Abstract
Dysfunctions affecting the connections of basal ganglia lead to major neurological and psychiatric disorders. We investigated levels of mRNA for three neurexins (Nrxn) and three neuroligins (Nlgn) in the globus pallidus, subthalamic nucleus, and substantia nigra, in control conditions and after short-term exposure to cocaine. The expression of Nrxn2β and Nlgn3 in the substantia nigra and Nlgn1in the subthalamic nucleus depended on genetic background. The development of short-term cocaine appetence induced an increase in Nrxn3β expression in the globus pallidus. Human NRXN3 has recently been linked to several addictions. Thus, NRXN3 adhesion molecules may play an important role in the synaptic plasticity of neurons involved in the indirect pathways of basal ganglia, in which they regulate reward-related learning.
Keywords: Cocaine; Mice, Inbred C57BL; Microdissection; Nerve Tissue Proteins; Neural Cell Adhesion Molecules; RNA, Messenger; Reverse Transcriptase Polymerase Chain Reaction; Up-Regulation; Animals; Cocaine-Related Disorders; Dopamine Uptake Inhibitors; Gene Expression; Globus Pallidus; Lasers; Male; Membrane Proteins; Mice
Introduction
Addiction raises major public health issues [1]. The underlying pathophysiology of addiction may be seen as the usurpation of neural processes that normally serve reward-related learning, changing the synaptic plasticity of the subgroups of neurons involved [2]. Adhesion molecules are involved in synaptic plasticity in both the development and function of synapses [2,3]. Neural cell adhesion molecules, neuronal cell adhesion molecules, and L1 cell adhesion molecules have been shown to be deregulated by various types of addiction, including cocaine, opiate, and alcohol dependence, respectively [4–6].
We investigated the direct and indirect circuits of basal ganglia [7], which are known to be involved in motor control, habit, and reward learning [8,9]. We analyzed two families of cell adhesion molecules, neurexins (Nrxn) and neuroligins (Nlgn) [10]. The molecular basis of the differential function of direct and indirect circuits has begun to be elucidated with the demonstration of differential patterns of gene expression resulting in different forms of synaptic plasticity [11,12]. Nrxn1 was recently shown to be differentially expressed between the direct and indirect pathways of basal ganglia [11].
We first analyzed molecular differences in the globus pallidus (GP) and subthalamic nucleus (STN) of the indirect pathway and the susbtantia nigra (SN) (both reticulata and compacta parts) of the direct pathway, microdissected with the help of a laser from mice of two different genetic backgrounds. We used outbred (OF1) and inbred (C57BL/6J) mice to investigate if gene expression for Nlgn and Nrxn could depend on mice strain. We applied a short-term protocol of cocaine-induced place preference [13,14] to characterize an increase in the expression of Nrxn3β in the GP of C57BL/6J mice.
Methods
Animals
We used 3-week-old male OF1 and C57BL/6J mice (Charles Rivers Laboratories, 5 mice per group) for strain comparisons of Nrxn/Nlgn gene levels expression and C57BL/6J (18–20 g; male) mice for cocaine-induced place preference.
Cocaine-induced place preference
We followed the 5-day paradigm described in two previous studies [13,14]. C57BL/6J (18–20 g; male) mice received daily intraperitoneal injections of cocaine (15 mg/kg) or saline over the course of 3 days. An unbiased conditioning protocol was used. The place preference apparatus consisted of two main square conditioning compartments (15 × 15 × 15 cm) with distinct walls separated by a triangular central division. Mice were tested for 18 min in the apparatus on day 1 to ensure that they displayed no bias toward any chamber of the apparatus (preconditioning phase). Mice that spent more than 11 min in any one compartment were excluded from the experiment. On days 2, 3, and 4, mice received a saline injection on one side of the apparatus in the morning. Four hours later, they received a cocaine injection on the other side of the apparatus. Each conditioning session lasted 30 min (conditioning phase). On day 5, mice were placed in the apparatus for 18 min, and the time spent on either side of the apparatus was determined (testing phase). We calculated a score for each mouse by subtracting the time spent in the cocaine or saline compartment on day 5 from that spent in the same compartment on day 1.
