs during stress revealed that a widespread relocalization of mRNA-binding proteins occurs concluding that subcellular rearrangement of PBs and SGs is a major and global response to stress (Mitchell et al., 2013).
Apart from SGs, PBs can interact with other RNA granules found in specific cellular subsets, like neuronal transport granules and germ granules in embryonic cells, involved in spatiotemporal control of translation of neuron-tethered and maternal mRNAs, respectively (Gallo et al., 2008, Zeitelhofer et al., 2008). It is notable that PBs as well as their related SGs and germ granules have been found to associate with membrane-bound organelles such as mitochondria and endosomes, linking mRNA silencing and decay with these organelle functions (Weil and Hollien, 2013). Two other types of RNA granules that have been reported to regulate mRNA stability and decay are the yeast UV granules, containing UV-damaged mRNAs and the mammalian exosome granules harboring ARE-mRNAs (Gaillard and Aguilera, 2008, Lin et al., 2007). The dynamic and reversible segregation of cytoplasmic components into these RNA granules and the interactions observed between them outline a complex model for the life-cycle of cytoplasmic mRNAs; specific transcripts can quickly change their binding partners, forming different mRNPs, while granule components can shuttle between RNA granules and active polysomes. All together these interactions ultimately determine the fate of mRNAs concerning translation, localization, storage and decay.
3. Ageing mechanisms and pathways
Ageing is generally considered as long-term accumulation of cellular damage, evoked by intrinsic or extrinsic assaults, which interferes with the normal functioning of cellular networks and disturbs organismal homeostasis. Over the past few years, genetic studies of longevity in classic model organisms have identified many genes, mechanisms and pathways that cause or slow ageing, and are often highly conserved through evolution. They can modulate lifespan mostly by enhancing cellular stress resistance, maintaining cellular homeostasis and altering metabolism of the organism. In addition, most of them are of vital importance to counteract age-associated disability and diseases like Parkinson’s, Alzheimer’s and Huntington’s disease.
3.1. Signal transduction pathways
Multiple signaling pathways that integrate hormonal, nutrient and stress signals have emerged as important modulators of ageing or age-related phenotypes. The first pathway shown to almost double the lifespan of C. elegans was the insulin/insulin-like growth factor-1 (IGF-1) signaling (IIS) pathway (Kenyon, 2010). It is evolutionary conserved and its down-regulation promotes longevity in organisms as diverse as worms, flies and mice; moreover genetic variations in IIS components have been associated with longevity and healthy ageing in humans (Bartke et al., 2013, Milman et al., 2014). The IIS in worms is initiated when insulin-like peptides bind to the single receptor DAF-2 and activate a downstream kinase cascade, leading to the phosphorylation of DAF-16, a homolog of the mammalian FOXO family of transcription factors. Phosphorylated DAF-16 is retained in the cytoplasm while reduced IIS, due to genetic mutations or hormonal/stress signals, negatively regulates the phosphorylation of DAF-16 and allows its translocation to the nucleus. Changes in expression of the DAF-16 gene targets, involved in stress resistance, immune defense and metabolism, can ultimately prolong lifespan. Nuclear translocation of DAF-16 can be induced by key mediators of stress and immune signal transduction pathways, such as c-JUN N-terminal kinase (JNK) and p38 kinases, linking these pathways to longevity and stress resistance in C. elegans (Kondo et al., 2005, Oh et al., 2005).
An evolutionary conserved pathway that interacts with IIS and integrates stress, nutrient, energy and hormone signals is the mTOR (mechanistic target of rapamycin) signaling pathway (Wullschleger et al., 2006, Zoncu et al., 2011). Like IIS, genetic mutations or drugs that reduce mTOR signaling can extend lifespan in model organisms and protect against neurodegeneration (Cornu et al., 2013, Johnson et al., 2013). The effects of mTOR in longevity are closely connected to its downstream regulated processes, such as protein translation, ribosome biosynthesis, lipid synthesis, autophagy, stress response etc. (Fonseca et al., 2014). Direct genetic manipulations of mTOR pathway components or downstream targets can alter lifespan in many different organisms, whereas treatment with rapamycin, an inhibitor of mTOR, has been shown to increase lifespan in yeast, worms, flies and, even in mice when initiated late in life (Bjedov et al., 2010, Blagosklonny, 2013, Kapahi et al., 2010, Vellai et al., 2003). Interestingly, at the metabolic and molecular level, mTOR inactivation mimics the effects of dietary restriction (DR), a reduction in nutrient intake without malnutrition (Kapahi et al., 2010). DR prolongs lifespan and opposes the late-onset disability and diseases, in diverse species, whereas studies in primates indicate that DR provides an overall health benefit (Mirzaei et al., 2014). The underlying mechanisms seem to be relevant to common strategies of cells and organisms that have evolved to cope with nutrient scarcity and stress. Consistent with this, the activation of SIR2 pathway in response to DR or starvation has been linked to longevity. SIR2 in yeast and its functional orthologs in other organisms, that make up the sirtuins family, are NAD+-dependent protein deacetylases that act as sensor of the metabolic state of the cell and organism; thus, they have important functions in multiple physiological processes, affecting several human diseases and ageing-associated metabolic and cognitive disorders (Imai and Guarente, 2014).
3.2. Protein synthesis and homeostasis
Organisms are constantly exposed to a range of stressful stimuli that perturb their homeostasis and, an important part of the coordinated cellular response that takes place is the control of protein synthesis. Upon nutrient limitation or environmental stress conditions, mTOR signaling, a master regulator of protein synthesis is inhibited whereas, the eIF2α (eukaryotic initiation factor 2α) kinase GCN2 (general amino acid control non-derepressible) is activated; although they utilize distinct mechanisms, both are able to initiate a general shutdown of protein synthesis accompanied by specific mRNAs’ translation. This translational control elicits an adaptive response that allows cells to survive by saving energy and by selective induction of stress-resistance proteins (Spriggs et al., 2010). Evidence from several model organisms has established that regulation of global protein synthesis is a common determinant of longevity, as altering the expression of translation factors to reduce mRNA translation can increase lifespan and stress resistance in animal models (Arnsburg and Kirstein-Miles, 2014, Syntichaki et al., 2007). The same mechanisms of translational regulation may contribute to lifespan extension and metabolic fitness conferred by DR (Gallinetti et al., 2013). Quantitative proteomic studies further support this idea as the proteome profile of both dietary restricted and IIS mutant worms is characterized by the decreased abundance of proteins involved in mRNA translation and protein metabolism (Depuydt et al., 2013, Dong et al., 2007, Stout et al., 2013).
A global reduction in mRNA translation could also preserve protein homeostasis (or proteostasis) by preventing the production of excessive amounts of proteins, some of which might be undesirable for normal cellular function. It has been shown that not only the intrinsic tendencies of proteins to aggregate but also their high cellular levels can drive their aggregation into non-functional and sometimes cytotoxic structures (Ciryam et al., 2013, Ellis and Minton, 2006, Walther et al., 2015). Although cells have evolved an elaborate network of proteolytic systems to safeguard proteome integrity and ensure proteostasis, the functionality of these systems is challenged by stress, ageing or pathologies, resulting in the widespread aggregation of proteome that occurs under these conditions (David et al., 2010, Olzscha et al., 2011, Reis-Rodrigues et al., 2012, Walther et al., 2015). In aged cells, increased oxidative damage of proteins can also lead to aggregates such as lipofuscin and advanced glycation end-products (AGEs) (Squier, 2001). A decline in proteostatic control with age further aggravates the ageing phenotypes and a causal link between ageing and proteotoxicity has been established by several studies, as genes and signaling pathways that extend lifespan can also restore proteostasis (Cohen et al., 2006, David et al., 2010, Hsu et al., 2003, Morley et al., 2002). The cellular protein quality control networks include molecular chaperones, in the cytosol or organelles, which facilitate the proper folding of proteins but can also recognize misfolded proteins and assist in their refolding, at a first level of proteome protection (Buchberger et al., 2010, Hartl et al., 2011).
