Abstract
Four dogs with poor semen quality, low seminal plasma superoxide dismutase (SOD) activity and low blood plasma testosterone (T) levels were orally administered one vitamin E tablet containing 50 mg α-tocopheryl acetate per dog daily for 4 weeks. The mean values of semen quality were temporarily improved after the start of vitamin E treatment and the values of 4, and 5 weeks after that were significantly different from those before the treatment (P<0.05–0.001). The mean blood plasma T and seminal plasma SOD activity values slightly increased in the 4 dogs after the treatment. The results of the present study indicate that poor semen quality in dogs with low seminal plasma SOD can be improved by vitamin E treatment.
Keywords: canine, spermatogenic dysfunction, superoxide dismutase, testosterone, vitamin E
Superoxide dismutase (SOD) is the main antioxidant enzyme in seminal plasma in humans [1] and dogs [3]. Seminal plasma SOD is produced by the testis, epididymis and accessory reproductive organs [1, 4]. Low SOD activity in seminal plasma causes poor quality of ejaculated semen in humans [12] and dogs [7, 8].
Vitamin E is one of the major antioxidants in the body and has an important role in protecting many different organs against oxidative stress [10, 11]. It has been reported that supplemental vitamin E protects the testis from oxidative damage and improves semen quality in sheep [15], goats [6] and rabbits [2, 14]. In the present study, we investigated the efficacy of vitamin E supplementation as a means of treating poor semen quality in 4 dogs with low SOD activity (low-SOD dogs) in the seminal plasma. We evaluated semen quality, plasma testosterone (T) levels and seminal plasma SOD activity in the low-SOD dogs before and after vitamin E treatment.
The dogs supplemented with vitamin E were 2 beagles and 2 mongrels aged 3–7 years (body weight: 8–12 kg). The 4 dogs had previously been diagnosed with poor semen quality based on examinations of semen collected three times at 1-week intervals. Two beagles and 3 mongrels (3–6 years old and 7–12 kg body weight) with normal semen quality were used as controls. The normal semen quality was evaluated according to a report on semen quality (total volume of semen (ml): 11.2 ± 4.2, total number of sperm (×106): 558.5 ± 72.4, actively motile sperm (%): 82.3 ± 9.1 and morphologically abnormal sperm (%): 3.6 ± 1.5) in healthy beagles [8]. All of these dogs were cared for in our university and housed in pens with ample runs and were maintained according to the guidelines of the Animal Care and Use Committee of the Nippon Veterinary and Life Science University.
The vitamin E used in this study was a tablet containing 50 mg α-tocopheryl acetate (Juvela, Eisai Pharmaceutical Co., Tokyo, Japan) for humans. The administrated dose of vitamin E was decided according to a report on the treatment of vitamin E to dogs [5]. The low-SOD dogs were orally administered one tablet per dog daily for 4 weeks.
Semen specimens from the low-SOD dogs were collected once weekly from 2 weeks before to 10 weeks after the start of vitamin E supplementation by digital manipulation without a teaser bitch. Semen collection from the 5 control dogs was performed 3 times at 1-week intervals. Each sample was examined for total semen volume, total number of sperm and percentages of actively motile sperm and morphologically abnormal sperm by previously described methods [9].
The sperm-rich fraction of the semen collected from the low-SOD dogs at 2-week intervals was used for measurement of SOD activity. One semen sample was collected from each control dog. The specimens were centrifuged at 1,500 × g for 20 min, and the supernatant was collected. The SOD activity in the supernatant was measured using an SOD Assay Kit (Trevigen, Gaithersburg, MD, U.S.A.) and a spectrophotometer (UV-160A, Shimadzu, Tokyo, Japan) at an absorbance of 550 nm.
Heparinized blood samples were collected from superficial leg veins of low-SOD dogs at 2-week intervals. Blood samples were collected at 4 different times during the day (09:00, 10:00, 15:00 and 18:00), because of diurnal fluctuations in blood plasma T levels in dogs [13]. The plasma T levels were measured with a Testosterone Enzyme-Immunoassay Kit (Cayman Chemical, Ann Arbor, MI, U.S.A.), which has a detection limit of 10 pg/ml.
All semen and endocrine data were averaged for each dog, and data are expressed as means ± standard error (SE). Differences between means were analyzed for statistical significance by the Student’s t-test.
The mean total semen volume, total number of sperm and percentage of actively motile sperm in semen ejaculated by the low-SOD dogs before the start of vitamin E supplementation were significantly lower than those of the control dogs (P<0.01, 0.05 and 0.001, respectively) (Table 1). The mean percentage of morphologically abnormal sperm (mainly sperm with bent or coiled tails) in the low-SOD dogs was significantly higher than that of the control dogs (P<0.05).
