Abstract
Changes in thyroid status are associated with profound alterations in biochemical and physiological functioning of cardiac muscle, although its impact on cardiac energy metabolism is still debated. Similarities between the changes in cardiac gene expression in pathological hypertrophy leading to heart failure, and hypothyroidism prompted scientists to suggest a role for thyroid hormone status in the development of metabolic and functional alterations in this disease. We thus investigated the effects of hypothyroidism on cardiac energy metabolism.
Hypothyroid state (HYPO) was induced by thyroidectomy and propyl-thio-uracyl in male rats for three weeks. We examined the effects of hypothyroid state on oxidative capacity and mitochondrial substrate utilization by measuring oxygen consumption of saponin permeabilized cardiac fibers, mitochondrial biogenesis by RT-PCR and energy metabolism and energy transfer enzymes by spectrophotometry. The results show that maximal oxidative capacity of the myocardium was decreased from 24.9±0.9 in control (CT) to 19.3±0.7 μmole O2/min/g dw in HYPO. However, protein content and mRNA of PGC-1α and mRNA of its transcription cascade that is thought to control mitochondrial content in normal myocardium and heart failure, were unchanged in HYPO. Mitochondrial utilization of glycerol-3P (−70%), malate (−45%) and octanoate (−24%) but not pyruvate was decreased in HYPO. Moreover, the creatine kinase system and energy transfer were hardly affected in HYPO. Besides, hypothyroidism decreased the activation of other signaling pathways like p38 MAPK, AMPK and calcineurin. These results show that cellular hypothyroidism can hardly account for the specific energetic alterations of HF.
INTRODUCTION
Thyroid hormone and energy metabolism
It is generally accepted that changes in thyroid status are associated with profound alterations in biochemical and physiological functioning of cardiac muscle impacting heart rate, contractility and cardiac mass. Extensive work has been done to delineate the effect of thyroid hormones (TH) on proteins responsible for calcium homeostasis and contraction (reviewed in [22,25]). In addition, animals given TH have increased oxygen consumption and mitochondrial ATP production rate, markedly increased blood flow, and increased oxygen extraction [12,47], suggesting a marked effect on cardiac metabolism. TH can control mammalian mitochondrial biogenesis through direct and indirect pathways. The direct pathway involves expression of nuclear-encoded mitochondrial proteins through thyroid hormone receptors (TR a and β) and their recognition sites TREs on nuclear target genes and activation of the mitochondrial genome via a truncated form of TRα called p43 [58]. The indirect pathway involves the nuclear-encoded transcriptional co-activator-1α of peroxisome proliferator activated receptor gamma (PGC-1a) [57], the respiratory factors (NRF-1 and 2) and the mitochondrial transcription factor (mtTFA), which itself activates the replication and transcription of mitochondrial DNA. However, the effect of TH on cardiac mitochondrial content and function, and energy transfer is less clear. For example, T3 treatment in rats was able to increase cardiac oxygen consumption, mitochondrial bioenergetic capacity and markers of mitochondrial biogenesis such as PGC-1α and its transcription cascade [12], but another study reported no change in PGC-1α in heart of T3 treated animals [20].
Heart failure and thyroid state
The thyroid state has been suspected to participate in the pathophysiology of heart failure (HF). Free triiodothyronine level, is below normal values in chronic heart failure [9]. Moreover, thyroid hormone (TH) metabolism is frequently altered in advanced congestive heart failure and is an independent predictor of mortality [13]. The hypothyroid state, and in particular a low triiodothyronine level, has been associated with a reduced cardiac performance and poor prognosis in HF [18,42]. In addition to changes in circulating hormone, the local thyroid state was also shown to be altered in HF. Enzyme activity of the type III deiodinase (D3), which converts T4 and T3 to inactive compounds is stimulated up to five fold in hypertrophied heart, with D3 activity significantly higher in those animals in which hypertrophy progresses to heart failure [54]. In parallel, levels of type I deiodinase (D1, the deiodinase which converts T4 in active T3) was decreased in CHF animals. The induction of a TH-degrading and decrease of a TH-generating deiodinase is expected to result in reduced cellular levels of T3 and thereby contribute to a local hypothyroid state in the failing ventricle [54]. Moreover, the nuclear thyroid hormone receptors, (TRα1, TRβ1, TRβ2), are also altered in heart failure. In failing human heart, TRα1 is downregulated, whereas TRα2, a splice variant that does not bind thyroid hormone but inhibits responses to liganded TRs, is increased, suggesting local attenuation of thyroid hormone signaling in the failing human heart [24]. However, other authors rather observed a coordinated increment in the expression of the TR isoforms in CHF patients [4]. Thus low or subclinical blood levels of thyroid hormone, altered expression of thyroid receptors, and increased cellular degradation of thyroid hormones in heart failure suggest that alterations in the thyroid status may participate in the physiopathology of the failing cardiomyocyte.
