Abstract
Polycystic ovary syndrome is a multi-factorial disease. Its etiopathogenesis has not been elucidated in detail. It is the most common endocrine disorder in women of child-bearing age. This disease entity is primarily characterized by disrupted ovulation and hyperandrogenism, but the clinical picture can be diversified and symptom intensity can vary. Currently, the sonographic assessment of ovaries is one of the obligatory criteria for the diagnosis of PCOS according to the Rotterdam consensus (2003) and Androgen Excess & PCOS Society (2006). This criterion is determined by the presence of ≥12 follicles within the ovary with a diameter of 2–9 mm and/or ovarian volume ≥10 cm3. Such an ultrasound image in one gonad only is sufficient to define polycystic ovaries. The coexistence of polycystic ovaries with polycystic ovary syndrome is confirmed in over 90% of cases irrespective of ethnic factors or race. However, because of the commonness of ultrasound features of polycystic ovaries in healthy women, the inclusion of this sign to the diagnostic criteria of polycystic ovary syndrome is still questioned. The development of new technologies has an undoubted influence on the percentage of diagnosed polycystic ovaries. This process has caused an increase in the percentage of polycystic ovary diagnoses since the Rotterdam criteria were published. It is therefore needed to prepare new commonly accepted diagnostic norms concerning the number of ovarian follicles and the standardization of the technique in which they are counted. The assessment of anti-Müllerian hormone levels as an equivalent of ultrasound features of polycystic ovaries is a promising method. However, analytic methods have to be standardized in order to establish commonly accepted diagnostic norms.
Keywords: polycystic ovary syndrome, PCOS, ultrasound, ovarian follicles
Abstract
Zespół policystycznych jajników jest chorobą wieloczynnikową. Szczegółowa jego etiopatogeneza wciąż nie została wyjaśniona. Jest to najczęstsze schorzenie endokrynologiczne kobiet w wieku reprodukcyjnym. Ta jednostka chorobowa charakteryzuje się przede wszystkim zaburzeniem przebiegu owulacji i hiperandrogenizacją, ale obraz kliniczny może być zróżnicowany, o różnym nasileniu symptomów. Obecnie sonograficzna ocena jajników należy do obowiązujących kryteriów rozpoznania zespołu według konsensusu z Rotterdamu (2003) oraz Androgen Excess & PCOS Society (2006). Kryterium to jest uwarunkowane obecnością w obrębie jajnika ≥12 pęcherzyków o średnicy 2–9 mm i/lub objętością jajnika ≥10 cm3. Opisany obraz ultrasonograficzny dotyczący tylko jednej gonady wystarczy do zdefiniowania policystyczności. Współistnienie policystyczności jajników z zespołem policystycznych jajników potwierdzane jest w ponad 90% przypadków, niezależnie od czynników etnicznych czy rasowych. Jednak ze względu na powszechną obecność ultrasonograficznych cech policystyczności jajników u kobiet zdrowych nadal kwestionuje się włączenie tego objawu do kryteriów diagnostycznych zespołu policystycznych jajników. Niewątpliwy wpływ na odsetek rozpoznań policystyczności jajników ma rozwój nowych technologii. Proces ten powoduje zwiększenie odsetka rozpoznań cech policystyczności jajników od czasu publikacji kryteriów rotterdamskich. Zatem istnieje potrzeba ustanowienia nowych, powszechnie akceptowanych norm diagnostycznych dotyczących liczby pęcherzyków jajnikowych, jak również standaryzacji techniki ich liczenia. Duże nadzieje wiązane są z oceną stężenia hormonu antymüllerowskiego jako równoważnego markera ultrasonograficznych cech policystyczności jajników, jednakże do wyznaczenia powszechnie akceptowanych norm diagnostycznych potrzebna jest standaryzacja metod analitycznych.
The coexistence of hirsutism, oligoovulation, infertility and bilateral enlargement of the ovaries was first reported by Stein and Leventhal in 1935(1). This syndrome was even named by these two physicians for some time. The term polycystic ovary syndrome (PCOS) was first used in the 1960s and gradually replaced its former name (Stein–Leventhal syndrome). The detailed etiopathogenesis of PCOS has not been elucidated so far. However, the individual susceptibility probably depends on prenatal, genetic and environmental risk factors. This disease entity is primarily characterized by disrupted ovulation and hyperandrogenism, but the clinical picture can be diversified and symptom intensity can vary(2, 3). This results from the fact that PCOS is modulated by multiple factors, such as: genetic factors, ethnic origin, nutrition and prenatal androgen exposure, insulin resistance in adolescence and/or more intense adrenarche and body mass changes(4–6). Environmental factors, such as obesity, seem to exacerbate genetic predispositions. As for ethnic factors, hirsutism is more rarely observed in individuals of Asian origin (approximately 10%) compared with the Caucasian race (approximately 70%)(6).
