Abstract
Tonic GABA currents mediated by high-affinity extrasynaptic GABAA receptors, are increasingly recognized as important regulators of cell and neuronal network excitability. Dysfunctional GABAA receptor signaling that results in modified tonic GABA currents is associated with a number of neurological disorders. Consequently, developing compounds to selectively modulate the activity of extrasynaptic GABAA receptors underlying tonic inhibition is likely to prove therapeutically useful. Here, we examine the GABAA receptor subtype selectivity of the weak partial agonist, 5-(4-piperidyl)isoxazol-3-ol (4-PIOL), as a potential mechanism for modulating extrasynaptic GABAA receptor-mediated tonic currents. By using recombinant GABAA receptors expressed in HEK293 cells, and native GABAA receptors of cerebellar granule cells, hippocampal neurons, and thalamic relay neurons, 4-PIOL evidently displayed differential agonist and antagonist-type profiles, depending on the extrasynaptic GABAA receptor isoforms targeted. For neurons, this resulted in differential modulation of GABA tonic currents, depending on the cell type studied, their respective GABAA receptor subunit compositions, and critically, on the ambient GABA levels. Unexpectedly, 4-PIOL revealed a significant population of relatively low-affinity γ2 subunit-containing GABAA receptors in the thalamus, which can contribute to tonic inhibition under specific conditions when GABA levels are raised. Together, these data indicate that partial agonists, such as 4-PIOL, may be useful for modulating GABAA receptor-mediated tonic currents, but the direction and extent of this modulation is strongly dependent on relative expression levels of different extrasynaptic GABAA receptor subtypes, and on the ambient GABA levels.
SIGNIFICANCE STATEMENT A background level of inhibition (tonic) is important in the brain for controlling neuronal excitability. Increased levels of tonic inhibition are associated with some neurological disorders but there are no specific ligands capable of selectively reducing tonic inhibition. Here we explore the use of a GABA partial agonist as a selective chemical tool in three different brain regions. We discover that the activity of a partial agonist is heavily dependent upon the GABAA receptor subunit composition underpinning tonic inhibition, and on the ambient levels of GABA in the brain.
Keywords: 4-PIOL, extrasynaptic GABA receptors, GABA, partial agonist, synaptic inhibition, tonic inhibition
Introduction
GABAA receptors are the major inhibitory ligand-gated ion channels in the mammalian CNS. To date, eight classes of GABAA receptor subunits have been identified, one-half of which exhibit multiple isoforms: α(1–6), β(1–3), γ(1–3), δ, ε, θ, π, ρ(1–3) (Smart, 2015). They mediate their physiological effects via two temporally and spatially distinct forms of signaling, denoted as phasic and tonic inhibition. Phasic inhibition involves the activation of synaptically located, usually α(1–3)βγ2 GABAA receptors, by transiently high concentrations (mm) of GABA (Maconochie et al., 1994; Jones and Westbrook, 1995), whereas tonic inhibition relies on the continuous activation of extrasynaptic (typically α4/6βδ) GABAA receptors, by ambient GABA concentrations (nm –μm; Farrant and Nusser, 2005), though other isoforms will also contribute, such as α5βγ, α1β, and α1βδ assemblies (Caraiscos et al., 2004; Mortensen and Smart, 2006; Glykys et al., 2007, 2008).
Imbalanced tonic inhibition is associated with a wide range of neurological disorders (Belelli et al., 2009; Brickley and Mody, 2012). In particular, enhanced tonic currents, arising from elevated ambient GABA levels in the brain, are implicated in the pathology of absence seizures, cognitive disorders, and motor deficits following stroke (Cope et al., 2009; Clarkson et al., 2010; Jo et al., 2014; Wu et al., 2014). Consequently, emerging evidence indicates that antagonists and/or inverse agonists that selectively inhibit extrasynaptic α5- and/or δ-containing GABAA receptors may prove therapeutically useful for such conditions (Navarro et al., 2002; Atack et al., 2006; Ballard et al., 2009; Cope et al., 2009; Atack, 2010; Clarkson et al., 2010; Braudeau et al., 2011; Martínez-Cué et al., 2013). Unfortunately, although several compounds selectively enhance δ- or α5-mediated tonic currents, such as THIP, DS2, AA29504, and Thio-4-PIOL (Stórustovu and Ebert, 2006; Wafford et al., 2009; Hoestgaard-Jensen et al., 2010, 2013; Jensen et al., 2013), only one compound (DPP-4-PIOL) appears to selectively inhibit δ-mediated tonic currents (Boddum et al., 2014). Moreover, clinical trials involving α5-selective inverse agonists (α5IA and L-655,708) have foundered due to adverse effects (Rudolph and Möhler, 2014).
As an alternative to GABAA receptor antagonists, we hypothesized that low-efficacy partial agonists may act as “functional antagonists”, given their ability to compete with GABA for the orthosteric binding site, and their reduced ability to activate GABAA receptors. Moreover, low-efficacy partial agonists may be less likely to induce convulsions, or unwanted side effects (Krogsgaard-Larsen et al., 2002). Here the subtype-selective profile of the low-efficacy partial agonist, 5-(4-piperidyl)isoxazol-3-ol (4-PIOL; Kristiansen et al., 1991; Frølund et al., 1995; Mortensen et al., 2002, 2004) was assessed on recombinant and neuronal GABAA receptors. The activity profile of 4-PIOL on tonic and phasic currents varied between three selected brain regions. This revealed a strong dependence on GABAA receptor subunit composition, and on ambient GABA levels, and helped uncover a population of largely silent GABAA receptors in the thalamus that can contribute to tonic inhibition under specific conditions. Overall, partial agonists may be useful as therapeutic agents, but their effectiveness will critically depend on which GABAA receptor isoforms are present and the extent of their activation.
Materials and Methods
Transient receptor expression in HEK293 cells.
Human embryonic kidney (HEK) 293 cells were cultured in DMEM, as previously described (Wooltorton et al., 1997). HEK cells were plated onto poly-l-lysine-coated coverslips and transfected using a calcium phosphate protocol. Briefly, GABAA receptor pRK5 cDNAs, with enhanced green fluorescent protein (eGFP) cDNA, were mixed with 340 mm CaCl2, and an equal volume of HEPES-buffered saline (50 mm HEPES, 280 mm NaCl and 2.8 mm Na2HPO4, pH 7.2). One microgram of each cDNA was used, and a total of 4 μg cDNA was used for each transfection. The cDNA-calcium phosphate suspension was applied to cells, which were incubated overnight, and used for electrophysiology 18–48 h after transfection.
Culturing of cerebellar granule cells.
Cerebellar cultures were prepared, as described previously (Houston and Smart, 2006) using postnatal day (P)4 Sprague-Dawley rats, prepared in accordance with the UK Animals Scientific Procedures Act (1986). Cells were dissociated using 0.1% w/v trypsin and mechanical trituration before plating onto glass coverslips coated with 500 μg/ml poly-d-ornithine, in basal medium eagle (BME) supplemented with 10% v/v heat-inactivated fetal calf serum (FCS). After 1 h, the medium was replaced with BME containing 5% v/v heat-inactivated horse serum (HS; Invitrogen), 20 U/ml penicillin G, 20 μg/ml streptomycin, 2 mm l-glutamine, 0.5% v/v glucose and a growth mixture (5 mg/L insulin, 5 mgl/L transferrin, 5 mg/L selenium; Sigma-Aldrich). Electrophysiological recordings were performed from cultured cerebellar granule cells (CGCs), between 7–19 d in vitro (DIV).
Culturing hippocampal neurons.
Hippocampal cultures were prepared from E18 rat embryos, as previously described (Thomas et al., 2005). Cells were dissociated, as above, before plating onto glass coverslips coated with 100 μg/ml poly-d-lysine (Sigma-Aldrich) in minimum essential media (Invitrogen) supplemented with 5% v/v FCS, 5% v/v HS, 10 U/ml penicillin-G, 10 μg/ml streptomycin, 2 mm l-glutamine, and 20 mm glucose. After 2 h, the media was replaced with Neurobasal-A (Invitrogen) media supplemented with 1% v/v B-27 (Invitrogen), 50 U/ml penicillin-G, 50 μg/ml streptomycin, 0.5% v/v Glutamax (Invitrogen), and 35 mm glucose. Electrophysiological recordings were performed between 11 and 21 DIV.
Acute brain slice preparation.
Young rats (P14) were terminally anesthetized with isoflurane. The brain was rapidly removed and immersed in ice-cold slicing solution composed of the following (in mm): 130 K-gluconate, 15 KCl, 0.05 EGTA, 20 HEPES, 4 Na-pyruvate, 25 glucose, and 2 kynurenic acid, pH 7.4. Coronal (thalamus and hippocampus) or parasagittal (cerebellum) slices (all 250 μm) were made using a Leica VT 1200s vibroslicer, and transferred to a holding chamber incubated at 37°C. The solution was slowly exchanged for artificial CSF (aCSF) containing the following (in mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, 1 MgCl2, 25 glucose, and 2 kynurenic acid (pH 7.4 when bubbled with 95% O2 and 5% CO2). Slices were maintained in the holding chamber at room temperature until required for electrophysiology.
Drug solutions.
For HEK293 cells, GABA was applied alone, or in combination with other drugs using a Y-tube application system (Mortensen and Smart, 2007). The 10–90% solution exchange times of the application system were within 20–30 ms as measured in open pipette tip recordings. GABA and bicuculline were obtained from Sigma-Aldrich, THIP and DS2 were purchased from Tocris Biosciences, and diazepam was sourced from Roche. Drug solutions were either prepared from water or DMSO concentrated stocks, or dissolved directly into the extracellular medium, depending on the final concentration. Drug solutions were corrected for pH before use.
Data acquisition.
Whole-cell currents were recorded using an Axopatch 200B amplifier. Currents were filtered at 5 kHz, digitized at 50 kHz via a Digidata 1332A (Molecular Devices), and recorded to disk (Dell Pentium Dual Core-Optiplex 960). Series resistances were monitored throughout each experiment and deviations >20% resulted in the data being excluded from further analysis.
HEK293 cell electrophysiology.
HEK293 cells were continuously perfused with Krebs solution containing the following (in mm): 140 NaCl, 4.7 KCl, 1.2 MgCl2, 2.52 CaCl2, 11 glucose, and 5 HEPES, adjusted to pH 7.4 with 1 m NaOH. Patch pipettes were fire polished to 2–4 MΩ and filled with an intracellular solution containing the following (in mm): 120 KCl, 1 MgCl2, 11 EGTA, 10 HEPES, 1 CaCl2, and 2 adenosine triphosphate, adjusted to pH 7.2 with 1 m NaOH. HEK293 cells were voltage-clamped between −20 and −60 mV, depending on peak current size.
Analysis of GABA concentration–response curves.
GABA-activated currents (IGABA) were normalized to the maximal current evoked by a saturating concentration of GABA (IMax,GABA). The normalized concentration–response curves were fitted using a modified Hill equation (Eq. 1), using a least-squares method.
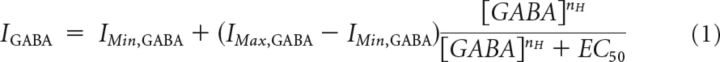 |
EC50 is the concentration of GABA, [GABA], which produced 50% of the maximal response, and nH, is the Hill coefficient. IMin,GABA is the minimum “plateau” response induced in the presence of 4-PIOL. For the GABA concentration–response curve constructed in the absence of 4-PIOL, IMin,GABA is zero. The parameters obtained from individual curve-fittings were collated and the data were expressed as the mean ± SEM.