Heart failure and energy metabolism
Energetic failure of the failing heart is now increasingly recognized. Four main features can be outlined. First of all, the failing heart, as the hypertrophied heart, exhibits a profound change in substrate utilization, with a metabolic switch from fatty acid to glucose utilization and subsequent dysregulation of fatty acid oxidation enzyme gene expression [16,48]. The second feature is a decrease in oxidative capacity of the myocardium [7,35,44], that appears related to alteration in mitochondrial biogenesis and the PGC-1α transcription cascade [11,51]. Thirdly, HF also impairs energy transfer and utilization. A generalized alteration of the creatine kinase (CK) system has long been observed with both cytosolic and mitochondrial isoenzymes of CK being affected (for review see [8,19,51]). Finally, the failing heart has reduced mechanical efficiency and increased energy cost of contraction [39] leading to energy wastage. Energetic imbalance of the failing heart is the major cause for the lower PCr/ATP ratio that turned out to be a valuable predictor of mortality in CHF [31]. However, the signals triggering the drop in creatine kinase and mitochondrial biogenesis in heart failure are at present undefined.
As energy metabolism is one of the main targets for thyroid hormones, we sought to investigate whether hypothyroidism can explain energetic failure of the failing myocardium. Hypothyroid state was induced by thyroidectomy and propyl-thio-uracyl in male rats for three weeks and we examined the effects of hypothyroid state on oxidative capacity and mitochondrial biogenesis, mitochondrial substrate utilization, and creatine kinase system of energy transfer. Moreover, signaling pathways known to be involved in the control of energy metabolism were assessed. The results show that hypothyroidism induces unique changes in cardiac bioenergetics that differ from heart failure.
MATERIALS AND METHODS
Animals
All procedures were performed in accordance with the principles and guidelines established by the European Convention for the Protection of Laboratory Animals. Adult male Wistar rats (~ 220–280g) were randomly divided into two groups housed 2 or 3 per cage in a temperature-controlled room (22°C), with a 12/12h light/dark cycle. The hypothyroid rats (HYPO, n=8) were thyroidectomized by Janvier (Le Genest ST Isle, France) and received 6-propyl-2-thio-uracil (PTU, 0.04%) in water during 3 weeks in order to maintain the low level of plasmatic T3. The control group (CT, n=16) was kept in the same conditions for three weeks. All animals were given food ad libitum. The rats were anesthetized by intraperitoneal injection of Pentothal (150mg/kg) and the heart was removed. Left ventricular tissue was isolated, part of which was immediately used for mitochondrial function measurement, and other part was rapidly frozen and kept at −80° C.
Study of in situ mitochondrial respiration
Oxygen consumption measurements of saponin-skinned fibers from left ventricle have been described previously [50]. Different experimental protocols were used based on substrate utilization pathways as previously described [1]. The first protocol was designed to study the different complexes of the respiratory chain in the presence of 2 mM ADP; complex I was investigated with 4 mM glutamate + 10 mM malate; complex I was then inhibited with 2 mM amytal and complex II was measured with 10 mM succinate; complex IV was finally assessed with 0.5 mM tetramethyl phenylenediamine (TMPD) + 0.5 mM ascorbate on n=8 CT and n=8 HYPO. The second protocol determined the sensitivity of mitochondrial respiration to various substrates in the presence of 2 mM ADP, by cumulative addition of 4 mM glycerol-3P (G3P), 4 mM malate, 0.4 mM octanoyl-carnitine, and 1 mM pyruvate as described previously [1] on n=8 CT and n=8 HYPO. In order to determine the relative contribution of each substrate to respiration rate, the effect of the substrate added previously was subtracted from the cumulative value. The third protocol was aimed at determining the dependency of respiration on external [ADP] and [creatine] [49], with glutamate+malate as substrates. The coupling of phosphorylation to oxidation was determined by calculating the acceptor control ratio (ACR) as the ratio between ADP stimulated respiration (Vglu/mal) over basal respiration without ADP (V0). As no difference was observed with or without creatine for V0, Vglu/mal and ACR, the data were pooled. Rates of respiration are given in μmoles O2.min−1.g dry weight−1 (dw). Two to three fiber bundles were assayed for each heart and each protocol.