Polycystic ovary syndrome is the most common endocrine disease in women of child-bearing age. The prevalence ranges from 9% when the NIH (National Institutes of Health) criteria are used to even 18% according to the guidelines of the Rotterdam consensus(2, 3, 7). Studies conducted among women with PCOS have revealed increased activity of cytochrome P450c17, which is a catalyst of a biochemical synthesis of ovarian and adrenal androgens, and is determined by CYP17 gene located on 10q24.3. It is unlikely, however, that a single gene is responsible for PCOS. It is probably associated with gene polymorphism that causes metabolic disorders. Chronic androgen hypersecretion by theca cells of the ovarian follicle can also be caused by increased luteinizing hormone (LH) pulse frequency and amplitude as well as increased insulin secretion determined by insulin resistance of tissues. Its etiology in women with PCOS is multi-factorial. The most important causes include: increased serine phosphorylation of insulin receptor, insulin receptor gene mutations, changes in nuclear gamma receptors: Pro12Ala polymorphism of the PPARG2 gene and GLUT4 glucose transporter defect in the adipose tissue(8). The influence of insulin on ovarian function is possible thanks to a large number of receptors for this hormone and structurally similar IGF-1 receptors in the region of the ovaries. Insulin induces hyperandrogenism by: increasing 17α-hydroxylase activity, decreasing aromatase activity, increasing LH secretion and decreasing the synthesis of sex hormone-binding globulin (SHBG) and insulin-like growth factor-binding protein (IGFBP)(9). The relevance of the morphological structure of female gonads in the diagnosis of polycystic ovary syndrome is a controversial issue. The first commonly acknowledged definition of PCOS diagnostic criteria, prepared during NIH consensus meeting in 1990, did not include the criterion concerning ovarian morphology. It was agreed then that PCOS can be diagnosed based on the presence of clinical and/or biochemical signs of hyperandrogenism as well as oligo- and anovulation(10). Ovarian morphology was included in the PCOS-defining criteria during the Rotterdam 2003 consensus by ESHRE/ASRM. At the same time, breakthrough publications in “Fertility and Sterility” and „Human Reproduction” included the new PCOS diagnostic recommendations(11, 12). According to them, the diagnosis can be established when at least two of the three following criteria are present: clinical and/ or biochemical signs of hyperandrogenism, oligo- and anovulation and polycystic ovaries (PCO) observed in an ultrasound examination. The last criterion is determined by the presence of ≥12 follicles within the ovary with a diameter of 2–9 mm and/or ovarian volume ≥10 cm3. Such an ultrasound image in one gonad only is sufficient to define polycystic ovaries. The diagnosis can be established when other endocrine diseases, such as congenital adrenal hyperplasia, androgen-producing tumors or Cushing's syndrome, are ruled out(11). The Rotterdam consensus criteria were based on the results reported by the following authors: Pache et al., van Santbrink et al. and Jonard et al.(13–15) In 2006, Androgen Excess and PCOS Society agreed that androgen excess is crucial in the pathogenesis of PCOS. They accepted this basic criterion as obligatory, with the following accompanying signs: oligo- and anovulation and/or polycystic ovaries observed in a US examination(16). The criteria described above are presented collectively in Tab. 1. Before the year 2003, the most common PCO criteria were those described by Adams in 1986: 10 or more follicles with a diameter of 2–8 mm in a single section of an ovary, arranged either peripherally around the core of the stroma or scattered throughout the increased amount of ovarian stroma(17).
Tab. 1.
Diagnostic PCOS criteria
| NIH 1990 | Rotterdam 2003 | AE-PCOS Society 2006 |
|---|---|---|
(Exclusion of other etiologies) Both criteria are necessary to establish diagnosis |
(Exclusion of other etiologies) Two of three criteria are necessary to establish diagnosis |
(Exclusion of other etiologies) Both criteria are necessary to establish diagnosis |
Excess of ovarian follicles – a definition of polycystic ovarian morphology
The ultrasonographic assessment of the ovarian structure is associated with certain difficulties resulting from the size and shape of follicles, particularly the small ones with a diameter ≤2 mm or those adjacent to one another(18). Moreover, a cyst or cysts can be overlooked or measured twice, particularly during a real-time examination when cysts that have already been counted cannot be marked. To prevent it, Lujan et al. proposed a method for marking individual follicles using a medical grid system that divides ovaries into compartments thus facilitating the assessment of follicles within these compartments(19). This method is characterized by high reproducibility of counts when used by different examiners and during repeated examinations. Unfortunately, it can only be used offline, and therefore it is mainly applied in academic studies rather than in daily clinical practice. Counting follicles is also possible with a computer analysis of three-dimensional images. Imaging in three perpendicular planes enables simultaneous identification of a high number of ovarian follicles. Another method of calculating and assessing follicles is the system enabling three-dimensional reconstruction with marking fluid-filled spaces (e.g. VOCAL, SonoAVC). Thanks to this, follicle volume measurements are more accurate and the possibilities of differences between examiners are limited(20, 21). However, this system requires the processing of numerous variables. That is why the reliability of results closely depends on the quality of images, and discrepancies between various imaging methods are particularly noticeable when one assesses multiple follicles (especially more than 15) or follicles <5 mm in diameter(20, 22). The available literature contains few reports on the comparison of gonad imaging in PCOS using two- and three-dimensional techniques. Deb et al. compared the assessment of ovarian follicles in patients with PCOS using the three-dimensional SonoAVC technique and two-dimensional sonography. They demonstrated that SonoAVC examinations are faster, but the number of visualized follicles is greater when the two-dimensional technique is used(20). Battaglia et al., however, report the lack of differences in the number of follicles in PCOS patients examined with these two methods(23).
