Abstract
Nineteen cases of histiocytic sarcomas in Pembroke Welsh Corgi were examined histopathologically. Focal or multiple masses were detected in the lung or in regional lymph nodes, or in both lung and nodes. All neoplastic lesions had common histological features characterized by the proliferation of pleomorphic histiocytic cells combined with various inflammatory cells. Most of the pleomorphic neoplastic cells were immunopositive for human leukocyte antigen (HLA)-DR and Iba-1. The median survival time for all dogs was 133 days. In the present study, several prognostic factors, such as gender, age, single or multiple lesions, lymph node involvement at the time of diagnosis, surgical resection status and additional chemotherapy, were examined, although none of these factors approached statistical significance. Histiocytic sarcoma must be considered in the differential diagnosis of dogs with pulmonary masses, especially in the canine breed.
Keywords: histiocytic sarcoma, lung, Pembroke Welsh Corgi
Canine proliferative histiocytic diseases are implicated in a range of disorders with marked differences in clinical manifestations and pathologic features [1, 3, 8]. Canine histiocytic neoplasia occurs as a localized tumor or a disseminated neoplastic process [1, 8]. Disseminated histiocytic sarcoma was first described as malignant histiocytosis of Bernese Mountain Dog [1, 8]. Localized histiocytic sarcomas develop from a single site [1, 5]. They are locally invasive and metastasize to draining lymph nodes. They arise most often in the subcutis, but may be seen in other primary locations, such as the lung, spleen and liver. The prevalence in dogs is primarily found within a narrow range of breeds, especially the Bernese Mountain Dog (for systemic HS) and Flat-Coated Retriever (for localized type) [1, 2, 5, 8]. Pembroke Welsh Corgi is one of the most popular breeds in Japan; for several years, there has been a suspicion of increased neoplasia in this breed [4, 7, 10]. Although pulmonary involvement has been recognized in dogs with disseminated (secondary) HS, published reports of HS confined to the lung (primary HS) are uncommon, and most describe small numbers of dogs [6]. The objective of the present study was to examine the characteristic of canine pulmonary histiocytic sarcomas that were examined in 19 dogs.
Pulmonary histiocytic sarcomas were collected from 19 dogs between 2010 and 2012. The typical radiographic appearance of a pulmonary mass is solitary, well-defined and of variable size; the mass may be comprised of a single nodule or of multiple nodules (Fig. 1). These sarcomas—isolated by surgical removal or biopsied specimens—were confirmed histopathologically to be histiocytic sarcoma. We excluded a case with lesions other than the lungs and regional lymph node. Distant metastasis was not detected in any dog at the time of initial staging. Dogs received treatment consisting of surgery, chemotherapy or a combination of the two; all dogs lived longer than 7 days following first treatment. The sex, age, primary site, lymph node involvement, treatment and survival time are shown in Table 1.
Fig. 1.
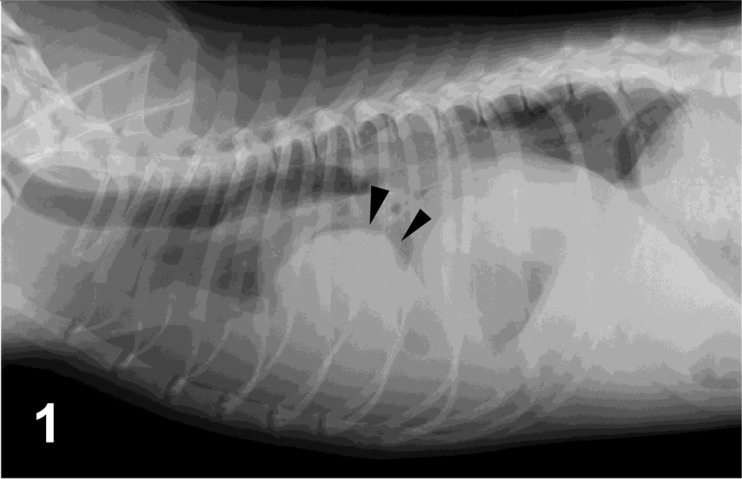
Case No 1. Right lateral thoracic radiograph. There is a large mass in the right middle and caudal lung lobes (arrowheads), obscuring the apex of the cardiac silhouette on the lateral view.
