Abstract
A sensitive and reproducible enzyme-linked immunosorbent assay (ELISA) using two monoclonal antibodies directed against a synthetic peptide with an amino-acid sequence related to the C-terminus of bovine myoglobin and the whole molecule of sodium dodecyl sulphate (SDS)-denatured bovine myoglobin was adapted for detecting bovine myoglobin in contaminated feeds. The ELISA employed bovine meat extract of a known myoglobin concentration as a calibration standard and had an limit of detection (LOD) of 3.54 ng/ml and an limit of quantification (LOQ) of 11.0 ng/ml corresponding to 0.022% and 0.067% (wt/wt) bovine meat-and-bone-meal (MBM) mixed in 20-fold-diluted feed extracts, respectively. A cut-off threshold of 20.6 ng/ml bovine myoglobin was set to simplify ELISA and facilitate quick assessment of test results without a tedious calibration process. The ELISA was able to detect bovine MBM in artificially prepared model feeds, mixed botanical feeds, mixed botanical feeds with skimmed milk, fish meal, pork meal and pork/chicken meal at 0.1% (wt/wt). It was also able to detect sheep MBM in test feeds, but showed no reactivity to swine MBM, chicken MBM, skimmed milk or gelatine of bovine origin. The advantages of this method are the quick and easy extraction protocol of proteins from test feeds, using 100 mM sodium sulphide and 0.6% sodium dodecyl sulphate in the extraction solution and the effective detection of bovine and sheep MBM at 0.1% (wt/wt).
Keywords: BSE, cut-off threshold, ELISA, monoclonal antibodies, myoglobin
Bovine spongiform encephalopathy (BSE) is a neurodegenerative disease caused by prions, which are disease-causing forms of normal proteins. Prions are transmitted through contaminated animal feeds containing meat-and-bone meal (MBM) that originated from infected animals [5, 11, 12, 15]. MBM supplements are manufactured by autoclaved, heat-dried or pulverized residual parts of livestock. To prevent BSE transmission, the Japanese government amended The Feed Safety Law in 2001 that prohibited the addition of any animal protein, including bovine, swine or poultry MBM to feeds. Guidelines to prevent cross-contamination of feed with animal proteins urged manufacturers to separate milling equipment and production lines from equipment and lines used for non-ruminant feeds that contained proteins prohibited in feeds [8]. Legislation was revised in 2005 and permitted the addition of swine and poultry MBM in swine and poultry feeds, respectively; however, the restriction remained unchanged for ruminant feeds. The eight major policies implemented by the Japanese government since 2001 were discussed in various reviews; 1) Surveillance in farm by veterinarian, 2) Prion test at healthy 1.3 million cows/year, by veterinarian, 3) Elimination of specified risk material (SRM), 4) Ban of MBM for production, sale use, 5) Prion test for fallen stocks, 6) Transparent information and traceability, 7) New Measures, such as Food Safety Basic Law, and 8) Establish of Food Safety Commission in the Cabinet Office [3, 10, 16].
The official methods of feed analysis for animal protein contamination were reviewed by Kusama et al. [7]. The Official Method of Feed Analysis stipulates that the results of three different tests have to be taken into consideration: (1) Microscopic and morphological examinations for the presence of bone debris, (2) a polymerase chain reaction (PCR) test for the presence of a DNA sequence specific to the target animal species and (3) an enzyme-linked immunosorbent assay (ELISA) for the presence of a protein reactive to an antibody specific to the target animal species. Our group previously developed the ELISA kit, which was listed in 2003 as an Official Method of Feed Analysis. The conventional ELISA kit employed a rabbit polyclonal antibody against heat-denatured bovine serum albumin (BSA); BSA was selected as the target protein, because it is universally distributed in cow’s bodies and remains soluble even after heat-denaturation. Additionally, the amino acid sequence of BSA has significant homology with serum albumin from swine. The advantage of the conventional ELISA kit was its ability to detect bovine and swine MBM with no cross-reactivity to fish meal, chicken meal or mixed botanical feeds. The disadvantage was that the detection of milk BSA and the reaction with gelatine produced false-positive results.
Kotoura et al. [6] produced hybridoma cell lines secreting monoclonal antibodies specific to bovine myoglobin and developed an ELISA for quantifying bovine meat in food, in order to reduce the risk of food-allergies. However, Kotoura’s method has been developed to determine the beef content in model processed foods and some commercial foods. The objective of this study was to adapt the ELISA system for detecting bovine MBM in feed, thereby circumventing the disadvantages of the conventional ELISA kit, which detect bovine serum albumin.