On the next level, the irreversibly damaged proteins can be targeted for degradation by the ubiquitin-proteasome system (UPS) or the autophagosome-lysosome pathway. The UPS-mediated proteolysis of proteins relies on their conjugation to several ubiquitin units and subsequently their recognition, translocation and degradation by the proteasome, in an ATP-dependent manner (Finley, 2009). The second proteolytic pathway, the lysosomal guided by autophagy, can degrade damaged cytoplasmic proteins and organelles, providing both cytoprotection and recycling of macromolecular constituents, especially under energy-limiting or stress conditions (He and Klionsky, 2009). These proteolytic pathways are also involved in normal aspects of cell development and physiology and must be properly regulated to maintain cellular homeostasis; defects in both processes are associated with a range of diseases in humans, while their activities are affected by ageing in several mammalian tissues and cells (Vilchez et al., 2014). Strikingly, studies on several experimental systems have established a link between increased proteasome activity and longevity or protection against age-related diseases (Chondrogianni et al., 2015). Likewise, activation of autophagy by genetic or pharmacological means promotes longevity and protects against neurodegeneration (Harris and Rubinsztein, 2011). In agreement with this, DR is a potent inducer of autophagy in all species, and many of the anti-ageing effects of DR are attributed to autophagy induction (Hansen et al., 2008). Similarly, other known ageing modulators, such as ISS and sirtuin/SIRT1, can stimulate autophagy (Lee et al., 2008, Melendez et al., 2003) expanding the cytoprotective role of autophagy in lifespan extension.
3.3. miRNA functions
MicroRNAs (miRNAs) are a class of small, non-coding RNAs (∼22 nucleotides long), which typically down-regulate gene expression at the post-transcriptional level, by base pairing to mRNAs and targeting them for degradation or translational repression. They are conserved, key regulators of many biological processes, as more than half of all mRNAs are estimated to be targets of miRNAs and each miRNA is predicted to regulate the expression of hundreds of target genes (Bartel, 2009). Due to this functional redundancy, less than 30% of the ∼150 identified miRNAs in C. elegans have known specific functions and this shortcoming is exaggerated in humans where more than a thousand miRNAs exist (stand for ∼3% of the total number of genes). However, it is now well-appreciated that miRNAs regulate a broad range of gene expression, even if their impact may be seen only in specific developmental stages, tissues, sensitized genetic background or stress; ultimately they help to confer robustness in nearly all biological processes, including development, growth, metabolism, tissue homeostasis, stress responses and ageing (Ebert and Sharp, 2012, Leung and Sharp, 2010). As expected, alterations in miRNA expression have been linked to several human diseases, such as cancer, metabolic disease and viral pathogenesis, making miRNAs promising candidates for diagnostic markers and therapeutic targets (Hammond, 2015, Mendell and Olson, 2012). Interestingly, recent data associate many miRNA families with various nutrient interventions that act through the above mentioned nutrient sensing pathways to modulate healthy lifespan in most organisms (Garcia-Segura et al., 2013, Palmer et al., 2014).
The first two miRNAs, lin-4 and let-7, were discovered in C. elegans, where they function in the temporal control of cell fates, regulating developmental timing events, but can also modulate adult lifespan (Boehm and Slack, 2005, Shen et al., 2012). Analysis of the miRNA expression during ageing using microarrays (Ibanez-Ventoso et al., 2006) or deep-sequencing (de Lencastre et al., 2010, Kato et al., 2011), revealed that a significant number of miRNAs change (most are down-regulated) during ageing, and their predicted targets are involved in cellular homeostasis and stress response signaling. Likewise, differential expression during ageing has been demonstrated for many miRNAs in mice, humans and primates, in a tissue-specific pattern (Inukai et al., 2012, Mercken et al., 2013). A decline of DICER RNase and miRNA processing with age has been observed in both worms and mice, while loss of DICER or other miRNA-associated factors reduces lifespan and stress tolerance, indicating a crucial role of miRNA function in ageing and stress response (Kato et al., 2011, Lehrbach et al., 2012, Mori et al., 2012). Studies in invertebrates and mammals revealed that miRNAs can modulate lifespan and age-associated processes, positively or negatively, by targeting components of known longevity modulators such as IIS, mTOR, DR, steroid signals and mitochondrial function, linking miRNA function and ageing in humans (Garg and Cohen, 2014, Jung and Suh, 2015, Smith-Vikos and Slack, 2012).
4. The connection between ageing modulators and cytoplasmic mRNA turnover
In recent years, we have put plenty of emphasis on the effects of various signaling pathways on the regulation of transcription of specific mRNAs that can modulate ageing and age-related disorders. However, gene expression is equally determined by the rate of mRNA translation and degradation. Post-transcriptional control is also an integral part of cellular stress response, where RNA granules that regulate mRNA turnover seem to have important functions. Mechanisms and signaling pathways that further control protein levels have gained much attention as lifespan determinants in view of maintaining protein homeostasis during ageing. The impact of mRNA decay factors and related RNA particles in controlling mRNA and protein levels during ageing and stress though remains largely unexplored, while the influence of ageing modulators on mRNA decay factors or their interacting proteins has been underappreciated.
4.1. Signal transduction pathways and mRNA turnover
A growing body of evidence suggests that different signal transduction pathways with pivotal role in lifespan and stress response of diverse organisms can influence the levels or activity of mRNA decay factors in response to cellular or external stimuli. In both C. elegans and humans, the scavenger mRNA decapping enzyme DCS1, is coordinately expressed with a cytosolic NADPH-dependent flavin reductase, known as FRE-1 in worms and NR1 in humans. Induction of dcs-1 and fre-1 genes by heat-shock suggests a role of both proteins in stress response of worms (Kwasnicka et al., 2003), whereas menadione-induced cytotoxicity in human cells that overexpress NR1 can be significantly reduced by DCS1, which physically interacts and inhibits NR1 activity, promoting cell survival (Kwasnicka-Crawford et al., 2005). Likewise, nutrient stress in S. cerevisiae induces transcription of the DCS1 gene via the cAMP-PKA signaling pathway, with DCS1 to inhibit the activity of neutral trehalase, NTH1, an enzyme regulating trehalose hydrolysis (Malys et al., 2004, Schepers et al., 2012). The disaccharide trehalose is accumulated during various stress conditions and stabilizes cellular components in many invertebrates. Remarkably, trehalose levels are elevated in several long-lived worms, such as daf-2 mutants of the IIS pathway or ife-2/eIF4E translation factor mutants and are considered as one of the metabolic signatures of long-life in C. elegans (Fuchs et al., 2010).
Major signal transduction pathways can affect the localization and function of mRNA turnover factors, constituents of cytoplasmic RNA granules. In yeast, phosphorylation of DCP2 decapping enzyme in response to oxidative stress or glucose deprivation was shown to affect its localization to PBs, to promote SG formation and to stabilize a subset of mRNAs encoding ribosomal proteins (Yoon et al., 2010). Similarly in mammalian cells, phosphorylation of DCP1a by JNK in response to stress or inflammatory stimuli regulates localization of DCP1a to PBs affecting the mRNA levels of NF-kB target genes (Rzeczkowski et al., 2011). Human DCP1a is also phosphorylated via the ERK pathway during early differentiation of preadipocytes, enhancing its association with DCP2 (Chiang et al., 2013). In addition, several types of stresses that induce eIF2α phosphorylation and translational repression promote SG formation and alter gene expression.
A number of signaling pathways can also target various RNA-binding proteins (RBPs) that regulate mRNA stability and translation by promoting conventional or specific decay pathways such as NMD or ARE-mediated mRNA decay (Buchan and Parker, 2009, Schoenberg and Maquat, 2012, Venigalla and Turner, 2012). Several SG-associated RBPs undergo post-translational modifications such as phopshorylation, ubiquitination, N-acetyl glucosamination, methyl-argininylation and poly ADP-ribosylation, which in turn promote assembly of stalled mRNPs into SGs. Their assembly could alter signaling activity by interrupting and sequestering signaling components or by regulating cellular responses to various stimuli (Kedersha et al., 2013). For example, sequestration of RACK1 scaffold protein at SGs in mammalian cells inhibits apoptotic death by suppressing the stress-induced activation of p38 and JNK signaling pathways (Arimoto et al., 2008). Interestingly enough, SGs assembly in both yeast and human cells alters mTOR signaling during nutrient- or heat-stress by recruiting and inactivating TOR complex 1 (TORC1) and downstream kinases (Takahara and Maeda, 2012, Wippich et al., 2013). During recovery from stress, TORC1 reactivation correlates with SG disassembly (Takahara and Maeda, 2012). The vast majority of mTOR targets are mRNAs that contain 5’-terminal oligopyrimidine tracts and one mechanism of translation repression of such mRNAs during amino acid starvation involves the binding of such tracts by the SG-associated TIA-1 and TIAR proteins (Damgaard and Lykke-Andersen, 2011).
