Abstract
MicroRNAs (miRNAs) are a class of short endogenous, single-stranded, non-coding small RNA molecules, about 19–25 nucleotides in length that regulate gene expression at the translation level and influence many physiological process, such apoptosis, metabolism, signal transduction, and occurrence and development of diseases. In this study, we constructed a library from the ovine luteal phase ovary by using next-generation sequencing technology (Solexa high-throughput sequencing technique) and identified 267 novel miRNAs by bioinformatics. One of the novel miRNAs (ovis_aries_ovary-m0033_3p), which expressed in the sheep ovary and testis, was confirmed by real time PCR and northern blot. Ovis_aries_ovary-m0033_3p was 21 nucleotides in length and located on chromosome 12, and it had 100% similarity to hsa-miR-214-3p, mmu-miR-214-3p, dre-miR-214and ssc-miR-214. Meanwhile, the pre-miRNA was 82 nucleotides in length and had a standard hairpin stem-loop structure. From the consistency of the sequence and structure, we speculated that ovis_aries_ovary-m0033_3p had a function similar to hsa-miR-214-3p, which is involved in the fine regulation of cell survival, embryonic development, breeding activities and resistance to ovarian cancer, so we defined it as oar-miR-214-3p. These experimental results will enrich the miRNA database for ovis aries and provide the basis for researching the regulation mechanism of miRNA in relation to breeding activities of seasonal breeding animals.
Keywords: microRNA, next-generation sequencing, northern blot, ovine, real-time PCR
MicroRNAs (miRNAs) are a class of short endogenous, single-stranded, non-coding functional RNA molecule [26] about 19–25 nucleotides (nt) in length that are widely present in animals and plants [10, 31] and act as posttranscriptional regulators in eukaryotes [6]. It has been estimated that miRNAs account for 1% of predicted genes in higher eukaryotic genomes and that up to 10–30% of genes may be regulated by miRNAs [8]. Since the first miRNA (Lin-4) was identified [13, 32], more than 28,645 miRNAs have been discovered in the eukaryote using various experimental approaches and computational predictions (http://www.mirbase.org/index.shtml; 7.1.2014). At present, little is known about the posttranscriptional mechanisms involved, and the multitude of reported miRNA-related studies has been drug-related and disease-related studies in either humans or rodents [11, 38]. However, the importance of miRNAs in other mammals, especially ruminants, has not yet been fully elucidated.
The ovary maintains and nurtures the germinal cells by delicate interactions with ovarian somatic cells until oocytes mature and are fertilized. Detailed morphologic and biochemical information has been generated regarding various aspects of cellular communications between these cells from the initiatory period up to establishment of cyclic follicular development after estrus [1, 3, 4, 18]. At the molecular level, these processes are regulated by several intraovarian gene products whose precise expression is fundamental for healthy maturation of oocytes until ovulation [19]. The role of small RNA in the ovary is indicated by the fact that knockout of Dicer, the ribonuclease III that processes pre-small RNA to mature functional small RNA in the ovary fertilization [9, 14, 22, 24], resulted in the dysfunction of folliculogenesis, oocyte maturation, estrus and ovulation. However, at present, it is not clear that specific miRNAs regulate the ovary, and no specific regulatory mechanism has been reported.
In recent years, the development of sequencing techniques bioinformatics has resulted in a large number of miRNAs being discovered and deposited in miRBase (http://www.mirbase.org/) or GEO (http://www.ncbi.nlm.nih.gov/geo/). But, compared with other mammals, little is known about the functional involvement of miRNAs and regulating effects on the ovary of sheep. In this study, we identified a novel miRNA from the ovine ovary by the Solexa high-throughput sequencing technique and bioinformatics and then confirmed its expression in the ovary and testis by real-time PCR and northern blot. As our knowledge advances, it is becoming clear that the discoveries in the field of miRNAs are likely to make significant contributions to the biomedical sciences, including the possibility of novel therapeutic for diseases and regulation of animal reproduction.
MATERIALS AND METHODS
Ethics statement: All animal experiments were approved by the Institutional Animal Care and Use Ethics Committee of Gansu Agricultural University (No. 2008004) and performed in accordance with the “Guidelines for Experimental Animals” of the Ministry of Agriculture (Beijing, PR China).
Samples and total RNA extraction: Luteal phase ovaries were collected after three sheep (8 months of age) were slaughtered during the breeding season and immediately frozen in liquid nitrogen, and three testes were also collected and frozen at the same age. Total RNAs, including miRNAs, were extracted using TRIzol reagent (Takara China, Dalian, China) according to the manufacturer’s instructions. They were homogenized and pooled for library construction and Solexa high-throughput sequencing. The purity and integrity of the total RNA were assessed using an IMPLEN P-360 ultraviolet spectrophotometer (Schatzbogen, Germany). Then, total RNA was stored at −80°C until use.
Library construction and high-throughput sequencing: The small RNA library was structured using a Small RNA Sample Preparation Kit (Illumina, San Diego, CA, U.S.A.) according to the manufacturer’s instructions. Briefly, we excised the 18-to 30-nucleotide (nt) fractions of total RNA and purified them following 15% denaturing polyacrylamide gel electrophoresis (PAGE). Then, 3′ and 5′ RNA adaptors were respectively connected using T4 RNA ligase enzyme. The adaptor-connected small RNAs were subjected to reverse transcription, and the cDNA was amplified with 25 PCR cycles. Then, PCR products of about 90 bp were purified using agarose gels and sequenced at the Beijing Genomics Institute (BGI, Shen zhen, PR China). Following a process to produce digital-quality data, the clean reads were processed for computational analysis after removing the adaptor sequences and contamination.