Neuronal whole-cell electrophysiology.
Thalamic relay neurons of the dorsal lateral geniculate nucleus (dLGN), CA1 pyramidal neurons in the hippocampus, and CGCs were visualized using infrared differential interference contrast optics and a Basler scA750–60fm camera. Cells were perfused with aCSF or Krebs, supplemented with 2 mm kynurenic acid, or a mixture of 20 μm D-AP5 (Tocris Bioscience) and 10 μm CNQX (Abcam) to block glutamatergic currents. Recordings were made using patch pipettes (2–4 MΩ) filled with a solution containing the following (in mm): 140 CsCl, 2 NaCl, 10 HEPES, 5 EGTA, 2 MgCl2, 0.5 CaCl2, 2 Na-ATP, and 2 QX-314 bromide. pH was adjusted using 1 m CsOH. In a subset of recordings, thalamic slices were preincubated for 30 min, in aCSF containing the GABA transporter (GAT) inhibitors, NNC-711 (10 μm; Abcam) and SNAP-5114 (20 μm; Tocris Bioscience). Both compounds were also present throughout the recordings. A saturating concentration of bicuculline (20 μm; Ueno et al., 1997) was bath-applied at the end of all electrophysiological recordings, to confirm that all synaptic events were GABAergic, and to reveal GABA-mediated tonic currents. All recordings were performed at room temperature.
For tonic currents, the average holding current for a 30 s epoch in each drug condition was measured using WinEDR software (v3.1; John Dempster, University of Strathclyde, Glasgow, UK). Changes in holding current were calculated by subtracting the average holding current after drug application, from the average holding current before drug application. In addition, the root mean square (rms) baseline noise was measured over a 30 s epoch, sampled every 100 ms. Because sIPSCs increase rms baseline noise, Microsoft Excel was used to calculate a threshold for eliminating contaminated 100 ms epochs. A running threshold (routinely the median) was calculated at 5 s time intervals, over a 30 s recording period, and any rms value greater than the calculated threshold, was automatically excluded from further analysis. Effective thresholding was validated by manually analyzing a small section of each recording (∼10 s), and manually eliminating 100 ms epochs contaminated by synaptic currents.
Synaptic current analysis.
The sIPSC frequency was determined using MiniAnalysis software (Synaptosoft). During 4-PIOL application, a significant increase in rms current noise was observed that might mask smaller sIPSCs, thereby introducing a bias toward larger events, compared with control. To limit the bias, only the largest hundred amplitude events from each condition were compared. The average decay kinetics for sIPSCs for each cell was determined by fitting uncontaminated events (>50 events for each condition) with either a mono- or bi-exponential decay function. To combine data obtained for mono- or bi-exponentially fitted events, decay times were transformed to a weighted decay time, τw, according to the following:
where τ1 and τ2 represent decay time constants, and A1 and A2 are the relative amplitude contributions of τ1 and τ2. For mono-exponential decaying events, A2 and τ2 are zero. The mean sIPSC frequency, amplitude, 10–90% rise time, and τw were calculated for each cell.
Results
Functional properties of synaptic-type GABAA receptors activated by a partial agonist
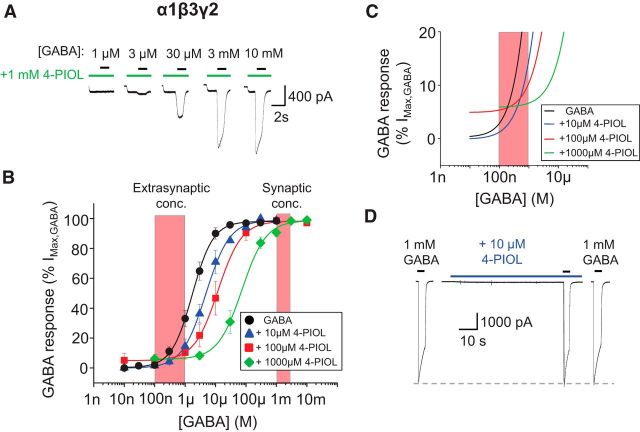
To evaluate the functional profile of 4-PIOL at synaptic-type GABAA receptors, peak whole-cell GABA currents were recorded from α1β3γ2-expressing HEK293 cells, in the absence or presence of 10, 100, or 1000 μm 4-PIOL (Fig. 1A). At these concentrations, 4-PIOL induced a rightward shift in the GABA concentration–response curve, with a discernible crossover with the control GABA curve (Fig. 1B). The GABA EC50 for α1β3γ2 receptors was increased from 4.5 ± 1.9 μm in control, to 9.4 ± 3.4, 15.4 ± 5.4, and 126.7 ± 55.6 μm in the presence of 10, 100, and 1000 μm 4-PIOL, respectively. Pre-application of 4-PIOL to α1β3γ2-expressing cells revealed a small agonist response, particularly with 100 or 1000 μm 4-PIOL (Fig. 1A). This agonist activity appeared on the concentration–response curves by an elevated minimum response (Fig. 1B,C) with 100 and 1000 μm 4-PIOL inducing agonist currents that were 5.5 ± 3.2% and 6.8 ± 1.3% of the maximum GABA response (Fig. 1C). Crucially for this synaptic GABAA receptor subtype, neither 10 nor 100 μm 4-PIOL inhibited the maximum responses to higher, synaptic concentrations of GABA (percentage control: 98.8 ± 1.2% and 98.7 ± 1.3% for 10 and 100 μm 4-PIOL at 1 mm GABA; Fig. 1D), indicating that at these concentrations of 4-PIOL, the partial agonist is potentially capable of inhibiting responses to predicted extrasynaptic concentrations of GABA, without depressing synaptic GABA currents.
Figure 1.
Functional properties of recombinant α1β3γ2 GABAA receptors activated by 4-PIOL. A, Representative whole-cell current traces elicited by GABA in the presence of preapplied 4-PIOL (1 mm), for α1β3γ2-expressing HEK293 cells. In this, and subsequent figures, the horizontal bars indicate the duration of drug applications; here representing GABA (black) and 4-PIOL (green) applications. B, Mean peak GABA concentration–response curves constructed in the absence (●), or presence of 10 μm (▴), 100 μm (■) or 1000 μm (♦) 4-PIOL (n = 4–13; mean ± SEM). The peak response to each concentration of GABA was measured in relation to the holding current before 4-PIOL preapplication, and each dataset was normalized to the maximum response achieved by a saturating concentration of GABA, in the absence of 4-PIOL. The normalized concentration–response curves were fitted using a modified Hill equation (black and colored lines), to account for the elevated curve minima induced by 4-PIOL. The red hatched boxes represent concentrations of GABA proposed to exist at extrasynaptic (100 nm–1 μm) and synaptic sites (>1 mm). C, An expanded part of the GABA concentration–response curves presented in B. Note the elevated curve minima induced by 100 and 1000 μm 4-PIOL. D, Example whole-cell current traces elicited by 1 mm GABA in the absence, or presence of preapplied 10 μm 4-PIOL (blue bar).
Functional properties of extrasynaptic-type GABAA receptors activated by a partial agonist
Given the minimal efficacy displayed by 10 μm 4-PIOL at α1βγ2 receptors, and its potential to not inhibit synaptic currents in neurons, we further characterized the effects of 10 μm 4-PIOL on extrasynaptic-type GABAA receptors expressed in HEK293 cells. Low ambient concentrations of GABA (0.1, 0.3, and 1 μm), were preapplied to α4β2δ-, α6β2δ-, and α5β3γ2-expressing HEK293 cells, until a steady-state response was achieved, and subsequently, GABA was coapplied with 10 μm 4-PIOL (Fig. 2A–C).
Figure 2.
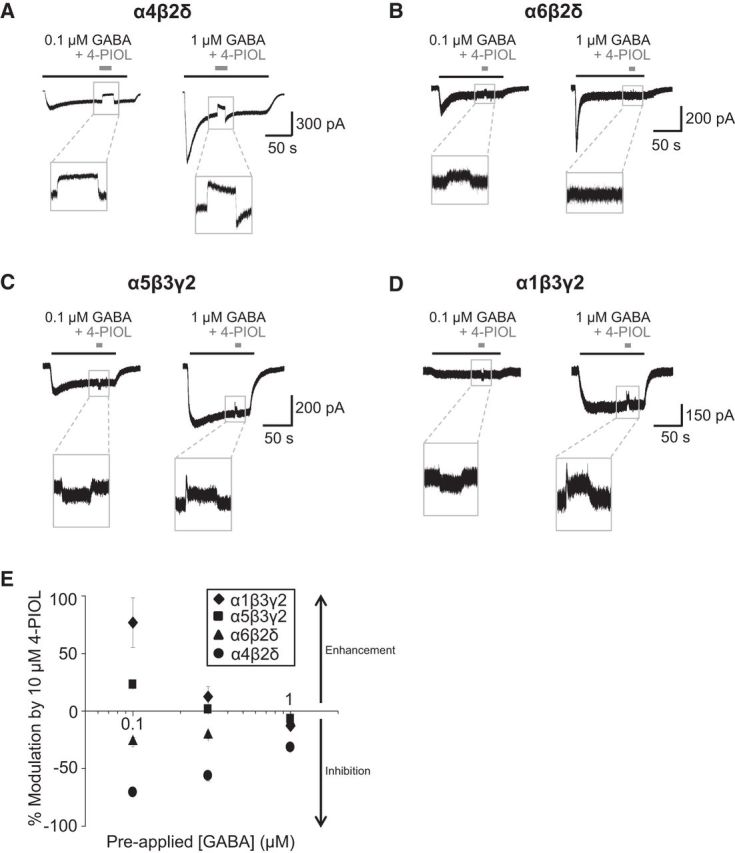
Functional effects of 4-PIOL on steady-state GABA currents. Representative whole-cell currents induced by long applications of 0.1 and 1 μm GABA to α4β2δ (A), α6β2δ (B), α5β3γ2 (C), and α1β3γ2 (D) receptors. 4-PIOL (10 μm) was briefly coapplied once a steady-state GABA current was achieved. E, Quantitative analysis of data depicted in A–D. Data points for α1β3γ2 (♦), α5β3γ2 (■), α6β2δ (▴), and α4β2δ (●) receptors are mean ± SEM (n = 4–5 cells). For each concentration (0.1, 0.3, and 1 μm) of preapplied GABA, the change in holding current produced by 4-PIOL is expressed as a percentage of the steady-state current to GABA alone. Negative values represent an inhibition of the steady-state GABA current.
For α4β2δ receptors, coapplication of 4-PIOL significantly inhibited the steady-state GABA current for 0.1, 0.3, and 1 μm GABA by 70.6 ± 2.7%, 56.1 ± 4.5%, and 31.5 ± 1.3%, respectively (Fig. 2A,E; p = 0.03, 0.04, and 0.02, respectively). By contrast, only a modest inhibition of steady-state currents was produced by 4-PIOL at recombinant α6β2δ receptors for the lowest concentrations of GABA (by 26.5 ± 3.9% and 20.9 ± 3.0 for 0.1 and 0.3 μm; p = 0.007 and 0.006; Fig. 2B), but not for 1 μm GABA (percentage inhibition: 9.6 ± 4.0%; p = 0.31). For recombinant α5β3γ2 receptors, the functional profile of 10 μm 4-PIOL varied, depending on the ambient GABA concentration (Fig. 2C). 4-PIOL enhanced the steady-state current elicited by 0.1 μm GABA (by 23.0 ± 1.5%; p = 0.004), was ineffective at 0.3 μm GABA (a 1.5 ± 0.8% enhancement; p = 0.2), and produced a small inhibition (6.8 ± 0.9%; p = 0.02) of steady-state GABA currents induced by 1 μm GABA.