Biochemical studies
Frozen tissue samples were weighed, homogenized (Bertin Precellys 24) in ice-cold buffer (50mg/ml) containing (mM) 5 HEPES (pH 8.7), 1 EGTA, 1 DTT, and 5 MgCl2, and 0.1% Triton X-100 and incubated for 60 min at 0°C to ensure complete enzyme extraction. Citrate synthase (CS), cytochrome c oxidase (COX), creatine kinase (CK) and lactate dehydrogenase (LDH) activities were measured at 30°C (pH 7.5) using coupled enzyme systems as previously described [7]. CK isoenzymes were separated using agarose 1% gel electrophoresis at 200 V for 90 min. Individual isoenzymes were resolved through incubation of the gels with a coupled enzyme system. Total [2] and mitochondrial [46] malate dehydrogenase (MDH) activities were measured at 30°C (pH 7.5) using coupled enzyme systems.
Real-Time Quantitative RT-PCR Analysis
Total cardiac RNA was extracted using standard procedures. Oligo-dT first strand cDNA was synthesized from 5 μg total RNA using superscript II reverse transcriptase (Invitrogen). Real-time PCR was performed using the SYBR®Green method on a LightCycler rapid thermal cycler (Roche Diagnostics) as previously described [11]. PCR amplification was performed in duplicate in a total reaction volume of 15 μl. The reaction mixture consisted of 5 μl diluted template, 1.5 μl FastStart DNA Master SYBR Green I kit (x10), 3 mM MgCl2 (except for mCPTI MCAD and PPARa with 4 mM) and 0.5 μM forward and reverse primers (Table 1). After an 8 min activation of Taq polymerase, amplification was allowed to proceed for 30–40 cycles, each consisting of denaturation at 95°C for 10s, annealing at specific temperature (Table 1) for 5s (except 6s for D2 and 10s for COX I), and extension at 72°C for 5–25 s, depending on the length of the PCR product (25 bp per sec). Fivefold serial dilution from cardiac total RNA were analyzed for each target gene and allowed us to construct linear standard curves from which the concentration of the test sample was calculated. Primers were designed in a different exon of the target gene sequence, eliminating the possibility of amplifying genomic DNA. A basic local alignment search tool (BLAST) search performed for each set of primers revealed that sequence homology was obtained only for the target gene. Cyclophilin A (CycA) was chosen as housekeeping gene for normalization as its expression did not differ between the two groups. Results were first normalized to cyclophilin A transcription in order to compensate for variation in input RNA amounts and efficiency of reverse transcription, then they were multiplied by total RNA per amount of tissue (mg wet weight−1) to compare expression level in different conditions [11].
Table 1.
Primers and conditions for real-time PCR amplification
| Gene | GenBank accession number | Forward Primer (5′-3′) | PCR product size (bp) | Annealing temperature (°C) |
|---|---|---|---|---|
| Reverse Primer (5′-3′) | ||||
| PGC-1α | NM_008904 | CACCAAACCCACAGAGAACAG | 210 | 65 |
| GCAGTTCCAGAGAGTTCCACA | ||||
| NRF 1 | NM_010938 | TTACTCTGCTGTGGCTGATGG | 92 | 60 |
| CCTCTGATGCTTGCGTCGTCT | ||||
| NRF 2 | XM_344002 | CACCACACTCAACATTTCGG | 244 | 58 |
| CCTTGGGGACCTTTGAACTT | ||||
| Tfam | NM_031326 | GAAAGCACAAATCAAGAGGAG | 175 | 55 |
| CTGCTTTTCATCATGAGACAG | ||||
| COX I | NC_001665 | AGCAGGAATAGTAGGGACAGC | 520 | 55 |
| TGAGAGAAGTAGTAGGACGGC | ||||
| COX IV | NM_017202 | TGGGAGTGTTGTGAAGAGTGA | 273 | 58 |
| GCAGTGAAGCCGATGAAGAAC | ||||
| MCIP 1 | NM_153724 | AGCGAAAGTGAGACCAGGGC | 264 | 60 |
| GGCAGGGGGAGAGATGAGAA | ||||
| MCIP 2 | NM_175518 | CGGACCTATGACGAATGTGTG | 235 | 60 |
| AAGGGGGTGAGATGAGGAACT | ||||