MATERIALS AND METHODS
Test feeds and chemicals: Bovine MBM (typical composition, 48–52% protein, 33–35% ash, 8–12% fat and 4–7% moisture), BSE-free feed (mixed botanical feed, fish meal, pork meal and pork/chicken meal) and artificial model feed, with or without bovine MBM, were kindly provided by The Food and Agricultural Materials Inspection Center (FAMIC), Japan. Bovine and swine gelatines were purchased from Nitta Gelatin Co. (Osaka, Japan), and bovine skimmed milk was procured from Morinaga Milk Industries Co., Ltd. (Tokyo, Japan). A 4:1 mixture of mixed botanical feed and bovine skimmed milk was prepared in the laboratory. Bovine, swine, chicken and sheep meats were obtained from the local market. Sodium dodecyl sulphate (SDS), sodium sulphide and Tween 20 were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). BSA was obtained by the Wako Pure Chemical Industries Co., Ltd. (Osaka, Japan; cat. no. 019-0749). Horseradish peroxidase was purchased from Toyobo Co., Ltd. (Osaka, Japan; cat. no. PE0-131) and 3,3′,5,5′-tetramethylbenzidine (TMB) from BioFX Laboratories, Inc. (Owings Mills, MD, U.S.A.; cat. no. TMBS-0013-01). Protein samples were quantified with the 2D Quant Protein Assay Kit (GE Healthcare UK Ltd., Buckinghamshire, U.K.).
Protein extraction: Prior to protein extraction, all test feeds were finely ground using a Millser IFN-700G homogenizer (Iwatani International Corp., Osaka, Japan). Proteins were extracted from ground test feeds by using 19 volumes (vol/wt) of extraction buffer [150 mM Tris-HCl, pH 7.4, containing 0.04% Tween 20, 0.1% BSA, 2 mM ethylenediaminetetraacetic acid trisodium salt solution (EDTA-3Na), 0.6% SDS and 100 mM sodium sulphide] and three repeated cycles of vigorous vortexing (each cycle consisting of a 30-sec run and a 30-sec cease), and boiled for 10 min. The approximately neutral pH (6.0–8.0) of the homogenates was verified using pH test paper. The homogenates were centrifuged at 3,000 × g at 25°C for 10 min, and the supernatant was filtered through Advantec No. 5A filter paper (Advantec, Tokyo, Japan). The filtrate was denoted as the feed extract. Prior to ELISA, the feed extract was diluted 20-fold with dilution buffer (150 mM Tris-HCl, pH 7.4, containing 0.04% Tween 20, 0.1% BSA and 2 mM EDTA-3Na).
The meat (bovine, swine, chicken and sheep) extracts were prepared from fresh meat using the same method as described for the preparation of the feed extract with some modifications. The meat was autoclaved at 135°C for 40 min before homogenization, and the extraction was performed with nine volumes (vol/wt) of extraction buffer. The meat extract was diluted 2,000-fold with dilution buffer and stored at −80°C until use.
ELISA: Our method was modified from the ELISA developed by Kotoura et. al. [6], which is established as Japan patent JP 4597172 B2. The capture monoclonal antibody 11H (FERM AP-21336) directed against a synthetic peptide with an amino-acid sequence related to the C-terminus of bovine myoglobin and the detection monoclonal antibody 11E against the whole molecule of sodium dodecyl sulphate (SDS)-denatured bovine myoglobin were purchased from Marudai Food Co., Ltd. (Osaka, Japan).
Microtiter plates (F8 Maxisorp Nunc-Immuno module, Thermo Fisher Scientific Inc., Waltham, MA, U.S.A.) were coated with 100 µl of 5 µg/ml monoclonal antibody 11H in 50 mM sodium carbonate, pH 9.6, and the non-specific binding sites were blocked with 20 mM Tris-HCl, pH 7.4, containing 150 mM NaCl, 0.05% Tween 20 and 0.1% BSA. The plates were incubated for 4 hr at 25°C. After incubation, the blocking solution was removed, and the plate was dried and stored at 4°C until use.