In addition, the effects of 4-PIOL were assessed on α1β3γ2 receptors at low extrasynaptic GABA concentrations (Fig. 2D), because their presence at extrasynaptic sites has also been suggested in several neuronal cell types (Nusser et al., 1998; Mangan et al., 2005; Thomas et al., 2005; Kasugai et al., 2010). 4-PIOL significantly enhanced the response to 0.1 μm GABA by 76.9 ± 21.5% (Fig. 2E; p = 0.02). However, when coapplied with 1 μm GABA, 4-PIOL produced a small inhibition of the steady-state GABA current (12.9 ± 3.3%; Fig. 2E; p = 0.05).
There is some evidence for the expression of native αβ receptors lacking either a γ or δ subunit at extrasynaptic sites in cerebellar and hippocampal neurons (Brickley et al., 1999; Mortensen and Smart, 2006). Therefore, we also assessed the effects of 4-PIOL on α1β3 receptors. Similar to the δ-containing receptors, 10 μm 4-PIOL showed no agonist behavior at these receptors, but instead inhibited steady-state currents activated by low GABA concentrations (by 69 ± 7%, 75 ± 2%, and 75 ± 2% at 0.1, 0.3, and 1 μm GABA, respectively).
Overall, these data indicate that 10 μm 4-PIOL may potently inhibit α4β2δ-mediated tonic currents, without affecting α1β3γ2-mediated phasic currents. Moreover, 10 μm 4-PIOL is expected to produce only a modest effect on α6β2δ-mediated tonic currents (eg, in the cerebellum), whereas any inhibition of extrasynaptic γ2-containing receptors, although minimal, will be strongly dependent on the ambient GABA concentration in neuronal preparations. Although our earlier studies suggested that αβ receptors represent only a modest component of the extrasynaptic GABAA receptor population in hippocampal pyramidal cells (∼10%; Mortensen and Smart, 2006), their contribution to the tonic current will be inhibited by 10 μm 4-PIOL.
Examining GABAA receptor-mediated tonic and phasic inhibition with a partial agonist
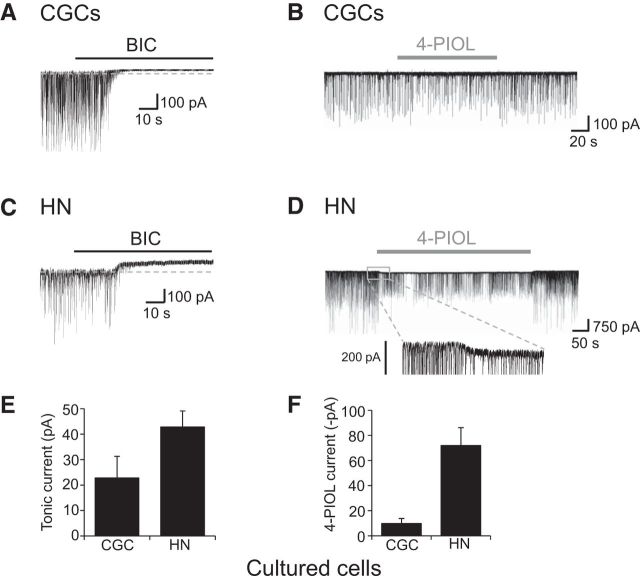
As our observations indicated that 4-PIOL may have differential effects on synaptic and extrasynaptic GABAA receptors, we chose to explore the functional profile of 4-PIOL on tonic and phasic GABA currents in neurons. Initially, whole-cell recordings were performed on cultured hippocampal and cerebellar granule (CGC) neurons. Under control recording conditions, a high frequency of sIPSCs were recorded from CGCs (7.2 ± 1.0 Hz) and hippocampal neurons (6.9 ± 1.7 Hz; Table 1), which displayed mean sIPSC amplitudes of 409 ± 113 and 361 ± 101 pA (Table 1), respectively. A saturating concentration of bicuculline (20 μm) was applied at the end of the recordings to confirm that all synaptic events were GABAergic, and to measure the amplitudes of GABAA receptor-mediated tonic currents. As expected, for both cell types, bicuculline abolished the sIPSCs and induced outward shifts in the membrane holding current, indicative of GABA tonic currents (Fig. 3A,C). For CGCs and hippocampal neurons, the basal tonic current amplitudes were as follows: 22.8 ± 8.5 and 42.8 ± 6.3 pA (Fig. 3E; Table 2).
Table 1.
sIPSC parameters for cultured cerebellar granule and hippocampal neurons
| CGCs |
Hippocampal neurons |
|||
|---|---|---|---|---|
| Control | +4-PIOL | Control | +4-PIOL | |
| Frequency, Hz | 7.2 ± 1.0 | 4.5 ± 1.3 | 6.9 ± 1.7 | 4.0 ± 1.6* |
| Amplitude, pA | 409 ± 113 | 374 ± 123 | 361 ± 101 | 243 ± 63** |
| 10–90% rise time, ms | 1.1 ± 0.1 | 1.2 ± 0.1 | 2.0 ± 0.3 | 2.1 ± 0.3 |
| Decay tau, ms | 17.7 ± 1.6 | 19.8 ± 1.7 | 31.4 ± 3.0 | 31.7 ± 3.7 |
All data are presented as mean ± SEM (n = 5–6). Control and 4-PIOL data were compared using a paired t test.
p < 0.05,
p < 0.01.
Figure 3.
Characterizing GABAA receptor-mediated tonic and 4-PIOL-activated currents in cultured neurons. Representative currents from cultured CGCs (A) and hippocampal neurons (HNs; C) in the presence of CNQX (10 μm) and D-APV (20 μm). Bicuculline (BIC; 20 μm) was applied for the duration indicated. All recordings were performed at room temperature and the holding potentials were −60 mV. B, D, Representative recordings from a CGC (B) and a hippocampal neuron (D), showing the effect of 10 μm 4-PIOL application on sIPSCs and holding current. E, F, Bar graphs for GABAA receptor-mediated tonic currents (E) and 4-PIOL-activated currents (F) from CGCs (n = 5) and hippocampal neurons (n = 6; bars are mean ± SEM). Tonic currents were calculated by subtracting the average holding current after BIC application from the average holding current in control Krebs.
Table 2.
4-PIOL and tonic currents for cultured cerebellar granule and hippocampal neurons
| CGCs | Hippocampal neurons | |
|---|---|---|
| 4-PIOL current, pA | −9.8 ± 4.0 | −72.0 ± 14.2** |
| (pA/pF) | (−0.8 ± 0.3) | (−0.8 ± 0.2) |
| Tonic current, pA | 22.8 ± 8.5* | 42.8 ± 6.3** |
| (pA/pF) | (1.8 ± 0.9) | (0.3 ± 0.1) |
Data shown are the mean currents (±SEM, n = 5–6) evoked by 10 μm 4-PIOL and 20 μm bicuculline (tonic current). According to normal convention, inward and outward currents have negative and positive polarities, respectively. Currents normalized to cell capacitance (pA/pF) are shown in parentheses below the absolute values. Statistical significance was assessed using a paired t test.
p < 0.05,
p < 0.01.
To assess the effects of 4-PIOL on the tonic and phasic currents, 10 μm 4-PIOL was applied to cells following a period of control recording (Fig. 3B,D). For CGCs, 4-PIOL showed a very small tendency to enhance the tonic current by 9.8 ± 4.0 pA (Fig. 3B,F), however, this was not statistically significant (p = 0.058). 4-PIOL exerted no significant effect on sIPSC amplitude (percentage control: 93.2 ± 14.9; p = 0.66), rise time (percentage control: 107 ± 4.1; p = 0.24), or frequency (percentage control: 64.0 ± 14.2; p = 0.057) in CGCs (Table 1).
By comparison, in hippocampal neurons, 10 μm 4-PIOL significantly enhanced the GABA-mediated tonic current by 72.0 ± 14.2 pA (p = 0.0025; Fig. 3D,F). Given that 10 μm 4-PIOL did not inhibit the synaptic-type responses of recombinant α1βγ2 receptors, it was unexpected that 4-PIOL also significantly inhibited both the frequency (percentage control: 49.8 ± 9.5%; p = 0.002) and the amplitude of sIPSCs (percentage control: 58.6 ± 16.8%; p = 0.047, Wilcoxon matched pairs test), but did not affect the rise time (percentage control: 110 ± 12.1; p = 0.50) or τw (percentage control: 100 ± 3.4; p = 0.75) of sIPSCs (Table 1).
For both types of cultured neurons, we used the δ-subunit selective agonist, THIP, to assess the presence of δ-containing GABAA receptors. Application of 1 μm THIP evoked significant inward currents, confirming the surface expression of extrasynaptic δ-GABAA receptors in the cultured cell preparations (CGCs: 22.4 ± 8.7 pA, n = 9; hippocampal neurons: 40.8 ± 5.8 pA, n = 16).
To characterize the effects of 4-PIOL under more physiological conditions, we recorded from CGCs, hippocampal CA1 pyramidal neurons and thalamic relay neurons of the dorsal lateral geniculate nucleus in acute brain slices. These three cell types were selected because their GABAA receptor-mediated tonic currents are thought to be mediated largely by α6βδ, α5βγ, and α4βδ GABAA receptors, respectively (Jones et al., 1997; Brickley et al., 2001; Caraiscos et al., 2004; Cope et al., 2005; Glykys et al., 2008).
Application of bicuculline (20 μm) blocked the sIPSCs (parameters shown in Table 3) and revealed GABAA receptor-mediated tonic currents in all three cell types (Fig. 4A,C,E,G). For CGCs, hippocampal neurons and dLGN relay neurons, the basal tonic current amplitudes were as follows: 9.8 ± 4.3, 19.2 ± 2.7, and 23.8 ± 2.1 pA (Fig. 4G; Table 4).
Table 3.
sIPSC parameters for cerebellar granule, CA1 hippocampal, and dLGN relay neurons in acute slices
| CGCs |
CA1 pyramidal neurons |
dLGN relay neurons |
||||||
|---|---|---|---|---|---|---|---|---|
| Inward 4-PIOL |
Outward 4-PIOL |
|||||||
| Control | +4-PIOL | Control | +4-PIOL | Control | +4-PIOL | Control | +4-PIOL | |
| Frequency, Hz | 1.5 ± 0.3 | 1.1 ± 0.2* | 2.0 ± 0.5 | 1.1 ± 0.3* | 0.6 ± 0.1 | 0.6 ± 0.1 | 11.5 ± 1.6 | 1.5 ± 0.3** |
| Amplitude, pA | 185 ± 36 | 120 ± 14* | 49 ± 13 | 31 ± 4 | 20 ± 2 | 17 ± 2* | 56.4 ± 4.3 | 40.2 ± 2.7* |
| 10–90% rise time, ms | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.2 | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.0 ± 0.2 | ||
| Decay tau, ms | 18.4 ± 3.1 | 18.7 ± 2.5 | 23.9 ± 2.2 | 22.7 ± 3.0 | 21.5 ± 1.6 | 19.7 ± 2.1 | ||
All data are presented as mean ± SEM (n = 4–8). Values for CA1 pyramidal neurons are divided according to direction of 4-PIOL response. Control and 4-PIOL data were compared using a paired t test.
p < 0.05,
p < 0.01.
Figure 4.
