Abstract
Patients with symptoms of thyroid-associated orbitopathy are classified on the basis of the clinical activity score (CAS) proposed by Mourits in 1989. Despite its undoubted clinical usefulness, it has several limitations which can decide about the success or failure of the implemented treatment. Numerous reports mention the presence of hemodynamic changes in orbital and bulbar vessels in the course of an orbitopathy called Graves’ disease. The usage of Doppler sonography in the diagnosis of numerous ophthalmologic vascular diseases suggests that changes in thyroid-associated orbitopathy can correlate with the activity and severity of the disease. This paper presents the overview of the state-of-the-art concerning the usefulness of Doppler imaging in patient selection for the treatment of thyroid-associated orbitopathy. It has been shown that the velocity of blood flow in the superior ophthalmic vein, which is the most susceptible to changes in anatomical conditions in the enclosed orbital space, decreases in a statistically significant way. A decrease in blood flow velocity is closely associated with the active stage of the disease whereas reverse flow or its absence attest to severe orbitopathy and constitute a risk factor of ocular neuropathy. The activity of the inflammatory process in the eyeball is also confirmed by an increase in peak systolic velocity (PSV) in the ophthalmic artery and central retinal artery as well as end-diastolic velocity (EDV) in the ophthalmic artery. Resistance index values decrease in the ophthalmic artery and increase in the central retinal artery mainly in cases with considerable expansion of the extraocular muscles.
Keywords: Graves’ disease, Graves’ ophthalmopathy, color Doppler imaging, ophthalmic artery
Abstract
Podstawą kwalifikacji do leczenia pacjentów z objawami orbitopatii tarczycowej jest skala aktywności choroby (clinical activity score, CAS), zaproponowana przez Mouritsa w 1989 roku. Mimo jej niewątpliwej przydatności klinicznej cechuje ją kilka ograniczeń, które mogą stanowić o sukcesie lub porażce zastosowanego leczenia. W licznych doniesieniach wykazywano zmiany hemodynamiczne, do jakich dochodzi w naczyniach oczodołu i gałki ocznej w przebiegu orbitopatii związanej z chorobą Gravesa. Wykorzystanie ultrasonografii dopplerowskiej w diagnostyce wielu schorzeń okulistycznych o podłożu naczyniowym pozwala sądzić, że również w orbitopatii tarczycowej zmiany te mogą korelować z aktywnością i ciężkością choroby. W poniższym opracowaniu przedstawiamy przegląd aktualnej wiedzy dotyczącej przydatności ultrasonografii dopplerowskiej w kwalifikacji pacjentów do leczenia orbitopatii tarczycowej. Wykazano statystycznie znamienne obniżenie prędkości przepływu krwi w żyle ocznej górnej, która jest naczyniem najbardziej podatnym na zmianę warunków anatomicznych w zamkniętej przestrzeni oczodołu. Obniżenie prędkości było ściśle związane z aktywną fazą choroby, natomiast przepływ odwrócony lub brak przepływu świadczyły o ciężkiej postaci orbitopatii i stanowiły czynnik ryzyka wystąpienia neuropatii nerwu wzrokowego. Aktywność procesu zapalnego w oczodole potwierdzają również: wzrost prędkości skurczowej (PSV) w tętnicy ocznej i tętnicy środkowej siatkówki oraz prędkości końcoworozkurczowej (EDV) w tętnicy ocznej. Natomiast wartości wskaźnika oporu ulegają obniżeniu w tętnicy ocznej, a rosną w tętnicy środkowej siatkówki, głównie w tych przypadkach, w których dochodzi do znacznego powiększenia objętości mięśni gałkoruchowych.
Introduction
The autoimmune inflammatory process that takes place in the orbit in the course of Graves’ disease results in multiple clinical signs, which can even lead to the loss of sight. Despite many years of study and search for the methods to classify patients in order to distinguish groups at a high risk of overt thyroid-related orbitopathy, no method has been found to ensure reliable treatment control. Appropriate classification is the basis for selecting treatment, with respect to both the time at which therapy is implemented and its type.