Table 1. Sex, age, primary site, lymph node involvement, treatment and survival time of 19 dogs.
| Case | Age | Sex | Lung lesions | Lymph node involvement | Treatment | Survival time (days) | |
|---|---|---|---|---|---|---|---|
| Occurrence | Location | ||||||
| 1 | 5 | F | multiple | right middle | + | C | 60 |
| right caudal | |||||||
| 2 | 8 | F | multiple | right lobe | – | C | 48 |
| 3 | 8 | MC | focal | right middle | + | C | 150 |
| 4 | 9 | MC | focal | left cranial | – | S+C | 60 |
| 5 | 9 | F | focal | right middle | – | S+C | 154 |
| 6 | 9 | MC | focal | right cranial | + | C | 75 |
| 7 | 9 | SF | focal | left caudal | + | S+C | 345 |
| 8 | 10 | F | multiple | right cranial | – | C | 30 |
| 9 | 10 | M | multiple | right caudal | – | C | 101 |
| left cranial | |||||||
| 10 | 10 | SF | focal | right middle | – | C | 184 |
| 11 | 10 | M | focal | right caudal | + | C | 93 |
| 12 | 11 | SF | focal | right caudal | – | C | 160 |
| left caudal | |||||||
| 13 | 11 | SF | multiple | left cranial | + | C | 66 |
| 14 | 11 | SF | focal | right middle | + | S+C | 135 |
| 15 | 12 | SF | focal | right middle | – | S+C | 177 |
| 16 | 12 | SF | focal | accessory lobe | + | C | 20 |
| 17 | 13 | MC | focal | right caudal | – | C | 133 |
| 18 | 14 | MC | multiple | left cranial | + | C | 209 |
| 19 | 14 | MC | focal | right middle | + | S+C | 263 |
S: surgically removed, C: chemotherapy
Tissue lesion samples were fixed in 10% phosphate buffered formalin solution before being embedded in paraffin. The paraffin sections (4 µm thick) then were stained with hematoxylin and eosin, and immnohistochemical staining was conducted. An automated immunohistochemistry system (Histostainer, Nichirei, Tokyo, Japan) was used to process the deparaffinized and antigen-retrieval tissues. The immunohistochemical antibodies were human leukocyte antigen (HLA)-DR (1:400, Dako, Tokyo, Japan), Iba-1 (1:200, Wako, Osaka, Japan), CD3 (1:200, Dako), CD20 (1:400, Thermo Scientific, Yokohama, Japan) and Epithelial cadherin (1:400, BD Bioscience, Tokyo, Japan). Peroxidase activity was demonstrated using diaminobenzidine solution, and slides were counterstained with hematoxylin. Histologically, in all cases, tumors were composed of densely arranged, pleomorphic, round cells with various degrees of small lymphocyte infiltration (Fig. 2). Neoplastic cells contained abundant eosinophilic cytoplasm with fairly well-delineated cell margins, eccentric nuclei of various sizes and many mitotic figures (Fig. 3). Anisokaryosis and anisonucleosis were prominent. Multinucleated giant cells often were scattered throughout the lesion. Phagocytic findings were rarely observed. Immunohistochemically, tumor cells were diffusely positive for HLA-DR (Fig. 4) and Iba-1. Many CD3 and CD20 were infiltrated in the lesions. None of the cases were stained positive for E-cadherin.
Fig. 2.
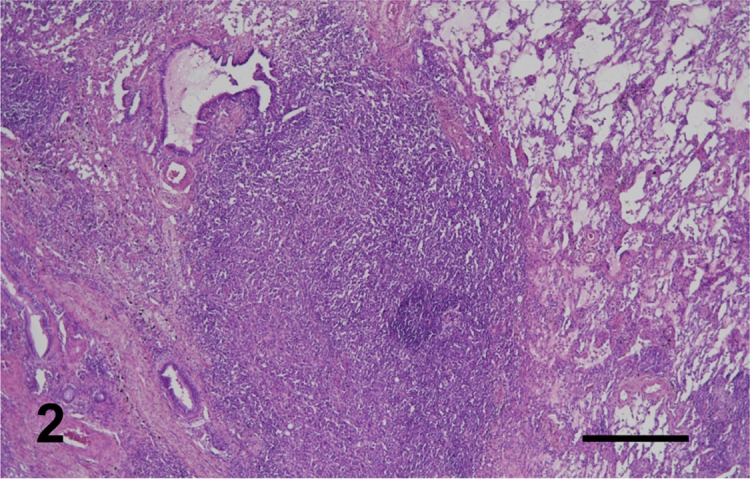
Case No. 10. A nodular mass is composed of densely arranged, round cells proliferation in the lung. H.E. Bar=500 µm.
Fig. 3.