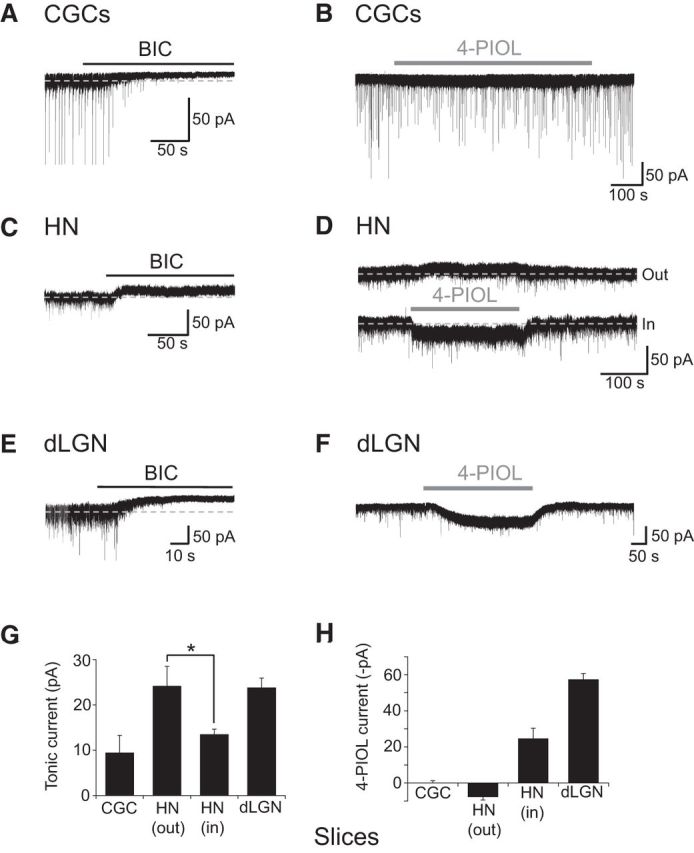
Characterizing GABAA receptor-mediated tonic and 4-PIOL-activated currents recorded from neurons in acute slices. Representative whole-cell currents for CGCs (A, B), CA1 hippocampal neurons (HNs; C, D), or dLGN relay neurons (E, F), showing the effects of 20 μm bicuculline (BIC) and 10 μm 4-PIOL application (indicated by black and gray bars, respectively) on sIPSCs and holding currents. Bicuculline blocks sIPSCs and reveals tonic GABAA currents in all three cell types. 4-PIOL has little effect on holding current in CGCs, induces outward or inward currents in hippocampal neurons, and causes large inward currents in dLGN relay neurons. G, H, Bar charts showing the tonic currents (G) and 4-PIOL-evoked currents (H), for CGCs (n = 9), CA1 hippocampal neurons (n = 7–8), and dLGN relay neurons (n = 42). All recordings were performed at room temperature, in the presence of 2 mm kynurenic acid. The holding potential was −60 mV.
Table 4.
4-PIOL and tonic currents for cerebellar granule, CA1 hippocampal, and dLGN relay neurons in acute slices
| CGCs | CA1 pyramidal neurons |
dLGN relay neurons | ||
|---|---|---|---|---|
| Inward 4-PIOL | Outward 4-PIOL | |||
| 4-PIOL current, pA | 0.05 ± 1.44 | −24.7 ± 5.7** | 7.8 ± 1.6** | −57.4 ± 3.3** |
| (pA/pF) | (0.18 ± 0.38) | (−0.20 ± 0.06) | (0.05 ± 0.01) | (−0.34 ± 0.03) |
| Tonic current, pA | 9.8 ± 4.3* | 13.5 ± 1.1*** | 24.2 ± 4.4*** | 23.8 ± 2.1** |
| (pA/pF) | (2.57 ± 1.76) | (0.10 ± 0.01) | (0.13 ± 0.03) | (0.13 ± 0.01) |
Data shown are the mean currents (±SEM, n = 7–42) evoked by 10 μm 4-PIOL and 20 μm bicuculline (tonic current). According to normal convention, inward and outward currents have negative and positive polarities, respectively. Currents normalized to cell capacitance (pA/pF) are shown in parentheses below the absolute values. Values for CA1 pyramidal neurons are divided according to direction of 4-PIOL response. Statistical significance was assessed using a paired t test.
p < 0.05,
p < 0.01,
p < 0.001.
As with the cultured cells, 10 μm 4-PIOL had little impact on the tonic current recorded from CGCs in slices (change in holding current 0.05 ± 1.44 pA; Fig. 4B,H). However, in contrast to the cultured cells, 4-PIOL significantly inhibited both the frequency (percentage control: 76.7 ± 6.1%; p = 0.006; Table 3) and the amplitude of sIPSCs (percentage control: 71.2 ± 4.2%; p = 0.0002, Wilcoxon matched-pairs test), but did not affect the rise time (percentage control: 101.6 ± 3.3; p = 0.63) or decay τw (percentage control: 105.6 ± 5.2; p = 0.32).
By comparison, in hippocampal CA1 pyramidal neurons, 10 μm 4-PIOL evoked a dichotomous response, with cells either displaying an inward current (−24.7 ± 5.7 pA, n = 7, p = 0.002) or a small, but significant outward current (7.8 ± 1.6 pA, n = 8, p = 0.001; Fig. 4D,H). Interestingly, when we divided cells according to their 4-PIOL response, we found that neurons showing an outward current had a larger basal tonic current than those cells producing an inward 4-PIOL current (outward 24.2 ± 4.4 pA, inward 13.5 ± 1.1 pA, p = 0.04, unpaired t test; Fig. 4G). Dividing cells in this way, also revealed a difference in the modulation of synaptic inhibition by 4-PIOL. For the inward current cohort, 4-PIOL caused a significant reduction in IPSC frequency (percentage control: 54.2 ± 8.1%, p = 0.04), but had no significant effect on synaptic GABA release for the outward current cohort (percentage control: 150.0 ± 43.0%, p = 0.91; Table 3).
Applying 4-PIOL to dLGN slices significantly enhanced tonic currents by 57.4 ± 3.3 pA (Fig. 4F,H), and notably reduced both the frequency (percentage control: 13.3 ± 2.2%; p = 0.001) and amplitude of sIPSCs (percentage control: 71.8 ± 2.7%; p = 0.01; Table 3), relative to synaptic events measured in control aCSF. Because of the low frequency and amplitude of sIPSCs in the presence of 4-PIOL, and the increase in rms baseline noise induced by 4-PIOL (13.2 ± 1.6 pA), no detailed analysis of sIPSC decay or rise times was performed for dLGN relay neurons.
To further investigate the modulation of synaptic inhibition by 4-PIOL, we repeated these experiments in the presence of TTX (500 nm) to block action potential-dependent GABA release. Under these conditions, dLGN relay neurons displayed a smaller basal tonic current compared with aCSF control recordings (TTX: 13.5 ± 1.7 pA; aCSF: 23.8 ± 2.1 pA; p = 0.0005, unpaired t test) consistent with a reduced ambient GABA concentration (Bright et al., 2007). Application of 10 μm 4-PIOL induced a similar increase in tonic current as in aCSF (TTX: 43.2 ± 7.0 pA; aCSF: 57.4 ± 3.3 pA; p = 0.09, unpaired t test) but had less effect on IPSC frequency (percentage control: 76.5 ± 9.3%; p = 0.03) and amplitude (percentage control: 89.7 ± 4.7%; p = 0.04, n = 9).
Probing the identity of GABAA receptors in dLGN relay neurons
According to our recombinant expression studies, 10 μm 4-PIOL showed no discernible agonist activity at α4βδ receptors, and was predicted to reduce GABA-mediated tonic currents at these receptors, assuming that the ambient GABA concentration in slices is ∼ 0.1–1 μm GABA (Fig. 2E). Because tonic currents in dLGN relay neurons are thought to be mediated by α4βδ receptors (Cope et al., 2005; Bright et al., 2007; Nani et al., 2013; Ye et al., 2013), the finding that 4-PIOL showed agonist behavior, by enhancing tonic currents in dLGN relay neurons, was unexpected. To verify the GABAergic origin of this current, we used bicuculline. Current responses to 10 μm 4-PIOL were abolished in the presence of coapplied bicuculline (percentage control 4-PIOL response: 2.2 ± 0.5; Fig. 5A), indicating that 4-PIOL was exclusively activating GABAA receptors to induce an inward current.
Figure 5.
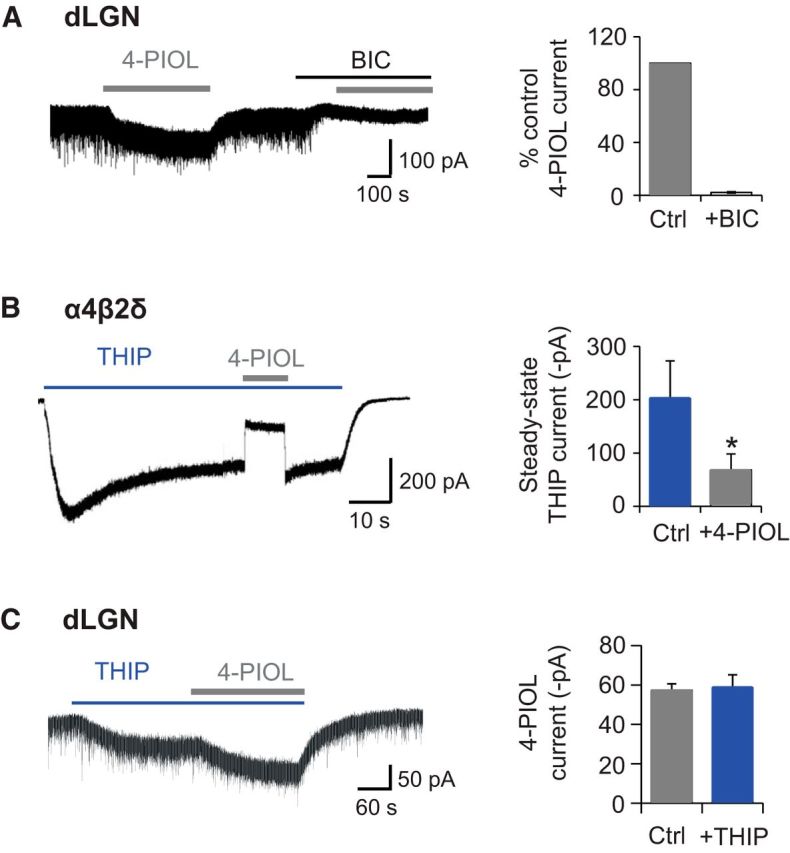
THIP and bicuculline modulation of 4-PIOL currents. A, Representative membrane current recorded from a dLGN relay neuron (left) in response to applied 4-PIOL (10 μm), in the absence or presence of BIC (20 μm). The bar chart (right) depicts the mean ± SEM (n = 4) for the 4-PIOL current in BIC (white) expressed as a percentage of the control 4-PIOL response (gray) recorded from the same dLGN relay neurons. B, Membrane currents recorded from a α4β2δ-expressing HEK293 cell in response to THIP (1 μm, blue bar) and 4-PIOL (10 μm, gray bar). Bar chart shows the steady-state THIP current in the absence or presence of 4-PIOL (n = 4). Significance was assessed using a paired t test,*p < 0.05. C, Membrane currents recorded from a dLGN relay neuron in response to THIP (1 μm) and 4-PIOL (10 μm). Bar chart shows the magnitude of the 4-PIOL current in the absence or presence of THIP (n = 5).
