Abstract
Whole-genome sequencing of 24 Proteus mirabilis isolates revealed the clonal expansion of two cefoxitin-resistant strains among patients with community-onset infection. These strains harboured blaCMY-2 within a chromosomally located integrative and conjugative element and exhibited multidrug resistance phenotypes. A predominant strain, identified in 18 patients, also harboured the PGI-1 genomic island and associated resistance genes, accounting for its broader antibiotic resistance profile. The identification of these novel multidrug-resistant strains among community-onset infections suggests that they are endemic to this region and represent emergent P. mirabilis lineages of clinical significance.
Keywords: blaCMY-2, genomics, ICE, PGI-1, Proteus mirabilis
Introduction
Proteus mirabilis is a significant cause of urinary tract infections and is a leading cause of catheter-associated urinary tract infections [1]. Treatment is complicated by the acquisition of antibiotic resistance genes affording P. mirabilis a selective advantage during therapy. Horizontal acquisition of AmpC-type β-lactamase genes has been an important driver of resistance in Europe and has been associated with the clonal expansion of resistant strains [2], [3]. Since the publication of the P. mirabilis HI4320 reference genome, additional genome sequences have become available providing a framework for genomic epidemiology in this species [4], [5], [6], [7], [8], [9]. Here, we applied whole-genome sequencing to investigate the genomic epidemiology of emergent cefoxitin-resistant P. mirabilis isolates causing community-onset infections in Ireland.
Methods
Clinical P. mirabilis isolates, recovered by the Microbiology Department of St James's Hospital (Dublin, Ireland), were subjected to antimicrobial sensitivity testing on a Vitek 2 system (bioMérieux, Marcy l’Étoile, France). Infections with onset in the community were categorized as either community acquired, when onset of illness occurred outside a healthcare facility with no reported discharge from a healthcare facility within the previous 12 weeks, or healthcare associated, when onset of illness occurred within 4 weeks of discharge from a healthcare facility. Whole-genome sequencing of P. mirabilis isolates was performed on an Illumina MiSeq platform at the TrinSeq sequencing facility (Trinity College Dublin, Ireland). Sequencing reads were aligned to the P. mirabilis HI4320 genome (AM942759) using the Burrows-Wheeler short-read aligner, while de novo assembly was performed using the NSilico Simplicity pipeline (Simplicity v1.2) [2], [10]. Sequence data for the P. mirabilis integrative and conjugative element ICEPmiJpn1 (AB525688.1) and Proteus Genomic Island-1 (KJ411925) were also used as reference sequences for read mapping and contig alignment. Single-nucleotide variants (SNVs) were resolved using SAMtools [11]. Phylogenetic trees were generated by neighbour-joining (BIONJ) using PhyML and visualized with iTOL [12], [13]. Raw short-read data have been deposited at the European Nucleotide Archive under study accession number PRJEB7631. Draft assemblies of representative cefoxitin-resistant strains PM655 and PM593 have been deposited at DDBJ/EMBL/GenBank under accession numbers JSUO00000000 and JSUP00000000, respectively.
Results
Between December 2012 and November 2013, 33% of P. mirabilis isolates (n = 79) recovered by the Microbiology Department of St James's Hospital were found to exhibit resistance to cefoxitin (minimum inhibitory concentration ≥16 mg/L). Twenty-one cefoxitin-resistant isolates, confirmed as being from community-onset infections, were further investigated by whole-genome sequencing. Cefoxitin-resistant isolates had multidrug-resistance (MDR) phenotypes, exhibiting coresistance to other antibiotics including amoxicillin–clavulanic acid (100%), gentamicin (91%), ceftazidime (76%) and ciprofloxacin (52%) but remained sensitive to piperacillin–tazobactam, with the three control isolates exhibiting susceptibility to all antibiotics tested (Fig. 1(A)). No epidemiologic links between cases could be established on analysis of patient records, suggesting that potential strain bias due to patient-to-patient transmission was minimal.
