Abstract
Adrenal glands are paired endocrine glands located over the upper renal poles. Adrenal pathologies have various clinical presentations. They can coexist with the hyperfunction of individual cortical zones or the medulla, insufficiency of the adrenal cortex or retained normal hormonal function. The most common adrenal masses are tumors incidentally detected in imaging examinations (ultrasound, tomography, magnetic resonance imaging), referred to as incidentalomas. They include a range of histopathological entities but cortical adenomas without hormonal hyperfunction are the most common. Each abdominal ultrasound scan of a child or adult should include the assessment of the suprarenal areas. If a previously non-reported, incidental solid focal lesion exceeding 1 cm (incidentaloma) is detected in the suprarenal area, computed tomography or magnetic resonance imaging should be conducted to confirm its presence and for differentiation and the tumor functional status should be determined. Ultrasound imaging is also used to monitor adrenal incidentaloma that is not eligible for a surgery. The paper presents recommendations concerning the performance and assessment of ultrasound examinations of the adrenal glands and their pathological lesions. The article includes new ultrasound techniques, such as tissue harmonic imaging, spatial compound imaging, three-dimensional ultrasound, elastography, contrast-enhanced ultrasound and parametric imaging. The guidelines presented above are consistent with the recommendations of the Polish Ultrasound Society.
Keywords: adrenal glands, adrenal masses, ultrasound, standards
Abstract
Nadnercze to parzysty gruczoł endokrynny położony nad górnymi biegunami nerek. Choroby nadnerczy prezentują różne obrazy kliniczne. Mogą przebiegać z nadczynnością w zakresie poszczególnych warstw kory nadnercza albo rdzenia, niedoczynnością kory nadnercza lub z zachowaną prawidłową czynnością hormonalną. Najczęściej spotykanymi zmianami nadnerczy są przypadkowo stwierdzane w badaniach obrazowych (ultrasonografia, tomografia, rezonans magnetyczny) guzy, określane terminem incydentaloma. Obejmują one szereg jednostek histopatologicznych, jednak najczęściej są to gruczolaki kory nadnerczy bez nadmiernej czynności hormonalnej. Każde badanie ultrasonograficzne jamy brzusznej dziecka albo osoby dorosłej powinno obejmować ocenę pól nadnerczowych. W przypadku uwidocznienia w polu nadnerczowym wcześniej nieopisywanej, przypadkowej litej zmiany ogniskowej o średnicy >1 cm (incydentaloma) konieczne jest jej potwierdzenie i różnicowanie za pomocą badania tomografii komputerowej lub obrazowania rezonansu magnetycznego oraz określenie stanu czynnościowego guza. Badanie ultrasonograficzne wykorzystywane jest również do kontrolowania przypadków incydentaloma nadnerczy niezakwalifikowanych do leczenia operacyjnego. W pracy przedstawiono zalecenia dotyczące wykonywania i oceny badań ultrasonograficznych nadnerczy oraz ich zmian patologicznych. Opracowanie uwzględnia nowe techniki ultrasonograficzne, takie jak tkankowe obrazowanie harmoniczne, obrazowanie złożone przestrzennie, ultrasonografia trójwymiarowa, elastografia oraz obrazowanie z zastosowaniem ultrasonograficznych środków kontrastujących i obrazowanie parametryczne. Zawarte w publikacji wytyczne są przedstawione zgodnie z zaleceniami Polskiego Towarzystwa Ultrasonograficznego.
Introduction
Adrenal glands are paired endocrine glands located over the upper renal poles. They consist of two main layers: medullar and cortical. The latter is divided into three layers: zona glomerulosa, fasciculata and reticularis. The medullar layer is responsible for catecholamine production, and the cortical layer produces mineralocorticoids, glucocorticoids and sex hormones, respectively for each zone.
Adrenal diseases can be accompanied by medullar hyperfunction (as in pheochromocytoma) or cortical hyperfunction (as in primary hyperaldosteronism, Cushing's syndrome and congenital adrenal hyperplasia). Insufficiency of the adrenal cortex, or Addison's disease, is a separate entity. In developed countries, it is caused by autoimmunization. It can also be caused by tuberculosis, metastases to both adrenal glands and destruction by a neoplastic process (lymphoma). Anatomic changes in the adrenal glands can also be accompanied by normal hormonal function. This group of pathologies should include the most common incidentally detected adrenal tumors, called incidentalomas. They include a range of histopathological entities but cortical adenomas without hormonal hyperfunction are the most common. The most frequent sources of metastases to the adrenal glands (via vascular spread) are lung and kidney cancers(1, 2).