Analysis of the sequence data: The raw reads were generated by Solexa high-throughput sequencing, and clean reads were obtained eliminating some of the contaminant reads with reference to the process of Ji et al. [11]. The clean reads were mapped to the Ovis aries genome constructed by the International Sheep Genomics Consortium (ISGC) [5], and then, selected sequences with perfect matches were used for the following analysis. The clean reads were aligned against the small RNAs (rRNAs, tRNAs, snRNAs, snoRNA and miRNA) to annotate the small RNA sequences using tag 2 miRNA annotation software (developed by BGI). To ensure each unique small RNA mapped to only one annotation, we used the following priority rule: rRNA etc. (in which GenBank >Rfam) >known miRNA >repeat >exon >intron [11]. The unannotated sequences were selected to predict novel miRNAs.
To further analyze the structures of potential novel miRNAs, we used the Mfold 3.2 software [20] to predict the novel RNA hairpin structure characteristic, and subjected each sequence to further analysis with the MIREAP v0.2 software [16] under previously reported parameter settings [11, 39].
Quantitative RT-PCR of known and novel miRNAs: Differential expressions profiles of known and novel miRNAs were validated with quantitative RT-PCR using a SYBR® Prime Script™ miRNA RT-PCR Kit (Takara, Code No. RR716) according to the manufacturer’s protocol. The forward primers were designed based on the mature miRNA sequences (Table 1), and the reverse primer was provided in the kit. The housekeeping gene U6 was used as an endogenous control. The reverse transcription reaction system contained 10 µl 2 × miRNA Reaction Buffer Mix, 2 µl 0.1% BSA, 2 µl miRNA Prime Script RT Enzyme Mixand 1 µl total RNA (10 pg/ml–1 µg/ml) and RNase-Free H2O (dH2O) was added to adjust the volume to 20 µl. The mixture was incubated at 37°C for 60 min and then at 85°C for 5 sec. The cDNA products were stored at −20°C.
Table 1. Summary of miRNA primers used in real-time RT-PCR.
| Name | Sequence (5′→3′) | Length (nt) | GC (%) | Tm |
|---|---|---|---|---|
| U6 | AGGATGTGAAGACACCAAGACA | 22 | 45.45 | 59.62 |
| oar_miR_3958_3p | AGATATTGCACGGTTGATCTCT | 22 | 40.9 | 55.9 |
| oar_miR_432 | TCTTGGAGTAGGTCATTGGGTGG | 23 | 52.2 | 62.8 |
| oar_miR_379_5p | TGGTAGACTATGGAACGTAGGC | 22 | 50.0 | 56.6 |
| oar_miR_485_5p | AGAGGCTGGCCGTGATGAAT | 20 | 55.0 | 61.8 |
| oar_miR_127 | ATCGGATCCGTCTGAGCTTG | 20 | 55.0 | 59.9 |
| ovis_aries_ovary-m0005_5p | GCGAAAGGTTCATTTGGGTT | 20 | 45.0 | 59.2 |
| ovis_aries_ovary-m0032_5p | CAGGCTAGGAGAAATGATTGG | 21 | 47.6 | 56.8 |
| ovis_aries_ovary-m0033_3p | ACAGCAGGCACAGACAGGC | 19 | 61.1 | 58.4 |
| ovis_aries_ovary-m0070_5p | GCTGGAAGACTAGTGATTTTGTTG | 24 | 41.7 | 58.1 |
| ovis_aries_ovary-m0083_3p | TCACAGTGAACCGGTCTCTTT | 21 | 47.6 | 57.0 |
| ovis_aries_ovary-m0088_5p | CAACGGAATCCCAAAAGCAGC | 211 | 52.4 | 64.4 |
| ovis_aries_ovary-m0098_3p | TTCACCACCTTCTCCACCC | 19 | 52.9 | 52.1 |
| ovis_aries_ovary-m0105_5p | GCAAAGAATTCTCCTTTTGGGC | 22 | 45.5 | 62.2 |
| ovis_aries_ovary-m0132_5p | AGGCGGAGACTTGGGCAATT | 20 | 55.0 | 63.4 |
| ovis_aries_ovary-m0161_3p | TCACAGTGAACCGGTCTCTTT | 21 | 47.6 | 57.0 |
The qRT-PCR was performed with a SYBR® PrimeScript™ miRNA RT-PCR Kit (Takara, Code No. RR716) on a FTC-3000 real-time quantitative PCR analyzer (Funglyn Biotech Inc., Toronto, ON, Canada). The reaction solution contained 10 µl SYBR Premix Ex Taq (2×), 0.8 µl PCR Forward Primer (10 mM), 0.8 µl Uni-miR q PCR Primer (10 mM), 1 µl RO× Reference Dye (50×) and 2 µl cDNA, and dH2O was added up to adjust the final volume to 20 µl. The reaction conditions were as follows: initial denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec, then 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec. All reactions were performed in triplicate, and the reaction solution was prepared on ice. The relative quantity of expression was calculated using the 2-ΔΔCT method [15] after the threshold cycle (Ct) and control with the Ct of U6. The miRNA expression level in the ovary and testis was determined individually. The primers of the miRNAs for qRT-PCR are shown in Table 1.