Aliquots containing 100 µl bovine meat extract (calibration standard sample) or feed extract (test sample) were dispensed into the microtiter plate wells. All wells were sealed with a self-adhesive plastic film and incubated for 1 hr at 25°C. The plates were washed six times with 300 µl of 20 mM Tris-HCl at pH 7.4, containing 150 mM NaCl and 0.5% Tween 20 (TBS-Tween). Aliquots containing 100 µl horseradish peroxidase-labeled monoclonal antibody 11E (0.6 µg/ml antibody in TBS containing 0.1% BSA) were dispensed into the microtiter plate wells and the plates incubated for 1 hr at 25°C. The plates were washed six times with 300 µl of TBS-Tween. Aliquots containing 100 µl TMB were added and incubated for 20 min at 25°C. The reaction was stopped with 100 µl of 1 M H2SO4, and the absorbance was measured at 450 nm with a microplate reader (Model 680 microplate reader, Bio-Rad, Hercules, CA, U.S.A.).
Purified bovine myoglobin at a concentration of 1.4 mg/ml, as determined with the 2D Quant Protein Assay Kit, was denatured at 95°C for 10 min in the presence of 0.6% SDS and 100 mM sodium sulphide. Myoglobin concentration in the bovine meat extract was 660 µg/ml, as determined by the ELISA using denatured myoglobin as a calibration standard. Obtained concentration corresponded to 6.6 mg myoglobin/g meat, assuming 100% recovery yield in the extraction, which was within the reported range of myoglobin content in bovine meat [13]. To express the test results in terms of bovine meat content in test feed rather than myoglobin content, the 2,000-fold diluted bovine meat extract (myoglobin concentration 330 ng/ml) was used as the calibration standard for the ELISA and defined as 2% bovine meat content in test feed.
The performance of this ELISA was examined using several kinds of model feeds prepared with and without bovine MBM (0.05% and 0.1% wt/wt). These included a mixed botanical feed, a 4:1 (wt/wt) mixture of mixed botanical feed and skimmed milk, fish meal, pork meal and pork/chicken meal. The model feeds with and without bovine MBM were assayed by this ELISA for the presence or absence of bovine meat contamination. The assay was repeated three times, each with duplicate determinations.
Statistical analyses: All statistical analyses were performed using SPSS Statistics 17.0 (IBM, Armonk, NY, U.S.A.).
RESULTS
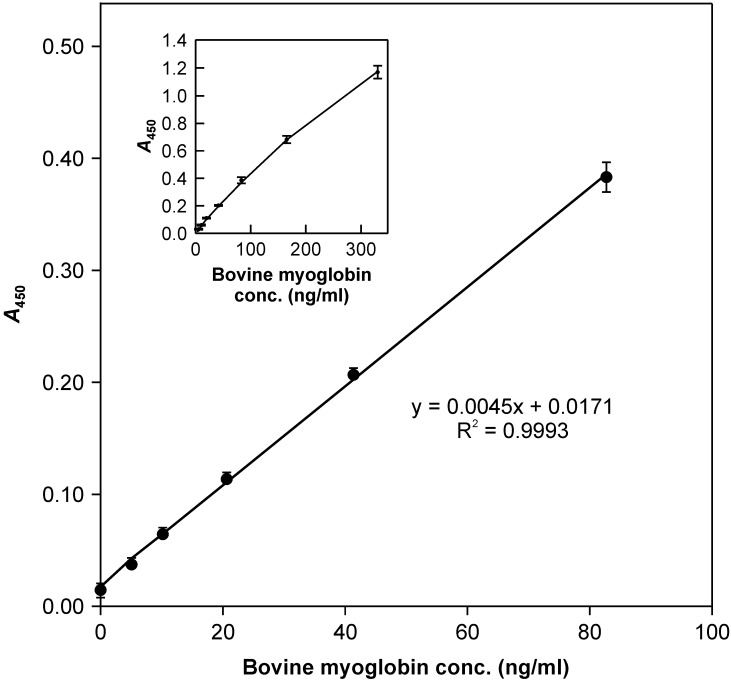
Calibration and precision of the ELISA: Calibration of the ELISA with bovine meat extract at varying dilutions showed a linear dose response at relatively low concentrations (Fig. 1), while the slope gradually fell off with increased concentration above 82.5 ng/ml myoglobin. The overall shape of the calibration line was slightly convex (Fig. 1, inset). Since precision and the limit of quantitation (LOQ) are of primary importance for the ELISA applicability, the intermediate precision was assessed at lower calibration concentrations using 8,000- to 128,000-fold diluted bovine meat extract and repeating the ELISA 10 times using different microtiter plates from the same batch. At each time, the duplicate calibration points were calculated backwards using the least-square regression equation of the straight calibration line. The raw data from the backward calculations were processed statistically for each level following the ISO 5725-2 method [2], and the limit of detection (LOD) and LOQ of the ELISA were deduced according to the method described by Currie [1] and were 3.54 and 11.0 ng/ml myoglobin, respectively [corresponding to 0.022% and 0.067% (wt/wt) bovine meat-and-bone meal (MBM) contamination in 20-fold-diluted feed extracts, respectively].
