Abstract
To clarify the pathogenicity of Japanese type 1 porcine reproductive and respiratory syndrome virus (PRRSV) isolate in experimentally infected pigs, we evaluated clinical signs and monitored viremia for 21 days post-inoculation (dpi). Lungs were mottled, tanned and reddish in appearance; had lesions predominantly in the cranial, middle and accessory lobes; and failed to collapse at 10 dpi. Although microscopic lesions of lungs were reproduced using the Japanese emerging type 1 PRRSV isolate under experimental conditions, no significant differences were noted between the challenge and control groups regarding mean rectal temperature and daily weight gain. These results provide useful insights into the limited pathogenicity of single infection with the Japanese type 1 PRRSV isolate in piglets, which differ from findings in reported field cases.
Keywords: porcine reproductive and respiratory syndrome virus, swine, type 1 PRRSV
Two major porcine reproductive and respiratory syndrome virus (PRRSV) genotypes have been identified, based on the genetic differences between the prototype strains of Lelystad [10] and VR-2332 [12]: type 1 and type 2. Type 1 PRRSVs (European-like strains; prototype Lelystad) are approximately 60% similar to type 2 PRRSVs (North American-like strains; prototype VR-2332) at the nucleotide level [3, 8, 13]. In Japan, type 2 PRRSV was first isolated in 1994, subsequently expanding throughout the country [5, 11, 14]. In 2009, type 1 PRRSVs were first isolated from pigs with respiratory disorders in a farrow-to-finish pig farm and exhibited unique characteristics in genetic analysis [6]. While the farm had a high number of symptomatic weaning pigs, elucidation of the pathogenicity of Japanese type 1 PRRSV in the field was difficult. Here, to determine the pathogenicity of Japanese type 1 PRRSV isolate in experimentally infected pigs, we evaluated clinical signs, viremia and pathological lesions.
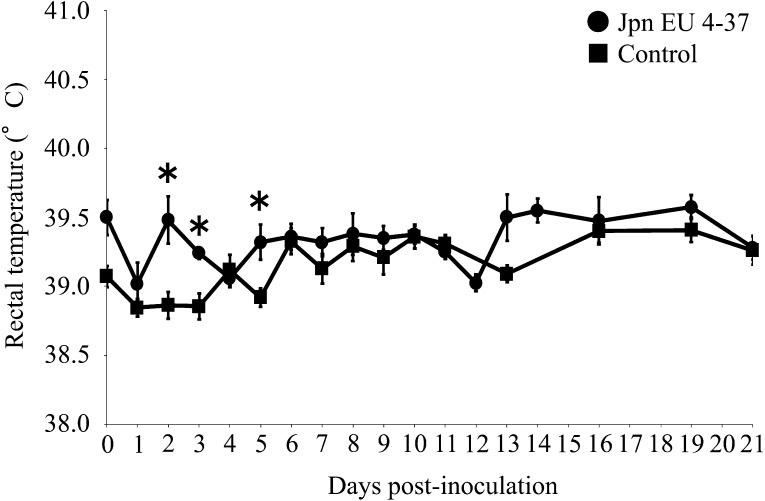
Thirteen specific-pathogen-free (SPF) pigs aged 4 weeks were randomly allocated to one of the following two groups: challenge (n=8) or control (n=5). This study was conducted in compliance with the animal experimentation code of the National Institute of Animal Health. Pigs were negative for antibodies of porcine circovirus type 2 and PRRSV before the experiment. The challenge group was inoculated with 1 ml of nasal spray containing 1 × 105 TCID50 of Japanese type 1 PRRSV isolate, Jpn EU 4–37 [6], which is the amount of virus required to induce pathogenicity according to previous reports of challenge experiments [1, 2, 4]. The isolate was propagated 3 times with swine alveolar macrophage (SAM) culture and stored at −80°C until use, and then amplified by one passage with SAM culture before the inoculation. Pigs were kept in an animal facility where they received a commercial diet and were monitored for rectal temperature, clinical signs and body weight throughout the experimental period. Animal monitoring revealed no significant differences between the challenge and control groups in mean daily weight gain (data not shown). Mean rectal temperature in the inoculated group increased transiently until 5 dpi (Fig. 1). Overall, mean rectal temperatures in the inoculated group were significantly higher than in the control group at 2, 3 and 5 dpi. The mean ± standard error (SE) of rectal temperatures of the inoculated group were as follows: 39.32 ± 0.09°C at 2 dpi, 39.25 ± 0.03°C at 3 dpi and 39.25 ± 0.03°C at 5 dpi. Post-inoculation, the mean ± SE of rectal temperatures of the control group were as follows: 38.72 ± 0.15°C at 2 dpi, 38.86 ± 0.16°C at 3 dpi and 38.94 ± 0.14°C at 5 dpi. Only a slight degree of tachypnea was observed at 4 to 12 dpi in a proportion of pigs in the inoculated group, and despite inoculating animals with the amount of virus suggested in previous reports to cause infection [1, 2, 4], no remarkable clinical symptoms were observed. Body temperature, weight and viral RNA values described below were analyzed using the unpaired t-test. P<0.05 was considered statistically significant.