The identified prognostic factors that enable risk assessment of clinically overt thyroid-related orbitopathy include: age, sex(1), smoking(2), serum anti-TSH receptor antibody (TSHRAb) levels(3) and genetic predisposition, i.e. the presence of CTLA-4 gene(4). However, the efficacy of immunosuppressive glucocorticosteroid therapy is merely 50–79%(5). There is a large group of patients who, apart from the currently available diagnostic methods, require different evaluation criteria to enable the implementation of an effective therapy.
The aim of the paper is to assess hemodynamic changes in orbital vessels in patients with Graves’ disease and to evaluate the usefulness of Doppler sonography in improving the diagnosis of the activity and severity of inflammatory processes in the orbit.
The gold standard in the assessment of thyroid-associated orbitopathy activity is CAS (clinical activity score), and patients are selected for treatment based on the obtained score(6). The correlation of CAS with the level of anti-TSH receptor antibodies also attests to the usefulness of this scale in the assessment of Graves’ orbitopathy(7). At the same time, it is common knowledge that this scale, despite being very useful, has certain limitations. First of all, it mainly allows for a qualitative rather than quantitative assessment. Secondly, all symptoms are scored in the same way whereas their diagnostic value probably varies. Thirdly, this scale is not of much use for the monitoring of changes. Finally, both eyeballs are examined simultaneously. Furthermore, the cut-off point of “4” in the CAS scale has been selected arbitrarily. This means that there is a group of patients who, despite having a score lower than 4, still has the signs of the disease activity. These limitations result in a decrease in its sensitivity and prompt the search for other diagnostic tools to improve the efficacy of immunosuppression. Based on literature reports, it is estimated that oral steroid therapy in patients with active thyroid-associated orbitopathy brings about effects in merely 56% of cases, and the intravenous use of glucocorticosteroids increases this value to 79%(5).
It should be asked what hemodynamic changes take place in orbital vessels of patients with thyroid-associated orbitopathy, whether color Doppler ultrasound can supplement the CAS score and become an additional criterion in patient selection for immunosuppressive treatment, and whether it can be a prognostic tool of treatment outcomes.
If it was possible to identify hemodynamic changes characteristic of and specific for persons with thyroid-associated orbitopathy in the course of Graves’ disease, Doppler ultrasound would become a valuable tool enabling early selection for treatment and strict control of patients at a high risk of overt orbitopathy even before clinical signs develop.
Doppler ultrasound is the only method of non-invasive measurement of blood flow in the orbital and retrobulbar circulation. Moreover, it is relatively safe assuming that the mechanical index (MI) does not exceed 0.23 and the thermal index (TI) is lower than 2.0(8).
Doppler ultrasound of retrobulbar vessels is used in the diagnosis of numerous ophthalmologic diseases, such as: central retinal artery occlusion, central retinal artery thrombosis, diabetic retinopathy, glaucoma, ocular ischemic syndrome, intrabulbar inflammatory diseases, tumors and vascular malformations(9–11).
The retrobulbar vascular bed is visualized using probes with the frequency of 7–10.5 MHz. Sporadically, probes of lower frequency are used to visualize the posterior fragment of the ophthalmic artery(12). The examination is typically conducted in the supine position through closed eyelids. Attention should be paid to various factors that can cause false results. They depend of the examiner and scanning conditions, and include: intensity of external lighting, compression on the eyeball, tightening eyelids, blinking, width of the Doppler gate and examiner's experience(13).