The diagnostic triad in adrenal diseases includes: interview and physical examination, biochemical tests and imaging. Imaging techniques used in the diagnosis of adrenal pathologies are presented in Tab. 1(3).
Tab. 1.
Contemporary imaging modalities applied to the diagnostics of adrenal diseases
|
Ultrasound imaging of the adrenal glands
Each abdominal ultrasound examination of a child or adult should include the assessment of the suprarenal areas.
Ultrasonography is a method of choice in the assessment of the adrenal glands in neonates and young children. It is particularly useful in the assessment of adrenal masses as hematoma and neuroblastoma.
Owing to the availability, cost and non-invasiveness, it is often used for the assessment of the suprarenal areas in adolescents and adults with extra-adrenal tumors. Moreover, it is recommended in patients with arterial hypertension or hyperadrenalism or adrenal insufficiency, and in the monitoring of adrenal tumors diagnosed in computed tomography or magnetic resonance imaging as benign adenoma-like neoplasms. This examination enables the differentiation between a solid tumor and adrenal cysts, which are rarely encountered.
Although literature reports claim that normal adrenal glands can be visualized in most examinations in adults (80%)(4), it is not that easy in practice. This is because of the fact that the echogenicity of the adrenal glands is similar to that of the retroperitoneal fat. Moreover, a number of other factors have to be taken into account, such as the acoustic window, quality of equipment and examiner's experience. Because of these limitations and difficulties in visualizing certain slight diffuse or focal adrenal lesions, and due to the impossibility to differentiate between adrenal tumors (with few exceptions), the methods of choice in the assessment of adrenal pathologies are computed tomography and magnetic resonance imaging (with appropriate indications, including the detection of a solid mass in the adrenal area on an ultrasound examination).
Equipment
Examinations of adrenal glands in neonates and young children are conducted with the use of broadband transducers of high frequency (up to 10 MHz) and a scanner equipped with the sensitive color or power Doppler option (diagnosis of neuroblastoma tumors).