Table 1. Mean (± S.E.) semen quality values in specimens collected 3 times at one-week intervals from 4 low SOD-dogs before vitamin E supplementation and 5 control dogs.
| Experimental groups (n) |
Semen volume (ml) |
Sperm count (×106) |
Motile sperm (%) |
Abnormal sperm (%) |
|---|---|---|---|---|
| Low SOD-dogs (4) | 4.1 ± 1.0 b) | 278.7 ± 93.2 c) | 58.0 ± 4.5 a) | 11.7 ± 2.5 c) |
| Control dogs (5) | 8.9 ± 0.9 | 536.6 ± 27.3 | 89.0 ± 1.7 | 5.6 ± 0.3 |
a) P<0.001, b) P<0.01 and c) P<0.05, compared with the control dogs.
Both the mean blood plasma T level and the seminal plasma SOD activity in the low-SOD dogs before vitamin E supplementation were significantly lower than those of the control dogs (P<0.001) (Table 2).
Table 2. Mean (± S.E.) blood plasma testosterone levels (ng/ml) and seminal plasma SOD (unit/g protein) in specimens collected from 4 low SOD-dogs before vitamin E supplementation and 5 control dogs.
| Experimental groups (n) | Testosterone | SOD |
|---|---|---|
| Low SOD-dogs (4) | 2.2 ± 0.2a) | 10.7 ± 1.0 a) |
| Control dogs (5) | 4.3 ± 0.3 | 35.7 ± 0.1 |
a) P<0.001, compared with the control dogs.
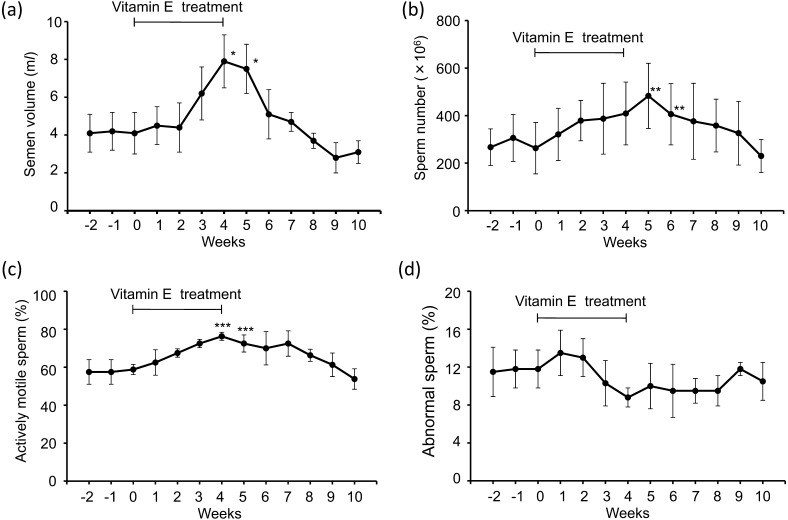
The mean semen volume, sperm number and sperm motility increased temporarily between 3 and 7 weeks after the start of vitamin E supplementation and peaked 4 or 5 weeks after that. The peak values were significantly different from the mean values of week 0 just before the start of vitamin E treatment (P<0.05 or 0.001) (Fig. 1a, 1b,1c). The mean percentage of abnormal sperm slightly decreased between 3 and 8 weeks after the start of vitamin E supplementation (Fig. 1d). As the results of improvement of semen quality in the low-SOD dogs, there were no significant differences between the peak values of semen volume and sperm number of the low-SOD dogs and those mean values of the 5 control dogs. However, there were significant differences in the percentages of actively motile sperm and morphologically abnormal sperm (P<0.05).
Fig. 1.
Changes in mean (± S.E.) total volume (ml) of semen (a) and total number (×106) of sperm (b) and mean (± S.E.) percentages of actively motile sperm (c) and morphologically abnormal sperm (d) collected from 4 low SOD-dogs weekly intervals from 2 weeks to 10 weeks after the start of vitamin E supplementation. ***P<0.001, **P<0.01 and *P>0.05, compared with the values of 0 week.