Hemodynamic changes in the superior ophthalmic vein
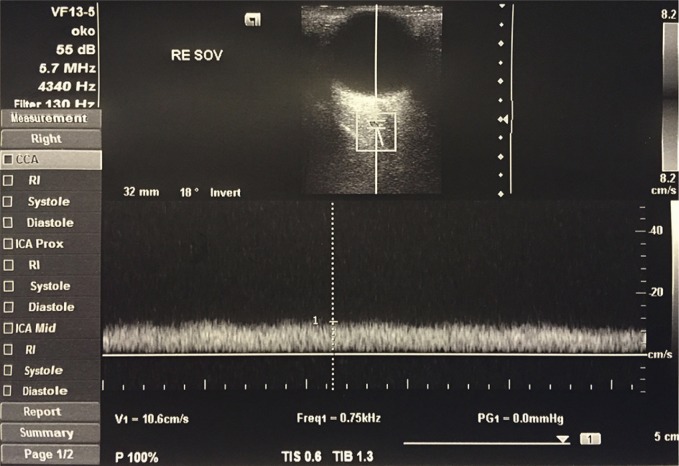
Hemodynamic changes in orbital vessels can be observed in thyroid-associated orbitopathy(14). The main venous out-flow pathway from the eyeball to the cavernous sinus is the superior ophthalmic vein. In a color Doppler examination, it can be localized in the superomedial part of the orbit (Fig. 1). Even in physiological conditions, the velocity of blood flow in the superior ophthalmic vein varies depending on the cardiac and respiratory activity(15). Because of the flaccidity of the wall and the lack of valves, the flow in this vessel largely depends on the external conditions in the orbit. The apical crowding of tissues in the orbit, which is observed in the course of thyroid-associated orbitopathy, is the main cause of stasis in the superior ophthalmic vein(16).
Fig. 1.
Flow velocity waveform in the superior ophthalmic vein
Thanks to Doppler examinations, hemodynamic changes in thyroid-associated orbitopathy, such as decreased flow velocity and stasis in the superior ophthalmic vein, are well-documented. They are detected in both active (congestive) and passive (fibrotic) stage of the disease(17). A few clinical and experimental studies have revealed that venous congestion (stasis) is a probable cause of extraocular muscle expansion, orbital tissue edema, conjunctival chemosis and optic neuropathy(18). Moreover, the apical orbital crowding, superior ophthalmic vein compression and decreased flow velocity can cause the dilatation of slight venous vessels that drain the adipose tissue thus increasing its volume and leading to eyeball protrusion. The induction of stasis in the superior ophthalmic vein in experimental settings causes clinical and histological signs of thyroid-associated orbitopathy(19).
Apart from decreased flow velocity in the superior ophthalmic vein in patients with thyroid-associated orbitopathy, reverse venous flow has also been observed. Monteiro et al. reported that 14% of patients with the congestive stage of the disease presented posteroanterior (reverse) flow in the superior ophthalmic artery(16). These data are confirmed in the studies of Nakase et al.(20) and Alpa et al.(21) Nakase observed reverse venous flow in 36% of patients with thyroid-associated orbitopathy and features of apical orbital crowding, 23% of whom presented features of nerve II neuropathy. Reverse venous flow was observed in 15% of patients with thyroid-associated orbitopathy without features of neuropathy. Based on the foregoing, a hypothesis was put forward that stasis in the superior ophthalmic vein is a risk factor of nerve II neuropathy. The compression of the superior ophthalmic vein is caused by enlarged extraocular muscles in the region of the orbital apex(16–18, 20, 21).
Benning et al. have also observed decreased flow velocity in the superior ophthalmic vein, and the degree of the reduction correlated with the severity of the disease. No reverse flow has been observed(14).
Sommer et al. examined 48 orbits (24 patients) and did not observe reverse flow. Reduced flow in the superior ophthalmic vein was noted in 62% of patients, and the lack of flow was observed in 37.5% of the orbits examined. Moreover, computed tomography conducted in these patients also revealed the signs of increased orbital tissue volume, features of apical crowding and superior ophthalmic vein compression. This confirms the hypothesis that the lack of flow in the superior ophthalmic vein in patients with thyroid-associated orbitopathy is connected with venous stasis due to vascular compression(22).
