Abstract
The common marmoset is widely used in neuroscience and regenerative medicine research. However, information concerning common marmoset disorders, particularly infectious diseases, is scarce. Here, we report a case of a female common marmoset that died suddenly due to gas gangrene. The animal presented with gaseous abdominal distention at postmortem, and Clostridium perfringens type A was isolated from several tissues. Vacuoles, a Gram-positive bacteremia and intravascular hemolysis were observed microscopically in the muscles, liver and lungs. On the basis of these findings, we diagnosed nontraumatic gas gangrene caused by Clostridium perfringens type A in this common marmoset.
Keywords: Clostridium perfringens, common marmoset, gas gangrene
The common marmoset (Callithrix jacchus) is a small primate species that is widely used in neuroscience and regenerative medicine research. However, information concerning common marmoset disorders, particularly infectious diseases, is scarce. Here, we report a case involving a common marmoset that developed Clostridium perfringens infection and unexpectedly succumbed to gas gangrene.
At our institute, common marmosets are obtained from CLEA Japan, Inc. (Tokyo, Japan) or bred in our facility. The animals are housed in a single, pairs or families in stainless cages (82 × 61 × 158 cm), fed a commercial diet supplemented with honey and vitamins A, C, D3 and E and provided ad libitum access to tap water. The animal room is maintained at a temperature of 25–27°C, with a relative humidity of 40–60% and a 12-hr light–dark cycle.
A 4-year-old female common marmoset, weighing 365 g, died suddenly. It had not been used in any experiment for 6 months and showed no apparent external injuries. It had recoverd from chronic watery diarrhea and associated weight loss 3 to 4 months before death and exhibited only facial erythema the day before death. At the time of discovery, the animal was believed to be dead for 6–12 hr. It showed uniform abdominal bloating with marked flatulence and unclear fluctuation (Fig. 1). The skin showed decreased turgor, with lightening of the color to light bronze (Fig. 1). At necropsy, severe straining of the abdominal wall due to gas buildup throughout the intestine was observed (Fig. 1), accompanied by a foul smell resembling that of rotten eggs and viscous, blood-tinged ascitic fluid. The skeletal muscles were grayish-yellow and watery. Generalized hemorrhage was observed in the subcutaneous tissues. The liver and spleen were dark red in color, and enlarged and scattered dark red spots were present on the lungs.
Fig. 1.
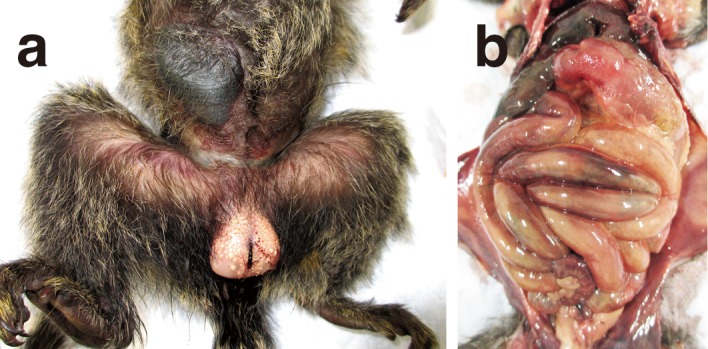
a) Postmortem findings in the abdominal and femoral regions. The abdominal skin is edematous and discolored with necrotic bullae. b) Necropsy findings in the abdominal cavity. Note the diffuse flatulence in the small and large intestines.
Stamp-smear samples of the spleen, liver, lungs and blood and tracheal swabs were inoculated onto 5% horse blood agar plates for aerobic and anaerobic bacteriological culture and observed after incubation at 37°C for 48 hr. The stamp-smear samples were stained with Giemsa and Gram stains. Following a catalase test and Gram staining, individual colonies were identified using biochemical tests and a polymerase chain reaction (PCR) assay [20]. All bacterial colonies were anaerobically and purely isolated and were gram-positive, catalase-negative, lecithinase-positive, large, nonmotile, grayish to grayish-yellow and rough with irregular edges. The isolates were identified as C. perfringens using biochemical tests and a PCR assay with species-specific primer pairs. Genotypes of C. perfringens isolates were determined by PCR to amplify the genes encoding enterotoxin and alpha, beta, epsilon, iota and beta2 toxins [6]. All isolates showed only the alpha toxin gene and were accordingly classified as C. perfringens type A. Giemsa and Gram-stained stamp-smear samples of the spleen, liver, lungs and peripheral blood demonstrated numerous boxcar-shaped, gram-positive organisms and few leukocytes (Fig. 2).
Fig. 2.