Laser-assisted microdissection
Mice were decapitated and their brains were rapidly removed, embedded in Tissue-Tek (Sakura, Tokyo, Japan), and frozen in isopentane chilled at −30°C with dry ice. Coronal sections (10 μm thick) were obtained at −20°C with a Leica CM3050S cryostat (Rueil-Malmaison, France) from the GP, STN, and substantia nigra, located 0.1–1.06, 1.7–2.3, and 2.54–3.88 mm posterior to bregma, respectively, according to a stereotaxic mouse brain atlas [15]. Laser-assisted microdissection was performed on these sections on poly-ethylene naphthalate membrane slides (Leica). The sections were allowed to dry for 3 min, were then stained with 1% cresyl violet (4 min) successively washed with absolute ethanol (1 min) and water (20 s), and were allowed to dry for 3 min. Laser-assisted microdissection was performed on a Leica ASLMD instrument with LMD 5.0 and IM1000 software (Leica). For each mouse, samples of each structure were collected and combined in a single tube cap, which was filled with extraction buffer used for total RNA extraction (Arcturus Picopure kit, Alphelys, Plaisir, France).
RNA extraction and reverse transcription from microdissected structures
RNA was extracted from samples with the Arcturus Picopure kit, used according to the manufacturer’s instructions (Alphelys). Extracts were also treated with DNase I (Qiagen, Courtaboeuf, France; 14 units per sample). Detailed protocol of reverse transcription is available on request.
Quality control for the RNA extract
The RNA extracted from the subregions was assessed by electrophoresis with an Agilent bioanalyzer triggered with 2100 expert software and the RNA pico chip 6000 kit (Agilent, Massy, France). The samples analyzed had similar RNA integrating number values, ranging from 5 (SN) to 6.5 (STN).
Primers and probes
The sequences used for real-time quantitative PCR (Q-PCR) are available on request.
Real-time quantitative PCR
For each gene, real-time Q-PCR assays were performed in triplicate for the three regions and normalized using mRNA for the corresponding β-2 microglobulin gene in an Opticon system (Biorad, Marnes-la-Coquette, France). RNA extracted from total brain was used to establish reference curves.
Statistical analysis
Student’s t-tests were used for analysis, with Welch’s correction when required.
Results and discussion
The GP, STN, and SN were microdissected using a laser capture device (the STN is shown in Fig. 1c and e; GP and SN data not shown). We first validated our microdissection procedure by studying Pitx2 transcripts, which are produced in large amounts specifically in the STN [17]. Real-time Q-PCR showed Pitx2 transcript levels to be high in this structure (data not shown), consistent with in-situ hybridization results for the Pitx2 gene (from Allen Brain Atlas data, Fig. 1b and d; high-resolution pictures provided by Dr Lein, Allen Brain Atlas Laboratories) [18]. The STN produced only small amounts of the Gfap transcripts encoding glial fibrillary acidic protein, but larger amounts of Nfl transcripts, encoding neurofilament light (68 kDa) protein, than the GP and SN, suggesting that STN is a neuronal structure with few Gfap-expressing glial cells (Fig. 2a and b).
Fig. 1.

(a) Schematic representation of the direct (striatonigral) pathway and indirect (striatopallidal) pathway in mouse (adapted from Ref. [16]) Glutamatergic, GABAergic, and dopaminergic neurons and synapses are shown in white, black, and gray, respectively. Note that rodent GP is homolog of the primate external pallidal segment. (b–e) Coronal brain slices showing the STN after in-situ hybridization of the Pitx2 gene [from the Allen Brain Atlas, (b, d)] or cresyl violet staining (c, e) before (c) or after (e) laser capture microdissection. The line indicates the zone cut by the laser (d) [scale 500 μM, (c, d, e)].CX, cortex; GABAergic, γ-amino-butyric acidergic; GP, globus pallidus; SNc, subtantia nigra compacta; SNr, subtantia nigra reticulata; St, striatum; STN, subthalamic nucleus.