With the development of sonographic techniques, one can observe the drive towards creating more and more exact definitions of the excess of ovarian follicles(17, 24). In order to distinguish a group of women with PCO from among healthy individuals, it is proposed that the limit values for the number of ovarian follicles within one ovary should be specified. This, however is problematic. To achieve this, various methods are used based on clinical experience, ROC curve analysis or marking a border on the 95th percentile. The difficulties in the application of these methods are associated with varied intensity of clinical signs of polycystic ovary syndrome and the consequent selection of controls. Tab. 2 presents possible phenotypes of patients depending on the definition of PCOS diagnosis. In the paper published in 1986, Adams et al. proposed the first broadly adapted ultrasound criteria for the diagnosis of the polycystic ovarian structure using a transabdominal probe(17). As the scanning technique developed, transabdominal probes were replaced with transvaginal transducers of high frequency and resolution, which has enabled exceptionally accurate assessment of the ovarian structure. It has ensured better visualization, particularly of smaller ovarian follicles, and enabled more accurate assessment of their number within the entire ovary.
Tab. 2.
Potential PCOS phenotypes according to the criteria of NIH 1990, Rotterdam 2003, AE-PCOS Society 200625 (modified original table)
| Diagnostic criteria | Potential PCOS phenotypes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | |
| Hyperandrogenemia | + | + | + | + | - | - | + | - | + | - |
| Hyperandrogenism | + | + | - | - | + | + | + | + | - | - |
| Oligoovulation anovulation | + | + | + | + | + | + | - | - | - | + |
| Polycystic ovaries | + | - | + | - | + | - | + | + | + | + |
| NIH 1990 | × | × | × | × | × | × | ||||
| Rotterdam 2003 | × | × | × | × | × | × | × | × | × | × |
| AE-PCOS Society 2006 | × | × | × | × | × | × | × | × | × | |
The most common ultrasound definition of a polycystic ovary image, which is included in the Rotterdam criteria, is based on the results obtained by, among others, Jonard et al. who have determined the diagnostic threshold of the number of ovarian follicles using the ROC curve analysis(15). In order to specify ultrasonographic criteria concerning the number of ovarian follicles in PCOS, Jonard et al. compared 112 healthy controls with a group of 214 patients with PCOS. All patients were examined with a transvaginal probe using the two-dimensional technique. The number of follicles measuring 2–9 mm was assessed between the 2nd and 7th day of the cycle. The study revealed a significantly higher mean number of ovarian follicles in PCOS patients compared with the controls (15.5 and 6.0, respectively). The ROC curve analysis demonstrated that the number of 12 follicles measuring 2–9 mm was the best border that differentiated the features of PCOS with the sensitivity of 99% and specificity of 75%. Furthermore, the number of ovarian follicles was also assessed in relation to their diameter in both groups. In this case, no significant differences were found between the groups when analyzing follicles measuring 6–9 mm. However, patients with PCOS presented significantly more follicles with a diameter of 2–5 mm(15). Dewailly et al. (2011) and Lujan et al. (2013) compared the ovarian structure in patients with PCOS and in controls, and specified diagnostic thresholds for follicle count at ≥19 and ≥26, respectively(26, 27) The conflicting results of both studies can result from the selection of controls. In the study of Dewailly et al., women with sonographic features of PCO but without diagnosed PCOS were excluded from the control group, whereas Lujan et al. did not use this exclusion criterion.