In turn, alterations in the activity of cytoplasmic RNA granules’ components could affect the function and outcomes of known ageing-associated signaling pathways. Defects in mRNA silencing/decay factors in PBs might influence the levels of proteins and miRNAs that are either targets or regulators of those signaling pathways. In C. elegans, loss-of-function mutations in genes encoding mRNA decay factors, such as the decapping complex or decapping activators, have negative effect on lifespan and stress resistance of wild-type animals but also reduce the long life, thermotolerance and pathogen resistance of daf-2 mutants with diminished IIS pathway (Cornes et al., 2015, Rousakis et al., 2014). Moreover, loss of decapping activity shortens lifespan of dietary restricted, translation defective or germline-deficient worms (Rousakis et al., 2014). In addition, disruption of other PB components, such as the 5’→3’ exonuclease XRN-1, the translation repressor GCH-1/DHH1/RCK or the core miRNA complex components ALG-1 or AIN-1/GW182 leads to reduced worm’s lifespan (Kato et al., 2011, Rousakis et al., 2014, Samuelson et al., 2007). It was also shown that deficiency of SIR-2.4, the worm homolog of mammalian SIRT6 and SIRT7 sirtuins, renders worms hypersensitive to heat and oxidative stress; SIR-2.4 promotes formation of P granules in germ cells of C. elegans or SGs in mammalian cells (Jedrusik-Bode et al., 2013) and modulates localization and function of DAF-16/FOXO transcription factor under stress (Chiang et al., 2012).
4.2. Protein synthesis/homeostasis and mRNA turnover
mRNA translation is a process closely coupled to, and often in competition with mRNA decay, but this interaction is affected by the status of the individual mRNA (translatable, repressed or aberrant) and its translocation into PBs or SGs (Anderson and Kedersha, 2009, Brengues et al., 2005). In response to environmental or cellular signals that inhibit translation, not-translating mRNAs accumulate in these granules for degradation or storage and recovery. Both granules are increased during stress in yeast, mammalian and Drosophila S2 cell cultures, but also in stressed or arrested oocytes of worms and flies (Jud et al., 2008, Noble et al., 2008, Schisa et al., 2001). In consistence, both PBs and SGs are formed in somatic cells of C. elegans in response to stress and the increased stress sensitivity of worm mutants for components of these granules reflect their crucial role under these conditions (Cornes et al., 2015, Rousakis et al., 2014, Sun et al., 2011).
Furthermore, the reduced lifespan of decapping mutants suggests a link of mRNA decay with ageing. Fluorescent protein markers of PBs have been monitored to accumulate in an age-dependent manner in somatic cells of adult worms (Rousakis et al., 2014) (Fig. 2). This accumulation can be attributed to a mild oxidative stress in aged tissues or to an overall reduction of mRNA translation/degradation or protein quality control mechanisms that accompany ageing. At least in C. elegans, ageing is associated with a progressive loss of proteome balance due to a decrease in ribosomal subunit proteins, along with a relief of miRNA-mediated translational repression (Walther et al., 2015). Both cause proteostasis stress and drive a widespread aggregation of the resulting aberrant proteins as part of a protective cellular response to maintain proteostasis, given that all C. elegans somatic cells are post-mitotic and have no self-renewal capacities (Walther et al., 2015). It is tempting to speculate that aggregation of PBs with age might contribute to proteostasis control by enhancing the efficiency of mRNA degradation, silencing or miRNA-mediated repression that take place into them. Maintaining of protein homeostasis is a key factor in the modulation of the ageing process but also in age-related neurodegenerative diseases, where specific peptides/proteins misfold and aggregate (Gidalevitz et al., 2011, Koga et al., 2011). Interestingly, current studies in yeast and mammalian cells have revealed a spatial sequestration of misfolded proteins into distinct protein quality control compartments (Q-bodies, JUNQ and IPOD) to undergo refolding or clearance of toxic species (Sontag et al., 2014). Thus, aggregation of cellular components in general might be protective, at least at the first stages of cellular dysfunction that is caused during ageing, stress or neurodegerative diseases (Miller et al., 2015).
The removal of potentially toxic protein aggregates is achieved by the ubiquitin-proteasome system (UPS) or the autophagosome-lysosome pathway. Latest data have unveiled an intimate relationship between these proteolytic mechanisms and cytoplasmic mRNA degradation. A recent study revealed that the ubiquitin proteasome system can regulate the levels of the mRNA degradation enzyme DCP2, to ensure proper control of its cellular activity. A C-terminal domain of the human DCP2 was found to be responsible not only for the interaction with a scaffold protein that stimulates the assembly of the decapping complex, but also for the degradation of unbound DCP2 by the proteasome; this might serve to maintain the appropriate levels of DCP2 protein relative to the remaining components of the decapping complex, or regulate its levels according to cellular cues (Erickson et al., 2015). In addition several mutations that disrupt autophagy were found to stabilize SGs and PBs in the cytoplasm of budding yeast, while blockades in the late steps of autophagic vesicle breakdown lead to the accumulation of PBs and SGs markers in an intravacuolar compartment. This targeting of RNA granules to the vacuole by autophagy was referred as “granulophagy” and may provide a mechanism for efficient PBs/SGs clearance, regulating their abundance during stress and development, serving in parallel as a means for the mass degradation of mRNA molecules that reside inside them (Buchan et al., 2013). A similar mechanism has been found to occur during C. elegans embryonic development, where germ-line specific mRNPs (P-granules) are selectively removed from somatic cells through autophagy (Zhang et al., 2009).
Conversely, the function of the mRNA decay machinery seems to affect the process of autophagy under stress conditions. Inactivation of TOR kinase pathway upon nutrient limitation in yeast was shown to prevent phosphorylation of the decapping enzyme DCP2, causing dissociation of the DHH1/RCK decapping activator from autophagy-related (atg) mRNAs. Thus, atg transcripts are not degraded by the 5’→3' mRNA decay pathway and induce autophagy and cell survival (Hu et al., 2015). What is more, stress induced inhibition of the NMD pathway, responsible for the degradation of aberrant mRNAs, can stimulate autophagy in several mammalian cell lines, due to the accumulation of the resulting aberrant protein products and/or the stabilization of NMD target mRNAs involved in the induction of autophagy (Wengrod et al., 2013). These observations suggest a role for NMD inhibition in the augmentation of cellular stress response, providing a link between the major mRNA and protein surveillance pathways in mammalian cells.
4.3. miRNA functions and mRNA turnover
In order to exert their regulatory effects in gene expression, mature miRNAs in the cytoplasm associate with the ribonucleoprotein complex miRISC (miRNA-induced silencing complex), the core of which is composed of the Argonaute (AGO) proteins (Bartel, 2009). The mechanisms used by miRISC to silence gene expression include inhibition of translation initiation or elongation, premature ribosomal drop-off and co-translational protein degradation, where the levels of target mRNA are not affected. Moreover, miRISC can accelerate mRNA decay by two distinct mechanisms; they direct endonucleolytic cleavage by AGOs, if the target mRNA is perfect complementary to the miRNA, or promote mRNA deadenylation and subsequently degradation by exoribonucleases, in cases of insufficient complementarity (Eulalio et al., 2008, Filipowicz et al., 2008). As most animal miRNAs are only partially complementary to their targets, translational repression and/or mRNA decay are the prevailing mechanisms of miRNA-mediated gene silencing. It is still under debate whether translational repression precedes mRNA deadenylation or there is interplay between the two regulatory mechanisms. Based on several in vitro, in vivo and genome-wide studies, a temporal model of miRNA-mediated gene silencing has been proposed (Fabian and Sonenberg, 2012). According to this model, miRNAs can inhibit translation initiation in a deadenylation-independent manner and subsequently can trigger deadenylation, destabilization and degradation through the typical mRNA decay pathways. It is also possible for translational repression and deadenylation to occur independently (Huntzinger et al., 2012).