One-way ANOVA was used to examine the significance of the difference in expression level of each miRNA between the ovary and testis.
Northern blot: Two novel miRNAs were chosen to validate their expression levels in the sheep ovary and testis by northern blot hybridization using a High Sensitive MiRNA Northern Blot Assay Kit (NB-1001, Signosis, Inc., Santa Clara, CA, U.S.A.) according to the manufacturer’s protocol, as well as by reference to the studies of Szarzynska et al. [29] and Lukasik et al. [17]. Briefly, RNA (45 µg) was resolved in 15% denaturing PAGE and transferred to a Hybond-NX nylon membrane, followed by UV cross-linking. Expression of a specific miRNA was detected with a biotin-labeled probe containing two moieties–a complementary sequence of the miRNA and a tag sequence. The tag sequence was then detected by an amplifier enriched with biotin molecules. A probe complementary to U6 was used as a control. Hybridization was performed overnight at 42°C. The membranes were washed twice with 1*NB wash buffer for 40 min at 42°C, then amplifiered and rotated at 42°C for 2 hr. The signals were recorded using an ultrasensitive chemiluminescence gel imaging system (ChemiDocTM XRS+System, Bio-Rad, Hercules, CA, U.S.A.). The probe sequences of miRNAs for northern blot are shown in Table 2. The experiment was repeated 3 times.
Table 2. Sequences of miRNAs for northern blot.
| Probe name | Number of reads | Sequences | Highly sensitive probe |
|---|---|---|---|
| U6 | – | 5′-biotin-ATCGTTCCAATTTTAGTATATGTGCTGCCGAAGCGAGCAC-3′ | Yes |
| ovis_aries_ovary-m0033_3p | 6478 | 5′-biotin- CTGCCTGTCTGTGCCTGCTGT-3′ | Yes |
| ovis_aries_ovary-m0161_3p | 625 | 5′-biotin-AAAGAGACCGGTTCACTGTGA-3′ | Yes |
RESULTS
Small RNA library construction and Solexa high-throughput sequencing: In this study, a small RNA library was constructed from the ovary using an Solexa high-throughput sequencer. The library contains a total of 9,600,000 raw reads. By removing the low-quality reads, adaptors and insufficient tags, there were ultimately 9,321,775 clean reads of 18–30 nt. Of these, 7,240,262 ovary sequences, which accounted for 75.42% of the total reads (Table 3), were perfectly mapped to the Ovis aries genome (http://www.ncbi.nlm.nih.gov/projects/mapview/map_search.cgi?taxid=9940&query=January 2013). All identical sRNA sequence reads were grouped together to simplify the sequencing data, and ultimately, a total of 369,105 unique sRNA sequences for the ovary remained for further analysis (Table 3).
Table 3. Summary of high-throughput sequencing data for small RNAs in the ovary.
| Categories | Unique sRNAs | Total sRNAs |
|---|---|---|
| Number of raw reads | – | 9,600,000 |
| Clear reads | 369,105 | 9,321,775 |
| Perfect match to ovis aries genome | 165,458 | 7,240,262 |
| Specific sequences | 314,145 | 486,908 |
| Common | Unique sRNAs: 54,960 | |
| Total | Unique sRNAs: 474,313 | |
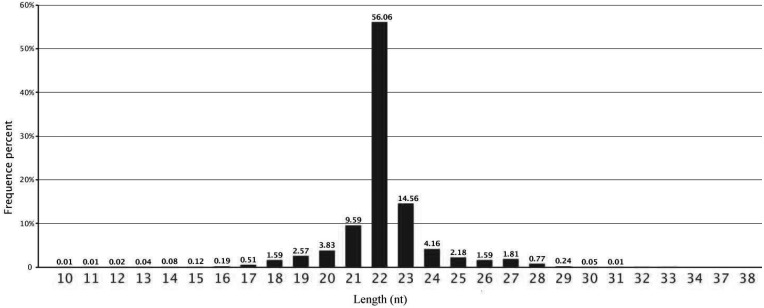
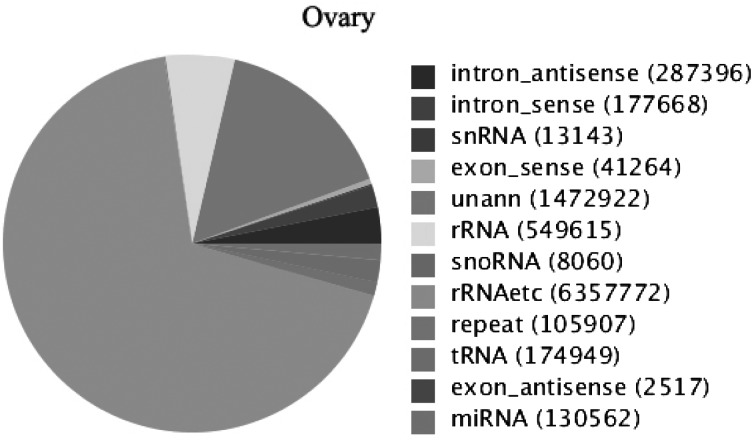
From Fig. 1, we can see that the lengths of the majority of small RNAs were from 20 to 24 nt and that the most common class was 22 nt in the small RNA sequence distribution, accounting for 56.06% of the small RNAs in the ovary, followed by those that were 23 nt (14.56%), 21 nt (9.59%) and 24 nt long (4.16%), which were typical of small RNA Dicer-processed products. We annotated and classified all sequence reads through alignment with the GenBank and Rfam databases to assess the efficiency of Solexa high-throughput sequencing for sRNA detection. The 9,321,775 clean reads were annotated and classified as introns, exons, miRNAs, rRNAs, tRNAs, snRNAs, snoRNAs, repeats, etc. (Fig. 2). Another 1,472,922 (accounting for 15.80% of the total reads) reads were unannotated in the ovary.