Fig. 2.
Validation of laser capture microdissection of the GP, STN, SN. Note that Vglut2 expression is restricted to the STN, as expected for glutamatergic neurons. Note that Gad1 expression is limited to the GP and SN, as expected. Qualitative-PCR measurements for Gfap, Nfl,Vglut2, and Gad1 were performed in two different genetic backgrounds (OF1 and C57). *, **P < 0.05 and P < 0.01, respectively, Student’s t-test. C57, inbred mouse; GP, globus pallidus; OF1, outbred; SN, substantia nigra; STN, subthalamic nucleus.
For further validation, we assessed the levels of expression of Vglut2, a bona fide marker for glutamatergic neurons, and Gad1, a bona fide marker for γ-aminobutyric acidergic neurons. As expected, only the STN expressed Vglut2, whereas Gad1 was expressed by the GP and SN (Fig. 2c and d; quantitative data are provided on request).
We also used Q-PCR to quantify Nrxn1β, Nrxn2β, Nrxn3β, Nlgn1, Nlgn2, and Nlgn3 expression in the GP, STN and SN (Fig. 3). Nrxn2β and Nlgn3 expression in the SN was significantly lower and Nlgn1 expression in the STN was significantly higher in C57 mice than in OF1 mice (P=0.03; P=0.03, and P=0.02, respectively, Fig. 3).
Fig. 3.
Comparison of the expression of Nrxn2β, Nlgn1, and Nlgn3 in the STN and SN. Qualitative-PCR assessments of Nrxn1β (a) Nlgn1 (b), and Nlgn3 (c) expression in the STN (b) and SN (a, c) for two different genetic backgrounds (OF1and C57). *P < 0.05, Student’s t-test. C57, inbred mouse; OF1, outbred; SN, substantia nigra; STN, subthalamic nucleus.
We then investigated the expression of Nrxn/Nlgn genes in the GP, STN, and SN in control conditions and after short-term cocaine administration. Mice were injected with saline or cocaine (15 mg/kg, i.p.) for 3 days and were selected on the basis of conditioned place preference using a paradigm similar to that described in two previous studies [13,14] (Fig. 4).
Fig. 4.

Cocaine-induced place preference score. C57BL/6J mice were given daily intraperitoneal injections of cocaine (15 mg/kg, C57 cocaine) or saline (C57 saline) for 3 days. The CPP score (in seconds) is the difference between the time spent in the compartment associated with cocaine or saline minus the time spent in the same compartment before injection (CPP score). *P < 0.05, Student’s t-test.