Fig. 1.
The ELISA dose response. The ELISA was calibrated using 8,000- to 128,000-fold diluted bovine meat extract. Bars indicate standard deviation (n=10).
Performance of the ELISA: Since the proportion of meat/bone in actual MBM varies from one batch to another, no confident MBM standard for the ELISA is available and the ELISA of bovine myoglobin does not reflect the exact amount of MBM in feeds. In this study, we used bovine meat extract with a known myoglobin concentration as a tentative measure for the ELISA calibration (Fig. 1), and the myoglobin concentration was directly converted to the percentage of bovine meat contamination in feed, despite the degree of MBM dryness. Given that our objective was to discriminate feeds with and without bovine MBM contamination, it was more practical to set a certain reasonable bovine myoglobin threshold for deciding the presence or absence of contamination and thereby avoid the tedious calibration process. In this context, we adapted the ELISA for bovine myoglobin, which quickly demonstrated the presence or absence of bovine MBM in feeds by using a pre-set cut-off threshold.
All model feeds without the bovine MBM showed A450 values much lower than the cut-off threshold value (Fig. 2A and Supplemental Table 1), and those containing 0.1% bovine MBM showed A450 values significantly higher than the cut-off threshold value (Fig. 2B). The significant difference between the cut-off threshold and test feed values in each set of data in Fig. 2A and 2B was analyzed by one-way ANOVA, followed by post-hoc multiple comparison Dunnett’s test using the cut-off threshold as the control. All the model feeds were classified into two groups based on the presence or absence of 0.1% MBM contamination (Supplemental Table 1), indicating that this method could reliably distinguish 0.1% bovine MBM contamination in feeds by comparing A450 values to the cut-off threshold. However, each model feed that contained 0.05% bovine MBM showed A450 values that did not differ significantly from the cut-off threshold (Fig. 2C, Supplemental Table 1). Therefore, this ELISA with a detection limit of 0.1% bovine MBM was established; however, paired t-test showed that accurate detection of 0.05% MBM in feeds was possible, if the relevant feed without MBM was used for comparison.
Fig. 2.
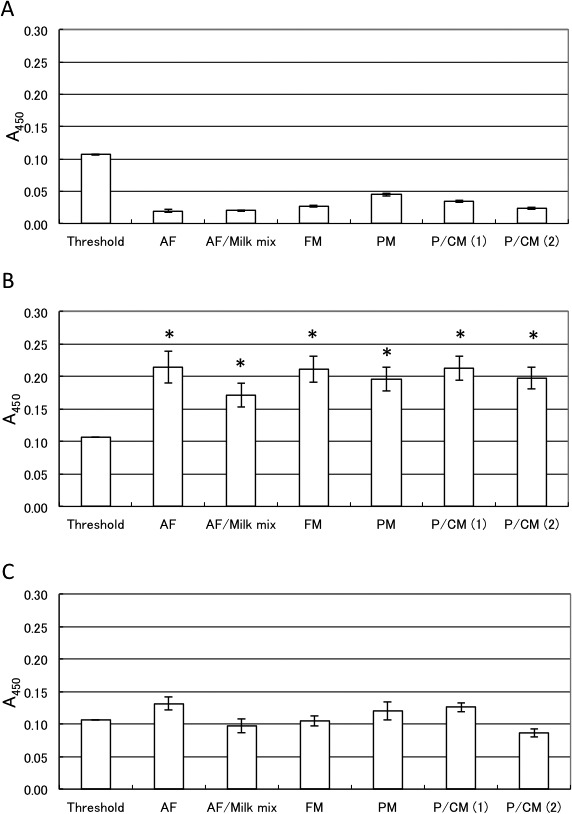
The ELISA applied to model feeds with varying bovine bone-and-meat-meal (MBM) content. A450 values of the model feeds without MBM (A), with 0.1% MBM (B), and with 0.05% MBM (C) were compared to the A450 value of the cut-off threshold. Data in Supplemental Table 1 are depicted. Bars indicate standard deviation (n=3). Comparison of the ELISA A450 values between the threshold reference and model feeds extracts, using Dunnett’s test. *P<0.05 (higher than threshold). Abbreviations are as follows: AF: mixed botanical feed; AF/Milk mix: a 4:1 mixture of a mixed botanical feed and skimmed milk; FM: fish meal; PM: pork meal; P/C M (1): pork/chicken meal (1); P/C M (2): pork/chicken meal (2).