To investigate whether the 4-PIOL current in dLGN relay neurons was mediated by δ-containing GABAA receptors, we assessed the ability of 4-PIOL to compete with a δ subunit-selective concentration of the GABAA receptor agonist, THIP (1 μm; Brown et al., 2002; Stórustovu and Ebert, 2006; Mortensen et al., 2010). If THIP and 4-PIOL compete for the same orthosteric binding site, we might expect the THIP-induced currents to be reduced by 4-PIOL, given the lower efficacy and potency displayed by 4-PIOL. THIP (1 μm) was preapplied to recombinant α4β2δ receptors expressed in HEK293 cells, until a steady-state current was achieved, and subsequently 10 μm 4-PIOL was coapplied with THIP (Fig. 5B). Indeed, under these conditions, 4-PIOL reduced the steady-state THIP current, from 202.3 ± 70.7 to 68.2 ± 30.1 pA (Fig. 5B; p = 0.02), suggesting that 4-PIOL is competing with THIP for the orthosteric binding site.
If 4-PIOL is acting on δ-containing receptors in dLGN relay neurons, we might also expect 4-PIOL (10 μm) to reduce the steady-state THIP (1 μm) current in dLGN relay neurons. As expected, THIP significantly enhanced the dLGN tonic current by 96.6 ± 12.6 pA, confirming the functional expression of δ subunit-containing receptors. However, coapplication of 4-PIOL with THIP generated a further inward current (58.6 ± 6.6 pA; Fig. 5C), with a mean magnitude that was similar to the control 4-PIOL current (57.4 ± 3.3 pA; p = 0.67). These data indicate that THIP and 4-PIOL may not be competing for the same δ subunit-containing receptors in dLGN relay neurons.
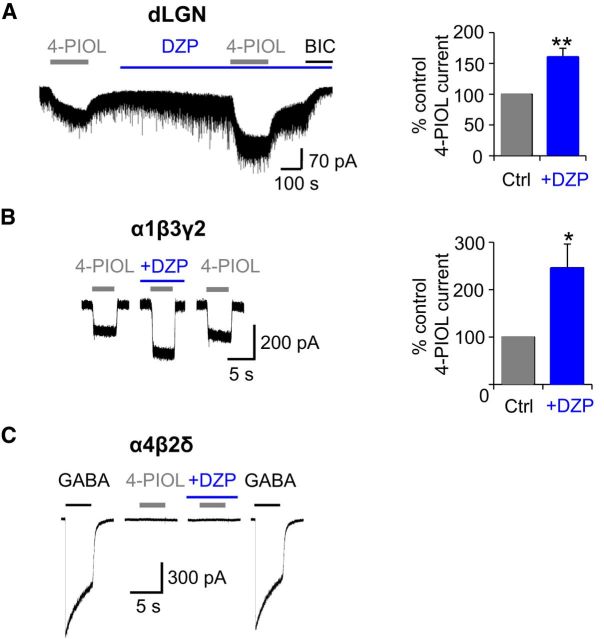
To further probe whether the 4-PIOL current in dLGN relay neurons was mediated by δ-containing receptors, we investigated whether the 4-PIOL current could be modulated by the δ subunit-selective positive allosteric modulator, DS2 (Wafford et al., 2009; Jensen et al., 2013). Whole-cell 10 μm 4-PIOL currents were recorded in the absence or presence of a δ-selective concentration of DS2 (10 μm; Fig. 6A). DS2 alone significantly enhanced dLGN tonic currents (Fig. 6A), giving rise to a bicuculline-sensitive current that was significantly greater (136 ± 23 pA) than that measured in control aCSF (23.8 ± 2.1 pA; p = 0.002). Intriguingly, DS2 also potentiated the 4-PIOL current (percentage control 4-PIOL response: 236.3 ± 28.9; Fig. 6A; p < 0.0001), indicating that the 4-PIOL current in dLGN relay neurons might be mediated by δ-containing receptors.
Figure 6.
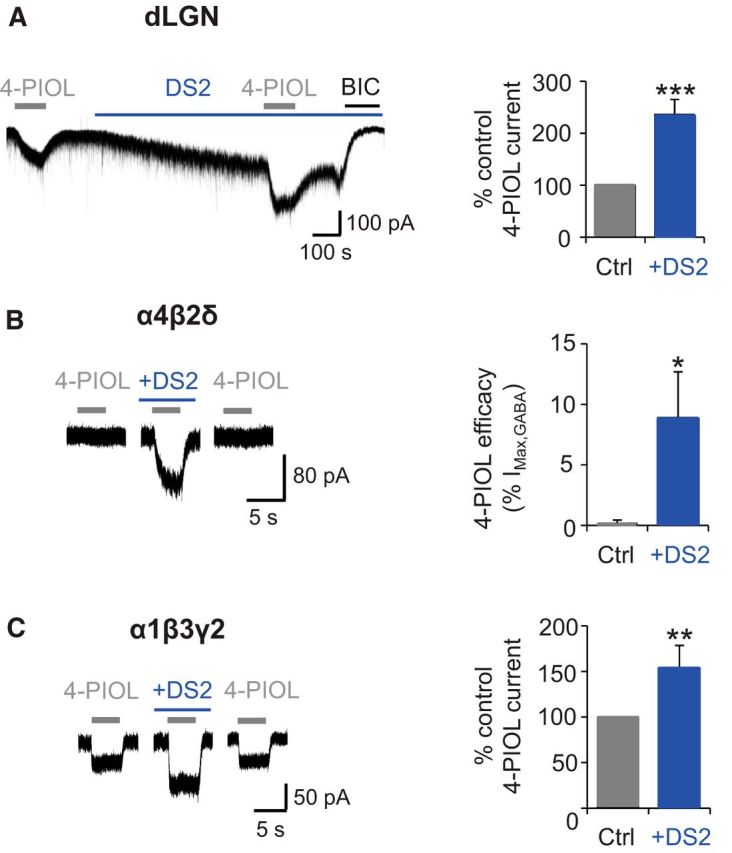
DS2 modulation of dLGN and recombinant receptor 4-PIOL currents. A, Representative membrane currents (left) recorded from a dLGN relay neuron in response to applied 4-PIOL (10 μm) in the absence or presence of DS2 (10 μm, blue bar). BIC (20 μm) was applied at the end of each experiment. Bar graph (right) of the 4-PIOL response in DS2 (blue), expressed as a percentage of the control 4-PIOL response (gray) recorded from the same dLGN relay neurons. B, Example 10 μm 4-PIOL currents (left) for recombinant α4β2δ receptors expressed in HEK293 cells in the absence or presence of DS2 (10 μm). Bar graph (right) illustrates the macroscopic efficacy of 4-PIOL, normalized to the current response evoked by a saturating concentration of GABA (1 mm). C, Example 10 μm 4-PIOL currents (left) recorded from recombinant α1β3γ2 receptors in the absence or presence of DS2 (10 μm). Bar graph (right) shows the mean 4-PIOL response in DS2 (blue), expressed as a percentage of the control 4-PIOL response (gray) recorded from the same α1β3γ2-expressing HEK293 cells. Data are presented as mean ± SEM (n = 5–6). *p < 0.05, **p < 0.01, and ***p < 0.001 from paired t tests.
Previously, the modulatory actions of DS2 have only been characterized on GABA-mediated currents (Wafford et al., 2009; Jensen et al., 2013), and not on responses evoked by other GABAA receptor agonists. To investigate, we monitored the effect of 10 μm DS2 on whole-cell 4-PIOL currents using recombinant α4β2δ (Fig. 6B) and α1β3γ2 (Fig. 6C) receptors, expressed separately in HEK293 cells. Under control conditions, 4-PIOL (10 μm) elicited no discernible agonist response at α4β2δ receptors (Fig. 6B), but induced a small inward current at α1β3γ2 receptors (Fig. 6C). However, coapplying DS2 with 4-PIOL, unveiled an agonist current at α4β2δ receptors, which was 8.9 ± 3.8% of the response to 1 mm GABA in the same cell (Fig. 6B). Unexpectedly, DS2 also potentiated the 4-PIOL current mediated at α1β3γ2 receptors (percentage control 4-PIOL current: 153.8 ± 24.7%; Fig. 6C; p = 0.017), albeit to a lesser extent than that observed at α4β2δ receptors. These findings complicate the interpretation of DS2-mediated potentiation of the 4-PIOL current in dLGN relay neurons, because DS2 may be modulating a population of γ2-containing GABAA receptors, or generating a δ-mediated component to the 4-PIOL current, which may not be present under control conditions.
Because 10 μm 4-PIOL activated γ2-containing receptors, but not δ-containing receptors in our recombinant expression studies, we explored the possibility that 4-PIOL was activating a population of γ2-containing receptors in dLGN relay neurons. The presence of γ2-containing receptors was examined using the benzodiazepine agonist, diazepam. Preapplication of 500 nm diazepam significantly increased the dLGN tonic current, giving rise to a bicuculline-sensitive tonic current (52.0 ± 5.6 pA) that was significantly greater than that measured in control aCSF (23.8 ± 2.1 pA; Fig. 7A; p = 0.003). Diazepam also increased the amplitude of the IPSCs suggesting that GABA release at these thalamic inhibitory synapses was not saturating. Coapplication of 4-PIOL with diazepam revealed a significantly larger inward current than the control 4-PIOL current (percentage control 4-PIOL response: 160.3 ± 14.3%; Fig. 7A; p = 0.003), indicating that a substantial component of the 4-PIOL current is most likely mediated by γ2-containing receptors in dLGN relay neurons.
Figure 7.
Diazepam modulation of dLGN and recombinant receptor 4-PIOL currents. A, Membrane currents (left) recorded from a dLGN relay neuron in response to: 4-PIOL (10 μm) in the absence or presence of diazepam (DZP; 500 nm) and to BIC (20 μm). Bar graph (right) of the 4-PIOL response in DZP (blue), expressed as a percentage of the control 4-PIOL response (gray) recorded from the same relay neurons. B, Example 10 μm 4-PIOL currents (left) for recombinant α1β3γ2 receptors expressed in HEK293 cells in the absence or presence of DZP (500 nm). Bar graph (right) for the mean 4-PIOL response in DZP (blue), expressed as a percentage of the control 4-PIOL response (gray) recorded from the same α1β3γ2-expressing HEK293 cells. C, Whole-cell currents for recombinant α4β2δ receptors in response to: GABA (1000 μm) or 4-PIOL (10 μm) in the absence or presence of 500 nm DZP. Data are presented as mean ± SEM (n = 5–6). *p < 0.05, **p < 0.01, paired t tests.
To confirm that diazepam would only potentiate the agonist responses of γ2 subunit-containing GABAA receptors (Pritchett et al., 1989), whole-cell currents were recorded from recombinant α1β3γ2 (Fig. 7B) or α4β2δ Fig. 7C) receptors in response to brief applications of 4-PIOL (10 μm) in the absence or presence of preapplied diazepam (500 nm). At α1β3γ2 receptors, diazepam significantly potentiated 4-PIOL responses (percentage control response: 246.6 ± 50.1; Fig. 7B; p = 0.03); and, as expected, 4-PIOL (10 μm) elicited no discernible agonist response at α4β2δ receptors, either in the absence or presence of diazepam (Fig. 7C). A saturating concentration of GABA (1 mm) was applied to each α4β2δ-expressing cell, to confirm the functional expression of α4β2δ receptors (Fig. 7C).
Together, these data indicate that although δ subunit-containing receptors are expressed in dLGN relay neurons, as confirmed by THIP and DS2 modulation of basal tonic currents, the 4-PIOL current appears to be largely mediated by γ2-containing receptors, with little, or no, contribution from δ subunit-containing receptors.