Fig. 1.
Change in rectal temperature following virus challenge. *Statistically significant difference between inoculated and control groups (P<0.05). Bars indicate SE of the mean.
To monitor antibodies against PRRSV and PRRSV RNA, serum was collected at 0, 1–8, 10, 12, 14, 16, 19 and 21 dpi. To quantify PRRSV RNA, oral fluid was collected from pigs at 0, 1–6, 8, 10, 12, 14, 16, 19 and 21 dpi. Antibodies against PRRSV were analyzed using a commercially available enzyme-linked immunosorbent assay (ELISA) (HerdChek PRRS ELISA; IDEXX Laboratories Inc., Westbrook, ME, U.S.A.). All inoculated pigs became seropositive (S/P>0.4) at 8 dpi. The S/P ratio then gradually rose until the end of the examination, and no antibodies against PRRSV were observed in the control group (data not shown). Viral RNAs were extracted using a QIAamp Viral RNA Mini kit (QIAGEN, Tokyo, Japan) for serum and oral fluid. Extracted RNAs were then used as a template for one-step real-time reverse transcriptase PCR (qRT-PCR) with One Step SYBR® PrimeScriptTM RT-PCR Kit II (Perfect Real Time) (Takara Bio Inc., Otsu, Japan). RNA extraction was conducted in accordance with the manufacturer’s instructions. Real-time RT-PCR was conducted as previously described [7]. Fluorescence data were analyzed using PE 7500 Sequence Detection System Software Version 1 (Life Technologies Inc., Carlsbad, CA, U.S.A.).
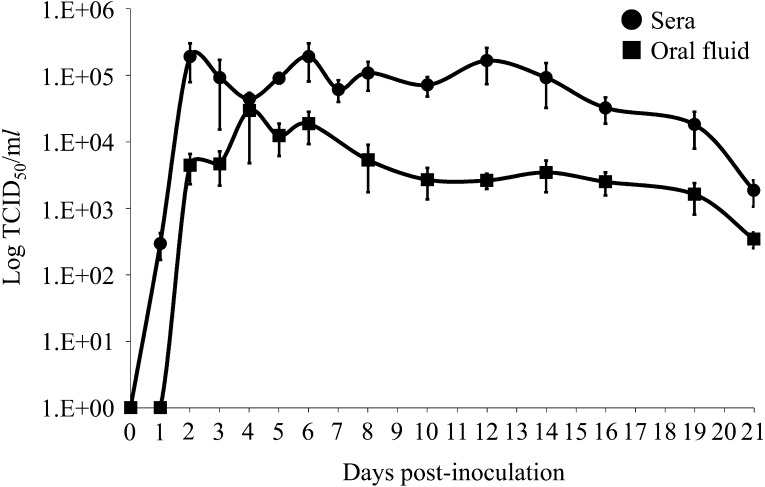
Mean serum viral load in the inoculated group sharply increased from 1 to 2 dpi and remained high (>1 × 104 TCID50/ml) until 19 dpi with peak viral load ranging from approximately 1 × 105 to 1 × 106 TCID50/ml, and viral RNA in oral fluid was detectable from 2 dpi and observed up to 21 dpi (Fig. 2). These data indicate that intranasal inoculation resulted in rapid viremia and dissemination of Japanese type 1 PRRSV isolate to several tissues. This finding is consistent with that of a previous report by Brockmeier et al., in which type 2 PRRSV inoculated intranasally was also able to be quickly detected following inoculation [1]. Further, although the average quantity of viral RNA in the oral fluid was 10 to 100 times lower than that of serum samples at peak viral load, the quantity of viral RNA was higher in the oral fluid than in serum samples in pigs at 3 and 4 dpi. This phenomenon is important for accurately interpreting the results of diagnostic tests using sera or oral fluid.