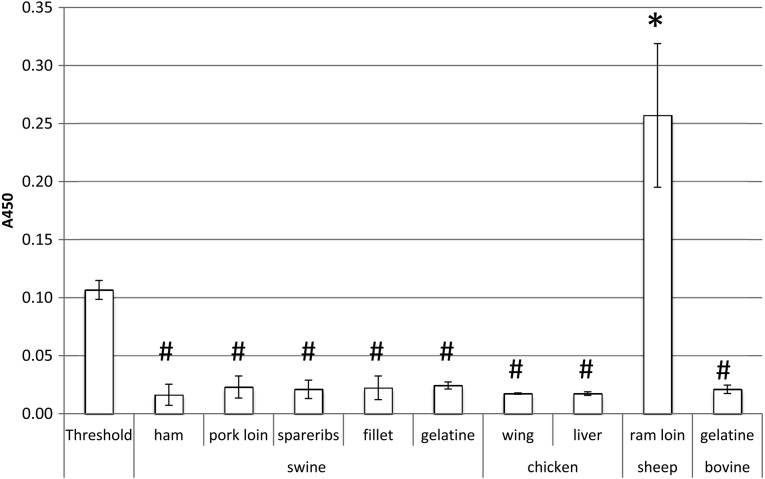
In addition, swine meat (ham, pork loin, spareribs and fillet), chicken meat (wing and liver), sheep meat (ram loin) and gelatines (bovine and swine origins) without bovine MBM were examined for possible false-positive reactivity of this method. All samples, except for sheep meat, showed A450 values far below the cut-off threshold (Fig. 3), indicating that this method could specifically detect 0.1% bovine MBM in a variety of feeds, except for those with sheep MBM.
Fig. 3.
The ELISA applied to each animal meat. A450 values of the each animal meat were compared to the A450 value of the cut-off threshold. Bars indicate standard deviation (n=3). Comparison of the ELISA A450 values between the threshold reference and each animal meat extracts, using Dunnett’s test. *P<0.05 (higher than threshold), #P<0.05 (lower than threshold)
DISCUSSION
A sensitive and reproducible ELISA using two monoclonal antibodies directed against a synthetic peptide with an amino-acid sequence related to the C-terminus of bovine myoglobin and the whole molecule of SDS-denatured bovine myoglobin was adapted for detecting bovine myoglobin in contaminated feeds. This ELISA employed bovine meat extract of a known myoglobin concentration as a calibration standard and an LOD of 3.54 ng/ml and an LOQ of 11.0 ng/ml corresponding to 0.022% and 0.067% (wt/wt) bovine MBM mixed in 20-fold-diluted feed extracts, respectively. The results of precision validation tests indicated that this ELISA was potentially useful for the quantification of bovine MBM contamination above 0.067% in feeds. The LOD and LOQ (3.54 and 11.0 ng/ml myoglobin, respectively) of this ELISA suggested that the bovine meat extract at a 32,000-fold dilution (20.6 ng/ml myoglobin; A450 approximately 0.11) (Fig. 1) provided a threshold with comfortable margins to discriminate feeds with and without bovine MBM contamination. This threshold was equivalent to 0.125% bovine MBM contamination in the preliminary ELISAs using a 20-fold diluted feed extract. This ELISA included a 2,000-fold diluted bovine meat extract (myoglobin concentration of 330 ng/ml) as a positive control, which confirmed a successful ELISA implementation by returning significantly high A450 values (around 1.2) and validated the absence of contamination in the test feeds. Although this ELISA did not use the calibration line, the bovine MBM contamination percentage in the test feed could be rationally estimated up to 0.5% by taking the ratio of A450 to the threshold (0.125%), because a linear dose dependency was warranted below this level (A450 approximately 0.4) (Fig. 1).