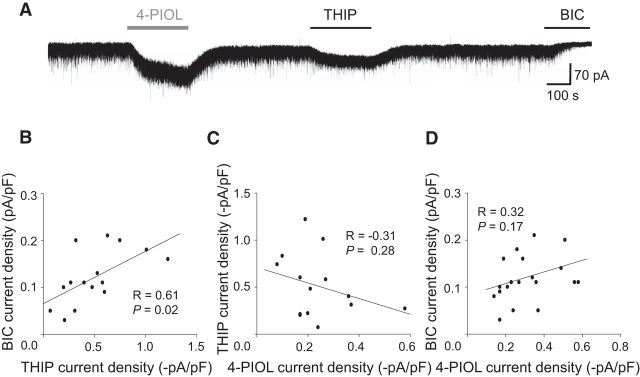
Given these findings, we explored which GABAA receptor isoforms underpin tonic currents in dLGN relay neurons. Since tonic currents in these cells are thought to be mediated by δ subunit-containing receptors (Cope et al., 2005; Bright et al., 2007; Nani et al., 2013; Ye et al., 2013), we investigated whether there was a correlation between currents induced by a δ-selective concentration of THIP (1 μm) and by bicuculline, for individual dLGN relay neurons. 4-PIOL and bicuculline were individually applied (Fig. 8A), and to account for variation in cell size, the holding currents were normalized to whole-cell capacitance (pF). A scatter plot comparing current densities revealed a positive correlation (Fig. 8B; r = 0.61; p = 0.02). Thus, cells with a larger THIP-induced current also displayed larger GABAA receptor-mediated tonic currents. These data indicate that a higher expression of δ subunit-containing receptors may underlie the larger tonic currents, although other factors, such as the ambient GABA concentration, will also be important.
Figure 8.
Correlating THIP, BIC, and 4-PIOL currents in dLGN neurons. A, Whole-cell currents recorded from a dLGN relay neuron in response to THIP (1 μm), 4-PIOL (10 μm), and BIC (20 μm). Scatter plots of: BIC current density versus the THIP current density (B); THIP current density versus 4-PIOL current density (C); and BIC current density versus 4-PIOL current density (D). Linear regression analyses are shown by the black lines, and R and P report Pearson's correlation coefficient and p values, respectively.
If, as our previous data indicates, THIP and 4-PIOL are acting at potentially different GABAA receptors (Fig. 5), no positive correlation would be expected between the THIP and 4-PIOL responses in dLGN relay neurons. Indeed, a scatterplot of THIP and 4-PIOL currents revealed no significant correlation (Pearson's correlation coefficient, r = −0.31; p = 0.28), supporting the notion that 4-PIOL was activating a distinct receptor population from the δ-containing receptors activated by THIP (Fig. 8C).
Because 4-PIOL and THIP are likely to be acting on different GABAA receptors in dLGN relay neurons, it was intriguing to explore whether the γ2-containing receptors that mediate the 4-PIOL current, also contribute to dLGN tonic currents. A scatterplot comparing 4-PIOL and bicuculline currents recorded from individual dLGN relay neurons showed poor correlation (Fig. 8D; r = 0.32; p = 0.17), indicating that the receptors that mediate the 4-PIOL current, are unlikely to contribute substantially to basal GABAA receptor-mediated tonic currents in dLGN relay neurons.
Modulation of tonic currents depends on ambient GABA levels
For recombinant α1β3γ2 receptors, 4-PIOL enhanced the steady-state GABA current when the GABA concentration was low (∼0.1 μm GABA), but produced a small inhibition when the ambient GABA level was raised to 1 μm (Fig. 2D). In dLGN relay neurons, the robust 4-PIOL enhancement of baseline tonic currents (mainly via γ2 subunit-containing receptors) indicates that the ambient GABA levels in this slice may be low (<1 μm).
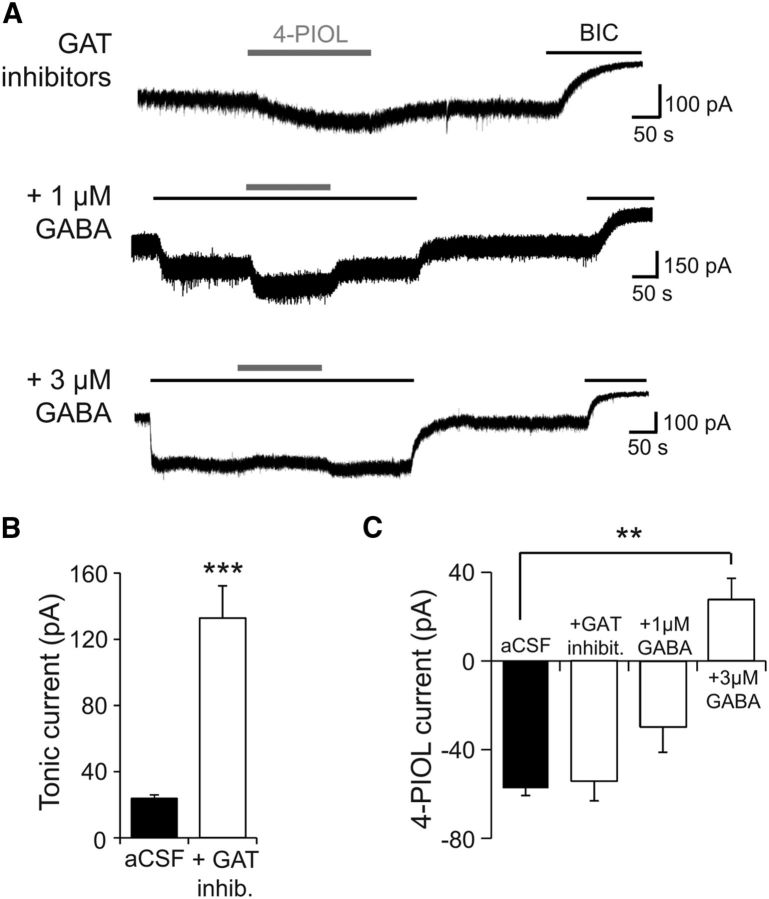
To determine whether 4-PIOL could switch from acting as an agonist (at low ambient GABA levels), to acting as an antagonist (at higher ambient GABA levels) in the native environment of dLGN relay neurons, GABA levels were raised in slices by inhibiting GABA uptake. Because GABA uptake in the thalamus is mediated by the GABA transporters, GAT1 and GAT3 (De Biasi et al., 1998), slices were preincubated (for 30 min) and subsequently recorded in aCSF supplemented with the GAT1 inhibitor, 10 μm NNC-711 (Borden et al., 1994) and the GAT2/3 inhibitor, 20 μm SNAP-5114 (Borden, 1996). Following a period of control recording (in the presence of the GAT inhibitors), 10 μm 4-PIOL was applied to dLGN relay neurons and subsequently washed out (Fig. 9A), before application of bicuculline to measure the GABA-mediated tonic current. As expected, the tonic current was significantly larger in the presence of the GAT blockers compared with control aCSF (132 ± 19.5 and 23.8 ± 2.1 pA, respectively; Fig. 9B), even when these currents were normalized to cell capacitance (0.7 ± 0.1 pA/pF and 0.1 ± 0.01 pA/pF, respectively; p < 0.0001). These data are consistent with elevated ambient GABA levels, in GAT-blocked slices, increasing the activation of extrasynaptic GABAA receptors.
Figure 9.
4-PIOL modulation of dLGN tonic currents depends on ambient GABA levels. A, Membrane currents recorded from dLGN relay neurons in response to 4-PIOL (10 μm), coapplied with GAT inhibitors (10 μm NNC-711 and 20 μm SNAP-5114), or GABA (1 or 3 μm) in the presence of GAT inhibitors. Slices were incubated in aCSF supplemented with GAT inhibitors for at least 30 min before electrophysiological recordings. All recordings were performed at room temperature. B, Bar graph of BIC current in control aCSF (black; n = 30), or in the presence of GAT inhibitors (white; n = 25). C, Bar graph of the mean 4-PIOL current measured in aCSF (black; n = 42), + GAT inhibitors (n = 8), or + GAT inhibitors with 1 μm (n = 11) or 3 μm GABA (n = 4). Data are presented as mean ± SEM. **p < 0.01, ***p < 0.001, unpaired t tests.
Coapplication of 10 μm 4-PIOL with GAT inhibitors enhanced the tonic current by 53.0 ± 10.1 pA (Fig. 9A,C). This 4-PIOL-induced current was similar to that observed in control aCSF (57.4 ± 3.3 pA; Fig. 9C; p = 0.47), and indicates that under control conditions, 4-PIOL was not a substrate for GAT1–3. Although the increased tonic current indicated that the ambient GABA level in the slice was raised, it may still be too low to alter the response profile for 4-PIOL. To further increase and normalize ambient GABA levels, 1 and 3 μm GABA were individually applied to GAT-inhibited slices, followed by coapplication with 10 μm 4-PIOL (Fig. 9A,C). Both 1 and 3 μm GABA enhanced the baseline tonic current by 139 ± 21.8 and 301 ± 35.2 pA, respectively (Fig. 9C). Coapplication of 10 μm 4-PIOL with 1 μm GABA also elicited an inward current (Fig. 9A), although the resultant 4-PIOL current was significantly smaller than that observed in control aCSF (29.6 ± 11.5 pA; Fig. 9C). By contrast, coapplying 10 μm 4-PIOL with 3 μm GABA produced an outward current (27.8 ± 9.5.1 pA; Fig. 9A). Thus, as observed for recombinant α1β3γ2 receptors, 4-PIOL exhibited a dominant agonist profile at low GABA concentrations (≤1 μm), but produced a small inhibition of dLGN tonic currents when the ambient GABA concentration was increased.
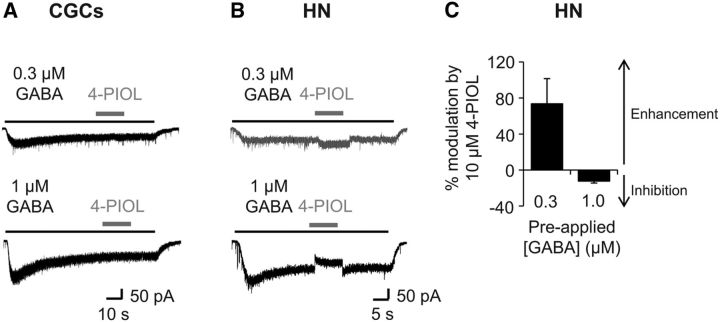
To determine whether ambient GABA levels also influence the 4-PIOL current in cultured CGCs and hippocampal neurons, low concentrations of GABA (0.3 and 1 μm) were preapplied until steady-state currents were achieved and subsequently, 4-PIOL was coapplied (Fig. 10A,B). Similar to its effects on endogenous CGC tonic currents, coapplication of 4-PIOL produced no significant shift in the holding current, even when the preapplied GABA concentration was raised to 1 μm (Fig. 10A).
Figure 10.
4-PIOL modulation of CGC and hippocampal neurons, at varying ambient GABA concentrations. Whole-cell currents induced by 0.3 or 1 μm GABA (black bars) with 4-PIOL (10 μm) coapplied once a steady-state GABA current was attained (gray bars) for CGCs (A) and hippocampal neurons (HNs; B). C, Bar graph showing the mean ± SEM percentage modulation induced by 10 μm 4-PIOL (n = 6). Note that positive and negative values, respectively, represent an enhancement or inhibition of the steady-state GABA current.