In one of few studies aiming to find correlations between the quantitative flow impairment in the superior ophthalmic vein and the stage of thyroid-associated orbitopathy, Monteiro et al. demonstrated that all patients with congestive-edematous stage of the disease presented considerably restricted venous flow; a similar situation was observed in the fibrotic stage in which extraocular muscle involvement prevailed. However, patients with fibrotic stage with prevailing adipose tissue hypertrophy did not present flow disturbances compared with healthy controls(16).
Also, Yanik et al. evaluated maximum flow velocity in the superior ophthalmic vein depending on the intensity of the inflammatory process in the orbit measured with the use of CAS. They found out that maximum flow velocity (MV) in patients with CAS >3 was considerably lower than in the group with CAS 0–2, but no statistically significant differences were found between CAS 0 and CAS 2 patients(23).
These hypotheses are indirectly confirmed in publications that assess venous flow following surgical orbit decompression. Apart from a simple increase in the volume in the orbit by removing the bony wall, the superior ophthalmic venous flow can be additionally improved by reducing the crowding since this lowers venous stasis thereby decreasing orbital tissue edema(24).
Reverse venous flow can be considered an indicator of more severe orbital flow disorders. Moreover, it has been demonstrated that it is correlated with increased incidence of ocular neuropathy(14, 17, 20, 21).
Hemodynamic changes in arteries
By contrast with relatively well-documented hemodynamic changes in the superior ophthalmic vein in patients with thyroid-associated orbitopathy, arterial flow evaluation is still rare.
Irrespective of the causes of hyperthyroidism, hemodynamic changes in arteries are associated with elevated systemic pressure, increased cardiac output, higher pulse pressure and tachycardia(25). Moreover, patients with Graves’ diseases, particularly with the signs of orbitopathy, present considerably higher concentration of markers attesting to the injury of the vascular endothelium (thrombomodulin)(26). The histochemical changes are accompanied by inflammatory markers in the orbital tissues caused by an autoimmune reaction. As a result, it is difficult to specify which of the hemodynamic changes observed in orbital arteries are the consequences of systemic changes and concern all vessels in patients with Graves’ disease, and which are isolated and present only in orbital vessels as a result of local changes.
In clinical studies involving orbital arteries, the following are assessed: ophthalmic artery, central retinal artery, short posterior ciliary arteries, more rarely lacrimal artery, long posterior ciliary arteries, supraorbital artery and supratrochlear artery.
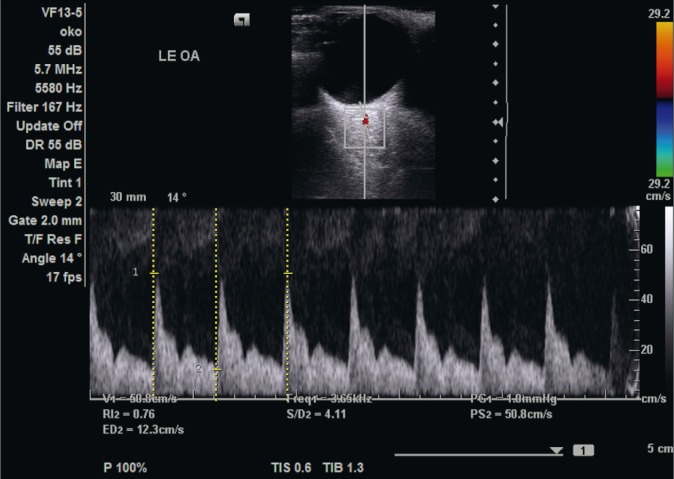
The ophthalmic artery is typically localized at a depth of 35–36 mm. The flow in this vessel is characterized by moderate resistance, rapidly increasing velocity, up to the maximum value in the systolic phase, and relatively rapidly decreasing diastolic velocity. Between these phases a socalled “dichotomous notch” can be observed(27) (Fig. 2).
Fig. 2.