In adolescents and adults, examinations of the adrenal glands are conducted with the use of convex, broadband transducers with the frequency of 2–5 MHz, which are used in abdominal scans. In patients of slight constitution, probes of higher frequencies can be used(3). The preparation for an examination is the same as for an abdominal scan.
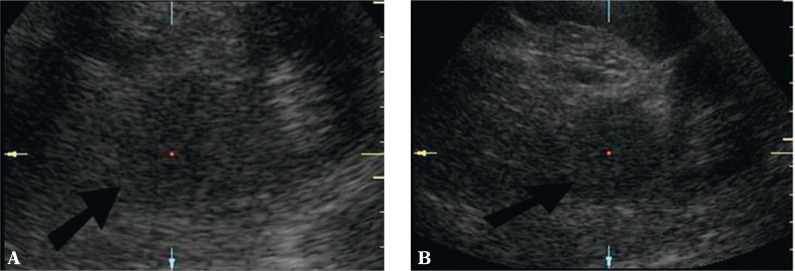
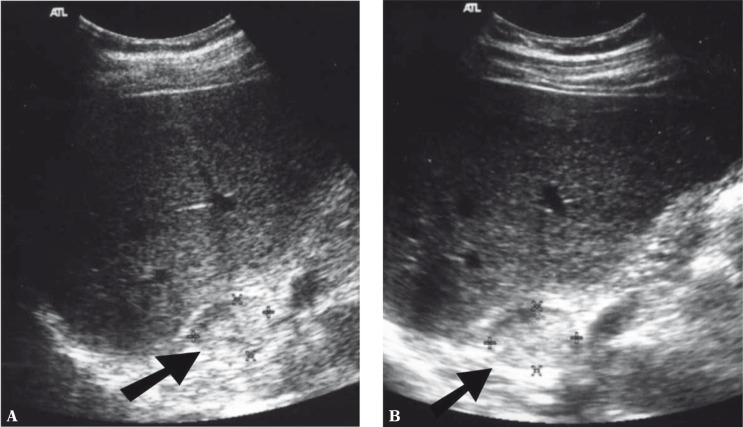
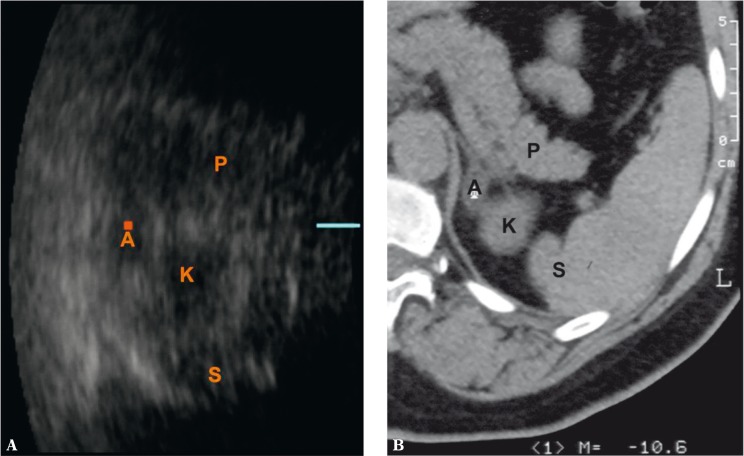
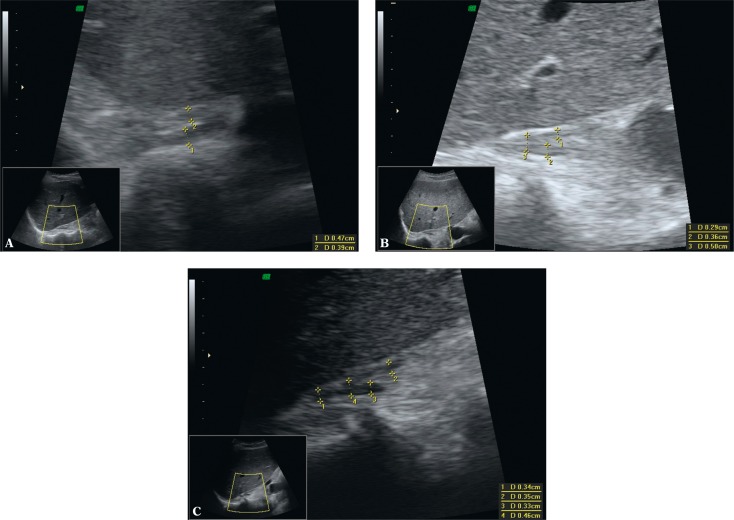
Of new ultrasound techniques to assess the suprarenal areas, harmonic imaging or spatial compound imaging are deemed useful and recommended. In numerous cases, these techniques improve the contrast between an adrenal tumor and adjacent tissues, and enable better visualization of tumor margins (Fig. 1 and 2). Harmonic imaging in particular enables better visualization (better contrast) of even normal adrenal glands, which can allow their measurements to be taken. Three-dimensional scans enable more thorough assessment of tumor sizes and better correlation with computed tomography (Fig. 3). In difficult cases, 3D US enables easier and more careful interpretation of complex anatomic relationships. A three-dimensional examination can be particularly useful for the visualization of tumors in the left adrenal gland since, because of the anatomic conditions, they are more difficult to visualize than tumors of the right adrenal gland (Fig. 3). In some cases, however, even the usage of the aforementioned new ultrasound techniques does not allow small adrenal tumors to be detected, particularly in the left gland.
Fig. 1.
Pheochromocytoma of the left adrenal gland (arrow). A. Conventional ultrasound in the B-mode B. B-mode with harmonic imaging. Compared with image A, fewer artefacts – clearer tumor margins and clearer contrast with surrounding structures
Fig. 2.
Pheochromocytoma of the right adrenal gland (arrow). A. Conventional ultrasound in the B-mode B. B-mode with spatial compound imaging. Compared with image A, fewer artefacts, smoother image (lower image speckles) – clearer tumor margins and clearer contrast with surrounding structures
Fig. 3.