Several studies have shown that miRNA-repressed mRNAs, miRNAs, AGO and GW182 proteins, apart from their cytoplasmic localization, can be also detected in PBs (Anderson and Kedersha, 2009, Eulalio et al., 2007a, Pillai et al., 2005). Although the localization of the silencing machinery into PBs is a consequence and not the cause of translational repression, in both human cells and Drosophila (Eulalio et al., 2007b, Pillai et al., 2005), the accumulation of miRNA-repressed mRNAs in RNA granules such us PBs and SGs, could provide a rapid or adaptive response to specific developmental or stress conditions. In support to this, miR-122-repressed CAT-1 mRNA in human cells can be released from PBs and return to active translation during amino acid deprivation (Bhattacharyya et al., 2006). Linked to the above is the finding that a subset of miRNAs and two miRISC components, PACT and AGO2, can associate with SGs and undergo dynamic exchange with polyribosomes in the cytoplasm of human cells under stress (Johnston et al., 2010, Leung et al., 2006, Pare et al., 2009). The chaperone Hsp90 is critical for this association, as well as post-translational modifications of AGO2 by stress-induced signaling pathways that affect the ageing process, connecting miRNA activity to cellular stress and ageing (Emde and Hornstein, 2014). miRNA activity during stress and ageing can be also modulated by specific RNA-binding proteins that bind adjacent to miRNA binding sites and control the expression of the same target mRNA. Furthermore, stress can affect the expression or the processing of miRNAs through changes in related transcription factors or signaling molecules (Leung and Sharp, 2010).
Although miRNA abundance is highly regulated at the biogenesis and processing levels, degradation mechanisms that control their half-lives are similarly important for miRNA homeostasis in all conditions. Several miRNAs display a dynamic expression pattern in a developmental stage- or tissue-specific manner without variation in the expression of miRNA precursors and various cellular conditions trigger rapid degradation of miRNAs (Krol et al., 2010, Ruegger and Grosshans, 2012). Both 5’→3’ and 3’→5’ exoribonucleases have been linked to miRNA degradation (Bail et al., 2010), whereas autophagic clearance of the miRNA-free miRISC components, DICER and AGO2 has been reported in human cells (Gibbings et al., 2012). In C. elegans, the 5’→3’ exoribonucleases XRN-1 and XRN-2 catalyze the degradation of AGO-unbound miRNAs (Chatterjee and Grosshans, 2009, Kai and Pasquinelli, 2010), whereas the scavenger decapping enzyme DCS-1 interacts with XRN-1 and stimulates miRNA degradation (Bosse et al., 2013). All together these data suggest a plausible link between the mRNA decay machinery and miRNA levels/function, which can regulate gene expression during both stress and ageing.
5. Concluding remarks
Regulation of mRNA stability in the cytoplasm represents an important means of post-transcriptional gene regulation in eukaryotes, in both normal and stress conditions. The mRNA decay rate or turnover determines the cytoplasmic abundance of all transcripts and is influenced by the assembly and function of specific RNA granules, termed PBs and SGs. They are sites of degradation or storage of un-translating mRNAs, with great importance under cellular or environmental conditions where a rapid and transient response is required. They have crucial roles during development and stress response/adaption but their role in ageing, a long-term process that may be regulated by short term stress responses, is also gradually emerging. A functional interplay between mRNA turnover mechanisms and well-known ageing modulators is depicted in Fig. 3.
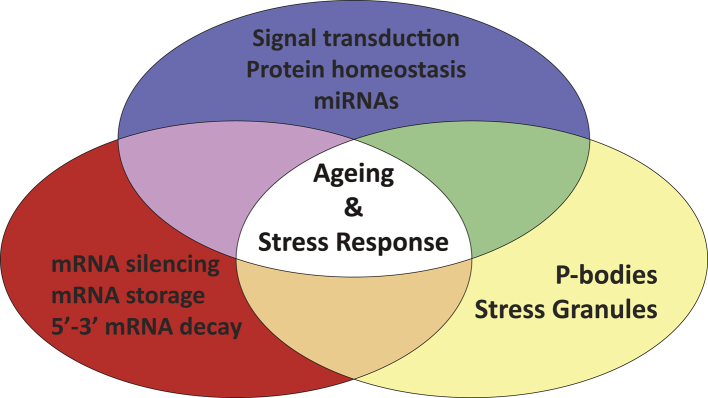
Fig. 3.
Functional interplay between modulators of ageing and cytoplasmic mRNA decay mechanisms. Key ageing modulators such as signal transduction pathways, protein synthesis/homeostasis and miRNAs can regulate the translational silencing, storage or degradation of mRNAs, which can be occurred to specific cytoplasmic RNA granules (PBs and SGs). In turn, mRNA turnover factors and RNA granules can influence the function and outcomes of ageing modulators.
Major signaling pathways that modulate ageing and stress survival in diverse species can affect mRNA turnover by directly altering the levels, activity or localization of decay factors as well as of trans-acting elements (RBPs and miRNAs) of transcripts. mRNA turnover factors and related RNA granules can in turn influence the function and outcome of such signaling pathways by targeting downstream effectors or regulators of them. Reduced mRNA translation is an important longevity determinant and a first point of the integrated stress response. A reduction in mRNA translation is achieved by proper induction of mRNA decay/repression mechanisms and preserves protein homeostasis by preventing the production of excessive amounts of proteins. The function of quality control systems in turn can affect the abundance of both mRNA turnover factors and their respective RNA granules. miRNAs regulate a broad range of gene expression and may help to confer robustness during stress adaptation and ageing. Also, the degradation of miRNAs as well as their mRNA targets has been linked to typical mRNA decay factors whereas storage of miRNA-repressed mRNAs in PBs and SGs could provide a rapid or adaptive response to specific developmental or stress conditions. Taken together, mRNA turnover can modulate many aspects of the ageing process and a better understanding of the involved factors and mechanisms is needed. Such investigation may allow us to develop novel and multidisciplinary approaches for the study of the ageing process that could drive anti-ageing interventions for extending healthy lifespan.
Acknowledgements
We apologize to investigators whose research could not be cited owing to space limitations. We thank George Kellaris and George Kanatouris for support and critical suggestions on the manuscript. Work in the laboratory of P.S. was supported by European Research Council under the European Union’s Seventh Framework Program (FP/2007-2013)/ERC Grant Agreement no. [201975].