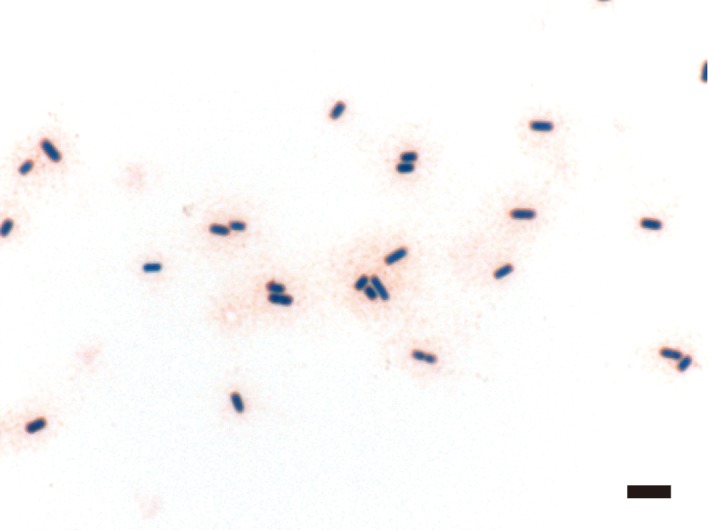
Photomicrograph of a peripheral blood smear sample with Gram stain. Several boxcar-shaped, Gram-positive bacilli can be seen. Bar: 5 µm.
Samples of the quadriceps femoris muscle, liver, pancreas, intestine and kidney were fixed in 20% neutral buffered formalin and embedded in paraffin. Sections of all samples were prepared using hematoxylin and eosin (HE), Giemsa and modified Gram stains [2].
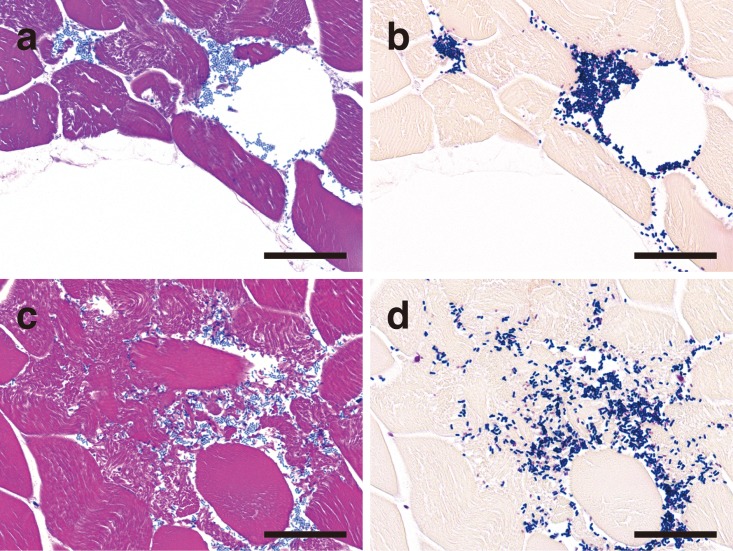
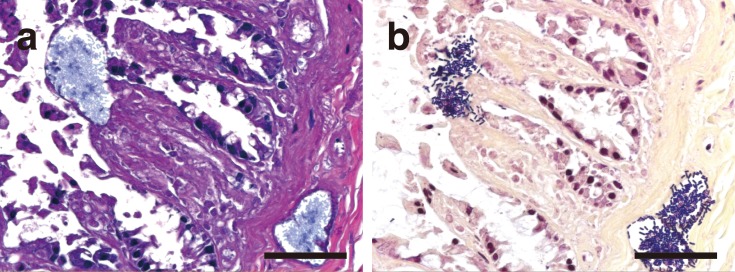
Microscopically, vacuoles were found in the skeletal muscle, intestine, liver, pancreas and lungs. In each of these tissues, intravascular hemolysis and bacteremia were observed. Numerous gram-positive bacilli were observed around the vacuoles, evoking a pauci-inflammatory response. Throughout the skeletal muscle tissue, there were vacuoles and interstitial edema with aggregated gram-positive bacilli and invading bacilli around rhabdomyolysis and coagulative myonecrosis (Fig. 3). In the small intestine, there was complete loss of the villi. Moreover, the choledochal duct showed severe mucosal necrosis and was impossible to observe histologically, although bacterial invasion was not observed in and around the duct. In the large intestine, there was diffuse mucosal necrosis. A striking number of gram-positive bacilli were observed in and around markedly dilated vessels in the lamina propria and submucosa (Fig. 4). However, inflammatory cell infiltration was sparse. In the liver, the central and interlobular veins were dilated, and the hepatic nuclei had almost disappeared. Similar changes were present in the pancreas. In the lungs, there were diffuse bronchiectasis, hyperemia and edema, with numerous gram-positive bacilli within the alveolar spaces and blood vessels. The kidney showed marked renal cortical necrosis and widely scattered granular tubular casts. On the basis of the above findings, a diagnosis of nontraumatic gas gangrene caused by C. perfringens type A was made.