Slight adenoma of the left adrenal gland – A. P – pancreas, K – upper renal pole, S – spleen. Three-dimensional ultrasound – axial image (A). This slight tumor of the left adrenal gland was not detected in a real-time 2D examination. 3D ultrasound enables the lesion to be visualized and its size to be compared with CT (B)
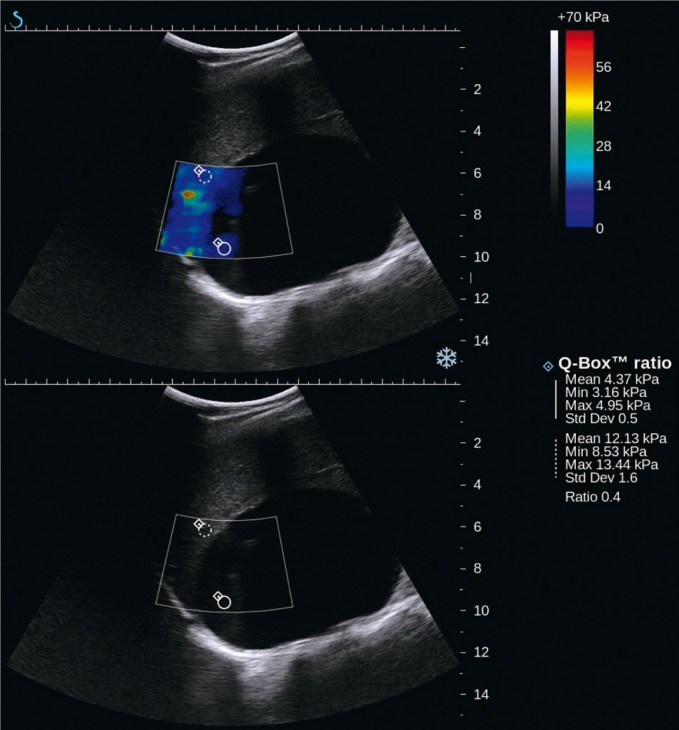
B-mode ultrasound imaging is useful in differentiating between rare cysts and solid tumors of the adrenal glands. This information is particularly significant if the diagnosis of adrenal adenoma in computed tomography has been established only on the basis of its low density (approximately 0 Hounsfield units) without the administration of iodinated contrast agent. Such an image can represent both cysts and adenomas that contain lipids. Tomography enables the differentiation between these lesions based on the observation of contrast enhancement. As for ultrasound imaging, if a hypoechoic lesion does not present all features of a simple cyst in a B-mode examination, a new dynamic elastography technique, called shear wave elastography, can be used. It enables the differentiation between a solid and cystic lesion. This technique allows convex probes for abdominal scans to be used by holding them motionlessly (by contrast with static elastography, no compression is applied). The results of tissue elasticity are presented in an absolute scale in kPa. This technique uses the analysis of the velocity of shear wave propagation in tissues. It is associated with tissue elasticity determined by the elastic Young's modulus. The analysis of this phenomenon is possible by using the method of rapid ultrasound image generation, with the velocity of approximately 20,000 images per second. Tissue elasticity is presented in real-time as a color map placed on a B-mode image. By contrast with solid tumors, cysts do not present a colored elastographic signal since shear waves do not propagate through fluids (Fig. 4)(5).
Fig. 4.