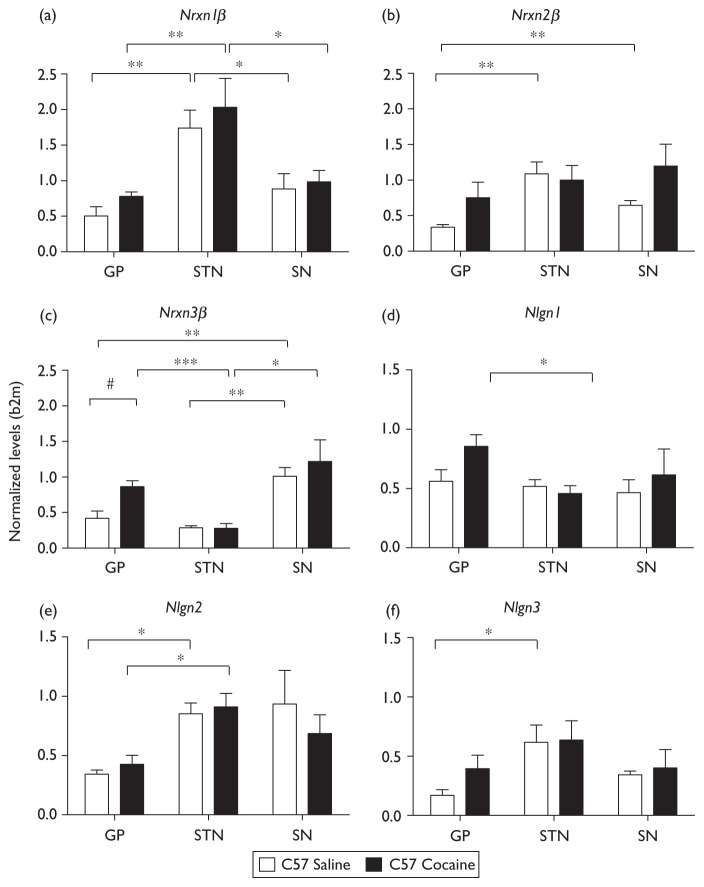
Nrxn3β expression in the GP of mice exposed to cocaine was twice that in control mice (P=0.01) (Fig. 5c). Furthermore, both Nrxn1β and Nlgn1 tended to be overexpressed in the GP (P=0.08 and P=0.07, respectively Fig. 5a and d). The difference in Nrxn3β expression levels in the GP of cocaine-treated and control mice is consistent with recent findings that the human ortholog of Nrxn3, NRXN3, is associated with nicotine, opioid, and alcohol dependence [19–21]. This gene has also recently been shown to be associated with polysubstance addiction [22]. These data suggest that NRXN3 deregulation in the GP neurons involved in the indirect pathway of basal ganglia may be a molecular trigger for the synaptic changes detected during the development of addiction [2]. Furthermore, the trend observed for the Nrxn1β and Nlgn1 genes suggests that short-term cocaine exposure may increase the expression of these two genes. An analysis of the levels of these transcripts after longer periods of exposure to cocaine might identify significant changes in expression. Modifications to genes encoding Nrxns/Nlgns have recently been reported, with mutations in NRXN1 identified in autism and schizophrenia [23,24] and mutations in NLGN3 identified in autism [25], reinforcing the interest to decipher their expression in specific brain regions from mice models.
Fig. 5.
Deregulation of gene expression induced by cocaine place preference. Each value corresponds to the amount of mRNA produced from the gene concerned, normalized with respect to the amount of mRNA produced from the β-2 microglobulin gene in C57BL/6J mice after saline (C57 saline) or cocaine (15 mg/kg, i.p., 3 days, C57 cocaine) administration. *, **, ***P < 0.05, P < 0.01, and P < 0.001, respectively, Student’s t-test, comparing the GP, NST, and SN in mice from the same group. #P < 0.05 for comparisons of C57 mice receiving saline with C57 mice receiving cocaine.
Conclusion
Our results suggest that short-term cocaine exposure is sufficient to induce changes in the Nrxn/Nlgn repertoire in the brain nuclei of the indirect pathway of the basal ganglia. Nrxn3 deregulation in the GP neurons may induce changes in both the presynaptic GP neurons and in the postsynaptic STN neurons, as Nlgns–Nrxns interactions link postsynaptic and presynaptic function [10]. As it is possible to manipulate gene expression in these neuronal structures in mice, the use of the approaches described here may be instrumental to unravel the molecular modifications induced early in cocaine appetence.
Acknowledgments
This study was supported by INSERM and MILDT grants. S.K. and G.M. were Jeune Chercheur INSERM and Fondation Orange fellows, respectively. The authors thank Dr Lein from the Allen Brain Institute for sharing high-resolution Pitx2, Nrxn1, Nlgn1, and Nlgn3 ISH data; and Corinne Canestrelli for technical assistance with the cocaine-induced place preference paradigm.