Fig. 2.
Quantity of viral RNA in serum and oral fluid following virus challenge. Quantity of porcine reproductive and respiratory syndrome virus (PRRSV) RNA (mean ± SE) was measured by one-step real-time reverse transcriptase PCR.
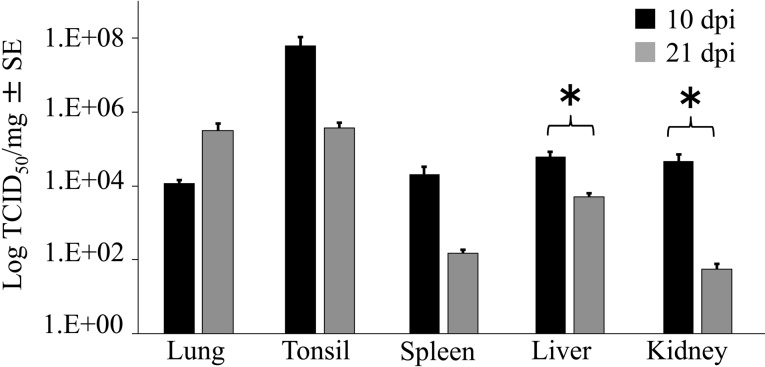
Four pigs inoculated with virus were necropsied at 10 dpi and the remaining pigs at 21 dpi for pathological and virological assays. At necropsy, the lungs, tonsils, liver, kidneys and spleen were collected to determine the quantity of PRRSV RNA in each tissue. Viral RNA was extracted using a QIAGEN RNeasy Mini kit (QIAGEN) for tissue and then amplified and analyzed via the same method as that used in sera and oral fluid. While the quantity of viral RNA in the spleen and kidneys was significantly lower at 21 dpi than at 10 dpi, no significant differences in quantity were noted for lungs, tonsils or liver between 10 and 21 dpi (Fig. 3). Further, no PRRSV RNA was observed in any control animals (data not shown). At necropsy, the organs of each pig were visually examined. Single sections of tissues were collected for microscopic examination as follows: lung lobes, brain, heart, ileum, tonsils, tracheobronchial lymph nodes, superficial inguinal lymph nodes, mandibular lymph nodes, mesenteric lymph nodes, thymus, liver, kidneys and spleen. Samples were suspended in 10% neutral buffered formalin and dehydrated, embedded in paraffin wax, sectioned at 4 µm and stained with hematoxylin and eosin. Detection of PRRSV antigen was performed in the lung tissues of infected pigs at 10 dpi using an immunohistochemical method with a monoclonal antibody against PRRSV (SDOW17; Research Technology Innovation LLC., Brookings, SD, U.S.A.) and a commercial kit (Histofine®; Nichirei Bioscience Inc., Tokyo, Japan), as previously described [9].
Fig. 3.
Porcine reproductive and respiratory syndrome virus (PRRSV) RNA load in infected pigs at 10 dpi (black) and 21 dpi (grey) (TCID50/mg). PRRSV RNA (mean ± SE) as measured by real-time RT-PCR. *Significant difference between 10 dpi and 21 dpi (P<0.05) as determined by the unpaired t-test.