The conventional extraction solution (150 mM Tris-HCl, pH 7.4, containing 0.04% Tween 20, 0.1% BSA, 2 mM EDTA-3Na, 0.5% SDS and 1% 2-mercaptoethanol) was modified (150 mM Tris-HCl, pH 7.4, containing 0.04% Tween 20, 0.1% BSA, 2 mM EDTA-3Na, 0.6% SDS and 100 mM sodium sulphide) following the guidelines of a revised legislation (2007) that banned 2-mercaptoethanol, as it was classified as a toxic material. The modified protocol in this study included a quick and efficient extraction of proteins from minced test feeds with denaturation at 95°C for 10 min and intense vortexing. The first ELISA reaction requires sealing of the microtiter wells with a self- adhesive plastic film before incubation, in order to avoid possible false-positive results, especially for swine and chicken meats.
A cut-off threshold of 20.6 ng/ml bovine myoglobin threshold was set to simplify ELISA and facilitate quick assessment of test results without the tedious calibration process. This ELISA was able to detect MBM in artificially prepared model feed, mixed botanical feed, mixed botanical feed with skimmed milk, fish meal, pork meal and pork/chicken meal at 0.1% (wt/wt) and also sheep MBM, but showed no reactivity to swine MBM, chicken MBM, skimmed milk or gelatine of bovine origin (Fig. 3). Statistical analysis showed that the cut-off threshold effectively discriminated feeds containing 0.1% bovine MBM from those without MBM (Fig. 2, Supplemental Table 1). The model feeds that contained 0.05% and 0.1% bovine MBM showed A450 values around 0.1 and 0.2, respectively (Fig.2, Supplemental Table 1), which were two times higher than the theoretical A450 values of approximately 0.05 and 0.1, respectively (Fig. 1). This discrepancy was described to the different matrix compositions of bovine meat and bovine MBM. The bovine meat extract that was used as a tentative standard was prepared using autoclaved edible bovine loin, and the water content was more than 60%; however, the industrially manufactured MBM is extensively heat-dried after autoclaving or crushing, and the water content is less than 10%. Meat is highly concentrated in MBM; therefore, the absolute myoglobin content is higher in MBM than it is in meat of the same weight. The bovine meat extract provides an arbitrary threshold standard, and the ELISA effectively discriminates feeds containing bovine MBM from feeds without MBM s.
This ELISA can be used to detect bovine MBM contamination exceeding 0.1% (wt/wt) in all kinds of feeds available in the current market. In addition, it detects sheep myoglobin in which the C-terminal amino acid sequence is almost identical to that of the bovine myoglobin, except for the penultimate residue. This is an advantage of the modified ELISA, because the legislations ban any ruminant MBM in feeds.
The ELISA showed false-negative results when 0.1% bovine MBM in 100% bovine skimmed milk was examined (data not shown), probably because the very high protein concentration inhibited the antigen-antibody reaction. Since the milk content in feeds is usually less than 20%, a 4:1 mixture of mixed botanical feed and skimmed milk (20% milk content) was examined by this method. The assay successfully discriminated the model feeds with and without the addition of 0.1% bovine MBM (Fig. 2 and Supplemental Table 1).
It would be convenient in the margin of the multi-evidence Official Method of Feed Analysis policy, both the ELISA and PCR methods to be sensitive enough to detect 0.1% bovine MBM in feeds. Although immunochemical methods, including ELISA and lateral flow, for detecting proteins of ruminant origin have been reported, they are of limited sensitivity and specificity when applied to mixed botanical feeds with bovine MBM [9] or utilize a rapid, but unquantifiable method [4]. Our modified ELISA detected 0.1% bovine MBM in all model feeds. The use of monoclonal antibodies in the ELISA is an advantage, because sufficient amounts of a constant quality can be supplied to manufacture a commercial kit. A collaborative validation of this ELISA kit for screening feeds with bovine MBM was conducted by FAMIC in 10 different laboratories. The results indicated that the ELISA was satisfactory for practical use [14]. Overall, the new ELISA kit detects bovine MBM in feeds at very low concentrations and might help to feed control for preventing the occurrence of BSE.
Supplementary Material
Acknowledgments
We thank Ph.D. Masami Takagi (FAMIC) for her advice in preparing this manuscript and Mr. Tsutomu Honjoh (Morinaga Institute of Biological Science) for his technical advice.