References
- 1.Uhl GR, Elmer GI, Labuda MC, Pickens RW. Genetic influences in drug abuse. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology the fourth generation of progress. New York: Raven Press; 1995. pp. 1793–1806. [Google Scholar]
- 2.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 3.Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maćkowiak M, Markowicz-Kula K, Fija K, Wedzony KM. Acute and repeated administration of cocaine differentially regulates expression of PSA-NCAM-positive neurons in the rat hippocampus. Brain Res. 2005;1055:149–155. doi: 10.1016/j.brainres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Tang N, He M, O’Riordan MA, Farkas C, Buck K, Lemmon V, et al. Ethanol inhibits L1 cell adhesion molecule activation of mitogen-activated protein kinases. J Neurochem. 2006;96:1480–1490. doi: 10.1111/j.1471-4159.2006.03649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishiguro H, Liu QR, Gong JP, Hall FS, Ujike H, Morales M, et al. NrCAM in addiction vulnerability: positional cloning, drug-regulation, haplotype-specific expression, and altered drug reward in knockout mice. Neuropsychopharmacology. 2006;31:572–584. doi: 10.1038/sj.npp.1300855. [DOI] [PubMed] [Google Scholar]
- 7.Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196:527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 9.Baunez C, Dias C, Cador M, Amalric M. The subthalamic nucleus exerts opposite control on cocaine and ‘natural’ rewards. Nat Neurosci. 2005;8:484–489. doi: 10.1038/nn1429. [DOI] [PubMed] [Google Scholar]
- 10.Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9:443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- 12.Lobo MK, Cui Y, Ostlund SB, Balleine BW, Yang XW. Genetic control of instrumental conditioning by striatopallidal neuron-specific S1P receptor Gpr6. Nat Neurosci. 2007;10:1395–1397. doi: 10.1038/nn1987. [DOI] [PubMed] [Google Scholar]
- 13.McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- 14.Benturquia N, Le Guen S, Canestrelli C, Lagente V, Apiou G, Roques BP, et al. Specific blockade of morphine- and cocaine-induced reinforcing effects in conditioned place preference by nitrous oxide in mice. Neuroscience. 2007;149:477–486. doi: 10.1016/j.neuroscience.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- 16.Beurrier C, Ben-Ari Y, Hammond C. Preservation of the direct and indirect pathways in an in vitro preparation of the mouse basal ganglia. Neuroscience. 2006;140:77–86. doi: 10.1016/j.neuroscience.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Martin DM, Skidmore JM, Philips ST, Vieira C, Gage PJ, Condie BG, et al. PITX2 is required for normal development of neurons in the mouse subthalamic nucleus and midbrain. Dev Biol. 2004;267:93–108. doi: 10.1016/j.ydbio.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 18.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 19.Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lachman HM, Fann CS, Bartzis M, Evgrafov OV, Rosenthal RN, Nunes EV, et al. Genomewide suggestive linkage of opioid dependence to chromosome 14q. Hum Mol Genet. 2007;16:1327–1334. doi: 10.1093/hmg/ddm081. [DOI] [PubMed] [Google Scholar]
- 21.Hishimoto A, Liu QR, Drgon T, Pletnikova O, Walther D, Zhu XG, et al. Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms. Hum Mol Genet. 2007;16:2880–2891. doi: 10.1093/hmg/ddm247. [DOI] [PubMed] [Google Scholar]
- 22.Liu QR, Drgon T, Walther D, Johnson C, Poleskaya O, Hess J, et al. Pooled association genome scanning: validation and use to identify addiction vulnerability loci in two samples. Proc Natl Acad Sci U S A. 2005;102:11864–11869. doi: 10.1073/pnas.0500329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Autism Genome Project Consortium; Szatmari P. Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirov G, Gumus D, Chen W, Norton N, Georgieva L, Sari M, et al. Comparative genome hybridization suggests a role for NRXN1 and APBA2 in schizophrenia. Hum Mol Genet. 2008;17:458–465. doi: 10.1093/hmg/ddm323. [DOI] [PubMed] [Google Scholar]
- 25.Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]