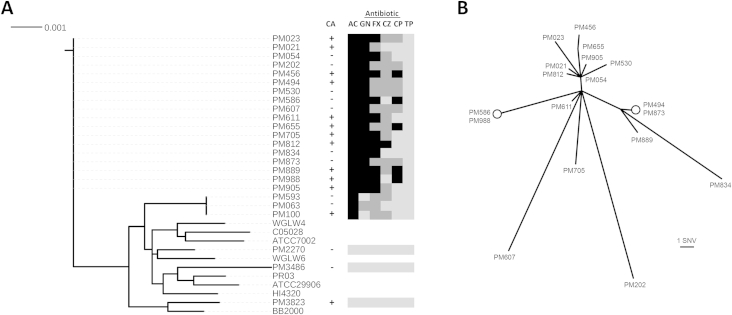
Fig. 1.
Phylogenetic comparison of Irish Proteus mirabilis isolates with previously sequenced P. mirabilis strains. (A) A neighbour-joining tree was generated on the basis of concatenated sequences of seven conserved genes present in all strains (adk; PMI0375, fumC; PMI0891, gyrB; PMI1296, icd; PMI2184, mdh; PMI1732, purA; PMI3370 and recA; PMI3400). Sequences from 24 P. mirabilis isolates from this study and eight representative P. mirabilis whole-genome data sets; C05028 (ANBT00000000), WGLW4 (AMGU00000000), HI4320 (AM942759), ATCC29906 (ACLE01000000), ATCC7002 (JOVJ00000000), PR03 (AORN00000000), WGLW6 (AMGT00000000) and BB2000 (CP004022) were compared. Black bar indicates average nucleotide substitutions per site across the seven genes analysed (9 kb). To the right of the tree, under label CA, plus and minus symbols denote either community-acquired or hospital-associated infections, respectively, while subsequent columns ‘AC,’ ‘GN,’ ‘FX,’ ‘CZ,’ ‘CP’ and ‘TP’ indicate the resistance profile of each isolate to amoxicillin–clavulanic acid, gentamicin, cefoxitin, ceftazidime, ciprofloxacin and piperacillin–tazobactam, respectively. Colour indicates resistance level: black, full resistance; dark grey, intermediate resistance; light grey, susceptible. (B) Unrooted neighbour-joining tree of the observed 18-strain clonal cluster based on whole-genome comparisons across 3,207,626 called sites relative to the P. mirabilis HI4320 genome. Circles indicate genetically indistinguishable strains. Bar below the tree indicates length corresponding to one single-nucleotide variant.
Whole-genome sequence data indicated that our resistant isolates exhibited significant genetic divergence from available P. mirabilis genome sequences present in the Refseq database and included a large cluster of 18 genetically related isolates (Fig. 1(A)). Genome-wide analysis of the 18-isolate cluster (cluster I) revealed isolates to be highly clonal, exhibiting a genetic divergence of between 0–30 SNVs (Fig. 1(B)). Similarly, within the smaller isolate cluster (cluster II, n = 3), isolates PM100 and PM593 were genetically indistinguishable, whereas PM063 diverged by 20 SNVs (isolates in cluster I diverged from those of cluster II by more than 19,000 SNVs).