Interestingly, statistically significant differences have been found when examining different ethnic groups. The results of studies conducted on the European or American population (Caucasian race) with high-frequency transvaginal probes revealed that the diagnostic threshold of the follicle number for PCOS women is about 25(26, 28). These results are different from the ones obtained in Asian patients. The results of studies conducted by Chen et al. among Chinese women and by Köşüş et al. among Turkish patients indicate lower values (8 and 12, respectively) (29, 30). Such discrepancies between populations can result from ethnic differences. The difficulties in the diagnosis of sonographic features of PCO are confirmed by studies conducted on the general population of women of childbearing age. The results of studies by Lujan et al. and Deb et al., published in 2013 and conducted among regularly menstruating women without sings of hyperandrogenism, prove that there are more than 12 ovarian follicles in most women, particularly younger than 30(27, 31). That is why, after over 10 years of establishing the Rotterdam criteria, it is emphasized that new ultrasonographic norms are needed in order to diagnose polycystic ovaries(30).
The differences in the number of ovarian follicles and PCO diagnostic norms can result from various methods of measuring, reporting and counting follicles(18, 28). The publication of Broekmans et al. from 2010 contains recommendations concerning the standardization of the simultaneous examination and assessment of the number of follicles within the ovary(18). The authors suggest that a scan of an ovary should be performed in two planes in order to specify its margins. Subsequently, the diameters of the largest follicles (above 10 mm) should be measured, preferably in the longitudinal section, followed by the measurement of smaller follicles (2–9 mm in diameter). Balen et al. have a different approach. They suggest that the number of follicles should be assessed in numerous planes only if there is no dominating follicle(28).
The assessment of the number of follicles in the same ovary conducted by different examiners gives controversial results(20, 32). In their prospective study, Lujan et al. prove that the results concerning ovarian follicle counts in PCOS patients differ when examinations are conducted by different examiners(33). Jayaprakasan et al., however, report high agreement of the results in women without PCOS(21). The causes of a better correlation of the results in healthy women could be the lower number of follicles, their slightly greater diameters and arrangement which is not as dense as in PCOS patients. The variability in the assessment of the number of ovarian follicles over the years also results from the considerable improvement of the quality of spatial resolution in imaging. Dewailly et al. have demonstrated that the application of high-frequency transducers considerably facilitates the detection of smaller ovarian follicles irrespective of the patient's age. Therefore, the authors recommend the usage of probes with the frequency greater than 8 MHz(24).
Size of an ovary in the assessment of its polycystic morphology
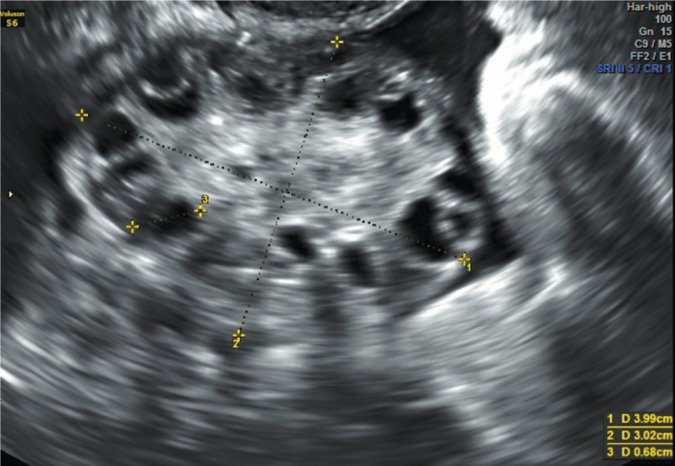
The results of various studies indicate that increased ovarian volume is one of PCO features(28, 33). Technical volume assessment seems less problematic than the assessment of the follicle number. Since the ovarian surface is irregular, the measurement of its volume as an elongated ellipsoid is only approximate (Fig. 1). To make such an assessment, the ovary should be visualized and measured in all three planes (longitudinal, sagittal and transverse). Currently available ultrasound systems enable the assessment of ovarian volume by marking the outlines and calculating the result using appropriate software. Ovarian volume is traditionally calculated with a formula for an elongated ellipsoid (π/6 × the highest size in each of the three planes). Since π/6 = 0.5233, it is possible to use a simplified formula for elongated ellipsoid volume (0.5 × the highest size in the longitudinal, sagittal and transverse view)(29). Volume assessment is included in the Rotterdam criteria in which the recommended threshold value is ≥10 ml. Several studies conducted after the Rotterdam consensus revealed considerably lower threshold values ranging from 6.4 to 7.5 ml(29, 30, 33). These differences can depend on the characteristics of the study population, particularly the ethnic factors, overweight or obesity and serum insulin level, which has been demonstrated by Carmina et al.(34) Ovarian volume changes over time. The highest values are observed in adolescents (1.3–3.8 years after menarche). Subsequently, this parameter gradually decreases to intensify considerably after menopause(28). Pavlik et al. have conducted the ultrasonographic assessment of ovarian volume in six age groups: <30, 30–39, 40–49, 50–59, 60–69 and ≥70 years of age, and obtained the following results: 6.6, 6.1, 4.8, 2.6, 2.1 and 1.8 ml, respectively(35). The available studies indicate that ovarian volume does not change much between the age of 20 and 39(27, 36). The results presented prove that there are natural, agerelated changes in ovarian volume, which should be taken into account when diagnosing PCO in adolescents and women older than 40 years of age.