Fig. 1.
Distribution of the lengths of the sequences in the ovary. Sequence length distribution of clean reads based on the abundance and distinct sequences; the most abundant size class was 22 nt, which accounted for over half of the total number of reads, followed by 23 nt, 21 nt, 24 nt and 20 nt.
Fig. 2.
Distribution of sRNAs categories in the ovary. The clean reads were annotated and classified as introns, exons, snRNAs, rRNAs, snoRNAs, tRNAs, miRNAs, etc., in the GenBank and Rfam databases. Some partial reads were not annotated and need further analysis.
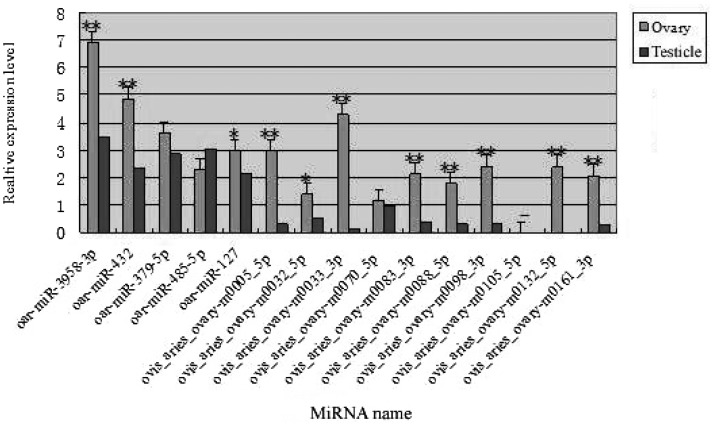
Quantitative RT-PCR validation of known and novel miRNAs expression: To validate the reliability of the Solexa high-throughput sequencing results, we applied relative quantitative RT-PCR to compare the expression levels of known and newly identified miRNAs in the ovary and testis. The 5 known miRNAs with the highest expression levels and 10 novel miRNAs with functions related to reproduction were selected to be validated by qRT-PCR. The experimental results are shown in Fig. 3. The miRNAs, including oar-miR-3958-3p, oar-miR-432, oar-miR-379-5p, oar-miR-485-5p, oar-miR-127, ovis_aries_ovary-m0005_5p, ovis_aries_ovary-m0032_5p, ovis_aries_ovary-m0033_3p, ovis_aries_ovary-m0070_5p, ovis_aries_ovary-m0083_3p, ovis_aries_ovary-m0088_5p, ovis_aries_ovary-m0098_3p, ovis_aries_ovary-m0132_5p and ovis_aries_ovary-m0161_3p, were differentially expressed in the ovary and testis, and the expression levels of some miRNAs, such as oar-miR-3958-3p, oar-miR-432, ovis_aries_ovary-m0005_5p, ovis_aries_ovary-m0033_3p, ovis_aries_ovary-m0083_3p, ovis_aries_ovary-m0088_5p, ovis_aries_ovary-m0098_3p, ovis_aries_ovary-m0132_5p and ovis_aries_ovary-m0161_3p, in the ovary were highly significantly different from those in the testis. Oar-miR-3958-3p showed the highest expression level in the ovary and testis, which was accordance with the date from sequencing, and ovis_aries_ovary-m0132_5p was only detected in the ovary; however, ovis_aries_ovary-m0105_5p was not detected in the ovary or testis (Fig. 3).
Fig. 3.
qRT-PCR validation of the identified known and potential novel miRNAs. Total RNAs pooled from the ovary and testis were used for qRT-PCR. The relative quantity of expression was calculated using the 2-ΔΔCT method after the threshold cycle (Ct) and control with the Ct of U6. The relative expression levels are described using the 2-ΔΔCT means ± SE. Each miRNA was replicated for three times. Five known miRNAs with the highest expression level and 10 novel miRNAs related to reproduction in the ovary. *P<0.05; **P<0.01.