An R391/SXT-family integrative and conjugative element (ICE), harbouring the blaCMY-2 AmpC-family β-lactamase gene, was identified in all cefoxitin-resistant strains (both in cluster I and cluster II). This ICE shared high sequence identity to ICEPmiJpn1 (AB525688.1) and similarly contained the blaCMY-2 gene within a composite transposon insertion [14]. The identified ICE region was integrated at the same chromosomal location in both strains (Fig. 2(A)). Cluster I isolates also harboured additional chromosomally integrated resistance genes; a Tn7-associated class 1 integron was present upstream of PMI3067 (glmS) carrying the aadA1 and dfrA1 resistance genes (Fig. 2(B)), while the Proteus Genomic Island 1 (PGI-1) was also identified [15]. PG1-1 harboured the resistance genes aadB, aad2, aphA1b, blaTEM-1 and sul1 associated with diverse mobile elements within its MDR region while lacking the “right-end” of the PGI-1 MDR region as well as the IS26-mediated recombination event originally described among French P. mirabilis isolates (Fig. 2(C)) [15]. The three antibiotic-susceptible isolates investigated lacked the R391/SXT-family ICE, class 2 integron-borne aadA1 and dfrA1, and PGI-1. Although we failed to identify mutations within the quinolone-resistance-determining regions of gyrA, gyrB, parC or parE among ciprofloxacin-resistant isolates, loss-of-function mutations in transcriptional regulators of efflux acrR (frameshift: c.90_93dup) and soxR (stop gain; p.Gln147Stop) were identified in four of five resistant isolates exhibiting ciprofloxacin minimum inhibitory concentrations of >4 mg/L. These mutations were absent from susceptible (n = 13) or intermediately resistant (n = 6) isolates.
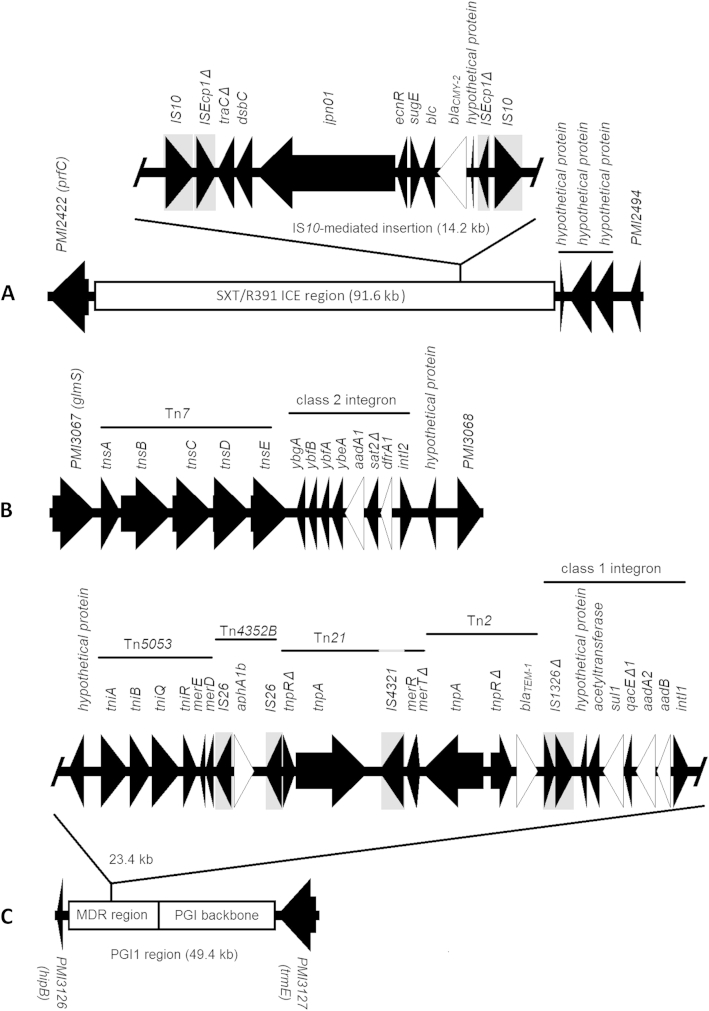
Fig. 2.
Observed mobile genetic elements associated with chromosomally integrated resistance genes among Proteus mirabilis strains identified in this study. Genetic environs (black) of detected resistance genes (white) among resistant P. mirabilis isolates are illustrated. Identified insertion sequences are highlighted in grey boxes. (A) Both strains harboured the described integrative and conjugative element (ICE) element ICEPmiJpn1, which was integrated between chromosomal genes PMI2422 (prfC) and PMI2949. The blaCMY-2 gene was present within an ICE-embedded composite transposon flanked by IS10 elements, as observed in ICEPmiJpn1 (AB525688.1). The ICE observed in cluster I isolates also included three additional genes encoding hypothetical proteins (underlined), which are absent from previously described ICE elements in P. mirabilis. (B) Cluster I isolates harboured the aadA1 and dfrA1 genes associated within a Tn7-associated class 2 integron, chromosomally located between PMI3067 (glmS) and PMI3068. (C) PGI-1 genomic island was identified among cluster I isolates, chromosomally integrated between PMI3127 (hipB) and PMI3127 (trmE). Resistance genes identified within the PGI-1 region included aphA1b, blaTEM-1, sul1, aadA2 and aadB, which were embedded among a mosaic of mobile genetic elements in the PGI-1 MDR region.