Recurring lymphatic cyst of the right adrenal gland of the maximum size of 10 cm: shear-wave elastography (SWE) – upper image, B-mode – lower image. In the upper SWE image, there is no colored signal since shear waves do not propagate through fluids. Inside the lesion at its periphery, an artefact-like colored signal can be seen, however indicating low stiffness values (approximately 4 kPa)
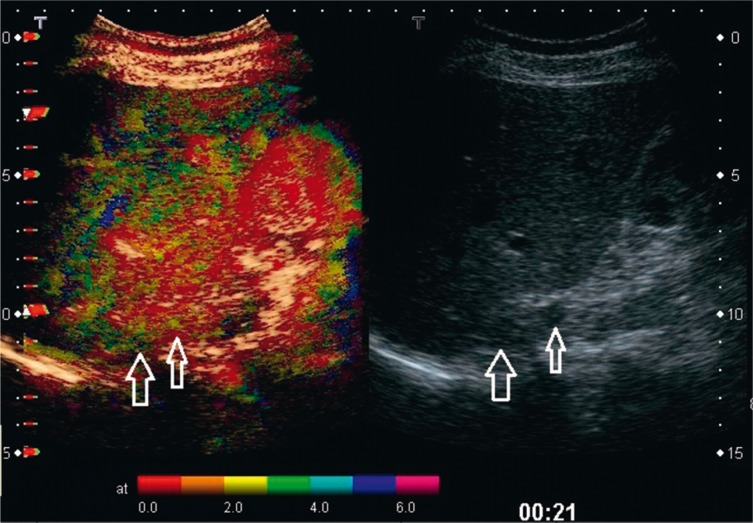
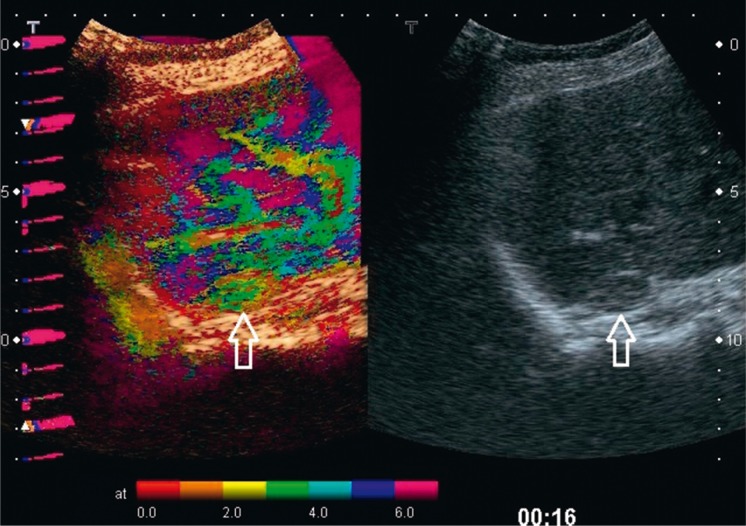
Contrast-enhanced ultrasound examinations have not demonstrated any features that would enable certain differentiation between benign and malignant adrenal tumors(6). The assessment of the vascularization pattern in the group of benign adrenal lesions has demonstrated that there are differences between nodular hyperplasia and adenomas. In nodular hyperplasia, the vascularization begins at the periphery of a lesion, which can be observed in parametric images of the contrast agent inflow (arrival) time. Adenomas, however, usually present mixed or central vascularization pattern (Fig. 5 and 6)(7).
Fig. 5.
Nodular hyperplasia of the adrenal cortex with two tumors masses of the right adrenal gland (arrows) with the mean diameter of 23 mm and 10 mm. B-mode image (on the right). The parametric image of the time of contrast agent (SonoVue) inflow (arrival) (on the left) shows vascularization of both tumors, starting from the periphery
Fig. 6.
Adenoma of the adrenal cortex (arrows) with the mean diameter of 16 mm. B-mode image (on the right). The parametric image of the time of contrast agent (SonoVue) inflow (arrival) (on the left) shows vascularization of the tumor, starting from the center and periphery at the same time
Anatomy of the adrenal region and scanning technique
The adrenal gland is a paired organ located cephaloanterio- medially from the upper renal poles. The right suprarenal area borders the inferior vena cava anteriorly; the upper and lateral border is the liver; the diaphragm is located medially and the kidney is positioned below it. The left suprarenal area borders the pancreatic tail, stomach and intestine anteriorly; the upper and lateral border is the spleen; the diaphragm and aorta are located medially and the kidney is positioned below it. Both adrenal glands are located in the perirenal adipose tissue surrounded by the Gerota's fascia.
Adrenal scans in neonates are usually conducted with a probe applied laterally in coronal, axial and intermediate planes. Normal adrenal glands are usually well-visible in neonates. This is associated with large sizes of adrenal glands compared with kidneys, low amount of the retroperitoneal fat tissue and short distance from the probe, which enables the usage of high-frequency linear transducers with higher resolution. The right adrenal gland can be visualized in 97% of neonates, and the left one in 83%. The cephalocaudal length of the adrenal gland in this group of patients ranges from 9–36 mm, mean 15 mm. The thickness of the adrenal limb ranges from 2–5 mm. In a neonate, there can be a hyperechoic center corresponding to the medullar layer and a hypoechoic edge that corresponds to the cortical layer. The ultrasound corticomedullar differentiation blurs with age(3, 8).