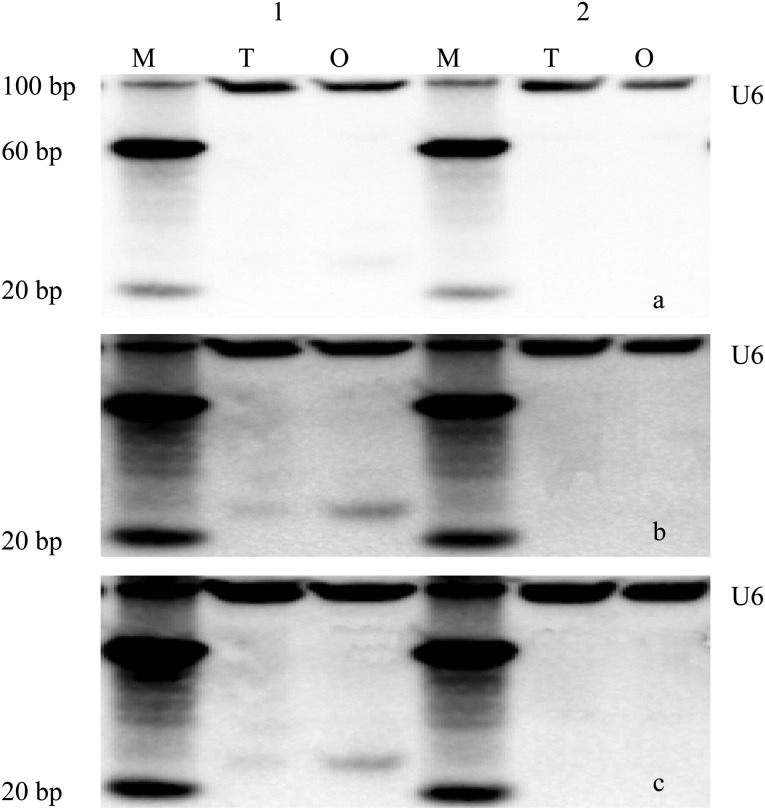
Northern blot analysis: In order to confirm the small RNA sequencing results and to determine the expression patterns of these miRNAs in the ovary and testis, we chose two novel miRNAs, ovis_aries_ovary-m0033_3p and ovis_aries_ovary-m0161_3p, that represented our sequencing data for further northern blot analysis. Figure 4 shows that ovis_aries_ovary-m0033_3p was detected in both the ovary and testis in triplicate experiments, but the abundance was lower in the testis according to real-time PCR. In this study, ovis_aries_ovary-m0161_3p was not detected in the ovary and testis. U6 was detected as flat bands and was abundant in the ovary and testis. Compared with U6, ovis_aries_ovary-m0033_3p was less abundant.
Fig. 4.
Northern blot analysis of selected novel miRNAs of sheep. a-c: Northern blot results for the three repeats of the experiment. M, marker; T, testis; O, ovary; 1, ovis_aries_ovary-m0033_3p; 2, ovis_aries_ovary-m0161_3p; U6, the control, which was detected as flat bands in the ovary and testis and in higher abundance. Ovis_aries_ovary-m0033_3p was detected from both the ovary and testis in the three repeats of the experiment, but its abundance was lower in the testis; ovis_aries_ovary-m0161_3p was not detected in the ovary and testis.
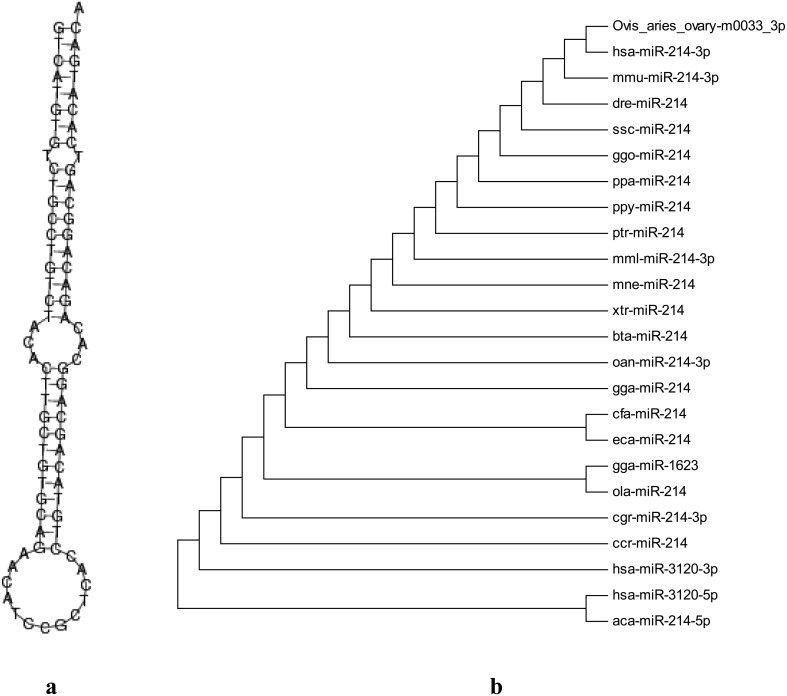
Secondary structure and homology analysis: To further analyze the structures of potential novel miRNAs, we used the Mfold 3.2 software [7] to predict the novel RNA hairpin structure characteristics and subjected each sequence to further analysis with the MIREAP v0.2 software [16] under previously reported parameter settings [11, 39]. Ultimately, the precursor structure (Fig. 5a) of ovis_aries_ovary-m0033_3p was very standard, having a typical hairpin stem-loop structure. We performed multiple alignment using the ClustalW software [7] for selected homologous sequences in various species and then constructed a phylogenetic tree using the MEGA 5 software by neithbor-joining (NJ) method (Fig. 5b). From the dendrogram, we can see that ovis_aries_ovary-m0033_3p clustered together with human hsa-mi214-3p and lay in the same evolutionary branch, which was 100% homologous to hsa-miR-214-3p (miRBase: MIMAT0000271), mmu-miR-214-3p (miRBase: MIMAT0000661), dre-miR-214 (miRBase: MIMAT0001283), ssc-miR-214 (miRBase: MIMAT0002147), etc. The results demonstrated that the new miRNA (ovis_aries_ovary-m0033_3p) identified was homologous to other species corresponding miRNAsandproved the reliability of our identification results.