| Dio 2 | NM_031720 | ACTCGGTCATTCTGCTCAAG | 315 | 59 |
| TAAAAGGTGGTCAGGTCGCT | ||||
| Dio 1 | NM_021653 | CATTCAAGGCAGCAGACCCC | 199 | 65 |
| GCTTCGGTGCTGCCTGATGT | ||||
| PPAR a | NM_013196 | ATGAGTCCCCTGGCAATG | 259 | 59 |
| GGCATTCTTCCAAAACGG | ||||
| PPAR β/d | NM_013141 | TCAGGCTTCCACTACGGGGT | 190 | 64 |
| AGCGGATAGCGTTGTGGGAC | ||||
| MCAD | NM_016986 | CCGTTCCCTCTCATCAAAAG | 129 | 60 |
| ACACCCATACGCCAACTCTT | ||||
| mCPTI | NM_013200 | TCACCTGGGCTACACGGAGA | 219 | 64 |
| TCGGGGCTGGTCCTACACTT | ||||
| SERCA 2A | J04023, X15635 | CACACCGCTGAATCTGAC | 129 | 56 |
| GGAAGCGGTTACTCCAGT | ||||
| β-MHC | NM_017240 | GGAAAAGCACGCAACAGAGA | 202 | 61 |
| ATCATCCACTTGCTGCTCCA | ||||
| Cyc A | NM_017101 | GAGCACTGGGGAGAAAGGAT | 259 | 65 |
| CTTGCCATCCAGCCACTCAG |
PGC-1α: peroxisome proliferator activated receptor gamma co-activator 1α; NRF: nuclear respiratory factor; Tfam, mitochondrial transcription factor A; COX: cytochrome oxidase; MCIP: myocyte calcineurin interacting protein; TR: thyroid hormone receptor; Dio: deiodinase; PPAR: peroxisome proliferator activated receptor; MCAD: middle chain Acylcoa dehydrogenase, mCPTI: muscle isoform of carnitine palmitoyl transferase I; SERCA: sarco-endoplasmic reticulum calcium ATPase; MHC: myosin heavy chain; CycA: cyclophilin A.
Western Blot Analysis
Protein extracts (50 μg) of cardiac muscles from CT and HYPO rats were loaded onto SDS-polyacrylamide gels and separated for 120 min at 120 V. After electrophoresis, the proteins were transferred to Hybond nitrocellulose membranes (Amersham) using a Bio-Rad blot system for 90 min at 150 V. Thereafter, the blots were blocked with 5% milk in PBS for 60 min at room temperature, followed by incubation with a primary antibody at 4°C overnight. Specific antibodies were used to measure the protein content of the OXPHOS complexes (MitoSciences LLC), AMPKa1, AMPKa2 and calcineurin (Upstate Biotechnology Inc), mi-CK (kind gift from Dr Z. Khuchua, Nashville USA), PGC-1α (Chemicon), phospho- and total p38 MAPK, and phospho- and total AMPK (Cell Signaling). After washing, the membranes were incubated with horseradish peroxidase secondary antibody for 60 min and revealed with enhanced chemiluminescent substrate (ECL, Amersham, France). Light emission was detected by autoradiography and quantified using an image-analysis system (Bio-Rad Geldoc 1000). Beta actin (Sigma) was used as reference. Quantification was performed using Quantity One software (Biorad) and expressed as a ratio of the signal obtained with the protein of interest relative to the β-actin.
Statistical analysis
Data are expressed as mean ± SEM. Statistical significance was defined as p<0.05 with a Student test.
RESULTS
Anatomical and physiological parameters
Table 2 summarizes the effects of thyroidectomy on reliable indicators of the thyroid state. Plasmatic levels of free T3 were decreased by 63% (p<0.001) in HYPO. The heart weight (HW) and body weight (BW) were lower in HYPO mainly due to a lower weight gain in HYPO, as the HW/BW ratio was not significantly decreased compared to CT. However when reported to tibia length, heart weight was significantly lower in HYPO. The expression of the cardiac isoform of the sarcoplasmic reticulum calcium ATPase SERCA2A was decreased by 45%. The deiodinase 1 and 2 (D1 and D2) activate thyroid hormone by converting T4 in T3 and by degrading the reverse T3 (inactive form of T3). As expected in HYPO [52] we found a significant increase of 34% of the D2 mRNA, while D1 was not expressed at detectable levels (result not shown). All these markers evidence the efficacy of the treatment.
Table 2.