Discussion
Proteus mirabilis exhibits a clonal population structure whereby the emergence of pathogenic strains is often observed in association with the acquisition of exogenous antibiotic resistance genes [3], [14], [16]. Here the application of whole-genome sequencing allowed fine-structure comparative genomic analysis of locally emergent P. mirabilis strains identifying two novel clonal lineages among community-onset infections. While both strains harboured the ICE-located blaCMY-2, accounting for observed cephamycin resistance, cluster I strains were distinguished by the presence of a chromosomally located Tn7-associated class 1 integron and the PGI-1 genomic island. These gene mobilization platforms harboured several additional antibiotic resistance genes accounting for the broader resistance profile of the cluster I strain such as invariable gentamicin resistance, which was accounted for by the presence of class 1 integron-associated aadB in the PGI-1 MDR region (Fig. 2(C)). Thus, the acquisition of multiple resistance genes via distinct gene mobilization platforms has likely been a key contributor to the clonal expansion of this novel strain among community-onset infections. Worryingly, a number of cluster I isolates were also resistant to ciprofloxacin, further limiting therapeutic scope for treatment of community-acquired infection. While the mechanism of fluoroquinolone resistance in these strains awaits formal validation, mutational disruptions in soxR and acrR were noted. Similar mutations in acrR and soxR are known to contribute to fluoroquinolone resistance in other pathogenic members of the Enterobacteriaceae by altering expression of the AcrAB efflux system, while increased expression of acrB has been confirmed among fluoroquinolone-resistant P. mirabilis isolates [17], [18], [19]. The predominance of the MDR strains described here among apparently epidemiologically unrelated patients suggests that they represent P. mirabilis lineages endemic to this region, warranting further local surveillance. It will be interesting to assess whether these strains are localized to this region or have a broader global distribution. In the absence of a widely accepted and portable typing scheme in P. mirabilis, whole-genome sequencing provides an important tool in addressing such questions.
Acknowledgements
Supported by the Department of Clinical Microbiology, Trinity College Dublin. The authors gratefully acknowledge C. Roche and L. Rose for their excellent technical assistance and NSilico Life Science Ltd for access to bioinformatic software.
Conflict of Interest
None declared.