Fig. 3.
Representative histopathological findings of gas gangrene in the skeletal muscle stained with HE (a, c) and Gram (b, d) stains. The lesions contain vacuoles and edema (a), Gram-positive bacilli accumulation (b) and myonecrosis (c) with invasion of the interstitial tissue by Gram-positive bacilli (d). Bars: 50 µm.
Fig. 4.
Histopathological findings in the cecum stained with HE (a) and Gram (b) stains. Several Gram-positive bacilli are distributed in clusters and have invaded the dilated vessles in the lamina propria and submucosa. Bars: 50 µm.
C. perfringens, formerly referred to as C. welchii, is an anaerobic, gram-positive, rod-shaped bacterium and the most common cause of gas gangrene. C. perfringens is widely distributed in nature and is a normal inhabitant of the intestinal tract in humans and animals, including nonhuman primates [10]. Clostridia multiply in an anaerobic environment, such as the large intestine. C. perfringens is classified as type A, B, C, D or E on the basis of the production of four major toxins, namely alpha, beta, epsilon and iota toxins [10]. Strains of C. perfringens can also produce enterotoxin (CPE) and beta2 toxin [10]. The alpha toxin (phospholipase C) is highly cytotoxic and myotoxic and can lead to hemolysis and the release of superoxide radicals and inflammatory cytokines [10, 15]. In this case, several tissues hence showed inflammatory cell infiltration, while the muscles showed myolysis and myonecrosis. CPE-negative C. perfringens type A has been associated with gas gangrene in humans [17, 18] and domestic animals [9, 13, 14]. However, little information is available on the pathogenesis of naturally occurring gas gangrene in animals. Furthermore, the role of C. perfringens type A in animal diseases, including yellow lamb disease in sheep [11], is not confirmed [19].
Gas gangrene is classified into three major types: post-traumatic, postoperative and spontaneous. The spontaneous type is associated with the highest mortality [8] and is mostly caused by clostridial infection following delivery, surgery or malignancy [1]. In addition, it is often observed in humans compromised by diabetes or liver cirrhosis [4]. Most cases of gas gangrene are traumatic; nontraumatic gas gangrene is rare. Underlying diseases are generally present when C. perfringens causes gas gangrene. It has been reported that C. perfringens is most likely to invade the hepatic–cystic system [7], with the following mechanism suggested by experts. When the mucosal reticuloendothelial system is damaged, C. perfringens easily invades the hepatic portal vein and causes bacteremia. In the present case, it is speculated that C. perfringens entered the bloodstream via the damaged mucosal barrier in the large intestine, considering the absence of trauma, and multiplied rapidly in the vessels and submucosa, which provide an anaerobic enviroment. Then, it invaded the muscles and finally caused myonecrosis.
Several studies have reported C. perfringens infection as a cause of gastric dilatation syndrome in nonhuman primates [3, 5, 16], including one report involving a common marmoset [16]. However, the condition of the present case differs from the pathological condition of acute gastric dilatation syndrome. With regard to nonhuman primates, only one case of C. perfringens infection in a rhesus macaque (Macaca mulatta) has been reported to date [12], although the primate presented with necrotizing clostridial infection caused by skin wounds [12]. Therefore, the present case is the first, to the best of our knowledge, to report nontraumatic gas gangrene caused by C. perfringens type A in nonhuman primates. This should also attract the attention of humans engaged in breeding nonhuman primates with regard to appropriate sanitary managements.