Fig. 5.
The secondary structure and phylogenetic tree of ovis_aries_ovary-m0033_3p. a: The secondary structure, which has a typical hairpin stem-loop structure. b: The phylogenetic tree. Ovis_aries_ovary-m0033_3p clustered together with human hsa-mi214-3p and lay in the same evolutionary branch, which was 100% homologous to hsa-mi214-3p, mmu-mi214-3p, dre-mi214 and ssc-miR-214.
DISCUSSION
Solexa high-throughput sequencing technology is used to identify miRNAs, and it has more special advantages for small RNA sequencing in various animals and plants, for example, high throughput, high repeatability and high accuracy. In this study, a small RNA library was constructed by using Solexa high-throughput sequencing technology to identify the small RNAs of sheep. We found that more than 84% of the small RNA reads in the ovary were 20–24 nt in length, which was consistent with the typical size of mature miRNAs and the findings of previous studies [28, 34]. Compared with traditional methods, high-throughput sequencing technology has a specific advantage for discovery of novel miRNAs with particular function . Many computational programs, such as miRSan, miRseeker, Mfold and findMiRNA, have been used for predicting unknown miRNAs [35], and these programs are based on minimal free energies and specific miRNA hairpin stem-loop structures [36]. In this study, the MIREAP software was used to predict the novel miRNAs by studying the minimum free energy, stem-loop structure and Dicer cleavage site [16]. Then, we eliminated the sequences with lots of less than two and then further analyzed their typical stem-loop structures with the Mfold and MiPred softwares. Finally, the predicted novel miRNAs and known miRNAs were further validated by real-time PCR (Fig. 3). Zhang et al. established an accurate and rapid real-time fluorescent PCR method to quantify human let-7a miRNA in human gastric carcinoma and normal tissue and then preliminarily discussed the relationship between the let-7a expression level determined by this PCR method and gastric carcinoma carcinogenesis [37]. In the present study, of the 5 known miRNAs and 10 predicted potential miRNAs, 13 were validated in the ovary and testis, 1 novel miRNA was detected only in the ovary, and 1 novel miRNA was not detected in both the ovary and testis. Lack of detection of miRNAs might be related to the very low abundance of an of unreasonable primers design, false-positive results or use the wrong detection method for potential miRNAs. At present, northern blot is still the gold standard for detection of miRNA. Lukasik et al. identified 13 selected miRNAs by northern blot hybridization in mature cabbage leaves [17]. Khudayberdiev et al. validated nuclear enrichment of specific candidate miRNAs (miR-25 and miR-92a), which could be independently validated by northern blot [12]. In this study, to further provide new insight regarding the regulation mechanisms of miRNAs related to the reproduction organs, as well as the accuracy of the results of high-throughput sequencing and real-time PCR, we performed northern blot to detect the expression of two novel miRNAs in the ovary and testis. Ovis_aries_ovary-m0033_3p was detected in two tissues, but the expression level was different, which was in accordance with the results of real-time PCR. Previous studies also documented that miRNAs were differentially expressed in the testis and ovary in diverse species [21, 27, 28], and suggested the important role of miRNAs in driving gonadal tissue development and function. But, ovis_aries_ovary-m0161_3p was not detected in the ovary and testis, and whether this was related to the level of abundance of false-positive results needs further examination. In our homology analysis, we found that ovis_aries_ovary-m0033_3p was 100% homologous to hsa-miR-214-3p, mmu-miR-214-3p, dre-miR-214 and ssc-miR-214. The secondary structure had a typical hairpin stem-loop structure and conformed to the structural characteristics of pre-miRNAs. A mature miRNA should be a distinct transcript of about 20–24 nt so that its expression level is detectable by qRT-PCR, northern blot or other experimental methods and should be generated from a precursor with typical characteristics in terms of secondary structure, for example, a stem-loop or fold-back structure. Ovis_aries_ovary-m0033_3p possessed all these characteristics.
A previous study reported that the PTEN/Akt pathway was a major target of miR-214 and largely mediated miR-214 antiapoptotic function, which played an important role in the pathogenesis of malignancy and was a potential target for ovarian cancer intervention [33]. MiR-214 was also upregulated in several human tumors, such as gastric cancer [30], Sezary syndrome [23] and melanoma [25]. Furthermore, elevated expression of miR-214 was associated with chemotherapy resistance [33] or tumor metastasis [25]. From the homology of the sequences and secondary structure, we speculated that ovis_aries_ovary-m0033_3p was miR-214 of the ovine, which was defined as oar-miR-214-3p based on the miRNA naming rules [2]. Taking the previous studies into consideration, we inferred that oar-miR-214-3p would play an important role in inducing cell survival (such as in luteal and granular cells) and cisplatin resistance, embryonic development, nasopharyngeal carcinoma (NPC) carcinogenesis and so on. In follow-up experiments, we will further confirm the regulation function of oar-miR-214-3p in the sheep ovary by gene knockout and overexpression.