Fig. 1.
Ovary with typical polycystic morphology, expressed as peripheral arrangement of multiple ovarian follicles with a diameter of 7 mm
Three-dimensional ultrasound is a recognized diagnostic modality to assess ovarian volume. The mean volume in patients with PCOS ranges from 10.6 and 16.7 ml whereas healthy women present values ranging from 5.2 and 8.7(23, 35). The comparison of ovarian volume measured in two- and three-dimensional images has been the subject of numerous studies. However, the presented outcomes indicate conflicting results(19, 23, 35). This could be caused by non-uniform technical standardization of examinations and different interpretation by different ultrasonographers.
It is believed that the assessment of ovarian volume belongs to the diagnostic criteria of PCO. However, it is characterized by a lower sensitivity compared with the assessment of ovarian follicles. The usage of such assessment is then recommended particularly when the visualization of the ovaries is difficult or it is not possible to conduct an examination with a transvaginal probe(28).
Other parameters used in the assessment of PCO
Vascularization of ovarian stroma
Three-dimensional sonography enables the assessment of the volume of the ovary and ovarian follicles. Using the difference between these two parameters, the volume of the ovarian stroma can be assessed. Fulghesu et al. demonstrate the usage of stromal volume to ovarian volume ratio as a diagnostic feature of PCOS that correlates with androgen concentration(36). However, stromal volume is a variable that is strictly correlated with the volume of the entire ovary. That is why, its assessment is of little use in clinical practice.
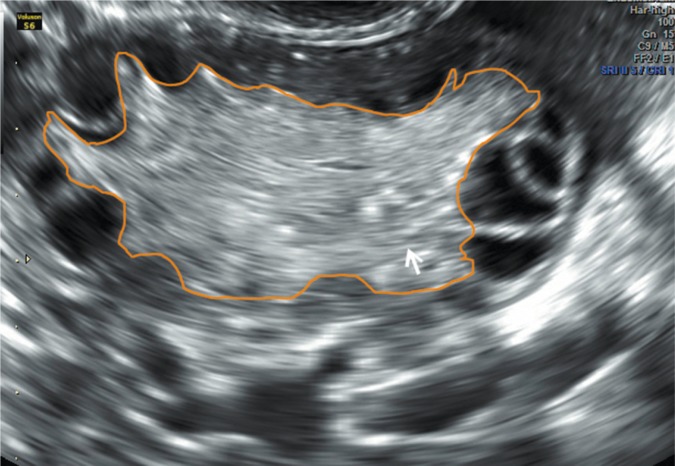
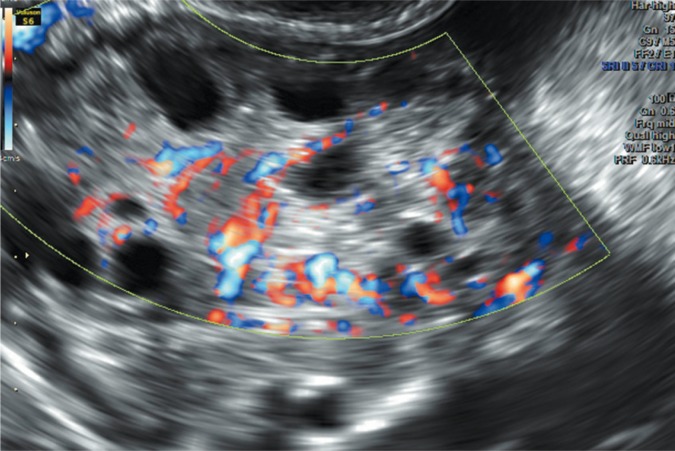
Increased ovarian volume not only correlates with increased stromal volume, but also with its increased vascularization (Fig. 2 and 3). However, studies that compare the intensity of vascularization using two- and threedimensional imaging techniques among patients with features of PCOS and healthy women indicate contradicting results(27, 37). Such discrepancies can result from the lack of the standardization of measurement methods and examinations conducted in small and diversified populations. Currently, because of non-uniform results and the lack of differentiating limit values, the assessment of stromal vascularization is not clinically used in the diagnosis of PCOS.
Fig. 2.
Ovary with increased stromal volume and peripherally arranged follicles. Arrow – ovarian stroma
Fig. 3.