References
- Anderson P., Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 2009;10:430–436. doi: 10.1038/nrm2694. [DOI] [PubMed] [Google Scholar]
- Andrei M.A., Ingelfinger D., Heintzmann R., Achsel T., Rivera-Pomar R., Luhrmann R. A role for eIF4E and eIF4E-transporter in targeting mRNPs to mammalian processing bodies. RNA. 2005;11:717–727. doi: 10.1261/rna.2340405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antic S., Wolfinger M.T., Skucha A., Hosiner S., Dorner S. General and microRNA-mediated mRNA degradation occurs on ribosome complexes in Drosophila cells. Mol. Cell. Biol. 2015;35:2309–2320. doi: 10.1128/MCB.01346-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimoto K., Fukuda H., Imajoh-Ohmi S., Saito H., Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat. Cell Biol. 2008;10:1324–1332. doi: 10.1038/ncb1791. [DOI] [PubMed] [Google Scholar]
- Arnsburg K., Kirstein-Miles J. Interrelation between protein synthesis, proteostasis and life span. Curr. Genomics. 2014;15:66–75. doi: 10.2174/1389202915666140210210542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribas-Layton M., Wu D., Lykke-Andersen J., Song H. Structural and functional control of the eukaryotic mRNA decapping machinery. Biochim. Biophys. Acta. 2013;1829:580–589. doi: 10.1016/j.bbagrm.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bail S., Swerdel M., Liu H., Jiao X., Goff L.A., Hart R.P., Kiledjian M. Differential regulation of microRNA stability. RNA. 2010;16:1032–1039. doi: 10.1261/rna.1851510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakheet T., Williams B.R., Khabar K.S. ARED 3.0: the large and diverse AU-rich transcriptome. Nucleic Acids Res. 2006;34:D111–D114. doi: 10.1093/nar/gkj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A., Sun L.Y., Longo V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol. Rev. 2013;93:571–598. doi: 10.1152/physrev.00006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm-Ansmant I., Rehwinkel J., Doerks T., Stark A., Bork P., Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006;20:1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S.N., Habermacher R., Martine U., Closs E.I., Filipowicz W. Stress-induced reversal of microRNA repression and mRNA P-body localization in human cells. Cold Spring Harb. Symp. Quant. Biol. 2006;71:513–521. doi: 10.1101/sqb.2006.71.038. [DOI] [PubMed] [Google Scholar]
- Bjedov I., Toivonen J.M., Kerr F., Slack C., Jacobson J., Foley A., Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny M.V. Rapamycin extends life- and health span because it slows aging. Aging (Albany NY) 2013;5:592–598. doi: 10.18632/aging.100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm M., Slack F. A developmental timing microRNA and its target regulate life span in C. elegans. Science. 2005;310:1954–1957. doi: 10.1126/science.1115596. [DOI] [PubMed] [Google Scholar]
- Bosse G.D., Ruegger S., Ow M.C., Vasquez-Rifo A., Rondeau E.L., Ambros V.R., Grosshans H., Simard M.J. The decapping scavenger enzyme DCS-1 controls microRNA levels in Caenorhabditis elegans. Mol. Cell. 2013;50:281–287. doi: 10.1016/j.molcel.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brengues M., Teixeira D., Parker R. Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science. 2005;310:486–489. doi: 10.1126/science.1115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan J.R., Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol. Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan J.R., Muhlrad D., Parker R. P bodies promote stress granule assembly in Saccharomyces cerevisiae. J. Cell Biol. 2008;183:441–455. doi: 10.1083/jcb.200807043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan J.R., Kolaitis R.M., Taylor J.P., Parker R. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell. 2013;153:1461–1474. doi: 10.1016/j.cell.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchberger A., Bukau B., Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol. Cell. 2010;40:238–252. doi: 10.1016/j.molcel.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Grosshans H. Active turnover modulates mature microRNA activity in Caenorhabditis elegans. Nature. 2009;461:546–549. doi: 10.1038/nature08349. [DOI] [PubMed] [Google Scholar]
- Chiang W.C., Tishkoff D.X., Yang B., Wilson-Grady J., Yu X., Mazer T., Eckersdorff M., Gygi S.P., Lombard D.B., Hsu A.L. C. elegans SIRT6/7 homolog SIR-2.4 promotes DAF-16 relocalization and function during stress. PLoS Genet. 2012;8:e1002948. doi: 10.1371/journal.pgen.1002948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang P.Y., Shen Y.F., Su Y.L., Kao C.H., Lin N.Y., Hsu P.H., Tsai M.D., Wang S.C., Chang G.D., Lee S.C., Chang C.J. Phosphorylation of mRNA decapping protein Dcp1a by the ERK signaling pathway during early differentiation of 3T3-L1 preadipocytes. PLoS One. 2013;8:e61697. doi: 10.1371/journal.pone.0061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondrogianni N., Voutetakis K., Kapetanou M., Delitsikou V., Papaevgeniou N., Sakellari M., Lefaki M., Filippopoulou K., Gonos E.S. Proteasome activation: an innovative promising approach for delaying aging and retarding age-related diseases. Ageing Res. Rev. 2015;23:37–55. doi: 10.1016/j.arr.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Ciryam P., Tartaglia G.G., Morimoto R.I., Dobson C.M., Vendruscolo M. Widespread aggregation and neurodegenerative diseases are associated with supersaturated proteins. Cell Rep. 2013;5:781–790. doi: 10.1016/j.celrep.2013.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho D.S., Domingos P.M. Physiological roles of regulated Ire1 dependent decay. Front. Genet. 2014;5(76) doi: 10.3389/fgene.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E., Bieschke J., Perciavalle R.M., Kelly J.W., Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- Cole S.E., LaRiviere F.J., Merrikh C.N., Moore M.J. A convergence of rRNA and mRNA quality control pathways revealed by mechanistic analysis of nonfunctional rRNA decay. Mol. Cell. 2009;34:440–450. doi: 10.1016/j.molcel.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornes E., Porta-De-La-Riva M., Aristizabal-Corrales D., Brokate-Llanos A.M., Garcia-Rodriguez F.J., Ertl I., Diaz M., Fontrodona L., Reis K., Johnsen R., Baillie D., Munoz M.J., Sarov M., Dupuy D., Ceron J. Cytoplasmic LSM-1 protein regulates stress responses through the insulin/IGF-1 signaling pathway in Caenorhabditis elegans. RNA. 2015 doi: 10.1261/rna.052324.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu M., Albert V., Hall M.N. mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev. 2013;23:53–62. doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Cougot N., Babajko S., Seraphin B. Cytoplasmic foci are sites of mRNA decay in human cells. J. Cell Biol. 2004;165:31–40. doi: 10.1083/jcb.200309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard C.K., Lykke-Andersen J. Translational coregulation of 5’TOP mRNAs by TIA-1 and TIAR. Genes Dev. 2011;25:2057–2068. doi: 10.1101/gad.17355911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D.C., Ollikainen N., Trinidad J.C., Cary M.P., Burlingame A.L., Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8:e1000450. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker C.J., Parker R. P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb. Perspect. Biol. 2012;4:a012286. doi: 10.1101/cshperspect.a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt G., Xie F., Petyuk V.A., Shanmugam N., Smolders A., Dhondt I., Brewer H.M., Camp D.G., 2nd Smith, Braeckman R.D., BP Reduced insulin/insulin-like growth factor-1 signaling and dietary restriction inhibit translation but preserve muscle mass in Caenorhabditis elegans. Mol. Cell. Proteomics. 2013;12:3624–3639. doi: 10.1074/mcp.M113.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doma M.K., Parker R. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature. 2006;440:561–564. doi: 10.1038/nature04530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M.Q., Venable J.D., Au N., Xu T., Park S.K., Cociorva D., Johnson J.R., Dillin A., Yates J.R., 3rd Quantitative mass spectrometry identifies insulin signaling targets in C. elegans. Science. 2007;317:660–663. doi: 10.1126/science.1139952. [DOI] [PubMed] [Google Scholar]
- Ebert M.S., Sharp P.A. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R.J., Minton A.P. Protein aggregation in crowded environments. Biol. Chem. 2006;387:485–497. doi: 10.1515/BC.2006.064. [DOI] [PubMed] [Google Scholar]