In adolescents and adults, an examination of adrenal glands is usually conducted in a supine position by applying the transducer laterally in the coronal sections along the long renal axis and in axial sections with the evaluation of the area located anteriorly, medialy and above the kidney. The suprarenal area examination may have to be extended to include additional views. When assessing the left suprarenal area, the right lateral position of the patient can cause the displacement of the stomach and intestine, thereby improving the its’ visualization. In this position, the probe can be applied more posteriorly so that intestinal gas does not conceal the suprarenal area. Depending on the conditions, adrenal examinations can be supplemented with axial and sagittal sections, viewed anteriorly. Sometimes, this position enables one to observe focal lesions in the adrenal glands that cannot be detected from the lateral approach.
The assessment of the suprarenal areas as well as the visualization of the adrenal glands and focal lesions can be difficult or impossible in the cases of considerable fatty liver (normal liver is a good acoustic window in the assessment of the right suprarenal area), large amount of gas in the intestine and stomach (left suprarenal area) and obesity (worse translucency for ultrasound wave).
In adults, normal adrenal glands are visualized as longitudinal organs that consist of the body and limbs and assume the shape of the letter V, Y or λ, a comma or triangle. By contrast with schematic figures presented in various anatomical atlases, the adrenal gland is a narrow organ with the limb diameter of 4–9 mm (5 ± 1 mm) and the body diameter of up to 10 mm. Its length reaches up to 50 mm(8). In an ultrasound examination, this organ is usually hypoechoic or its echogenicity resembles that of the perirenal fat tissue, which might make its visualization more difficult (Fig. 7). A focal lesion in the suprarenal area should be visualized in two perpendicular planes; three perpendicular maximum dimensions should be marked (adrenal tumors can grow irregularly in three directions). Sometimes, ultrasonic artefacts within the adipose capsule of the kidney can mimic focal lesions but their analysis in two perpendicular planes should enable the proper classification of the image.
Fig. 7.
Ultrasound B-mode image of the normal right adrenal gland with marked measurements of the thickness of the limbs and body (calipers). A. Axial plane with harmonic imaging. Hypoechoic adrenal gland of V shape. The echogenicity of the adrenal limb ends is similar to the echogenicity of the retroperitoneal fat tissue. B. Longitudinal section through the adrenal gland. In this view, the adrenal gland is of λ shape. It is only slightly hypoechoic in relation to the retroperitoneal fat tissue. C. Longitudinal section with harmonic imaging: increased contrast between the hypoechoic adrenal gland and the retroperitoneal fat tissue (compared with Fig. 7B). Harmonic imaging is recommended in the visualization of normal adrenal glands and their focal lesions
In the monitoring of adrenal masses, it is necessary to correlate findings with maximum dimensions found in computed tomography or magnetic resonance imaging (axial sections and height). An ultrasonologist should be familiar with previous ultrasound, computed tomography or magnetic resonance images of evaluated adrenal lesions. This should help to visualize them in ultrasound examination and enable better assessment of their growth dynamics (Fig. 3).
When examining adults using a convex probe with the frequency of 2–5 MHz, the mean diameter of adrenal lesions that can be visualized exceeds 10 mm. However, the literature mentions that focal lesions with the diameter of 5 mm can be visualized via the abdominal access and those with 2–3 mm in diameter can be detected in endoscopic ultrasound(8). Nevertheless, some tumors with their maximum diameter below 20 mm in the left adrenal gland can remain invisible for transabdominal ultrasonography. This depends on their localization in the gland as well as on anatomic and physiological conditions.
The suprarenal areas can also include objects that are normal structures (splenic vessels, lobulated spleen, accessory spleen), tumors or cysts of surrounding organs (kidneys, liver, pancreas), called adrenal pseudotumors.
If a focal lesion is detected in the suprarenal area, the remaining abdominal organs must also be assessed on ultrasound examination.
It must be emphasized that some forms of adrenal diseases, such as certain forms of adrenal hyperplasia, may remain invisible in sonography or even with modalities of choice in adrenal imaging, such as computed tomography or magnetic resonance imaging.
Test description
In neonates, the description of the examination should include the size of the gland and the presence of possible focal lesions. When describing focal lesions of the adrenal glands, the following should be taken into account: dimensions (two perpendicular maximum dimensions in the axial section and the third dimension – height), echogenicity, homogeneity and vascularization in color and power Doppler examinations in order to differentiate between adrenal hematoma and neuroblastoma tumor.