Flow velocity waveform in the ophthalmic artery
The central retinal artery can be visualized in the region of the head of the ocular nerve. The flow velocity is measured at a distance of 1–10 mm from the posterior wall of the orbit. It is worth noting that, depending on the Doppler gate placement, the flow velocity changes: the deeper it is, the lower the flow. This also concerns measurements conducted in the ophthalmic artery(28).
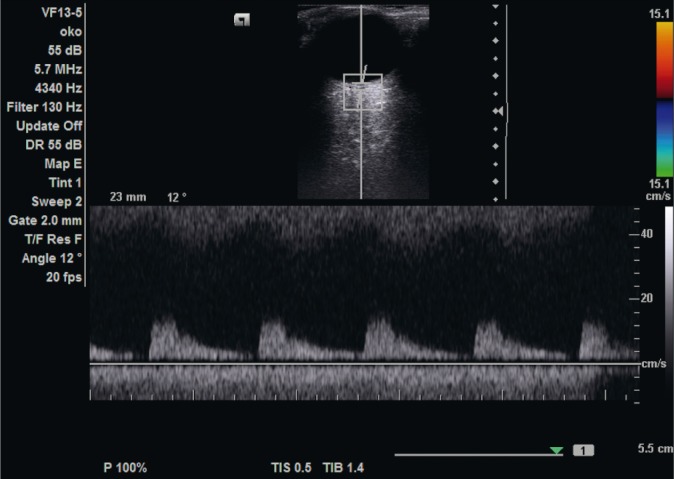
The flow in the central retinal artery is characterized by low resistance: low peak systolic velocity values and relatively rapid flow in the diastolic phase (Fig. 3).
Fig. 3.
Flow velocity waveform in the central retinal artery
Laterally and medially from the ocular nerve, there are short posterior ciliary arteries. The Doppler gate is positioned 1–3 mm from the posterior orbital pole. The flow in these vessels is characterized by low resistance and flow values in the systolic and diastolic phases are in between those obtained in the ophthalmic artery and central retinal artery(27).
Kurioka et al. have found a relationship between thyroid function and hormonal status of patients with Graves’ disease and flow values in the ophthalmic and central retinal arteries. The assessment mainly involved the resistance index (RI), which is defined as the difference between the maximum and minimum flow velocity divided by diastolic flow velocity. In Graves’ disease patients with euthyroidism and with no signs of overt orbitopathy, resistance index values in the ophthalmic artery were considerably higher than in healthy controls. Furthermore, resistance index values were positively correlated with the degree of exophthalmos and serum TRAB level in patients with active thyroid-associated orbitopathy. However, no correlation has been found between TRAB concentration and the intensity of exophthalmos despite the fact that the antibody level is considered a good sign of the disease activity. Also, an RI decrease in the central retinal artery has been observed: from 0.719 ± 0.041 in patients with hyperthyroidism to 0.661 ± 0.051 in patients with euthyroidism after thyreostatic treatment. However, these values were not decreased to the level observed in healthy controls (0.642 ± 0.052). RI values in the central retinal artery demonstrated no relationship with the intensity of exophthalmos and FT3 and FT4 levels. A slight reduction of RI after hormonal treatment was closely correlated with the β stiffness index in the carotid artery and pulse pressure(29).
In their study, Yanik et al. asked a question whether blood flow in orbital vessels of patients with thyroid-associated orbitopathy is connected with the activity of the disease measured with the use of the CAS score. The statistical analysis of studies conducted on 118 patients has revealed that systolic (PSV) and end-diastolic (EDV) velocities measured in the ophthalmic artery and central retinal artery were considerably higher in patients with CAS >3 compared with patients with CAS 0 and CAS 1–2 as well as healthy controls. The RI in the ophthalmic artery was considerably lower than in controls. There were no differences in systolic velocity, end-diastolic velocity and resistance index, both in the ophthalmic artery and in the central retinal artery, between the controls and groups with a low CAS score (0–2)(23).