- Emde A., Hornstein E. miRNAs at the interface of cellular stress and disease. EMBO J. 2014;33:1428–1437. doi: 10.15252/embj.201488142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson S.L., Corpuz E.O., Maloy J.P., Fillman C., Webb K., Bennett E.J., Lykke-Andersen J. Competition between decapping complex formation and ubiquitin-mediated proteasomal degradation controls human Dcp2 decapping activity. Mol. Cell Biol. 2015;35:2144–2153. doi: 10.1128/MCB.01517-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 2007;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Schweizer D., Izaurralde E. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol. Cell Biol. 2007;27:3970–3981. doi: 10.1128/MCB.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A., Huntzinger E., Izaurralde E. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat. Struct. Mol. Biol. 2008;15:346–353. doi: 10.1038/nsmb.1405. [DOI] [PubMed] [Google Scholar]
- Eystathioy T., Jakymiw A., Chan E.K., Seraphin B., Cougot N., Fritzler M.J. The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA. 2003;9:1171–1173. doi: 10.1261/rna.5810203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian M.R., Sonenberg N. The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat. Struct. Mol. Biol. 2012;19:586–593. doi: 10.1038/nsmb.2296. [DOI] [PubMed] [Google Scholar]
- Ferraiuolo M.A., Basak S., Dostie J., Murray E.L., Schoenberg D.R., Sonenberg N. A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay. J. Cell Biol. 2005;170:913–924. doi: 10.1083/jcb.200504039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W., Bhattacharyya S.N., Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca B.D., Smith E.M., Yelle N., Alain T., Bushell M., Pause A. The ever-evolving role of mTOR in translation. Semin. Cell Dev. Biol. 2014;36:102–112. doi: 10.1016/j.semcdb.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Fuchs S., Bundy J.G., Davies S.K., Viney J.M., Swire J.S., Leroi A.M. A metabolic signature of long life in Caenorhabditis elegans. BMC Biol. 2010;8:14. doi: 10.1186/1741-7007-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard H., Aguilera A. A novel class of mRNA-containing cytoplasmic granules are produced in response to UV-irradiation. Mol. Biol. Cell. 2008;19:4980–4992. doi: 10.1091/mbc.E08-02-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinetti J., Harputlugil E., Mitchell J.R. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem. J. 2013;449:1–10. doi: 10.1042/BJ20121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo C.M., Munro E., Rasoloson D., Merritt C., Seydoux G. Processing bodies and germ granules are distinct RNA granules that interact in C. elegans embryos. Dev. Biol. 2008;323:76–87. doi: 10.1016/j.ydbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura L., Perez-Andrade M., Miranda-Rios J. The emerging role of MicroRNAs in the regulation of gene expression by nutrients. J. Nutrigenet Nutrigenomics. 2013;6:16–31. doi: 10.1159/000345826. [DOI] [PubMed] [Google Scholar]
- Garg D., Cohen S.M. miRNAs and aging: a genetic perspective. Ageing Res. Rev. 2014;17:3–8. doi: 10.1016/j.arr.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Garneau N.L., Wilusz J., Wilusz C.J. The highways and byways of mRNA decay. Nat. Rev. Mol. Cell Biol. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- Gibbings D., Mostowy S., Jay F., Schwab Y., Cossart P., Voinnet O. Selective autophagy degrades DICER and AGO2 and regulates miRNA activity. Nat. Cell Biol. 2012;14:1314–1321. doi: 10.1038/ncb2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidalevitz T., Prahlad V., Morimoto R.I. The stress of protein misfolding: from single cells to multicellular organisms. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M., Chandra A., Mitic L.L., Onken B., Driscoll M., Kenyon C. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008;4:e24. doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H., Rubinsztein D.C. Control of autophagy as a therapy for neurodegenerative disease. Nat. Rev. Neurol. 2011;8:108–117. doi: 10.1038/nrneurol.2011.200. [DOI] [PubMed] [Google Scholar]
- Hartl F.U., Bracher A., Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien J., Lin J.H., Li H., Stevens N., Walter P., Weissman J.S. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A.L., Murphy C.T., Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Hu W., Sweet T.J., Chamnongpol S., Baker K.E., Coller J. Co-translational mRNA decay in Saccharomyces cerevisiae. Nature. 2009;461:225–229. doi: 10.1038/nature08265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Petzold C., Coller J., Baker K.E. Nonsense-mediated mRNA decapping occurs on polyribosomes in Saccharomyces cerevisiae. Nat. Struct. Mol. Biol. 2010;17:244–247. doi: 10.1038/nsmb.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G., McQuiston T., Bernard A., Park Y.D., Qiu J., Vural A., Zhang N., Waterman S.R., Blewett N.H., Myers T.G., Maraia R.J., Kehrl J.H., Uzel G., Klionsky D.J., Williamson P.R. A conserved mechanism of TOR-dependent RCK-mediated mRNA degradation regulates autophagy. Nat. Cell Biol. 2015;17:930–942. doi: 10.1038/ncb3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntzinger E., Kuzuoglu-Ozturk D., Braun J.E., Eulalio A., Wohlbold L., Izaurralde E. The interactions of GW182 proteins with PABP and deadenylases are required for both translational repression and degradation of miRNA targets. Nucleic Acids Res. 2012;41:978–994. doi: 10.1093/nar/gks1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez-Ventoso C., Yang M., Guo S., Robins H., Padgett R.W., Driscoll M. Modulated microRNA expression during adult lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:235–246. doi: 10.1111/j.1474-9726.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Imai S., Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai S., de Lencastre A., Turner M., Slack F. Novel microRNAs differentially expressed during aging in the mouse brain. PLoS One. 2012;7:e40028. doi: 10.1371/journal.pone.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrusik-Bode M., Studencka M., Smolka C., Baumann T., Schmidt H., Kampf J., Paap F., Martin S., Tazi J., Muller K.M., Kruger M., Braun T., Bober E. The sirtuin SIRT6 regulates stress granule formation in C. elegans and mammals. J. Cell Sci. 2013;126:5166–5177. doi: 10.1242/jcs.130708. [DOI] [PubMed] [Google Scholar]
- Johnson S.C., Rabinovitch P.S., Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M., Geoffroy M.C., Sobala A., Hay R., Hutvagner G. HSP90 protein stabilizes unloaded argonaute complexes and microscopic P-bodies in human cells. Mol. Biol. Cell. 2010;21:1462–1469. doi: 10.1091/mbc.E09-10-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas S., Izaurralde E. The role of disordered protein regions in the assembly of decapping complexes and RNP granules. Genes Dev. 2013;27:2628–2641. doi: 10.1101/gad.227843.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jud M.C., Czerwinski M.J., Wood M.P., Young R.A., Gallo C.M., Bickel J.S., Petty E.L., Mason J.M., Little B.A., Padilla P.A., Schisa J.A. Large P body-like RNPs form in C. elegans oocytes in response to arrested ovulation, heat shock, osmotic stress, and anoxia and are regulated by the major sperm protein pathway. Dev. Biol. 2008;318:38–51. doi: 10.1016/j.ydbio.2008.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H.J., Suh Y. Regulation of IGF-1 signaling by microRNAs. Front. Genet. 2015;5(472) doi: 10.3389/fgene.2014.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai Z.S., Pasquinelli A.E. MicroRNA assassins: factors that regulate the disappearance of miRNAs. Nat. Struct. Mol. Biol. 2010;17:5–10. doi: 10.1038/nsmb.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P., Chen D., Rogers A.N., Katewa S.D., Li P.W., Thomas E.L., Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Chen X., Inukai S., Zhao H., Slack F.J. Age-associated changes in expression of small, noncoding RNAs, including microRNAs, in C. elegans. RNA. 2011;17:1804–1820. doi: 10.1261/rna.2714411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N., Stoecklin G., Ayodele M., Yacono P., Lykke-Andersen J., Fritzler M.J., Scheuner D., Kaufman R.J., Golan D.E., Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N., Ivanov P., Anderson P. Stress granules and cell signaling: more than just a passing phase? Trends Biochem. Sci. 2013;38:494–506. doi: 10.1016/j.tibs.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C.J. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kervestin S., Jacobson A. NMD: a multifaceted response to premature translational termination. Nat. Rev. Mol. Cell. Biol. 2012;13:700–712. doi: 10.1038/nrm3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball S.R., Horetsky R.L., Ron D., Jefferson L.S., Harding H.P. Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am. J. Physiol. Cell Physiol. 2003;284:C273–C284. doi: 10.1152/ajpcell.00314.2002. [DOI] [PubMed] [Google Scholar]
- Koga H., Kaushik S., Cuervo A.M. Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res. Rev. 2011;10:205–215. doi: 10.1016/j.arr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M., Yanase S., Ishii T., Hartman P.S., Matsumoto K., Ishii N. The p38 signal transduction pathway participates in the oxidative stress-mediated translocation of DAF-16 to Caenorhabditis elegans nuclei. Mech. Ageing Dev. 2005;126:642–647. doi: 10.1016/j.mad.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- Kulkarni M., Ozgur S., Stoecklin G. On track with P-bodies. Biochem. Soc. Trans. 2010;38:242–251. doi: 10.1042/BST0380242. [DOI] [PubMed] [Google Scholar]