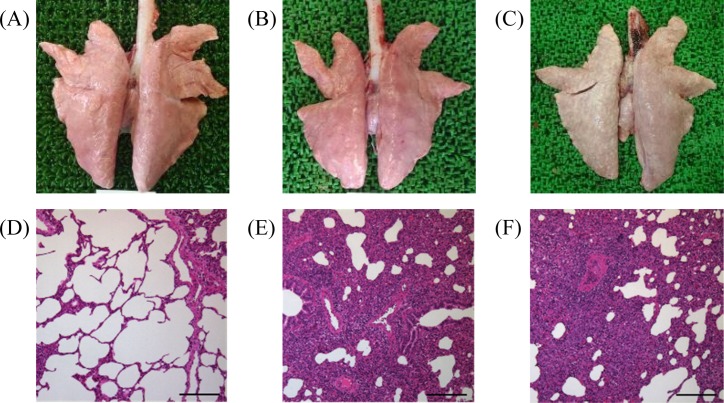
Lungs of inoculated pigs had a mottled, tanned and reddish appearance; had lesions predominantly in the cranial, middle and accessory lobes; and failed to collapse at 10 dpi (Fig. 4B). In all inoculated pigs, lungs showed discolored swollen consolidation at 21 dpi (Fig. 4C), and lymphadenopathy was observed in lymph nodes at 10 and 21 dpi (data not shown). Microscopically, pneumonic lesions at 10 dpi were characterized by multifocal mild to moderate interstitial pneumonia with septal thickening of histiolymphocytic infiltration and type II pneumocyte hypertrophy and hyperplasia (Fig. 4E). PRRSV antigen was detected in alveolar macrophages in the interstitial pneumonic lesions in 2 out of 5 infected pigs at 10 dpi. Pneumonic lesions at 21 dpi were similar to moderate interstitial pneumonia at 10 dpi, but type II pneumocyte hyperplasia in the multifocal alveolar walls was prominent (Fig. 4F). Germinal center hyperplasia and hypertrophy were observed in the lymph nodes at 10 and 21 dpi (data not shown). Notably, no gross or microscopic lesions were observed in the control group (Fig. 4A and 4D). Although respiratory disorders were not reproduced in conventional pigs by infection with Japanese type 1 PRRSV isolate, the virus spread throughout the respiratory and lymphoid tissues, and interstitial pneumonia was noted in all inoculated animals. Our findings are consistent with Halbur and Duan’s report that the pathogenicity of type 1 PRRSV is generally weaker than that of type 2 PRRSV [2, 4].
Fig. 4.
Gross and microscopic lung lesions. (A) Dorsal surface of lung in the control group. (B) Dorsal surface of lung at 10 dpi. (C) Dorsal surface of lung at 21 dpi. (D) No treatment (hematoxylin and eosin staining, bar=200 µm). (E) Mild interstitial pneumonia at 10 dpi (hematoxylin and eosin staining, bar=200 µm). (F) Interstitial pneumonia (discolored) at 21 dpi (hematoxylin and eosin staining, bar=200 µm).
The results of the present study clarify that the Japanese type 1 PRRSV isolate induces lung lesions but that symptoms are quite limited. However, the Japanese type 1 PRRSV outbreak resulted in a high mortality rate for pigs at all stages, as we previously reported [5]. This discrepancy might be due in part to the presence of other pathogens at the farm, such as Salmonella sp., porcine circovirus 2 and type 2 PRRSV. Thus, in the presence of other pathogens and types of environmental stress, Japanese type 1 PRRSV might cause severe symptomatic disease.
Acknowledgments
The authors thank the staff of the NIAH for animal handling and technical assistance. This research was supported by a Grant-in-Aid from the regulatory Research Project of the Ministry of Agriculture, Forestry and Fisheries.