For hippocampal neurons, coapplication of 10 μm 4-PIOL with 0.3 μm GABA significantly enhanced the tonic current (Fig. 10B) by 62.8 ± 9.9 pA (p = 0.03, Wilcoxon matched-pairs test), similar to the enhancement observed under control conditions (72.0 ± 14.2 pA; p = 0.90). However, when the preapplied GABA concentration was raised to 1 μm GABA, 10 μm 4-PIOL significantly reduced the steady-state 1 μm GABA current (Fig. 10B), by 52.5 ± 7.1 pA (p = 0.0004, paired t test). This corresponded to a 73.6 ± 28.1% enhancement of the steady-state 0.3 μm GABA current, and a 12.4 ± 2.0% inhibition of the 1 μm GABA current (Fig. 10C). Thus, the modulation of tonic currents by low-efficacy partial agonists, such as 4-PIOL, appears to be highly dependent on ambient GABA levels.
Discussion
GABAA receptor-mediated tonic inhibition is an important regulator of cell and network excitability (Mann and Mody, 2010), and its dysfunction is associated with several pathophysiological states (Belelli et al., 2009; Brickley and Mody, 2012). Selectively modulating the activity of extrasynaptic GABAA receptors may therefore be therapeutically useful for the treatment of such disorders. Given the absence of suitable subtype-selective antagonists, we took a different approach by investigating the selectivity profile of the weak partial agonist, 4-PIOL (Mortensen et al., 2002, 2004), to determine whether it could be used as a selective modulator of GABA-mediated tonic currents.
Partial agonist modulation of GABA synaptic currents
Our receptor expression studies predicted that at high GABA (synaptic) concentrations, 4-PIOL should not affect α1β3γ2-mediated currents. However, 4-PIOL variably reduced sIPSC amplitudes and frequencies in many of our neuronal preparations. Using TTX in relay neurons revealed that 4-PIOL reduced mIPSCs to a lesser extent than sIPSCs, which is in accord with both presynaptic and postsynaptic effects. The reduced sIPSC frequency is indicative of a presynaptic action, with 4-PIOL activating extrasynaptic γ2-GABAA receptors, reducing interneuron excitability and lowering GABA release (Axmacher and Draguhn, 2004). This is supported by the bidirectional 4-PIOL responses of CA1 neurons. Cells with inward 4-PIOL currents show reduced sIPSC frequencies, consistent with inhibition of presynaptic interneurons by 4-PIOL, whereas cells with outward currents show no effect on IPSC frequency, reflecting unaffected interneuron excitability. This suggests that 4-PIOL has congruent effects on extrasynaptic γ2-GABAA receptors on presynaptic interneurons and postsynaptic pyramidal neurons, exposed to similar GABA levels.
The residual block of mIPSCs would suggest a small inhibition of postsynaptic receptors. Although this was not resolved in recombinant α1β3γ2 receptors, native GABAA receptors might display a greater sensitivity to the actions of 4-PIOL, either due to endogenous factors (eg, receptor phosphorylation states), or the presence of distinct receptor subunit compositions (eg, α2 or α3βγ2 receptors).
Modulation of tonic currents depends on GABAA receptor subunit composition
As expected for a partial agonist, 4-PIOL exhibited both agonist- and antagonist-type behaviors at recombinant and native GABAA receptors. However, the direction of modulation depended on two critical factors: GABAA receptor composition; and critically, the ambient GABA concentration.
GABAA receptor-mediated tonic currents in CGCs, which are largely mediated by α6βδ receptors (Fritschy et al., 1992; Somogyi et al., 1996; Jones et al., 1997; Nusser et al., 1998; Brickley et al., 2001), were unaffected by 4-PIOL. This was unsurprising, given that 4-PIOL alone produced little if any modulation of steady-state GABA currents at recombinant α6β2δ receptors. By comparison, 4-PIOL bidirectionally modulated tonic currents in hippocampal neurons, which will express an array of GABAA receptor isoforms, including α5βγ2, α4βδ, and αβ (Mangan et al., 2005; Mortensen and Smart, 2006; Glykys et al., 2008). This might be expected, given that α5βγ2 receptors are important for tonic currents in these cells (Caraiscos et al., 2004; Glykys et al., 2008), and 4-PIOL had a bidirectional effect on GABA currents at recombinant α5βγ2 receptors. However, other α1–3βγ2 receptor isoforms may also mediate this modulation, because tonic currents, and the 4-PIOL current itself, were previously shown to be positively modulated by benzodiazepine agonists in hippocampal neurons (Kristiansen et al., 1995; Liang et al., 2004). Interestingly, 4-PIOL generated both inward and outward currents in CA1 neurons under control conditions, suggesting that the ambient GABA concentration in this slice is close to the threshold where 4-PIOL switches from agonist to antagonist behavior.
Extrasynaptic GABAA receptor isoforms in thalamic relay neurons
In relay neurons, the 4-PIOL-induced enhancement of tonic currents was unexpected, because α4β2δ receptors are thought to underlie tonic currents in these cells (Cope et al., 2005; Bright et al., 2007; Nani et al., 2013; Ye et al., 2013), and 4-PIOL potently inhibited the steady-state GABA currents of recombinant α4β2δ receptors (by ∼40–80%, when the ambient GABA concentration was varied between 0.1 and 1 μm). However, several observations suggest the 4-PIOL current in relay neurons was probably not mediated by δ-containing receptors. First, despite being able to compete with THIP for the orthosteric binding site at recombinant α4β2δ receptors, 4-PIOL and THIP appeared not to compete for the same δ subunit-containing receptors in dLGN relay neurons. Next, the ability of 4-PIOL to bidirectionally modulate tonic currents, strongly depended on the ambient GABA concentration, recapitulating its actions at recombinant α1βγ2 receptors, but not α4β2δ receptors. Third, the 4-PIOL current in dLGN relay neurons was potentiated by the benzodiazepine, diazepam, strongly implying an action at γ2 subunit-containing receptors.
To counter this, the 4-PIOL current in dLGN relay neurons was potentiated by the δ subunit-selective modulator DS2 (Wafford et al., 2009; Jensen et al., 2013). It was notable that this modulator unveiled a previously undetected 4-PIOL current at recombinant α4β2δ receptors and thus DS2 may act similarly at α4β2δ receptors in dLGN, by producing a δ-mediated component to the 4-PIOL current, which is absent under control conditions. However, DS2 also potentiated the 4-PIOL current at recombinant α1β3γ2 receptors, bringing into question its isoform selectivity. DS2 modulation of α1β3γ2-GABA currents has been seen previously, and significantly, a small residual DS2-induced current was apparent in thalamic relay neurons from δ subunit knock-out mice (Jensen et al., 2013). Thus, DS2 will modulate γ2-containing receptors, albeit to a lesser extent than δ-containing counterparts, and so may also be potentiating the 4-PIOL current at γ2-GABAA receptors in relay neurons.
Overall, the simplest explanation for these data are that functional effects of 4-PIOL on tonic currents in dLGN relay and hippocampal neurons, are largely dominated by its actions on γ2 subunit-containing receptors, though we cannot completely exclude a contribution from δ subunit-containing receptors. Although the significant presence of extrasynaptic γ2 subunit-containing receptors on dLGN relay neurons was unexpected, given that they are thought to accumulate at synaptic sites, immunohistochemical and functional studies indicate that a significant number of α1–α3 subunits, which associate with γ2 subunits, may also exist at extrasynaptic sites (Soltesz et al., 1990; Nusser et al., 1998; Mangan et al., 2005; Thomas et al., 2005; Kasugai et al., 2010). Although their functional significance remains to be established in native systems, a recent study has proposed that tonically active α1β3γ2 receptors might contribute to the clinical actions of positive allosteric modulators, such as etomidate and propofol (Li and Akk, 2015). Thus, the relative expression levels of extrasynaptic δ- and γ2-containing receptors may be important in determining the functional effects of compounds, such as 4-PIOL, which can modulate both receptor isoforms under distinct conditions.
Low ambient GABA levels in neuronal preparations
Under our experimental conditions, the ambient GABA concentration in all three neuronal preparations was estimated to be significantly <1 μm. In accord with these findings, although difficult to measure precisely in the structurally tortuous environment of the brain, microdialysis studies estimate that extracellular GABA concentrations in vivo range from 30 nm to 2.9 μm (Glaeser and Hare, 1975; Lerma et al., 1986; de Groote and Linthorst, 2007; Wlodarczyk et al., 2013), whereas the activity of GABA transporters predicts that ambient GABA levels are within 0.1–0.4 μm (Attwell et al., 1993; Richerson and Wu, 2003; Wu et al., 2007).
Given the low ambient GABA levels that we estimate in slices, and the low GABA sensitivity of γ2 subunit-containing GABAA receptors (Brown et al., 2002; Mortensen et al., 2010, 2011), it is unsurprising that the extrasynaptic population of γ2-containing receptors, detected in dLGN relay neurons, did not significantly contribute to basal tonic currents under our experimental conditions. However, this does not discount the possibility that γ2-containing receptors may contribute to tonic currents when ambient GABA levels are significantly increased, for instance, during behavioral or pathophysiological disease states. Although applying diazepam to dLGN relay neurons enhanced dLGN tonic currents, demonstrating that γ2-containing receptors can contribute to tonic currents, it is difficult to discount the possibility that diazepam may have increased the apparent affinity of these receptors for GABA (Gielen et al., 2012), thus recruiting a population of extrasynaptic γ2-containing receptors that may be inactive under control conditions.
The observation that ambient GABA levels strongly influence the functional profile of 4-PIOL is unsurprising given that both 4-PIOL and GABA act via the same binding site. Indeed, similar observations have been made for the agonist, THIP, whose enhancement of δ-mediated tonic currents in CGCs was attenuated at higher ambient GABA concentrations (Houston et al., 2012). Thus, when evaluating the potential effects of therapeutic compounds on tonic currents, an important consideration is how this modulation will be affected by variable ambient GABA concentrations.
Therapeutic potential of low-efficacy agonists
Overall, the therapeutic potential of a low-efficacy partial agonist, such as 4-PIOL, has merit, but its action will critically depend on a number of factors, including which GABAA receptor isoforms are expressed, their expression levels on the cell surface, and ambient GABA levels. Given its enhancement of tonic currents at low ambient GABA concentrations, a weak partial agonist like 4-PIOL might be most useful for neurological conditions where an increased tonic inhibition is desirable, for example in Fragile X syndrome and sleep disorders (Brickley and Mody, 2012; Whissell et al., 2015).
Footnotes
This work was supported by the Medical Research Council, and B.P. was supported by an MRC PhD studentship.
The authors declare no competing financial interests.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License Creative Commons Attribution 4.0 International, which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed.