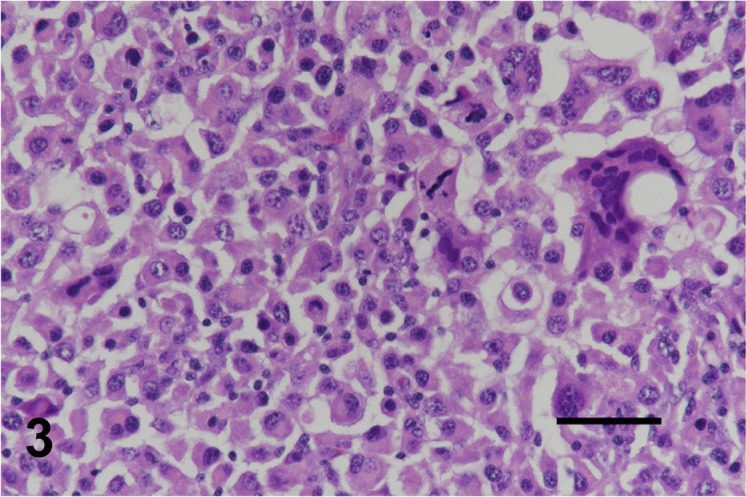
Case No. 3. Neoplastic cells contained abundant eosinophilic cytoplasm with eccentric nuclei of various sizes with various degrees of small lymphocyte infiltration. Multinucleated giant cells were occasionally observed. H.E. Bar=50 µm.
Fig. 4.
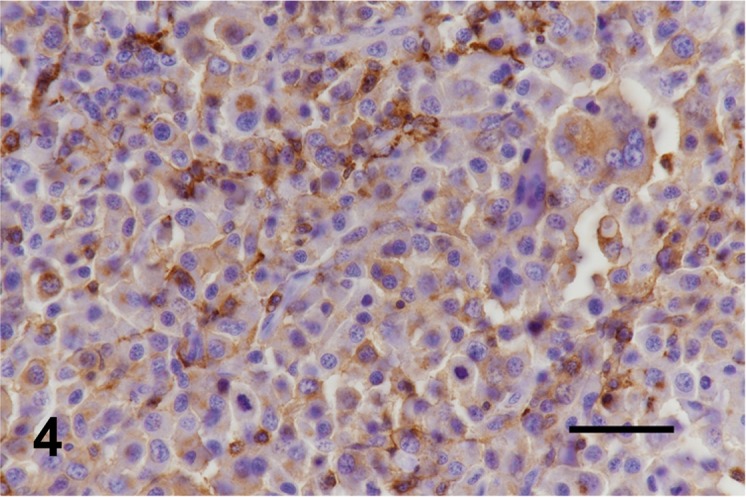
Case No. 3. Neoplastic cells are diffusely positive for HLA-DR. Bar=50 µm.
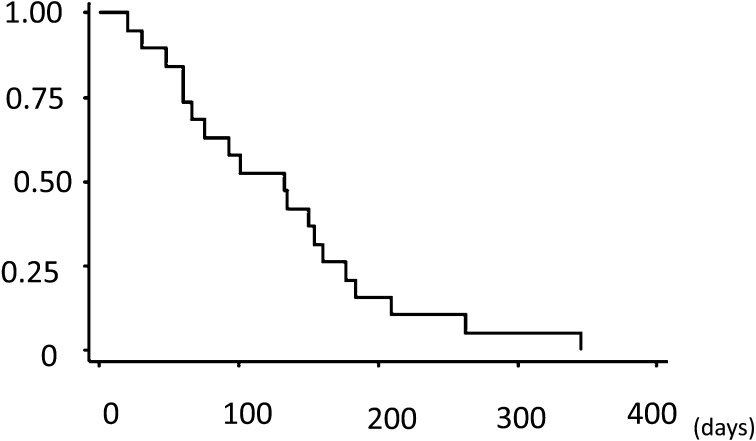
Statistical analysis: Survival probabilities were estimated using the Kaplan–Meier product limit method (Fig. 5). Survival time was calculated from the date of first treatment to the date of death. In the examination of prognostic factors, log-rank tests were used to determine whether each factor, as assessed at diagnosis, influenced survival. In addition, a forced-entry Cox proportional hazards model was developed to assess the independent contributions of various prognostic factors. Several prognostic factors yielding a P value of less than 0.1 were included in the hazards model. A value of P<0.05 was considered to be significant in all statistical tests. Data were analyzed using commercially available statistics software. In the present study, several prognostic factors (e.g., gender, age, single or multiple lesions, whether or not the sarcoma was removed surgically) were examined, although no factor demonstrated clear or statistically significant difference.
Fig. 5.
Kaplan–Meier plot of cumulative survival over time for 19 dogs with pulmonary HS. The median survival time is 133 days.
Histiocytic proliferative disorders are prevalent in dogs and are comprised of a variety of clinical and pathologic presentations, with recognized breed predilections [1, 2, 8]. Most dogs with histiocytic sarcoma in the lung have disseminated disease, which is associated with a worse prognosis compared with localized tumor. In the literature, localized histiocytic sarcomas arise generally from a single site, with predilection sites being subcutis and underlying tissues on extremities; however, tumors occurred in other locations, including spleen, lung, brain, nasal cavity and bone marrow [1, 3, 8]. The development of localized pulmonary histiocytic sarcoma is possible, although primary lung histiocytic sarcomas are not well documented. In our study, predominantly histiocytic subtype was consistent with previously described HS of the spleen, rather than the histiocytic–spindle–pleomorphic subtype as seen in periarticular histiocytic sarcoma (PAHS) [1, 2, 5, 8]. Dendritic cells (DCs) of either Langerhans cell or epithelial and interstitial DC lineages are the proliferating cells in all these diseases [1, 3, 8, 9]. Pulmonary HS can arise from dendritic cells; however, available markers limited our phenotypic analysis of formalin-fixed tissues.