Acknowledgments
This work was supported by the Departments of Gansu province, Department of Agriculture of China (grant numbers: 2009ZX08008-008B) and College of Veterinary Medicine, Gansu Agricultural University (grant number: JYCX-KX007).
REFERENCES
- 1.Albertini D. F., Barrett S. L.2003. Oocyte-somatic cell communication. Reprod. Suppl. 61: 49–54. [PubMed] [Google Scholar]
- 2.Ambros V., Bartel B., Bartel D. P., Burge C. B., Carrington J. C., Chen X., Dreyfuss G., Eddy S. R., Griffiths-Jones S., Marshall M., Matzke M., Ruvkun G., Tuschl T.2003. A uniform system for microRNA annotation. RNA 9: 277–279. doi: 10.1261/rna.2183803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amsterdam A.2003. Novel genes regulated by gonadotropins in granulosa cells: new perspectives on their physiological functions. Mol. Cell. Endocrinol. 202: 133–137. doi: 10.1016/S0303-7207(03)00074-1 [DOI] [PubMed] [Google Scholar]
- 4.Andreu-Vieyra C., Lin Y. N., Matzuk M. M.2006. Mining the oocyte transcriptome. Trends Endocrinol. Metab. 17: 136–143. doi: 10.1016/j.tem.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 5.Archibald A. L., Cockett N. E., Dalrymple B. P., Faraut T., Kijas J. W., Maddox J. F., McEwan J. C., Hutton Oddy V., Raadsma H. W., Wade C., Wang J., Wang W., Xun X., International Sheep Genomics Consortium2010. The sheep genome reference sequence: a work in progress. Anim. Genet. 41: 449–453. doi: 10.1111/j.1365-2052.2010.02100.x [DOI] [PubMed] [Google Scholar]
- 6.Bartel D. P.2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297. doi: 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 7.Chenna R., Sugawara H., Koike T., Lopez R., Gibson T. J., Higgins D. G., Thompson J. D.2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31: 3497–3500. doi: 10.1093/nar/gkg500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiou T. J., Aung K., Lin S. I., Wu C. C., Chiang S. F., Su C. L.2006. Regulation of phosphate homeostasis by MicroRNA in Arabidopsis. Plant Cell 18: 412–421. doi: 10.1105/tpc.105.038943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong X., Luense L. J., McGinnis L. K., Nothnick W. B., Christenson L. K.2008. Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology 149: 6207–6212. doi: 10.1210/en.2008-0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houbaviy H. B., Murray M. F., Sharp P. A.2003. Embryonic stem cell-specific MicroRNAs. Dev. Cell 5: 351–358. doi: 10.1016/S1534-5807(03)00227-2 [DOI] [PubMed] [Google Scholar]
- 11.Ji Z., Wang G., Xie Z., Wang J., Zhang C., Dong F., Chen C.2012. Identification of novel and differentially expressed MicroRNAs of dairy goat mammary gland tissues using solexa sequencing and bioinformatics. PLoS ONE 7: e49463. doi: 10.1371/journal.pone.0049463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khudayberdiev S. A., Zampa F., Rajman M., Schratt G.2013. A comprehensive characterization of the nuclear microRNA repertoire of post-mitotic neurons. Front. Mol. Neurosci. 6: 43. doi: 10.3389/fnmol.2013.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee R. C., Feinbaum R. L., Ambros V.1993. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843–854. doi: 10.1016/0092-8674(93)90529-Y [DOI] [PubMed] [Google Scholar]
- 14.Lei L., Jin S., Gonzalez G., Behringer R. R., Woodruff T. K.2010. The regulatory role of Dicer in folliculogenesis in mice. Mol. Cell. Endocrinol. 315: 63–73. doi: 10.1016/j.mce.2009.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak K. J., Schmittgen T. D.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT Method. Methods 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 16.Li Y., Zhang Z., Liu F., Vongsangnak W., Jing Q., Shen B.2012. Performance comparison and evaluation of software tools for microRNA deep-sequencing data analysis. Nucleic Acids Res. 40: 4298–4305. doi: 10.1093/nar/gks043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukasik A., Pietrykowska H., Paczek L., Szweykowska-Kulinska Z., Zielenkiewicz P.2013. High-throughput sequencing identification of novel and conserved miRNAs in the Brassica oleracea leaves. BMC Genomics 14: 801.http://www.biomedcentral.com/1471–2164/14/801. doi: 10.1186/1471-2164-14-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makabe S., Naguro T., Stallone T.2006. Oocyte-follicle cell interactions during ovarian follicle development, as seen by high resolution scanning and transmission electron microscopy in humans. Microsc. Res. Tech. 69: 436–449. doi: 10.1002/jemt.20303 [DOI] [PubMed] [Google Scholar]
- 19.Matzuk M. M., Burns K. H., Viveiros M. M., Eppig J. J.2002. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296: 2178–2180. doi: 10.1126/science.1071965 [DOI] [PubMed] [Google Scholar]