Assessment of the vascularization of the ovarian stroma using the color Doppler technique; numerous vessels with ordered arrangement
Anti-Müllerian hormone as a marker of polycystic ovaries
Anti-Müllerian hormone (AMH) is produced in granular cells in the follicular phase and participates in the early follicle recruitment process. AMH secretion is continued until follicles grow to a diameter of 8 mm. The secretion is negligible in larger follicles(38). There is then a good correlation between AMH levels and the number of small follicles as well as ovarian volume. The results of published studies indicate that the level of AMH is higher in patients with PCOS, which can be helpful in the diagnosis in this syndrome(28, 39, 40). Moreover, it has also been shown that there is a correlation between higher AMH concentration, rare menstruation and hyperandrogenism(38). However, due to the usage of various methods to analyze plasma AMH levels, it is difficult to compare previous studies and specify diagnostic norms that would be characterized by high sensitivity and specificity for patients with the features of PCO(28, 38).
Role of sonographic assessment of polycystic ovaries
Currently, the sonographic assessment of ovaries is one of the obligatory criteria in the diagnosis of PCOS according to the Rotterdam consensus (2003) and Androgen Excess & PCOS Society (2006)(11–13). However, because of the presence of ultrasound features of PCO in healthy women, the inclusion of this sign to the diagnostic criteria of polycystic ovary syndrome is still questioned(28). On the other hand, the available publications prove that PCO can be hereditary(41). It has also been confirmed that the coexistence of polycystic ovaries with PCOS is common (over 90% of cases) irrespective of ethnic factors or race(7, 28). The excess of ovarian follicles in this syndrome is strictly associated with hyperandrogenism, which has been demonstrated by Dewailly et al.(42) The authors of this publication also prove that there is a correlation between the presence of PCO features, increased AMH levels and ovulation disorders in patients with PCOS. That is why, the assessment of the features of PCO and increased AMH levels can be useful in the diagnosis of oligoovulation in PCOS patients(43, 44).
The sonographic features of PCO, as included in the Rotterdam criteria, are currently identified in 50% of the general population of women(28). Considering the results of studies, it has been shown that the presence of PCO features in healthy women of child-bearing age is not associated with significant metabolic disturbances, but a slight increase in AMH and androgen levels, compared with women with the normal ovarian structure, can be observed(43). The presence of PCO in the population of adolescent patients frequently coexists with menstrual disorders and acne. However, these symptoms are not sufficient to diagnose PCOS. However, the polycystic ovarian structure in this age group can be indicative of PCOS in further life. These patients should therefore be monitored clinically and sonographically, and the AMH levels should be controlled. The available studies on the commonness of sonographic signs of PCO have yielded conflicting results. On the one hand, they attest to the heterogeneity of phenotypes in completely healthy and normally ovulating women and in those with mild occult PCOS(44). On the other hand, they reveal the homogeneity of the female population with PCO features as a mild form of polycystic ovary syndrome(45).
To conclude, it must be emphasized that the influence of the development of new technologies in the sonographic assessment of PCO features is undoubtedly noticeable. This process has caused an increase in the percentage of diagnoses of PCO and PCOS since the Rotterdam criteria were published. It is therefore needed to prepare new commonly accepted diagnostic norms concerning the number of ovarian follicles and the standardization of the technique in which they are counted. However, the application of new examination techniques does not entail the need for the modification of diagnostic norms concerning ovarian volume, which are characterized by lower sensitivity compared with ovarian follicle count. Attention is paid to the need of determining diagnostic norms depending on patients’ age and ethnic origin in individual populations of women. The assessment of AMH levels as an equivalent of ultrasound features of PCO is a promising method. However, analytic methods have to be standardized in order to establish commonly accepted diagnostic norms. That is why, further studies, conducted on appropriately selected populations of women, are needed to investigate this non-uniform disease entity.