A previous study by Klahn and colleagues evaluated and compared the outcomes of dogs with PAHS and histiocytic sarcoma of other anatomic locations (non-PAHS) [5]. Dogs with the periarticular form of HS may have a more favorable outcome than dogs with HS of other anatomic locations. Non-PAHS carries a very poor prognosis, as shown in the Klahn study, where the overall median survival was 128 days [5]. In our study, we found outcomes similar to those of non-PAHS, with a median survival time of 133 days; the prognosis in non-PAHS when internal organs were involved was poor. Also, in this study, multivariate analysis failed to show statistically significant improvement in prognosis when the disease was treated aggressively with complete surgical excision or chemotherapy alone.
The histiocytic sarcoma complex generally has been more commonly observed in Bernese Mountain Dog and retrievers [1, 8], whereas in the present study, localized pulmonary histiocytic sarcomas were frequently detected in Pembroke Welsh Corgi. The Pembroke Welsh Corgi as a breed has unique anatomical localization of histiocytic sarcoma, which in the breed occurs in sites, such as dural and lung [4, 7]. The overrepresentation within the Pembroke Welsh Corgi breed would seem to indicate a genetic tendency [4, 7, 10]. Histiocytic sarcoma must be considered in the differential diagnosis of dogs with pulmonary masses, especially in Pembroke Welsh Corgi.
REFERENCES
- 1.Affolter V. K., Moore P. F.2002. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet. Pathol. 39: 74–83. doi: 10.1354/vp.39-1-74 [DOI] [PubMed] [Google Scholar]
- 2.Constantino-Casas F., Mayhew D., Hoather T. M., Dobson J. M.2011. The clinical presentation and histopathologic-immunohistochemical classification of histiocytic sarcomas in the Flat Coated Retriever. Vet. Pathol. 48: 764–771. doi: 10.1177/0300985810385153 [DOI] [PubMed] [Google Scholar]
- 3.Fulmer A. K., Mauldin G. E.2007. Canine histiocytic neoplasia: an overview. Can. Vet. J. 48: 1041–1043, 1046–1050. [PMC free article] [PubMed] [Google Scholar]
- 4.Ide T., Uchida K., Kagawa Y., Suzuki K., Nakayama H.2011. Pathological and immunohistochemical features of subdural histiocytic sarcomas in 15 dogs. J. Vet. Diagn. Invest. 23: 127–132. doi: 10.1177/104063871102300123 [DOI] [PubMed] [Google Scholar]
- 5.Klahn S. L., Kitchell B. E., Dervisis N. G.2011. Evaluation and comparison of outcomes in dogs with periarticular and nonperiarticular histiocytic sarcoma. J. Am. Vet. Med. Assoc. 239: 90–96. doi: 10.2460/javma.239.1.90 [DOI] [PubMed] [Google Scholar]
- 6.Kumagai K., Makino T., Maejima T., Manabe S., Teranishi M.2006. Pulmonary histiocytic sarcoma in two dogs of a Beagle colony. J. Toxicol. Pathol. 19: 143–146. doi: 10.1293/tox.19.143 [DOI] [Google Scholar]
- 7.Mariani C. L., Jennings M. K., Olby N. J., Borst L. B., Brown J. C., Jr, Robertson I. D., Seiler G. S., MacKillop E.2015. Histiocytic sarcoma with central nervous system involvement in dogs: 19 cases (2006–2012). J. Vet. Intern. Med. 29: 607–613. doi: 10.1111/jvim.12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore P. F.2014. A review of histiocytic diseases of dogs and cats. Vet. Pathol. 51: 167–184. doi: 10.1177/0300985813510413 [DOI] [PubMed] [Google Scholar]
- 9.Moore P. F., Affolter V. K., Vernau W.2006. Canine hemophagocytic histiocytic sarcoma: a proliferative disorder of CD11d+ macrophages. Vet. Pathol. 43: 632–645. doi: 10.1354/vp.43-5-632 [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M., Tomiyasu H., Hotta E., Asada H., Fukushima K., Kanemoto H., Fujino Y., Ohno K., Uchida K., Nakayama H., Tsujimoto H.2014. Clinical characteristics and prognostic factors in dogs with histiocytic sarcomas in Japan. J. Vet. Med. Sci. 76: 661–666. doi: 10.1292/jvms.13-0414 [DOI] [PMC free article] [PubMed] [Google Scholar]