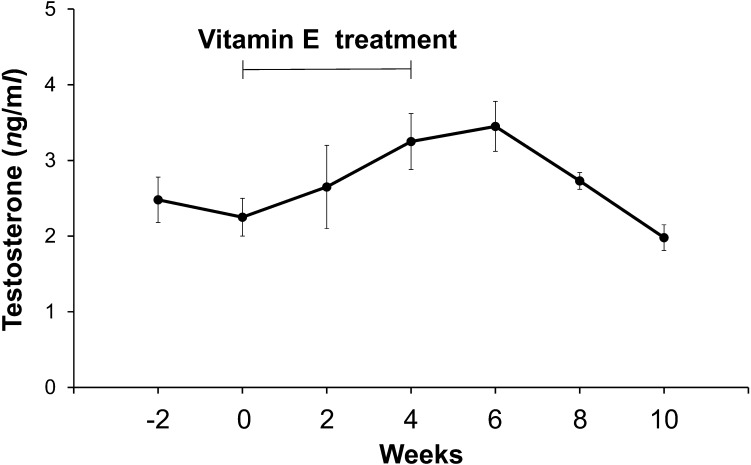
The mean blood plasma T levels slightly increased after the start of vitamin E treatment and peaked 6 weeks after that (Fig. 2). There was no significant difference between the peak value of the low-SOD dogs and the mean value of the 5 control dogs.
Fig. 2.
Changes in mean (± S.E.) T levels (ng/ml) in the peripheral blood plasma collected from 4 low SOD-dogs at 2 week intervals from 2 weeks to 10 weeks after the start of vitamin E supplementation.
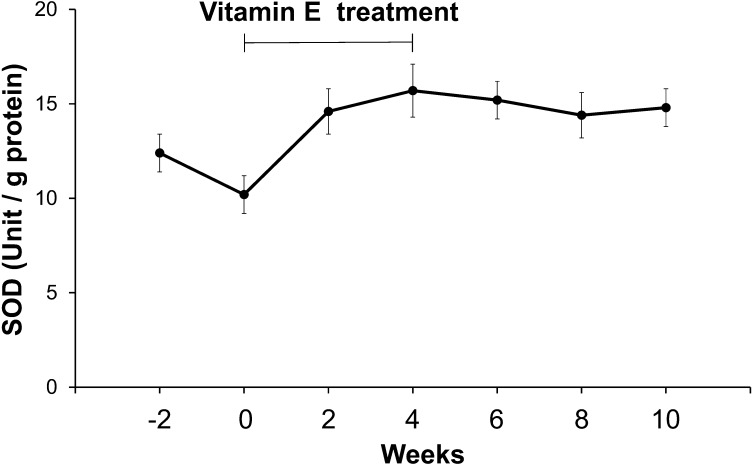
The mean seminal plasma SOD activities after the start of vitamin E treatment also slightly increased compared with those of the control (Fig. 3). However, there was significant difference between the peak value of the low-SOD dogs and the mean value of the 5 control dogs (P<0.01).
Fig. 3.
Changes in mean (± S.E.) SOD activities (unit/g protein) in the seminal plasma collected from 4 low SOD-dogs at 2 week intervals from 2 weeks to 10 weeks after the start of vitamin E supplementation.
The semen qualities of the 4 low-SOD dogs were temporarily improved by the antioxidant effect of vitamin E supplementation for 4 weeks. Vitamin E has antioxidant effects [10, 11]. In this study, we hypothesized that poor semen quality in the 4 low-SOD dogs is the result of increased oxidative stress in the testes, epididymides, prostatic gland and seminal plasma. Although the percentage of morphologically abnormal sperm during vitamin E treatment was not markedly decreased, vitamin E treatment for longer periods may be necessary to induce lower percentages of abnormal sperm in the ejaculated semen.
We postulate that the temporary increase in the mean blood plasma T levels after the start of vitamin E treatment was caused by improvements in the function of Leydig cells in the testes of the 4 low-SOD dogs due to reduction of oxidative stress after the vitamin E treatment. Furthermore, the increased plasma T levels could have induced the observed improvements in semen qualities. The small increase in the mean SOD activities in the seminal plasma collected after the start of vitamin E treatment also might have been induced by improvement of SOD production in the testes, epididymides and prostate of the low-SOD dogs due to vitamin E treatment. Further studies are necessary to investigate the factors of low SOD activity in seminal plasma and the efficacy of longer-term vitamin E treatment on the poor semen quality in low-SOD dogs.