Parameters of hypothyroid (HYPO) and control (CT) rats
| CT n=16 |
HYPO n=8 |
|
|---|---|---|
| Plasma T3 (pmol/L) | 5.32±0.40 | 1.99±0.26*** |
| Body weight, BW (g) | 434±10 | 232±7*** |
| Weight gain for 3 weeks (g) | 180±14 | 8.9±6.9*** |
| Heart weight, HW (g) | 1.17±0.04 | 0.61±0.01*** |
| HW/BW (mg/g) | 2.70±0.08 | 2.65±0.06 |
| HW/Tibia length (mg/cm) | 0.289±0.01 | 0.174±0.005*** |
| mmRNA expression (AU) | ||
| SERCA2A | 0.95±0.06 | 0.52±0.04*** |
| D2 | 0.68±0.09 | 1.03±0.06** |
Values are means ± S.E.M. SERCA: sarco-endoplasmic reticulum calcium ATPase; D2: deiodinase 2.
p<0.01,
p<0.001 versus CT.
Mitochondrial oxidative capacity
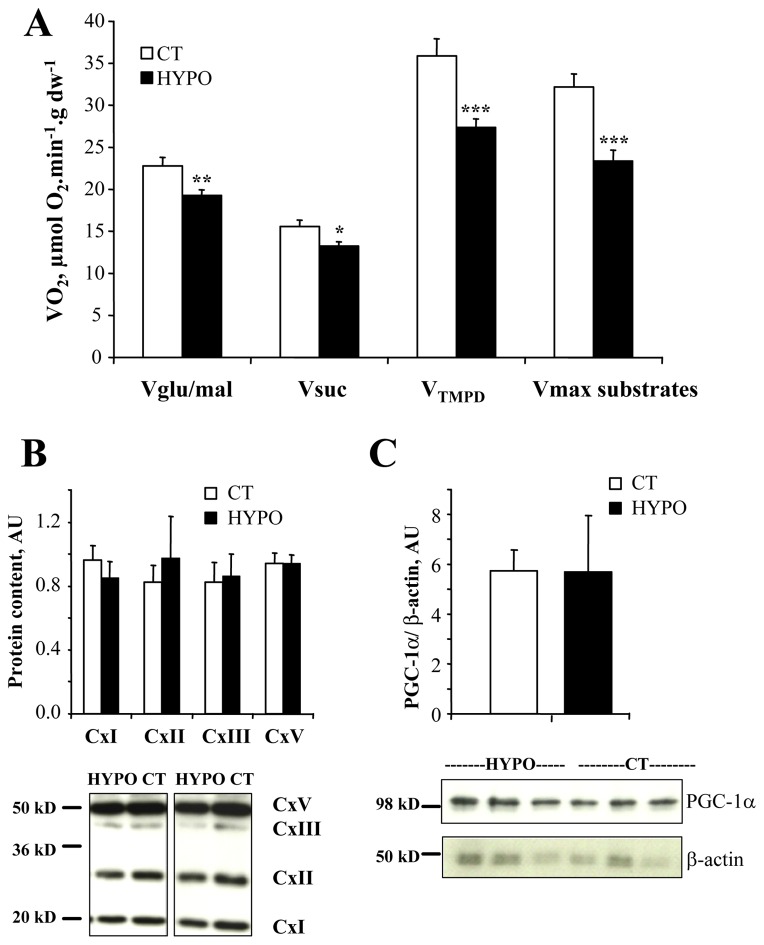
We determined the effects of hypothyroid state on maximal respiratory capacity of cardiac tissue using saponin permeabilized fibers. Basal respiration rate (without ADP) was not significantly changed in HYPO (3.28±0.23 μmol O2.min−1.g dw−1) compared to CT (3.86±0.23 μmol O2.min−1.g dw−1) rats. Oxidative capacity (Fig. 1A) either with glutamate/malate (Vglu/mal, respiration from complex I), with succinate/malate (Vsuc, respiration from complex II) or TMPD/ascorbate (respiration from complex IV) as substrates were all decreased in HYPO rats showing the lowering effects of hypothyroid state on cardiac muscle respiratory chain. Moreover, when all substrates were present (Vmax substrates, see below), oxygen consumption was still lower in HYPO compared to CT rats. Finally, the acceptor control ratio (ACR) was unchanged in HYPO rats (Table 4).
Figure 1. Effects of hypothyroidism on cardiac oxidative capacity.