- Kwasnicka D.A., Krakowiak A., Thacker C., Brenner C., Vincent S.R. Coordinate expression of NADPH-dependent flavin reductase, Fre-1, and Hint-related 7meGMP-directed hydrolase, DCS-1. J. Biol. Chem. 2003;278:39051–39058. doi: 10.1074/jbc.M306355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwasnicka-Crawford D.A., Carson A.R., Roberts W., Summers A.M., Rehnstrom K., Jarvela I., Scherer S.W. Characterization of a novel cation transporter ATPase gene (ATP13A4) interrupted by 3q25-q29 inversion in an individual with language delay. Genomics. 2005;86:182–194. doi: 10.1016/j.ygeno.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Lee I.H., Cao L., Mostoslavsky R., Lombard D.B., Liu J., Bruns N.E., Tsokos M., Alt F.W., Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach N.J., Castro C., Murfitt K.J., Abreu-Goodger C., Griffin J.L., Miska E.A. Post-developmental microRNA expression is required for normal physiology, and regulates aging in parallel to insulin/IGF-1 signaling in C. elegans. RNA. 2012;18:2220–2235. doi: 10.1261/rna.035402.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A.K., Sharp P.A. MicroRNA functions in stress responses. Mol. Cell. 2010;40:205–215. doi: 10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A.K., Calabrese J.M., Sharp P.A. Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18125–18130. doi: 10.1073/pnas.0608845103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Song M., Kiledjian M. Differential utilization of decapping enzymes in mammalian mRNA decay pathways. RNA. 2011;17:419–428. doi: 10.1261/rna.2439811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J., Ha M., Chang H., Kwon S.C., Simanshu D.K., Patel D.J., Kim V.N. Uridylation by TUT4 and TUT7 marks mRNA for degradation. Cell. 2014;159:1365–1376. doi: 10.1016/j.cell.2014.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.J., Duffy A., Chen C.Y. Localization of AU-rich element-containing mRNA in cytoplasmic granules containing exosome subunits. J. Biol. Chem. 2007;282:19958–19968. doi: 10.1074/jbc.M702281200. [DOI] [PubMed] [Google Scholar]
- Lubas M., Damgaard C.K., Tomecki R., Cysewski D., Jensen T.H., Dziembowski A. Exonuclease hDIS3L2 specifies an exosome-independent 3’-5’ degradation pathway of human cytoplasmic mRNA. EMBO J. 2013;32:1855–1868. doi: 10.1038/emboj.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecki M., Viegas S.C., Carneiro T., Golik P., Dressaire C., Ferreira M.G., Arraiano C.M. The exoribonuclease Dis3L2 defines a novel eukaryotic RNA degradation pathway. EMBO J. 2013;32:1842–1854. doi: 10.1038/emboj.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malys N., Carroll K., Miyan J., Tollervey D., McCarthy J.E. The ‘scavenger’ m7GpppX pyrophosphatase activity of Dcs1 modulates nutrient-induced responses in yeast. Nucleic Acids Res. 2004;32:3590–3600. doi: 10.1093/nar/gkh687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez A., Talloczy Z., Seaman M., Eskelinen E.L., Hall D.H., Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- Mendell J.T., Olson E.N. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken E.M., Majounie E., Ding J., Guo R., Kim J., Bernier M., Mattison J., Cookson M.R., Gorospe M., de Cabo R., Abdelmohsen K. Age-associated miRNA alterations in skeletal muscle from rhesus monkeys reversed by caloric restriction. Aging (Albany NY) 2013;5:692–703. doi: 10.18632/aging.100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milac A.L., Bojarska E., Wypijewska del Nogal A. Decapping Scavenger (DcpS) enzyme: advances in its structure, activity and roles in the cap-dependent mRNA metabolism. Biochim. Biophys. Acta. 2014;1839:452–462. doi: 10.1016/j.bbagrm.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Olivas W.M. Roles of Puf proteins in mRNA degradation and translation. Wiley Interdiscip. Rev. RNA. 2010;2:471–492. doi: 10.1002/wrna.69. [DOI] [PubMed] [Google Scholar]
- Miller S.B., Mogk A., Bukau B. Spatially organized aggregation of misfolded proteins as cellular stress defense strategy. J. Mol. Biol. 2015;427:1564–1574. doi: 10.1016/j.jmb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Milman S., Atzmon G., Huffman D.M., Wan J., Crandall J.P., Cohen P., Barzilai N. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. 2014;13:769–771. doi: 10.1111/acel.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei H., Suarez J.A., Longo V.D. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol. Metab. 2014;25:558–566. doi: 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S.F., Jain S., She M., Parker R. Global analysis of yeast mRNPs. Nat. Struct. Mol. Biol. 2013;20:127–133. doi: 10.1038/nsmb.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokas S., Mills J.R., Garreau C., Fournier M.J., Robert F., Arya P., Kaufman R.J., Pelletier J., Mazroui R. Uncoupling stress granule assembly and translation initiation inhibition. Mol. Biol. Cell. 2009;20:2673–2683. doi: 10.1091/mbc.E08-10-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M.A., Raghavan P., Thomou T., Boucher J., Robida-Stubbs S., Macotela Y., Russell S.J., Kirkland J.L., Blackwell T.K., Kahn C.R. Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab. 2012;16:336–347. doi: 10.1016/j.cmet.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley J.F., Brignull H.R., Weyers J.J., Morimoto R.I. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S.L., Allen B.L., Goh L.K., Nordick K., Evans T.C. Maternal mRNAs are regulated by diverse P body-related mRNP granules during early Caenorhabditis elegans development. J. Cell Biol. 2008;182:559–572. doi: 10.1083/jcb.200802128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.W., Mukhopadhyay A., Svrzikapa N., Jiang F., Davis R.J., Tissenbaum H.A. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohn T., Kedersha N., Hickman T., Tisdale S., Anderson P. A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat. Cell Biol. 2008;10:1224–1231. doi: 10.1038/ncb1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olzscha H., Schermann S.M., Woerner A.C., Pinkert S., Hecht M.H., Tartaglia G.G., Vendruscolo M., Hayer-Hartl M., Hartl F.U., Vabulas R.M. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell. 2011;144:67–78. doi: 10.1016/j.cell.2010.11.050. [DOI] [PubMed] [Google Scholar]
- Palmer J.D., Soule B.P., Simone B.A., Zaorsky N.G., Jin L., Simone N.L. MicroRNA expression altered by diet: can food be medicinal? Ageing Res. Rev. 2014;17:16–24. doi: 10.1016/j.arr.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Pare J.M., Tahbaz N., Lopez-Orozco J., LaPointe P., Lasko P., Hobman T.C. Hsp90 regulates the function of argonaute 2 and its recruitment to stress granules and P-bodies. Mol. Biol. Cell. 2009;20:3273–3284. doi: 10.1091/mbc.E09-01-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale A., Govoni S. The complex world of post-transcriptional mechanisms: is their deregulation a common link for diseases? Focus on ELAV-like RNA-binding proteins. Cell. Mol. Life Sci. 2012;69:501–517. doi: 10.1007/s00018-011-0810-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccarelli M., Kebaara B.W. Regulation of natural mRNAs by the nonsense-mediated mRNA decay pathway. Eukaryot Cell. 2014;13:1126–1135. doi: 10.1128/EC.00090-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelechano V., Wei W., Steinmetz L.M. Widespread co-translational RNA decay reveals ribosome dynamics. Cell. 2015;161:1400–1412. doi: 10.1016/j.cell.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai R.S., Bhattacharyya S.N., Artus C.G., Zoller T., Cougot N., Basyuk E., Bertrand E., Filipowicz W. Inhibition of translational initiation by Let-7 microRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- Preiss T., Hentze W.M. Starting the protein synthesis machine: eukaryotic translation initiation. Bioessays. 2003;25:1201–1211. doi: 10.1002/bies.10362. [DOI] [PubMed] [Google Scholar]