References
- Atack JR. Preclinical and clinical pharmacology of the GABAA receptor α5 subtype-selective inverse agonist α5IA. Pharmacol Ther. 2010;125:11–26. doi: 10.1016/j.pharmthera.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for α5-containing GABAA receptors. Neuropharmacology. 2006;51:1023–1029. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-H. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Draguhn A. Inhibition of GABA release by presynaptic ionotropic GABA receptors in hippocampal CA3. Neuroreport. 2004;15:329–334. doi: 10.1097/00001756-200402090-00024. [DOI] [PubMed] [Google Scholar]
- Ballard TM, Knoflach F, Prinssen E, Borroni E, Vivian JA, Basile J, Gasser R, Moreau JL, Wettstein JG, Buettelmann B, Knust H, Thomas AW, Trube G, Hernandez MC. RO4938581, a novel cognitive enhancer acting at GABAA α5 subunit-containing receptors. Psychopharmacology (Berl) 2009;202:207–223. doi: 10.1007/s00213-008-1357-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddum K, Frølund B, Kristiansen U. The GABAA antagonist DPP-4-PIOL selectively antagonises tonic over phasic GABAergic currents in dentate gyrus granule cells. Neurochem Res. 2014;39:2078–2084. doi: 10.1007/s11064-014-1397-9. [DOI] [PubMed] [Google Scholar]
- Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol. 1994;269:219–224. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Braudeau J, Delatour B, Duchon A, Pereira PL, Dauphinot L, de Chaumont F, Olivo-Marin JC, Dodd RH, Hérault Y, Potier MC. Specific targeting of the GABAA receptor α5 subtype by a selective inverse agonist restores cognitive deficits in Down syndrome mice. J Psychopharmacol. 2011;25:1030–1042. doi: 10.1177/0269881111405366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABAA receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Bright DP, Aller MI, Brickley SG. Synaptic release generates a tonic GABAA receptor-mediated conductance that modulates burst precision in thalamic relay neurons. J Neurosci. 2007;27:2560–2569. doi: 10.1523/JNEUROSCI.5100-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Di Giovanni G, Fyson SJ, Orbán G, Errington AC, Lorincz ML, Gould TM, Carter DA, Crunelli V. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med. 2009;15:1392–1398. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi S, Vitellaro-Zuccarello L, Brecha NC. Immunoreactivity for the GABA transporter-1 and GABA transporter-3 is restricted to astrocytes in the rat thalamus: a light and electron-microscopic immunolocalization. Neuroscience. 1998;83:815–828. doi: 10.1016/S0306-4522(97)00414-4. [DOI] [PubMed] [Google Scholar]
- de Groote L, Linthorst AC. Exposure to novelty and forced swimming evoke stressor-dependent changes in extracellular GABA in the rat hippocampus. Neuroscience. 2007;148:794–805. doi: 10.1016/j.neuroscience.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Möhler H. Five subtypes of type A gamma-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci U S A. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frølund B, Kristiansen U, Brehm L, Hansen AB, Krogsgaard-Larsen P, Falch E. Partial GABAA receptor agonists: synthesis and in vitro pharmacology of a series of nonannulated analogs of 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridin-3-ol. J Med Chem. 1995;38:3287–3296. doi: 10.1021/jm00017a014. [DOI] [PubMed] [Google Scholar]
- Gielen MC, Lumb MJ, Smart TG. Benzodiazepines modulate GABAA receptors by regulating the preactivation step after GABA binding. J Neurosci. 2012;32:5707–5715. doi: 10.1523/JNEUROSCI.5663-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser BS, Hare TA. Measurement of GABA in human cerebrospinal fluid. Biochem Med. 1975;12:274–282. doi: 10.1016/0006-2944(75)90129-5. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoestgaard-Jensen K, Dalby NO, Wolinsky TD, Murphey C, Jones KA, Rottländer M, Frederiksen K, Watson WP, Jensen K, Ebert B. Pharmacological characterization of a novel positive modulator at α4β3δ-containing extrasynaptic GABAA receptors. Neuropharmacology. 2010;58:702–711. doi: 10.1016/j.neuropharm.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Hoestgaard-Jensen K, O'Connor RM, Dalby NO, Simonsen C, Finger BC, Golubeva A, Hammer H, Bergmann ML, Kristiansen U, Krogsgaard-Larsen P, Bräuner-Osborne H, Ebert B, Frølund B, Cryan JF, Jensen AA. The orthosteric GABAA receptor ligand thio-4-PIOL displays distinctly different functional properties at synaptic and extrasynaptic receptors. Br J Pharmacol. 2013;170:919–932. doi: 10.1111/bph.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston CM, Smart TG. CaMK-II modulation of GABAA receptors expressed in HEK293, NG108–15 and rat cerebellar granule neurons. Eur J Neurosci. 2006;24:2504–2514. doi: 10.1111/j.1460-9568.2006.05145.x. [DOI] [PubMed] [Google Scholar]
- Houston CM, McGee TP, Mackenzie G, Troyano-Cuturi K, Rodriguez PM, Kutsarova E, Diamanti E, Hosie AM, Franks NP, Brickley SG. Are extrasynaptic GABAA receptors important targets for sedative/hypnotic drugs? J Neurosci. 2012;32:3887–3897. doi: 10.1523/JNEUROSCI.5406-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ML, Wafford KA, Brown AR, Belelli D, Lambert JJ, Mirza NR. A study of subunit selectivity, mechanism and site of action of the δ selective compound 2 (DS2) at human recombinant and rodent native GABAA receptors. Br J Pharmacol. 2013;168:1118–1132. doi: 10.1111/bph.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, Bae JY, Kim T, Lee J, Chun H, Park HJ, Lee da Y, Hong J, Kim HY, Oh SJ, Park SJ, Lee H, Yoon BE, Kim Y, Jeong Y, et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer's disease. Nat Med. 2014;20:886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Mäkelä R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJ, Wisden W. Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Kasugai Y, Swinny JD, Roberts JD, Dalezios Y, Fukazawa Y, Sieghart W, Shigemoto R, Somogyi P. Quantitative localisation of synaptic and extrasynaptic GABAA receptor subunits on hippocampal pyramidal cells by freeze-fracture replica immunolabelling. Eur J Neurosci. 2010;32:1868–1888. doi: 10.1111/j.1460-9568.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen U, Lambert JD, Falch E, Krogsgaard-Larsen P. Electrophysiological studies of the GABAA receptor ligand, 4-PIOL, on cultured hippocampal neurones. Br J Pharmacol. 1991;104:85–90. doi: 10.1111/j.1476-5381.1991.tb12389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen U, Barker JL, Serafini R. The low efficacy gamma-aminobutyric acid type A agonist 5-(4-piperidyl)isoxazol-3-ol opens brief Cl− channels in embryonic rat olfactory bulb neurons. Mol Pharmacol. 1995;48:268–279. [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Frølund B, Liljefors T. Specific GABAA agonists and partial agonists. Chem Rec. 2002;2:419–430. doi: 10.1002/tcr.10040. [DOI] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martín del Río R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus: a method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther. 2004;310:1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- Li P, Akk G. Synaptic-type α1β2γ2L GABAA receptors produce large persistent currents in the presence of ambient GABA and anesthetic drugs. Mol Pharmacol. 2015;87:776–781. doi: 10.1124/mol.114.096453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maconochie DJ, Zempel JM, Steinbach JH. How quickly can GABAA receptors open? Neuron. 1994;12:61–71. doi: 10.1016/0896-6273(94)90152-X. [DOI] [PubMed] [Google Scholar]
- Mangan PS, Sun C, Carpenter M, Goodkin HP, Sieghart W, Kapur J. Cultured hippocampal pyramidal neurons express two kinds of GABAA receptors. Mol Pharmacol. 2005;67:775–788. doi: 10.1124/mol.104.007385. [DOI] [PubMed] [Google Scholar]
- Mann EO, Mody I. Control of hippocampal gamma oscillation frequency by tonic inhibition and excitation of interneurons. Nat Neurosci. 2010;13:205–212. doi: 10.1038/nn.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cué C, Martínez P, Rueda N, Vidal R, García S, Vidal V, Corrales A, Montero JA, Pazos Á, Flórez J, Gasser R, Thomas AW, Honer M, Knoflach F, Trejo JL, Wettstein JG, Hernández MC. Reducing GABAA α5 receptor-mediated inhibition rescues functional and neuromorphological deficits in a mouse model of down syndrome. J Neurosci. 2013;33:3953–3966. doi: 10.1523/JNEUROSCI.1203-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic αβ subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Single-channel recording of ligand-gated ion channels. Nat Protoc. 2007;2:2826–2841. doi: 10.1038/nprot.2007.403. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Frølund B, Jørgensen AT, Liljefors T, Krogsgaard-Larsen P, Ebert B. Activity of novel 4-PIOL analogues at human α1β2γ2S GABAA receptors- correlation with hydrophobicity. Eur J Pharmacol. 2002;451:125–132. doi: 10.1016/S0014-2999(02)02271-9. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Kristiansen U, Ebert B, Frølund B, Krogsgaard-Larsen P, Smart TG. Activation of single heteromeric GABAA receptor ion channels by full and partial agonists. J Physiol. 2004;557:389–413. doi: 10.1113/jphysiol.2003.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Ebert B, Wafford K, Smart TG. Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J Physiol. 2010;588:1251–1268. doi: 10.1113/jphysiol.2009.182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Patel B, Smart TG. GABA potency at GABAA receptors found in synaptic and extrasynaptic zones. Front Cell Neurosci. 2011;6:1. doi: 10.3389/fncel.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nani F, Bright DP, Revilla-Sanchez R, Tretter V, Moss SJ, Smart TG. Tyrosine phosphorylation of GABAA receptor γ2-subunit regulates tonic and phasic inhibition in the thalamus. J Neurosci. 2013;33:12718–12727. doi: 10.1523/JNEUROSCI.0388-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro JF, Burón E, Martín-López M. Anxiogenic-like activity of L-655,708, a selective ligand for the benzodiazepine site of GABAA receptors which contain the α5 subunit, in the elevated plus-maze test. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1389–1392. doi: 10.1016/S0278-5846(02)00305-6. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol. 2003;90:1363–1374. doi: 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABAA receptor subtypes: therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu Rev Pharmacol Toxicol. 2014;54:483–507. doi: 10.1146/annurev-pharmtox-011613-135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG. GABAA receptors. In: Zheng J, Trudeau MC, editors. Handbook of ion channels. Boca Raton, FL: CRC; 2015. pp. 345–359. [Google Scholar]
- Soltesz I, Roberts JD, Takagi H, Richards JG, Mohler H, Somogyi P. Synaptic and nonsynaptic localization of benzodiazepine/GABAA receptor/Cl− channel complex using monoclonal antibodies in the dorsal lateral geniculate nucleus of the cat. Eur J Neurosci. 1990;2:414–429. doi: 10.1111/j.1460-9568.1990.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Fritschy JM, Benke D, Roberts JD, Sieghart W. The γ2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the α1 and β2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology. 1996;35:1425–1444. doi: 10.1016/S0028-3908(96)00086-X. [DOI] [PubMed] [Google Scholar]
- Stórustovu SI, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Thomas P, Mortensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat Neurosci. 2005;8:889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. Novel compounds selectively enhance δ subunit containing GABAA receptors and increase tonic currents in thalamus. Neuropharmacology. 2009;56:182–189. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Whissell PD, Lecker I, Wang DS, Yu J, Orser BA. Altered expression of δ GABAA receptors in health and disease. Neuropharmacology. 2015;88:24–35. doi: 10.1016/j.neuropharm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk AI, Sylantyev S, Herd MB, Kersanté F, Lambert JJ, Rusakov DA, Linthorst AC, Semyanov A, Belelli D, Pavlov I, Walker MC. GABA-independent GABAA receptor openings maintain tonic currents. J Neurosci. 2013;33:3905–3914. doi: 10.1523/JNEUROSCI.4193-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JR, Moss SJ, Smart TG. Pharmacological and physiological characterization of murine homomeric β3 GABAA receptors. Eur J Neurosci. 1997;9:2225–2235. doi: 10.1111/j.1460-9568.1997.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Díez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56:851–865. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Guo Z, Gearing M, Chen G. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer's disease model. Nat Commun. 2014;5:4159. doi: 10.1038/ncomms5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, McGee TP, Houston CM, Brickley SG. The contribution of δ subunit-containing GABAA receptors to phasic and tonic conductance changes in cerebellum, thalamus and neocortex. Front Neural Circuits. 2013;7:203. doi: 10.3389/fncir.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]