REFERENCES
- 1.Currie L. A.1995. Nomenclature in evaluation of analytical methods including detection and quantification capabilities (IUPAC recommendations 1995). Pure Appl. Chem. 67: 1699–1723. doi: 10.1351/pac199567101699 [DOI] [Google Scholar]
- 2.ISO 5725–2. 1994. Accuracy (trueness and precision) of measurement methods and results-Part 2: Basic method for the determination of repeatability and reproducibility of a standard measurement method. http://www.iso.org/iso/catalogue_detail.htm?csnumber=11834.
- 3.Kamisato T.2005. BSE crisis in Japan: A chronological overview. Environ. Health Prev. Med. 10: 295–302. doi: 10.1007/BF02897705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein F., Lupo T., Pielack D., Mozola M.2005. Validation study of a lateral-flow immunoassay for detection of ruminant by-product material in animal feeds and feed ingredients. J. AOAC Int. 88: 1583–1592. [PubMed] [Google Scholar]
- 5.Kong Q., Surewicz W. K., Petersen R. B., Zou W., Chen S. G., Gambetti P., Parchi P., Capellari S., Goldfarb L., Montagna P., Lugaresi E., Piccardo P., Ghetti B.2004. Inherited prion diseases. pp. 673–775. In: Prion Biology and Diseases, 2nd ed. (Prusiner, S. B. ed.), Cold Spring Harbor Laboratory Press, New York. [Google Scholar]
- 6.Kotoura S., Murakami-Yamaguchi Y., Kizu K., Nakamura M., Fuchu H., Miake K., Sugiyama M., Narita H.2012. Establishment of a sandwich ELISA for the determination of beef content in processed foods by using monoclonal antibodies to myoglobin. Food Agric. Immunol. 23: 289–301. doi: 10.1080/09540105.2011.624176 [DOI] [Google Scholar]
- 7.Kusama T., Hibino H., Onodera T., Sugiura K.2009. Animal feed controls implemented in Japan for the eradication of bovine spongiform encephalopathy. Vet. Ital. 45: 287–295. [PubMed] [Google Scholar]
- 8.MAFF2007. Handbook for Introduction of Food Traceability System http://www.maff.go.jp/j/syouan/seisaku/trace/pdf/handbook_en.pdf.
- 9.Myers M. J., Yancy H. F., Farrell D. E., Washington J. D., Deaver C. M., Frobish R. A.2007. Assessment of two enzyme-linked immunosorbent assay tests marketed for detection of ruminant proteins in finished feed. J. Food Prot. 70: 692–699. [DOI] [PubMed] [Google Scholar]
- 10.Onodera T., Kim C. K.2006. BSE situation and establishment of Food Safety Commission in Japan. J. Vet. Sci. 7: 1–11. doi: 10.4142/jvs.2006.7.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prusiner S. B.1982. Novel proteinaceous infectious particles cause scrapie. Science 216: 136–144. doi: 10.1126/science.6801762 [DOI] [PubMed] [Google Scholar]
- 12.Prusiner S. B.2004. An introduction to prion biology and diseases, and development of the prion concept. pp. 1–142. In: Prion Biology and Diseases, 2nd ed. (Prusiner, S. B. ed.), Cold Spring Harbor Laboratory Press, New York. [Google Scholar]
- 13.Rickansrud D. A., Henrickson R. L.1967. Total pigments and myoglobin concentration in four bovine muscles. J. Food Sci. 32: 57–61. doi: 10.1111/j.1365-2621.1967.tb01957.x [DOI] [Google Scholar]
- 14.Takeda Z., Hashimoto Y., Yamamoto T.2011. Assessment of Morinaga ELISA kit Ver.2 detecting bovine protein in feeds. Shiryo Kenkyu Houkoku 36: 91–100. [Google Scholar]
- 15.Will R. G., Ironside J. W., Zeidler M., Cousens S. N., Estibeiro K., Alperovitch A., Poser S., Pocchiari M., Hofman A., Smith P. G.1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347: 921–925. doi: 10.1016/S0140-6736(96)91412-9 [DOI] [PubMed] [Google Scholar]
- 16.Yamanouchi K., Yoshikawa Y.2007. Bovine spongiform encephalopathy (BSE) safety measures in Japan. J. Vet. Med. Sci. 69: 1–6. doi: 10.1292/jvms.69.1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.