- Reis-Rodrigues P., Czerwieniec G., Peters T.W., Evani U.S., Alavez S., Gaman E.A., Vantipalli M., Mooney S.D., Gibson B.W., Lithgow G.J., Hughes R.E. Proteomic analysis of age-dependent changes in protein solubility identifies genes that modulate lifespan. Aging Cell. 2012;11:120–127. doi: 10.1111/j.1474-9726.2011.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissland O.S., Norbury C.J. Decapping is preceded by 3’ uridylation in a novel pathway of bulk mRNA turnover. Nat. Struct. Mol. Biol. 2009;16:616–623. doi: 10.1038/nsmb.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousakis A., Vlanti A., Borbolis F., Roumelioti F., Kapetanou M., Syntichaki P. Diverse functions of mRNA metabolism factors in stress defense and aging of Caenorhabditis elegans. PLoS One. 2014;9:e103365. doi: 10.1371/journal.pone.0103365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger S., Grosshans H. MicroRNA turnover: when, how, and why. Trends Biochem. Sci. 2012;37:436–446. doi: 10.1016/j.tibs.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Rzeczkowski K., Beuerlein K., Muller H., Dittrich-Breiholz O., Schneider H., Kettner-Buhrow D., Holtmann H., Kracht M. c-Jun N-terminal kinase phosphorylates DCP1a to control formation of P bodies. J. Cell Biol. 2011;194:581–596. doi: 10.1083/jcb.201006089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson A.V., Carr C.E., Ruvkun G. Gene activities that mediate increased life span of C. elegans insulin-like signaling mutants. Genes Dev. 2007;21:2976–2994. doi: 10.1101/gad.1588907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers W., Van Zeebroeck G., Pinkse M., Verhaert P., Thevelein J.M. In vivo phosphorylation of Ser21 and Ser83 during nutrient-induced activation of the yeast protein kinase A (PKA) target trehalase. J. Biol. Chem. 2012;287:44130–44142. doi: 10.1074/jbc.M112.421503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisa J.A., Pitt J.N., Priess J.R. Analysis of RNA associated with P granules in germ cells of C. elegans adults. Development. 2001;128:1287–1298. doi: 10.1242/dev.128.8.1287. [DOI] [PubMed] [Google Scholar]
- Schoenberg D.R., Maquat L.E. Regulation of cytoplasmic mRNA decay. Nat. Rev. Genet. 2012;13:246–259. doi: 10.1038/nrg3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Wollam J., Magner D., Karalay O., Antebi A. A steroid receptor-microRNA switch regulates life span in response to signals from the gonad. Science. 2012;338:1472–1476. doi: 10.1126/science.1228967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U., Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 2003;300:805–808. doi: 10.1126/science.1082320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Vikos T., Slack F.J. MicroRNAs and their roles in aging. J. Cell Sci. 2012;125:7–17. doi: 10.1242/jcs.099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Hinnebusch A.G. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M.G., Li Y., Kiledjian M. Multiple mRNA decapping enzymes in mammalian cells. Mol. Cell. 2010;40:423–432. doi: 10.1016/j.molcel.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E.M., Vonk W.I., Frydman J. Sorting out the trash: the spatial nature of eukaryotic protein quality control. Curr. Opin. Cell Biol. 2014;26:139–146. doi: 10.1016/j.ceb.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs K.A., Bushell M., Willis A.E. Translational regulation of gene expression during conditions of cell stress. Mol. Cell. 2010;40:228–237. doi: 10.1016/j.molcel.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Squier T.C. Oxidative stress and protein aggregation during biological aging. Exp. Gerontol. 2001;36:1539–1550. doi: 10.1016/s0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Stalder L., Muhlemann O. Processing bodies are not required for mammalian nonsense-mediated mRNA decay. RNA. 2009;15:1265–1273. doi: 10.1261/rna.1672509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout G.J., Stigter E.C., Essers P.B., Mulder K.W., Kolkman A., Snijders D.S., van den Broek N.J., Betist M.C., Korswagen H.C., Macinnes A.W., Brenkman A.B. Insulin/IGF-1-mediated longevity is marked by reduced protein metabolism. Mol. Syst. Biol. 2013;9:679. doi: 10.1038/msb.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Yang P., Zhang Y., Bao X., Li J., Hou W., Yao X., Han J., Zhang H. A genome-wide RNAi screen identifies genes regulating the formation of P bodies in C. elegans and their functions in NMD and RNAi. Protein Cell. 2011;2:918–939. doi: 10.1007/s13238-011-1119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syntichaki P., Troulinaki K., Tavernarakis N. Protein synthesis is a novel determinant of aging in Caenorhabditis elegans. Ann. N.Y. Acad. Sci. 2007;1119:289–295. doi: 10.1196/annals.1404.001. [DOI] [PubMed] [Google Scholar]
- Takahara T., Maeda T. TORC1 of fission yeast is rapamycin-sensitive. Genes Cells. 2012;17:698–708. doi: 10.1111/j.1365-2443.2012.01618.x. [DOI] [PubMed] [Google Scholar]
- Taverniti V., Seraphin B. Elimination of cap structures generated by mRNA decay involves the new scavenger mRNA decapping enzyme Aph1/FHIT together with DcpS. Nucleic Acids Res. 2015;43:482–492. doi: 10.1093/nar/gku1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira D., Parker R. Analysis of P-body assembly in Saccharomyces cerevisiae. Mol. Biol. Cell. 2007;18:2274–2287. doi: 10.1091/mbc.E07-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira D., Sheth U., Valencia-Sanchez M.A., Brengues M., Parker R. Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA. 2005;11:371–382. doi: 10.1261/rna.7258505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T., Takacs-Vellai K., Zhang Y., Kovacs A.L., Orosz L., Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Venigalla R.K., Turner M. RNA-binding proteins as a point of convergence of the PI3K and p38 MAPK pathways. Front. Immunol. 2012;3(398) doi: 10.3389/fimmu.2012.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez D., Saez I., Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat. Commun. 2014;5:5659. doi: 10.1038/ncomms6659. [DOI] [PubMed] [Google Scholar]
- Vlasova I.A., Bohjanen P.R. Posttranscriptional regulation of gene networks by GU-rich elements and CELF proteins. RNA Biol. 2008;5:201–207. doi: 10.4161/rna.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle E., Winkler G.S. RNA decay machines: deadenylation by the Ccr4-not and Pan2-Pan3 complexes. Biochim. Biophys. Acta. 2013;1829:561–570. doi: 10.1016/j.bbagrm.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Walther D.M., Kasturi P., Zheng M., Pinkert S., Vecchi G., Ciryam P., Morimoto R.I., Dobson C.M., Vendruscolo M., Mann M., Hartl F.U. Widespread proteome remodeling and aggregation in aging C. elegans. Cell. 2015;161:919–932. doi: 10.1016/j.cell.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D., Hollien J. Cytoplasmic organelles on the road to mRNA decay. Biochim. Biophys. Acta. 2013;1829:725–731. doi: 10.1016/j.bbagrm.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Weill L., Belloc E., Bava F.A., Mendez R. Translational control by changes in poly(A) tail length: recycling mRNAs. Nat. Struct. Mol. Biol. 2012;19:577–585. doi: 10.1038/nsmb.2311. [DOI] [PubMed] [Google Scholar]
- Wengrod J., Martin L., Wang D., Frischmeyer-Guerrerio P., Dietz H.C., Gardner L.B. Inhibition of nonsense-mediated RNA decay activates autophagy. Mol. Cell. Biol. 2013;33:2128–2135. doi: 10.1128/MCB.00174-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczynska A., Aigueperse C., Kress M., Dautry F., Weil D. The translational regulator CPEB1 provides a link between dcp1 bodies and stress granules. J .Cell Sci. 2005;118:981–992. doi: 10.1242/jcs.01692. [DOI] [PubMed] [Google Scholar]
- Wippich F., Bodenmiller B., Trajkovska M.G., Wanka S., Aebersold R., Pelkmans L. Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell. 2013;152:791–805. doi: 10.1016/j.cell.2013.01.033. [DOI] [PubMed] [Google Scholar]
- Wullschleger S., Loewith R., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yoon J.H., Choi E.J., Parker R. Dcp2 phosphorylation by Ste20 modulates stress granule assembly and mRNA decay in Saccharomyces cerevisiae. J. Cell Biol. 2010;189:813–827. doi: 10.1083/jcb.200912019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitelhofer M., Macchi P., Dahm R. Perplexing bodies: the putative roles of P-bodies in neurons. RNA Biol. 2008;5:244–248. doi: 10.4161/rna.6948. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yan L., Zhou Z., Yang P., Tian E., Zhang K., Zhao Y., Li Z., Song B., Han J., Miao L., Zhang H. SEPA-1 mediates the specific recognition and degradation of P granule components by autophagy in C. elegans. Cell. 2009;136:308–321. doi: 10.1016/j.cell.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Zhou M., Bail S., Plasterer H.L., Rusche J., Kiledjian M. DcpS is a transcript-specific modulator of RNA in mammalian cells. RNA. 2015;21:1306–1312. doi: 10.1261/rna.051573.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R., Efeyan A., Sabatini D.M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lencastre A., Pincus Z., Zhou K., Kato M., Lee S.S., Slack F.J. MicroRNAs both promote and antagonize longevity in C. elegans. Curr. Biol. 2010;20:2159–2168. doi: 10.1016/j.cub.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoof A., Frischmeyer P.A., Dietz H.C., Parker R. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science. 2002;295:2262–2264. doi: 10.1126/science.1067272. [DOI] [PubMed] [Google Scholar]
- von Roretz C., Di Marco S., Mazroui R., Gallouzi I.E. Turnover of AU-rich-containing mRNAs during stress: a matter of survival. Wiley Interdiscip. Rev. RNA. 2010;2:336–347. doi: 10.1002/wrna.55. [DOI] [PubMed] [Google Scholar]