References
- 1.Armbruster C.E., Mobley H.L. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat Rev Microbiol. 2012;10(11):743–754. doi: 10.1038/nrmicro2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luzzaro F., Brigante G., D'Andrea M.M., Pini B., Giani T., Mantengoli E. Spread of multidrug-resistant Proteus mirabilis isolates producing an AmpC-type beta-lactamase: epidemiology and clinical management. Int J Antimicrob Agents. 2009;33(4):328–333. doi: 10.1016/j.ijantimicag.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 3.D'Andrea M.M., Literacka E., Zioga A., Giani T., Baraniak A., Fiett J. Evolution and spread of a multidrug-resistant Proteus mirabilis clone with chromosomal AmpC-type cephalosporinases in Europe. Antimicrob Agents Chemother. 2011;55(6):2735–2742. doi: 10.1128/AAC.01736-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson M.M., Sebaihia M., Churcher C., Quail M.A., Seshasayee A.S., Luscombe N.M. Complete genome sequence of uropathogenic Proteus mirabilis, a master of both adherence and motility. J Bacteriol. 2008;190(11):4027–4037. doi: 10.1128/JB.01981-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalid M.I., Teh L.K., Lee L.S., Zakaria Z.A., Salleh M.Z. Genome sequence of Proteus mirabilis strain PR03, isolated from a local hospital in Malaysia. Genome Announc. 2013;1(3) doi: 10.1128/genomeA.00327-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan N.L., Septer A.N., Fields A.T., Wenren L.M., Gibbs K.A. The complete genome sequence of Proteus mirabilis strain BB2000 reveals differences from the P. mirabilis reference strain. Genome Announc. 2013;1(5) doi: 10.1128/genomeA.00024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi X., Zhu Y., Li Y., Jiang M., Lin Y., Qiu Y. Genome sequence of Proteus mirabilis clinical isolate C05028. Genome Announc. 2014;2(2) doi: 10.1128/genomeA.00167-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minogue T.D., Daligault H.E., Davenport K.W., Bishop-Lilly K.A., Bruce D.C., Chain P.S. Draft genome assemblies of Proteus mirabilis ATCC 7002 and Proteus vulgaris ATCC 49132. Genome Announc. 2014;2(5) doi: 10.1128/genomeA.01064-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roach D.J., Burton J.N., Lee C., Stackhouse B., Butler-Wu S.M., Cookson B.T. A year of infection in the intensive care unit: prospective whole genome sequencing of bacterial clinical isolates reveals cryptic transmissions and novel microbiota. PLoS Genet. 2015;11(7):e1005413. doi: 10.1371/journal.pgen.1005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh P., Carroll J., Sleator R.D. Accelerating in silico research with workflows: a lesson in Simplicity. Comput Biol Med. 2013;43(12):2028–2035. doi: 10.1016/j.compbiomed.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guindon S., Dufayard J.F., Lefort V., Anisimova M., Hordijk W., Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 13.Letunic I., Bork P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics. 2007;23(1):127–128. doi: 10.1093/bioinformatics/btl529. [DOI] [PubMed] [Google Scholar]
- 14.Harada S., Ishii Y., Saga T., Tateda K., Yamaguchi K. Chromosomally encoded blaCMY-2 located on a novel SXT/R391-related integrating conjugative element in a Proteus mirabilis clinical isolate. Antimicrob Agents Chemother. 2010;54(9):3545–3550. doi: 10.1128/AAC.00111-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siebor E., Neuwirth C. Proteus genomic island 1 (PGI1), a new resistance genomic island from two Proteus mirabilis French clinical isolates. J Antimicrob Chemother. 2014;69(12):3216–3220. doi: 10.1093/jac/dku314. [DOI] [PubMed] [Google Scholar]
- 16.Nagano N., Shibata N., Saitou Y., Nagano Y., Arakawa Y. Nosocomial outbreak of infections by Proteus mirabilis that produces extended-spectrum CTX-M-2 type beta-lactamase. J Clin Microbiol. 2003;41(12):5530–5536. doi: 10.1128/JCM.41.12.5530-5536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H., Dzink-Fox J.L., Chen M., Levy S.B. Genetic characterization of highly fluoroquinolone-resistant clinical Escherichia coli strains from China: role of acrR mutations. Antimicrob Agents Chemother. 2001;45(5):1515–1521. doi: 10.1128/AAC.45.5.1515-1521.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kehrenberg C., Cloeckaert A., Klein G., Schwarz S. Decreased fluoroquinolone susceptibility in mutants of Salmonella serovars other than Typhimurium: detection of novel mutations involved in modulated expression of ramA and soxS. J Antimicrob Chemother. 2009;64(6):1175–1180. doi: 10.1093/jac/dkp347. [DOI] [PubMed] [Google Scholar]
- 19.Saito R., Sato K., Kumita W., Inami N., Nishiyama H., Okamura N. Role of type II topoisomerase mutations and AcrAB efflux pump in fluoroquinolone-resistant clinical isolates of Proteus mirabilis. J Antimicrob Chemother. 2006;58(3):673–677. doi: 10.1093/jac/dkl297. [DOI] [PubMed] [Google Scholar]