- 20.Zuker M.2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31: 3406–3415. doi: 10.1093/nar/gkg595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishima T., Takizawa T., Luo S. S., Ishibashi O., Kawahigashi Y., Mizuguchi Y., Ishikawa T., Mori M., Kanda T., Goto T., Takizawa T.2008. MicroRNA (miRNA) cloning analysis reveals sex differences in miRNA expression profiles between adult mouse testis and ovary. Reproduction 136: 811–822. doi: 10.1530/REP-08-0349 [DOI] [PubMed] [Google Scholar]
- 22.Murchison E. P., Stein P., Xuan Z., Pan H., Zhang M. Q., Schultz R. M., Hannon G. J.2007. Critical roles for Dicer in the female germline. Genes Dev. 21: 682–693. doi: 10.1101/gad.1521307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narducci M. G., Arcelli D., Picchio M. C., Lazzeri C., Pagani E., Sampogna F., Scala E., Fadda P., Cristofoletti C., Facchiano A., Frontani M., Monopoli A., Ferracin M., Negrini M., Lombardo G. A., Caprini E., Russo G.2011. MicroRNA profiling reveals that miR-21, miR486 and miR-214 are upregulated and involved in cell survival in Sézary syndrome. Cell Death Dis. 2: e151. doi: 10.1038/cddis.2011.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otsuka M., Zheng M., Hayashi M., Lee J. D., Yoshino O., Lin S., Han J.2008. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J. Clin. Invest. 118: 1944–1954. doi: 10.1172/JCI33680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penna E., Orso F., Cimino D., Tenaglia E., Lembo A., Quaglino E., Poliseno L., Haimovic A., Osella-Abate S., De Pittà C., Pinatel E., Stadler M. B., Provero P., Bernengo M. G., Osman I., Taverna D.2011. microRNA-214 contributes to melanoma tumour progression through suppression of TFAP2C. EMBO J. 30: 1990–2007. doi: 10.1038/emboj.2011.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Real F. M., Sekido R., Lupiáñez D. G., Lovell-Badge R., Jiménez R., Burgos M.2013. A microRNA (mmu-miR-124) prevents Sox9 expression in developing mouse ovarian cells. Biol. Reprod. 89: 78. doi: 10.1095/biolreprod.113.110957 [DOI] [PubMed] [Google Scholar]
- 27.Ro S., Park C., Sanders K. M., McCarrey J. R., Yan W.2007. Cloning and expression profiling of testis-expressed microRNAs. Dev. Biol. 311: 592–602. doi: 10.1016/j.ydbio.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ro S., Song R., Park C., Zheng H., Sanders K. M., Yan W.2007. Cloning and expression profiling of small RNAs expressed in the mouse ovary. RNA 13: 2366–2380. doi: 10.1261/rna.754207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szarzynska B., Sobkowiak L., Pant B. D., Balazadeh S., Scheible W. R., Mueller-Roeber B., Jarmolowski A., Szweykowska-Kulinska Z.2009. Gene structures and processing of Arabidopsis thaliana HYL1-dependent pri-miRNAs. Nucleic Acids Res. 37: 3083–3093. doi: 10.1093/nar/gkp189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueda T., Volinia S., Okumura H., Shimizu M., Taccioli C., Rossi S., Alder H., Liu C. G., Oue N., Yasui W., Yoshida K., Sasaki H., Nomura S., Seto Y., Kaminishi M., Calin G. A., Croce C. M.2010. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 11: 136–146. doi: 10.1016/S1470-2045(09)70343-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei Y., Chen S., Yang P., Ma Z., Kang L.2009. Characterization and comparative profiling of the small RNA transcriptomes in two phases of locust. Genome Biol. 10: R6. doi: 10.1186/gb-2009-10-1-r6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wightman B., Ha I., Ruvkun G.1993. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75: 855–862. doi: 10.1016/0092-8674(93)90530-4 [DOI] [PubMed] [Google Scholar]
- 33.Yang H., Kong W., He L., Zhao J. J., O’Donnell J. D., Wang J., Wenham R. M., Coppola D., Kruk P. A., Nicosia S. V., Cheng J. Q.2008. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 68: 425–433. doi: 10.1158/0008-5472.CAN-07-2488 [DOI] [PubMed] [Google Scholar]
- 34.Zhang B., Stellwag E. J., Pan X.2009. Large-scale genome analysis reveals unique features of microRNAs. Gene 443: 100–109. doi: 10.1016/j.gene.2009.04.027 [DOI] [PubMed] [Google Scholar]
- 35.Zhang B., Pan X., Wang Q., Cobb G. P., Anderson T. A.2006. Computational identification of microRNAs and their targets. Comput. Biol. Chem. 30: 395–407. doi: 10.1016/j.compbiolchem.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 36.Zhang B. H., Pan X. P., Cox S. B., Cobb G. P., Anderson T. A.2006. Evidence that miRNAs are different from other RNAs. Cell. Mol. Life Sci. 63: 246–254. doi: 10.1007/s00018-005-5467-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H. H., Wang X. J., Li G. X., Yang E., Yang N. M.2007. Detection of let-7a microRNA by real-time PCR in gastric carcinoma. World J. Gastroenterol. 13: 2883–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong S., Li W., Chen Z., Xu J., Zhao J.2013. MiR-222 and miR-29a contribute to the drug-resistance of breast cancer cells. Gene 531: 8–14. doi: 10.1016/j.gene.2013.08.062 [DOI] [PubMed] [Google Scholar]
- 39.Zuker M., Jacobson A. B.1998. Using reliability information to annotate RNA secondary structures. RNA 4: 669–679. doi: 10.1017/S1355838298980116 [DOI] [PMC free article] [PubMed] [Google Scholar]