Considering local factors (excluding mechanical ones) that can cause hemodynamic changes in orbital vessels of patients with Graves’ diseases, the most important is increased concentration of vascular injury markers. The markers that are well-documented are: high levels of thrombomodulin, coagulation factor VIII and von Willebrand factor as well as their relationship with hemodynamic disorders. At the same time, it has been shown that the levels of the above-mentioned parameters normalize following thyreostatic treatment. However, Kurioka has not found any differences in the concentration of vascular injury markers depending on the intensity of thyroidassociated orbitopathy. Moreover, it was also impossible to correlate the reduction of these markers with changes in vascular resistance caused be the implemented treatment. Serum concentrations of all vascular injury markers were not significantly different between patients with or without clinically overt thyroid-associated orbitopathy, both in those with hyperthyroidism and euthyroidism(29).
Conclusions
Despite various discrepancies between publications, the final conclusions suggest that color Doppler imaging can be useful in the diagnosis of thyroid-associated orbitopathy. A decrease in flow velocity in the superior ophthalmic vein is characteristic of the active stage of this pathology. A decrease in venous flow velocity has also been observed in the fibrotic (passive) stage. However, when this stage is characterized by predominant adipose tissue hypertrophy, flow velocities in the superior ophthalmic vein are not different from the values observed in healthy individuals.
The lack of flow, additionally confirmed in other examinations (NMR), attests to the severe course of the active stage and is a risk factor of optic neuropathy. Similarly, the reverse flow in the superior ophthalmic vein is an indicator of severe stasis and is usually correlated with considerable extraocular muscle enlargement.
As for flow velocity parameters in arteries, there are even more discrepancies in the literature. Most authors are of the opinion that peak systolic velocity (PSV) in the ophthalmic artery and central retinal artery as well as end-diastolic velocity (EDV) in the ophthalmic artery also indicate the activity of the disease.
The resistance index (RI) in the ophthalmic artery remains decreased in most studies. When combining this finding with increased arterial flow velocities, a hypothesis can be put forward that hemodynamic disorders are caused not only by tissue compression, but also by an inflammatory process in the orbit.
However, it must be emphasized that the resistance index in the central retinal artery in patients with considerable extraocular muscle expansion was higher than in patients with prevailing increase in the volume of orbital fat. Compared with healthy individuals, RI values are increased even in patients with Graves’ diseases without the features of thyroid-associated orbitopathy.
The assessment of flow velocity in the superior ophthalmic vein as a stasis function is a highly valuable parameter that can be used in the clinical evaluation of patients and their selection to thyroid-associated orbitopathy treatment. Among patients who show a poor response to immunosuppressive treatment with glucocorticosteroids, stasis is a probable cause of persisting clinical signs. Its earlier assessment with Doppler imaging should entail a surgical intervention and orbital decompression instead of a long-term therapy with high doses of methylprednisolone.
Color Doppler ultrasound is the supplementation and a source of important information enabling one to reasonably plan the treatment on the basis of quantitative data. Moreover, it is less expensive and more accessible than magnetic resonance imaging. A low number of studies, which are conducted on small groups of patients, have not enabled the authors to standardize the results and determine the norms that would serve as a reference in daily clinical practice. Nonetheless, color Doppler imaging currently enables the quantitative assessment of the vasculature in Graves’ orbitopathy.