A. Maximal respiration rates with glutamate and malate (respiration from complex I, Vglu/mal), succinate (respiration from complex II, Vsuc), TMPD/ascorbate (respiration from complex IV, VTMPD) or with malate+glutamate+glycerol-3P+octanoate (Vmax substrates) were obtained in the presence of 2 mM ADP. They were all decreased in hypothyroid rats (CT n=8, HYPO n=8). B. Protein content of complexes of the respiratory chain (Cx I, II, III, V); upper part, mean values normalized to β-actin; lower part, representative Western blots. C: protein content of PGC-1α; upper part, mean values normalized to β-actin; lower part, representative Western blot of PGC-1α (upper lane) and β-actin (lower lane). * p<0.05, ** p<0.01, *** p<0.001 versus CT.
Table 4.
Energy transfer system
| CT n=8 |
HYPO n=8 |
|
|---|---|---|
| Protein expression (A.U) | ||
| mi-CK | 0.99±0.12 | 0.60±0.14 |
| Enzymatic activity (IU/g prot) | ||
| Total AK | 1209±93 | 1477±82 |
| Total CK | 3742±230 | 3367±130 |
| mi-CK | 743±63 | 604±42 |
| M-CK | 2612±227 | 2600±170 |
| B-CK | 387±38 | 226±30** |
| Mitochondrial function1 | ||
| KmADP, μM | 189±12 | 228±12* |
| KmADP+Cr, μM | 65±5 | 101±8*** |
| mi-CK efficacy | 3.6±0.2 | 2.4±0.2** |
| ACR | 7.1±0.4 | 6.2±0.3 |
Values are means ± S.E.M. CK: creatine kinase; Km: Michaelis-Menten constant of respiration rate for ADP (μmol/l) without (KmADP) or with (KmADP+Cr) 20mM creatine; mi-CK efficacy: KmADP/KmADP+CR; ACR: acceptor control ratio (Vglu+mal/V0).
p<0.01, versus CT;
p<0.001.
(n=16 for CT and n=8 for HYPO).
In order to understand the molecular nature of these changes in the oxidative capacities we measured some mitochondrial markers (Table 3). The activity of citrate synthase (CS) a marker of mitochondrial mass, and of the cytochrome oxidase c (COX) were significantly decreased in HYPO. However neither the mRNA expression of COX I (mito-encoded) and IV (nucleus-encoded) subunits (Table 3) nor the protein content of complex V (α), III (core2), II (30kD) and I (20 kD) subunits of the respiratory chain complexes were changed (Fig. 1B). Moreover, no significant decrease in the protein (Fig. 1C) and mRNA contents of PGC1a, or in the mRNA content of the downstream effectors of mitochondrial biogenesis NRF1, NRF2 and Tfam were observed in HYPO rats (Table 3). So the low oxidative capacity of HYPO hearts cannot be explained by the reduction of the classical markers of mitochondrial biogenesis.
Table 3.
Gene expression and activity of proteins involved in oxidative capacity
| CT n=8 |
HYPO n=8 |
|
|---|---|---|
| mRNA (AU) | ||
| PGC1a | 0.80±0.07 | 0.69±0.04 |
| NRF1 | 0.25±0.02 | 0.25±0.03 |
| NRF2 | 0.20±0.02 | 0.20±0.02 |
| Tfam | 0.29±0.03 | 0.27±0.03 |
| COX I (mito-encoded) | 0.42±0.03 | 0.37±0.03 |
| COX IV (nucleus-encoded) | 0.58±0.04 | 0.49±0.03 |
| Enzymatic activity (IU.g prot−1) | ||
| CS | 933±48 | 777±33* |
| COX | 1787±165 | 1306±130* |
| Total LDH | 2422±82 | 2327±75 |
| H-LDH | 1487±51 | 1499±59 |
| M-LDH | 934±34 | 828±36* |
| H-LDH/M-LDH | 1.59±0.03 | 1.83±0.10 |
Values are means ± S.E.M. PGC-1α: peroxisome proliferator activated receptor gamma co-activator 1α; NRF: nuclear respiratory factor; Tfam, mitochondrial transcription factor A; COX: cytochrome oxidase; Cx I, II, III, V: complexes of respiratory chain; CS: citrate synthase; MDH: malate dehydrogenase; LDH: lactate dehydrogenase.
p<0.05 versus CT.