REFERENCES
- 1.Alkan I., Simşek F., Haklar G., Kervancioğlu E., Ozveri H., Yalçin S., Akdaş A.1997. Reactive oxygen species production by the spermatozoa of patients with idiopathic infertility: relationship to seminal plasma antioxidants. J. Urol. 157: 140–143. doi: 10.1016/S0022-5347(01)65307-2 [DOI] [PubMed] [Google Scholar]
- 2.Aydilek N., Aksakal M., Karakilçik A. Z.2004. Effects of testosterone and vitamin E on the antioxidant system in rabbit testis. Andrologia 36: 277–281. doi: 10.1111/j.1439-0272.2004.00618.x [DOI] [PubMed] [Google Scholar]
- 3.Cassani P., Beconi M. T., O’Flaherty C.2005. Relationship between total superoxide dismutase activity with lipid peroxidation, dynamics and morphological parameters in canine semen. Anim. Reprod. Sci. 86: 163–173. doi: 10.1016/j.anireprosci.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 4.Chen H., Chow P. H., Cheng S. K., Cheung A. L., Cheng L. Y., O W. S.2003. Male genital tract antioxidant enzymes: their source, function in the female, and ability to preserve sperm DNA integrity in the golden hamster. J. Androl. 24: 704–711. [DOI] [PubMed] [Google Scholar]
- 5.El-Daharawyn M., Hela I.2011. Human amniotic membrane and vitamin E/selenium for control of postoperative adhesion in dogs. Jpn. J. Vet. Res. 59: 165–171. [PubMed] [Google Scholar]
- 6.Hong Z., Hailing L., Hui M., Guijie Z., Leyan Y., Dubing Y.2010. Effect of vitamin E supplement in diet on antioxidant ability of testis in Boer goat. Anim. Reprod. Sci. 117: 90–94. doi: 10.1016/j.anireprosci.2009.03.016 [DOI] [PubMed] [Google Scholar]
- 7.Kawakami E., Kuroda D., Hori T., Tsutsui T.2007. Changes in the plasma testosterone level and testicular superoxide dismutase activity of 5 azoospermic beagles after GnRH analogue injections. J. Vet. Med. Sci. 69: 561–562. doi: 10.1292/jvms.69.561 [DOI] [PubMed] [Google Scholar]
- 8.Kawakami E., Takemura A., Sakuma M., Takano M., Hirano T., Hori T., Tsutsui T.2007. Superoxide dismutase and catalase activities in the seminal plasma of normozoospermic and asthenozoospermic Beagles. J. Vet. Med. Sci. 69: 133–136. doi: 10.1292/jvms.69.133 [DOI] [PubMed] [Google Scholar]
- 9.Kawakami E., Tsutsui T., Yamada Y., Yamauchi M.1984. Cryptorchidism in the dog: occurrence of cryptorchidism and semen quality in the cryptorchid dog. Jpn. J. Vet. Sci. 46: 303–308. doi: 10.1292/jvms1939.46.303 [DOI] [PubMed] [Google Scholar]
- 10.Kokcam I., Naziroglu M.2002. Effects of vitamin E supplementation on blood antioxidants levels in patients with Behçet’s disease. Clin. Biochem. 35: 633–639. doi: 10.1016/S0009-9120(02)00400-9 [DOI] [PubMed] [Google Scholar]
- 11.Packer L., Landvik S.1990. Vitamin E in biological system. pp.93–103. In: Antioxidants in Therapy and Preventive Medicine. Plenum Press, New York. [Google Scholar]
- 12.Siciliano L., Tarantino P., Longobardi F., Rago V., De Stefano C., Carpino A.2001. Impaired seminal antioxidant capacity in human semen with hyperviscosity or oligoasthenozoospermia. J. Androl. 22: 798–803. [PubMed] [Google Scholar]
- 13.Tsutsui T., Tsuji J., Kawakami E., Yamada Y., Amano T., Yamauchi M., Ogasa A.1987. Fluctuations in peripheral plasma androgen levels in peripubertal dogs. Nippon Juigaku Zasshi 49: 751–755. doi: 10.1292/jvms1939.49.751 [DOI] [PubMed] [Google Scholar]
- 14.Yousef M. I., Abdallah G. A., Kamel K. I.2003. Effect of ascorbic acid and Vitamin E supplementation on semen quality and biochemical parameters of male rabbits. Anim. Reprod. Sci. 76: 99–111. doi: 10.1016/S0378-4320(02)00226-9 [DOI] [PubMed] [Google Scholar]
- 15.Yue D., Yan L., Luo H., Xu X., Jin X.2010. Effect of Vitamin E supplementation on semen quality and the testicular cell membranal and mitochondrial antioxidant abilities in Aohan fine-wool sheep. Anim. Reprod. Sci. 118: 217–222. doi: 10.1016/j.anireprosci.2009.08.004 [DOI] [PubMed] [Google Scholar]