The description of examinations conducted in adolescents and adults should also include possible enlargement of the adrenals and presence of focal lesions. When describing focal lesions of the adrenal glands, the following should be taken into account: dimensions (two perpendicular maximum dimensions in the axial section and the third dimension – height), shape (regular, irregular), margins (well-circumscribed, uneven) echogenicity, homogeneity and nature (solid or cystic). The differential diagnosis should include the organ from which a lesion visible in the suprarenal area may arise.
If focal lesions in adrenal glands are visualized, other possible lesions in other abdominal organs should be described as well.
If a previously non-reported, incidental solid focal lesion exceeding 1 cm (incidentaloma) is detected in the suprarenal area, computed tomography or magnetic resonance imaging should be conducted to confirm its presence and for differentiation and the tumor functional status should be determined. Subsequently, the lesion should be monitored with ultrasound examinations. In lesions with diameters <4 cm and density <10 H.U., a follow-up ultrasound examination should be conducted after 3 months. If the tumor does not grow, the next follow-up scans should be conducted every 6 months for 2 years, and subsequently once a year. If the tumor grows, computed tomography is conducted. Biposy is indicated if adrenal metastasis from an unknown primary focus is suspected. Contraindications include the suspicion of pheochromocytoma or adrenal carcinoma. In adenomas, biopsy is of a limited diagnostic value.
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Funding/Support and role of the sponsor
The publication has been created thanks to the grant of the Polish Ministry of Science and Higher Education No N N402481239, carried out within 2010–2014.
References
- 1.Kasperlik-Załuska AA, Otto M, Cichocki A, Rosłonowska E, Słowińska-Srzednicka J, Jeske W, et al. Incidentally discovered adrenal tumors: a lesson from observation of 1,444 patients. Horm Metab Res. 2008;40:338–141. doi: 10.1055/s-2008-1073167. [DOI] [PubMed] [Google Scholar]
- 2.Kasperlik-Załuska AA, Otto M, Cichocki A, Rosłonowska E, Słowińska-Srzednicka J, Zgliczyński W, et al. 1,161 patients with adrenal incidentalomas: indications for surgery. Langenbecks Arch Surg. 2008;393:121–126. doi: 10.1007/s00423-007-0238-6. [DOI] [PubMed] [Google Scholar]
- 3.Słapa RZ, Jakubowski W, editors. Ultrasonografia i inne metody obrazowania w diagnostyce nadnerczy. Warszawa–Zamość: Roztoczańska Szkoła Ultrasonografii; 2003. [Google Scholar]
- 4.Dietrich CF, Wehrmann T, Hoffmann C, Herrmann G, Caspary WF, Seifert H. Detection of the adrenal glands by endoscopic or transabdominal ultrasound. Endoscopy. 1997;29:859–864. doi: 10.1055/s-2007-1004322. [DOI] [PubMed] [Google Scholar]
- 5.Słapa RZ, Kasperlik-Załuska AA, Migda B, Jakubowski WS. Shear wave elastography of adrenal masses is feasible and may help to differentiate between solid and cystic lesions – an initial report. Endokrynol Pol. 2014;65:119–124. doi: 10.5603/EP.2014.0017. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich CF, Ignee A, Barreiros AP, Schreiber-Dietrich D, Sienz M, Bojunga J, et al. Contrast-enhanced ultrasound for imaging of adrenal masses. Ultraschall Med. 2010;31:163–168. doi: 10.1055/s-0028-1109357. [DOI] [PubMed] [Google Scholar]
- 7.Slapa RZ, Kasperlik-Zaluska AA, Migda B, Otto M, Jakubowski WS. Application of parametric ultrasound contrast agent perfusion studies for differentiation of hyperplastic adrenal nodules from adenomas-Initial study. Eur J Radiol. 2015;84:1432–1435. doi: 10.1016/j.ejrad.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Jenssen C, Dietrich CF. [Ultrasound and endoscopic ultrasound of the adrenal glands] Ultraschall Med. 2010;31:228–247. doi: 10.1055/s-0029-1245449. [DOI] [PubMed] [Google Scholar]