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
References
- 1.Perros P, Crombie AL, Matthews J, Kendall-Taylor P. Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid-eye clinic. Clin Endocrinol (Oxf) 1993;38:367–372. doi: 10.1111/j.1365-2265.1993.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 2.Vestergaard P. Smoking and thyroid disorders – a meta-analysis. Eur J Endocrinol. 2002;146:153–161. doi: 10.1530/eje.0.1460153. [DOI] [PubMed] [Google Scholar]
- 3.Eckstein A, Plicht M, Lax H, Neuhäuser M, Mann K, Lederbogen S, et al. Thyrotropin receptor antibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91:3464–3470. doi: 10.1210/jc.2005-2813. [DOI] [PubMed] [Google Scholar]
- 4.Bednarczuk T, Gopinath B, Ploski R, Wall JR. Susceptibility genes in Graves’ ophthalmopathy: searching for a needle in a haystack? Clin Endocrinol (Oxf) 2007;67:3–19. doi: 10.1111/j.1365-2265.2007.02854.x. [DOI] [PubMed] [Google Scholar]
- 5.Wiersinga W, Kahaly G. A Multidiscpilinary Approach – Questions and Answers. Karger; 2010. Graves’ Orbitopathy; p. 152. [Google Scholar]
- 6.Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639–644. doi: 10.1136/bjo.73.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckstein AK, Plicht M, Lax H, Neuhäuser M, Mann K, Lederbogen S, Heckmann C, et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91:3464–3470. doi: 10.1210/jc.2005-2813. [DOI] [PubMed] [Google Scholar]
- 8.Nowicki A, Łewin AP, Łypacewicz G. Dopuszczalne dawki mocy akustycznych w ultradźwiękowych urządzeniach diagnostycznych. Ultrasonografia. 2000;4:17–25. [Google Scholar]
- 9.Ho AC, Lieb WE, Flaharty PM, Sergott RC, Brown GC, Bosley TM, et al. Color Doppler imaging of the ocular ischemic syndrome. Ophthalmology. 1992;99:1453–1462. doi: 10.1016/s0161-6420(92)31784-1. [DOI] [PubMed] [Google Scholar]
- 10.Tamaki Y, Nagahara M, Yamashita Y, Kikuchi M. Blood velocity in the ophthalmic artery determined by color Doppler imaging in normal subjects and diabetics. Jpn J Ophthalmol. 1993;37:385–392. [PubMed] [Google Scholar]
- 11.Kaiser HJ, Schoetzau A, Stümpfig D, Flammer J. Blood-flow velocities of the extraocular vessels in patients with high-tension and normaltension primary open-angle glaucoma. Am J Ophthalmol. 1997;123:320–327. doi: 10.1016/s0002-9394(14)70127-8. [DOI] [PubMed] [Google Scholar]
- 12.Modrzejewska M. Zastosowanie ultrasonografii doplerowskiej w okulistyce. Część I: metody ultrasonograficzne stosowane w diagnostyce schorzeń gałki ocznej i oczodołu. Ultrasonografia. 2006;26:9–10. [Google Scholar]
- 13.Ustymowicz A. Ultrasonografia dopplerowska z obrazowaniem przepływu krwi w kolorze (USG-kolor Doppler) w diagnostyce okulistycznej – doświadczenia własne i przegląd literatury. Klinika Oczna. 2008;110:108–111. [PubMed] [Google Scholar]
- 14.Benning H, Lieb W, Kahaly G, Grehn F. Color Doppler ultrasound findings in patients with thyroid ophthalmopathology. Ophthalmologe. 1994;91:20–25. [PubMed] [Google Scholar]
- 15.Hansen F, Bergqvist D, Mangell P, Rydén A, Sonnesson B, Länne T. Non-invasive measurement of pulsatile vessel diameter change and elastic properties in human arteries: a methodological study. Clin Physiol. 1993;13:631–643. doi: 10.1111/j.1475-097x.1993.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 16.Monteiro M, Angotti-Neto H, Benabou J, Betinjane AJ. Color Doppler imaging of the superior ophthalmic vein in different clinical forms of Graves’ orbitopathy. Jpn J Ophthalmol. 2008;52:483–488. doi: 10.1007/s10384-008-0594-y. [DOI] [PubMed] [Google Scholar]