REFERENCES
- 1.Bodey G. P., Rodriguez S., Fainstein V., Elting L. S.1991. Clostridial bacteremia in cancer patients. A 12-year experience. Cancer 67: 1928–1942. doi: [DOI] [PubMed] [Google Scholar]
- 2.Brown R. C., Hopps H. C.1973. Staining of bacteria in tissue sections: a reliable gram stain method. Am. J. Clin. Pathol. 60: 234–240. [DOI] [PubMed] [Google Scholar]
- 3.Campanile N., Rood P. P., Yeh P., Casu A., Bottino R., Cooper D. K.2007. Acute gastric dilatation after porcine islet transplantation in a cynomolgus monkey - case history and review of the literature. Xenotransplantation 14: 265–270. doi: 10.1111/j.1399-3089.2007.00406.x [DOI] [PubMed] [Google Scholar]
- 4.Chen Y. M., Lee H. C., Chang C. M., Chuang Y. C., Ko W. C.2001. Clostridium bacteremia: emphasis on the poor prognosis in cirrhotic patients. J. Microbiol. Immunol. Infect. 34: 113–118. [PubMed] [Google Scholar]
- 5.Christie R. J., King R. E.1981. Acute gastric dilation and rupture in Macaca arctoides associated with Clostridium perfringens. J. Med. Primatol. 10: 263–264. [DOI] [PubMed] [Google Scholar]
- 6.Ferrarezi M. C., Cardoso T. C., Dutra I. S.2008. Genotyping of Clostridium perfringens isolated from calves with neonatal diarrhea. Anaerobe 14: 328–331. doi: 10.1016/j.anaerobe.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Fry D. E., Klamer T. W., Garrison R. N., Polk H. C., Jr1981. Atypical clostridial bacteremia. Surg. Gynecol. Obstet. 153: 28–30. [PubMed] [Google Scholar]
- 8.Hart G. B., Lamb R. C., Strauss M. B.1983. Gas gangrene: I. A collective review. J. Trauma 23: 991–995. doi: 10.1097/00005373-198311000-00006 [DOI] [PubMed] [Google Scholar]
- 9.Harwood D. G.1984. Apparent iatrogenic clostridial myositis in cattle. Vet. Rec. 115: 412. doi: 10.1136/vr.115.16.412 [DOI] [PubMed] [Google Scholar]
- 10.McClane B. A., Robertson S. L., Li J.2013. Clostridium perfringens pp. 465–489. In: Food Microbiology: Fundamentals and Frontiers, 4th ed. (Buchanan, R. L. and Doyle, M. P. eds.), ASM press, Washington, DC. [Google Scholar]
- 11.McGowan B., Moulton J. E., Rood S. E.1958. Lamb losses associated with Clostridium perfringens type A. J. Am. Vet. Med. Assoc. 133: 219–221. [PubMed] [Google Scholar]
- 12.Meier T. R., Myers D. D., Jr, Eaton K. A., Ko M. H., Hankenson F. C.2007. Gangrenous Clostridium perfringens infection and subsequent wound management in a rhesus macaque (Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 46: 68–73. [PubMed] [Google Scholar]
- 13.Odani J. S., Blanchard P. C., Adaska J. M., Moeller R. B., Uzal F. A.2009. Malignant edema in postpartum dairy cattle. J. Vet. Diagn. Invest. 21: 920–924. doi: 10.1177/104063870902100631 [DOI] [PubMed] [Google Scholar]
- 14.Parish S., Valberg S.2009. Inflammatory myopathies Clostridial myonecrosis. pp. 1400–1492. In: Large Animal Internal Medicine. (Smith, B. P. ed.), Mosby-Elsevier, St Louis. [Google Scholar]
- 15.Sakurai J., Nagahama M., Oda M.2004. Clostridium perfringens alpha-toxin: characterization and mode of action. J. Biochem. 136: 569–574. doi: 10.1093/jb/mvh161 [DOI] [PubMed] [Google Scholar]
- 16.Stein F. J., Lewis D. H., Stott G. G., Sis R. F.1981. Acute gastric dilatation in common marmosets (Callithrix jacchus). Lab. Anim. Sci. 31: 522–523. [PubMed] [Google Scholar]
- 17.Stevens D. L., Rood J. I.2006. Histotoxic clostridia. pp. 715–725. In: Gram-Positive Pathogens, 2th ed. (Fischetti, V. A., Novick, R. P., Ferretti, J. J., Portnoy, D. A. and Rood, J. I. eds.), ASM press, Washington, DC, U.S.A.. [Google Scholar]
- 18.Titball R. W., Naylor C. E., Basak A. K.1999. The Clostridium perfringens alpha-toxin. Anaerobe 5: 51–64. doi: 10.1006/anae.1999.0191 [DOI] [PubMed] [Google Scholar]
- 19.Uzal F. A., Vidal J. E., McClane B. A., Gurjar A. A.2014. Clostridium perfringens toxins involved in mammalian veterinary diseases. Open Toxicol. J. 2: 24–42. [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J., Zhang W., Xie B., Wu M., Tong X., Kalpoe J., Zhang D.2009. Detection and toxin typing of Clostridium perfringens in formalin-fixed, paraffin-embedded tissue samples by PCR. J. Clin. Microbiol. 47: 807–810. doi: 10.1128/JCM.01324-08 [DOI] [PMC free article] [PubMed] [Google Scholar]