Substrate utilization by mitochondria
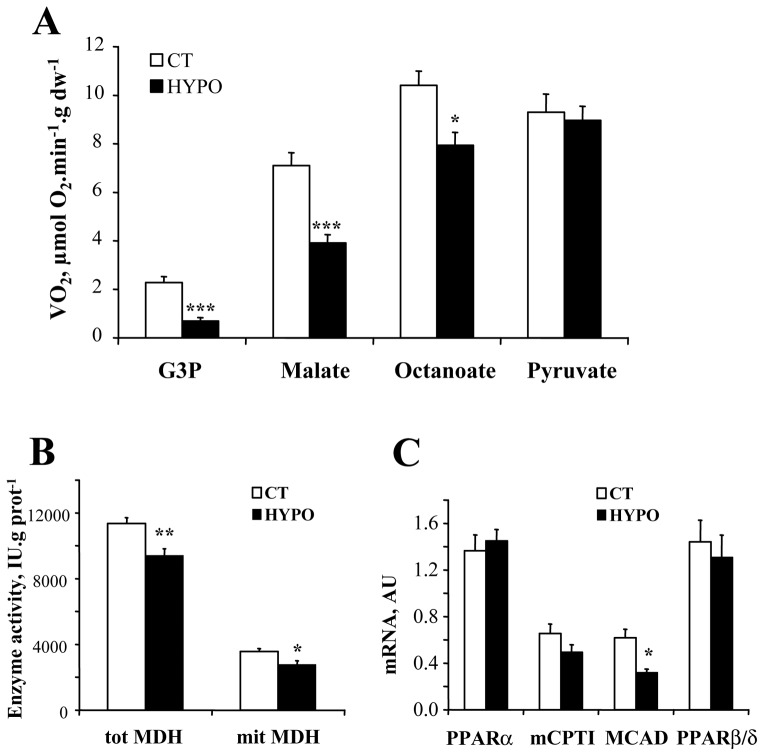
Respiration rates were measured with different substrates in order to study whether mitochondrial substrate utilization by mitochondria was sensitive to thyroid hormones (Fig. 2A). Respiration with glycerol-3-phosphate (G3P) was decreased by 55% in the HYPO (p<0.001). Malate stimulated respiration, was also significantly decreased in the HYPO. This is consistent with a decrease in total and mitochondrial malate dehydrogenase (MDH) activity in the HYPO (Fig. 2B). We also noticed a slight decrease of the respiration rate when octanoate was added in the HYPO and no change with pyruvate. This was associated with a decrease in mRNA expression of the middle chain AcylCoA dehydrogenase (MCAD) while the mitochondrial fatty acid translocase mCPTI (muscle isoform) and the peroxisome proliferator activated receptor α and β/δ (PPARα and PPARβ/δ) were unchanged (Fig. 2C). Moreover, hypothyroidism did not significantly affect total LDH and H-LDH activity but M-LDH was significantly decreased.
Figure 2. Effects of hypothyroidism on substrate utilization by mitochondria.
A. Respiration rates were obtained by cumulative addition of glycerol-3P (G3P, 4mM), malate (4 mM), octanoyl-carnitine (0.4 mM), and pyruvate (1 mM). The increments in respiration rates are plotted to show the substrate preference of control and hypothyroid cardiac fibers (CT n=16, HYPO n=8). B. Enzymatic activity of total and mitochondrial MDH. C. mRNA expression of proteins of the β-oxidation pathway. PPAR: peroxisome proliferator activated receptor; MCAD: middle chain AcylCoA dehydrogenase; mCPTI: muscle carnitine palmitoyl transferase I. * p<0.05, ** p<0.01, *** p<0.001 versus CT.
Energy transfer
Then we examined whether thyroid hormone may influence the energy transfer system (Table 4). The total CK and adenylate kinase (AK) activities were unchanged in HYPO while a 42% decrease of the B-CK activity could be observed. No significant change in mi-CK expression or activity was observed in HYPO. The sensitivity of mitochondrial respiration to phosphate acceptors (ADP and creatine) was assessed by measuring the Michaelis-Menten constant for ADP in the presence and the absence of creatine. The KmADP was significantly increased in HYPO. The KmADP+Cr of the HYPO was also increased by 64% compared to CT despite an unchanged expression of the mitochondrial CK isoenzyme, evidencing a decreased efficacy of mitochondrial creatine kinase (mi-CK efficacy).