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
References
- 1.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–191. [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Asunción M, Calvo RM, San Millán JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 4.Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009;71:776–784. doi: 10.1002/ajp.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberfield SE, Sopher AB, Gerken AT. Approach to the girl with early onset of pubic hair. J Clin Endocrinol Metab. 2011;96:1610–1622. doi: 10.1210/jc.2011-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang HY, Guo CX, Zhu FF, Qu PP, Lin WJ, Xiong J. Clinical characteristics, metabolic features, and phenotype of Chinese women with polycystic ovary syndrome: a large-scale case-control study. Arch Gynecol Obstet. 2013;287:525–531. doi: 10.1007/s00404-012-2568-z. [DOI] [PubMed] [Google Scholar]
- 7.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 8.Szydlarska D, Grzesiuk W, Bar-Andziak E. Kontrowersje wokół patogenezy zespołu policystycznych jajników. Endokrynologia, Otyłość i Zaburzenia Przemiany Materii. 2010;6:141–146. [Google Scholar]
- 9.Bachanek M, Sawicki W. Zespół metaboliczny a zespół policystycznych jajników. In: Mamcarz A, editor. Zespół metaboliczny. Warszawa: Medical Education; 2008. pp. 567–577. [Google Scholar]
- 10.Zawadski JK, Dunaif A. Polycystic ovary syndrome. Boston: Blackwell Scientific; 1992. [Google Scholar]
- 11.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 12.The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 13.Pache TD, Wladimiroff JW, Hop WC, Fauser BC. How to discriminate between normal and polycystic ovaries: transvaginal US study. Radiology. 1992;183:421–423. doi: 10.1148/radiology.183.2.1561343. [DOI] [PubMed] [Google Scholar]
- 14.van Santbrink EJ, Hop WC, Fauser BC. Classification of normogonadotropic infertility: polycystic ovaries diagnosed by ultrasound versus endocrine characteristics of polycystic ovary syndrome. Fertil Steril. 1997;67:452–458. doi: 10.1016/s0015-0282(97)80068-4. [DOI] [PubMed] [Google Scholar]
- 15.Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod. 2003;18:598–603. doi: 10.1093/humrep/deg115. [DOI] [PubMed] [Google Scholar]
- 16.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Androgen Excess Society: Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 17.Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed) 1986;293:355–359. doi: 10.1136/bmj.293.6543.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broekmans FJ, de Ziegler D, Howles CM, Gougeon A, Trew G, Olivennes F. The antral follicle count: practical recommendations for better standardization. Fertil Steril. 2010;94:1044–1051. doi: 10.1016/j.fertnstert.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 19.Lujan ME, Brooks ED, Kepley AL, Chizen DR, Pierson RA, Peppin AK. Grid analysis improves reliability in follicle counts made by ultrasonography in women with polycystic ovary syndrome. Ultrasound Med Biol. 2010;36:712–718. doi: 10.1016/j.ultrasmedbio.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deb S, Campbell BK, Clewes JS, Raine-Fenning NJ. Quantitative analysis of antral follicle number and size: a comparison of two-dimensional and automated three-dimensional ultrasound techniques. Ultrasound Obstet Gynecol. 2010;35:354–360. doi: 10.1002/uog.7505. [DOI] [PubMed] [Google Scholar]
- 21.Jayaprakasan K, Walker KF, Clewes JS, Johnson IR, Raine-Fenning NJ. The interobserver reliability of off-line antral follicle counts made from stored three-dimensional ultrasound data: a comparative study of different measurement techniques. Ultrasound Obstet Gynecol. 2007;29:335–341. doi: 10.1002/uog.3913. [DOI] [PubMed] [Google Scholar]
- 22.Scheffer GJ, Broekmans FJM, Bancsi LF, Habbema JD, Te Velde ER. Quantitative transvaginal two- and three-dimensional sonography of the ovaries: reproducibility of antral follicle counts. Ultrasound Obstet Gynecol. 2002;20:270–275. doi: 10.1046/j.1469-0705.2002.00787.x. [DOI] [PubMed] [Google Scholar]
- 23.Battaglia C, Battaglia B, Morotti E, Paradisi R, Zanetti I, Meriggiola MC, Venturoli S. Two- and three-dimensional sonographic and color Doppler techniques for diagnosis of polycystic ovary syndrome. The stromal/ovarian volume ratio as a new diagnostic criterion. J Ultrasound Med. 2012;31:1015–1024. doi: 10.7863/jum.2012.31.7.1015. [DOI] [PubMed] [Google Scholar]
- 24.Dewailly D, Lujan ME, Carmina E, Cedars MI, Laven J, Norman RJ, et al. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2014;20:334–352. doi: 10.1093/humupd/dmt061. [DOI] [PubMed] [Google Scholar]
- 25.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 26.Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26:3123–3129. doi: 10.1093/humrep/der297. [DOI] [PubMed] [Google Scholar]