- 17.Konuk O, Onaran Z, Ozhan Oktar S, Yucel C, Unal M. Intraocular pressure and superior ophthalmic vein blood flow velocity in Graves’ orbitopathy: relation with the clinical features. Graefes Arch Clin Exp Ophthalmol. 2009;247:1555–1559. doi: 10.1007/s00417-009-1144-0. [DOI] [PubMed] [Google Scholar]
- 18.Hudson HL, Levin L, Feldon SE. Graves exophthalmos unrelated to extraocular muscle enlargement. Superior rectus muscle inflammation may induce venous obstruction. Ophthalmology. 1991;98:1495–1499. doi: 10.1016/s0161-6420(91)32099-2. [DOI] [PubMed] [Google Scholar]
- 19.Saber E, McDonnell J, Zimmermann K, Yugar J, Feldon SE. Extraocular muscle changes in experimental orbital venous stasis: some similarities to Graves’ orbitopathy. Graefes Arch Clin Exp Ophthalmol. 1996;234:331–336. doi: 10.1007/BF00220709. [DOI] [PubMed] [Google Scholar]
- 20.Nakase Y, Osanai T, Yoshikawa K, Inoue Y. Color Doppler imaging of orbital venous flow in dysthyroid optic neuropathy. Jpn J Ophthalmol. 1994;38:80–86. [PubMed] [Google Scholar]
- 21.Alp MN, Ozgen A, Can I, Cakar P, Gunalp I. Colour Doppler imaging of the orbital vasculature in Graves’ disease with computed tomographic correlation. Br J Ophthalmol. 2000;84:1027–1030. doi: 10.1136/bjo.84.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somer D, Ozkan SB, Ozdemir H, Atilla S, Söylev MF, Duman S. Colour Doppler imaging of superior ophthalmic vein in thyroid-associated eye disease. Jpn J Ophthalmol. 2002;46:341–345. doi: 10.1016/s0021-5155(02)00485-9. [DOI] [PubMed] [Google Scholar]
- 23.Yanik B, Conkbayir I, Acaroglu G, Hekimoglu B. Graves’ ophthalmopathy: comparison of the Doppler sonography parameters with the clinical activity score. J Clin Ultrasound. 2005;33:375–380. doi: 10.1002/jcu.20154. [DOI] [PubMed] [Google Scholar]
- 24.Pérez-López M, Sales-Sanz M, Rebolleda G, Casas-Llera P, González- Gordaliza C, Jarrín E, Muñoz-Negrete FJ. Retrobulbar ocular blood flow changes after orbital decompression in Graves’ ophthalmopathy measured by color Doppler imaging. Invest Ophthalmol Vis Sci. 2011;52:5612–5617. doi: 10.1167/iovs.10-6907. [DOI] [PubMed] [Google Scholar]
- 25.Mercé J, Ferrás S, Oltra C, Sanz E, Vendrell J, Simón I, et al. Cardiovascular abnormalities in hyperthyroidism: a prospective Doppler echocardiographic study. Am J Med. 2005;118:126–131. doi: 10.1016/j.amjmed.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Morikawa Y, Morikawa A, Makino I. Relationship of thyroid states and serum thrombomodulin (TM) levels in patients with Graves’ disease: TM, a possible new marker of the peripheral activity of thyroid hormones. J Clin Endocrinol Metab. 1993;76:609–614. doi: 10.1210/jcem.76.3.7680353. [DOI] [PubMed] [Google Scholar]
- 27.Modrzejewska M. Zastosowanie ultrasonografii doplerowskiej w okulistyce. Część II: technika badania gałki ocznej i oczodołu, określenie norm pracownianych prędkości przepływu krwi w naczyniach oka dla zdrowych ochotników. Ultrasonografia. 2006;26:15–20. [Google Scholar]
- 28.Ustymowicz A. Ultrasonografia Duplex-Doppler w diagnostyce okulistycznej. Ultrasonografia. 2010;10:9–13. [Google Scholar]
- 29.Kurioka Y, Inaba M, Kawagishi T, Emoto M, Kumeda Y, Inoue Y, et al. Increased retinal blood flow in patients with Graves’ disease: influence of thyroid function and ophthalmopathy. Eur J Endocrinol. 2001;144:99–107. doi: 10.1530/eje.0.1440099. [DOI] [PubMed] [Google Scholar]