REFERENCES
- 1.Brockmeier S. L., Loving C. L., Vorwald A. C., Kehrli M. E., Jr, Baker R. B., Nicholson T. L., Lager K. M., Miller L. C., Faaberg K. S.2012. Genomic sequence and virulence comparison of four Type 2 porcine reproductive and respiratory syndrome virus strains. Virus Res. 169: 212–221. doi: 10.1016/j.virusres.2012.07.030 [DOI] [PubMed] [Google Scholar]
- 2.Duan X., Nauwynck H. J., Pensaert M. B.1997. Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV). Vet. Microbiol. 56: 9–19. doi: 10.1016/S0378-1135(96)01347-8 [DOI] [PubMed] [Google Scholar]
- 3.Forsberg R., Storgaard T., Nielsen H. S., Oleksiewicz M. B., Cordioli P., Sala G., Hein J., Bøtner A.2002. The genetic diversity of European type PRRSV is similar to that of the North American type but is geographically skewed within Europe. Virology 299: 38–47. doi: 10.1006/viro.2002.1450 [DOI] [PubMed] [Google Scholar]
- 4.Halbur P. G., Paul P. S., Frey M. L., Landgraf J., Eernisse K., Meng X. J., Lum M. A., Andrews J. J., Rathje J. A.1995. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 32: 648–660. doi: 10.1177/030098589503200606 [DOI] [PubMed] [Google Scholar]
- 5.Iseki H., Takagi M., Miyazaki A., Katsuda K., Mikami O., Tsunemitsu H.2011. Genetic analysis of ORF5 in porcine reproductive and respiratory syndrome virus in Japan. Microbiol. Immunol. 55: 211–216. doi: 10.1111/j.1348-0421.2010.00303.x [DOI] [PubMed] [Google Scholar]
- 6.Iseki H., Takagi M., Kawashima K., Shibahara T., Kuroda Y., Tsunemitsu H.2012. Type 1 porcine reproductive and respiratory syndrome virus emerged in Japan., ed., The 22nd International Pig Veterinary Society Congress. Proceedings p. 978.
- 7.Iseki H., Takagi M., Kuroda Y., Katsuda K., Mikami O., Tsunemitsu H., Yamakawa M.2014. Application of a SYBR®Green one step real-time RT-PCR assay to detect type 1 porcine reproductive and respiratory syndrome virus. J. Vet. Med. Sci. 76: 1411–1413. doi: 10.1292/jvms.14-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapur V., Elam M. R., Pawlovich T. M., Murtaugh M. P.1996. Genetic variation in porcine reproductive and respiratory syndrome virus isolates in the midwestern United States. J. Gen. Virol. 77: 1271–1276. doi: 10.1099/0022-1317-77-6-1271 [DOI] [PubMed] [Google Scholar]
- 9.Kawashima K., Yamada S., Kobayashi H., Narita M.1996. Detection of porcine reproductive and respiratory syndrome virus and Mycoplasma hyorhinis antigens in pulmonary lesions of pigs suffering from respiratory distress. J. Comp. Pathol. 114: 315–323. doi: 10.1016/S0021-9975(96)80053-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meulenberg J. J. M., de Meijer E. J., Moormann R. J. M.1993. Subgenomic RNAs of Lelystad virus contain a conserved leader-body junction sequence. J. Gen. Virol. 74: 1697–1701. doi: 10.1099/0022-1317-74-8-1697 [DOI] [PubMed] [Google Scholar]
- 11.Murakami Y., Kato A., Tsuda T., Morozumi T., Miura Y., Sugimura T.1994. Isolation and serological characterization of porcine reproductive and respiratory syndrome (PRRS) viruses from pigs with reproductive and respiratory disorders in Japan. J. Vet. Med. Sci. 56: 891–894. doi: 10.1292/jvms.56.891 [DOI] [PubMed] [Google Scholar]
- 12.Nelsen C. J., Murtaugh M. P., Faaberg K. S.1999. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J. Virol. 73: 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ropp S. L., Wees C. E. M., Fang Y., Nelson E. A., Rossow K. D., Bien M., Arndt B., Preszler S., Steen P., Christopher-Hennings J., Collins J. E., Benfield D. A., Faaberg K. S.2004. Characterization of emerging European-like porcine reproductive and respiratory syndrome virus isolates in the United States. J. Virol. 78: 3684–3703. doi: 10.1128/JVI.78.7.3684-3703.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu M., Yamada S., Murakami Y., Morozumi T., Kobayashi H., Mitani K., Ito N., Kubo M., Kimura K., Kobayashi M., Yamamoto K., Miura Y., Yamamoto T., Watanabe K.1994. Isolation of porcine reproductive and respiratory syndrome (PRRS) virus from Heko-Heko disease of pigs. J. Vet. Med. Sci. 56: 389–391. doi: 10.1292/jvms.56.389 [DOI] [PubMed] [Google Scholar]