- 27.Lujan ME, Jarrett BY, Brooks ED, Reines JK, Peppin AK, Muhn N, et al. Updated ultrasound criteria for polycystic ovary syndrome: reliable thresholds for elevated follicle population and ovarian volume. Hum Reprod. 2013;28:1361–1368. doi: 10.1093/humrep/det062. [DOI] [PubMed] [Google Scholar]
- 28.Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9:505–514. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Li L, Chen X, Zhang Q, Wang W, Li Y, et al. Ovarian volume and follicle number in the diagnosis of polycystic ovary syndrome in Chinese women. Ultrasound Obstet Gynecol. 2008;32:700–703. doi: 10.1002/uog.5393. [DOI] [PubMed] [Google Scholar]
- 30.Köşüş N, Köşüş A, Turhan NÖ, Kamalak Z. Do threshold values of ovarian volume and follicle number for diagnosing polycystic ovarian syndrome in Turkish women differ from western countries? Eur J Obstet Gynecol Reprod Biol. 2011;154:177–181. doi: 10.1016/j.ejogrb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Deb S, Campbell BK, Clewes JS, Pincott-Allen C, Raine-Fenning NJ. Intracycle variation in number of antral follicles stratified by size and in endocrine markers of ovarian reserve in women with normal ovulatory menstrual cycles. Ultrasound Obstet Gynecol. 2013;41:216–222. doi: 10.1002/uog.11226. [DOI] [PubMed] [Google Scholar]
- 32.Bentzen JG, Forman JL, Johannsen TH, Pinborg A, Larsen EC, Andersen AN. Ovarian antral follicle subclasses and anti-mullerian hormone during normal reproductive aging. J Clin Endocrinol Metab. 2013;98:1602–1611. doi: 10.1210/jc.2012-1829. [DOI] [PubMed] [Google Scholar]
- 33.Lujan ME, Chizen DR, Peppin AK, Dhir A, Pierson RA. Assessment of ultrasonographic features of polycystic ovaries is associated with modest levels of inter-observer agreement. J Ovarian Res. 2009;2:6. doi: 10.1186/1757-2215-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carmina E, Orio F, Palomba S, Longo RA, Lombardi G, Lobo RA. Ovarian size and blood flow in women with polycystic ovary syndrome and their correlations with endocrine parameters. Fertil Steril. 2005;84:413–419. doi: 10.1016/j.fertnstert.2004.12.061. [DOI] [PubMed] [Google Scholar]
- 35.Pavlik EJ, DePriest PD, Gallion HH, Ueland FR, Reedy MB, Kryscio RJ, et al. Ovarian volume related to age. Gynecol Oncol. 2000;77:410–412. doi: 10.1006/gyno.2000.5783. [DOI] [PubMed] [Google Scholar]
- 36.Fulghesu AM, Angioni S, Frau E, Belosi C, Apa R, Mioni R, et al. Ultrasound in polycystic ovary syndrome – the measuring of ovarian stroma and relationship with circulating androgens: results of a multicentric study. Hum Reprod. 2007;22:2501–2508. doi: 10.1093/humrep/dem202. [DOI] [PubMed] [Google Scholar]
- 37.Pascual MA, Graupera B, Hereter L, Tresserra F, Rodriguez I, Alcázar JL. Assessment of ovarian vascularization in the polycystic ovary by three-dimensional power Doppler ultrasonography. Gynecol Endocrinol. 2008;24:631–636. doi: 10.1080/09513590802308099. [DOI] [PubMed] [Google Scholar]
- 38.Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti-Mullerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab. 2013;98:3332–3340. doi: 10.1210/jc.2013-1393. [DOI] [PubMed] [Google Scholar]
- 39.Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, Dewailly D. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–5962. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- 40.Laven JS, Mulders AG, Visser JA, Themmen AP, DeJong FH, Fauser BC. Anti-Müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004;89:318–323. doi: 10.1210/jc.2003-030932. [DOI] [PubMed] [Google Scholar]
- 41.Govind A, Obhrai MS, Clayton RN. Polycystic ovaries are inherited as an autosomal dominant trait: analysis of 29 polycystic ovary syndrome and 10 control families. J Clin Endocrinol Metab. 1999;84:38–43. doi: 10.1210/jcem.84.1.5382. [DOI] [PubMed] [Google Scholar]
- 42.Dewailly D, Pigny P, Soudan B, Catteau-Jonard S, Decanter C, Poncelet E, et al. Reconciling the definitions of polycystic ovary syndrome: the ovarian follicle number and serum anti-Müllerian hormone concentrations aggregate with the markers of hyperandrogenism. J Clin Endocrinol Metab. 2010;95:4399–4405. doi: 10.1210/jc.2010-0334. [DOI] [PubMed] [Google Scholar]
- 43.Johnstone EB, Rosen MP, Neril R, Trevithick D, Sternfeld B, Murphy R, et al. The polycystic ovary post-Rotterdam: a common, age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965–4972. doi: 10.1210/jc.2010-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mortensen M, Ehrmann DA, Littlejohn E, Rosenfield RL. Asymptomatic volunteers with a polycystic ovary are a functionally distinct but heterogeneous population. J Clin Endocrinol Metab. 2009;94:1579–1586. doi: 10.1210/jc.2008-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Catteau-Jonard S, Bancquart J, Poncelet E, Lefebvre-Maunoury C, Robin G, Dewailly D. Polycystic ovaries at ultrasound: normal variant or silent polycystic ovary syndrome? Ultrasound Obstet Gynecol. 2012;40:223–229. doi: 10.1002/uog.11202. [DOI] [PubMed] [Google Scholar]