Signaling pathways
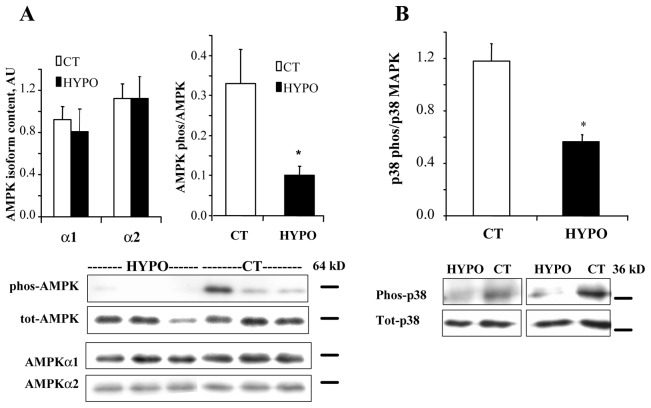
We then assessed whether thyroid hormone could regulate signaling pathways proposed to be involved in the control of cardiac energy metabolism. The AMP-activated protein kinase (AMPK) which is involved in mitochondrial biogenesis was shown to depend on thyroid hormone in skeletal muscle [33]. In the heart, the protein content of AMPK a1, a2 and total AMPK was unchanged in the HYPO versus the CT, but the phosphorylation state was significantly decreased (Fig. 3A).
Figure 3. Effects of hypothyroidism on AMPK and p38 MAPK phosphorylation.
A. upper part, mean values of AMPKα1 and α2 protein content and of the ratio of phosphorylated over total AMPK; lower part, representative Western blot showing the decrease in phosphorylated AMPK, without changes in total AMPK or α1 and α2 subunits. B. upper part, mean values of phosphorylated p38 MAPK over total p38; lower part, representative Western blot showing the decrease in phosphorylated p38 without significant changes in total p38. * p<0.05 versus CT.
The p38 MAPK has been involved in the regulation of PGC-1α expression and mitochondrial protein expression. We thus assessed the content and phosphorylation level of p38 MAPK (Fig. 3B). Hypothyroidism did not affect total p38 MAPK expression (1.92±0.46 in CT versus 1.96±0.29 AU in HYPO). However, the level of phosphorylation was decreased by 52% in HYPO rats (p<0.05).
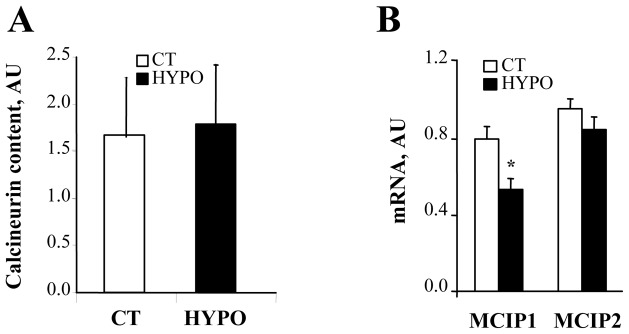
Finally, calcium-dependent phosphatase calcineurin is an important signaling molecule in cardiac hypertrophy and failure involved in the regulation of mitochondrial biogenesis. The calcineurin protein content (Fig. 4A) was unchanged in HYPO. The two calcineurin regulatory proteins MCIP1 and MCIP2 were shown to be regulated respectively by calcineurin and thyroid hormone [59]. Surprisingly, the level of MCIP1 but not MCIP2 was altered by 49% in HYPO (Fig. 4B). As MCIP1 promoter sequence contains 15 repeats of the NFAT binding site it has been shown to be the most sensitive indicator of calcineurin transcriptional activity [59]. Thus it appears that thyroid hormone may interfere to enhance calcineurin signaling in cardiac cells.
Figure 4. Effects of hypothyroidism on calcineurin pathway.
A. Western blot analysis of calcineurin content, normalized to β-actin. B. Real-time quantitative RT-PCR analysis of mRNA content of modulatory calcineurin interacting protein 1 (MCIP1) and 2 (MCIP2), normalized to cyclophilin A and expressed as arbitrary units (CT n=8, HYPO n=8). * p<0.05 versus CT.
DISCUSSION
The present results can be summarized as follows: Hypothyroidism 1) induces a decrease in the maximal oxidative capacity of cardiac fibers from complex I, II and IV together with CS activity and COX activities but no change in PGC-1α mRNA and protein content or in the PGC1α transcription cascade, suggesting a unique mode of mitochondrial respiration regulation by TH; 2) decreased the mitochondrial utilization of glycerol-3P, malate and octanoate but not pyruvate; 3) hardly affected creatine kinase system and energy transfer; 4) affected the level of p38 MAPK and AMPK phosphorylation as well as calcineurin activation (MCIP1 expression), showing the capacity of thyroid hormone to interact with different signaling pathways.
Physiological parameters
Hypothyroidism was confirmed by the low thyroid hormone plasma level, decreased mRNA content of SERCA2A [22] and increased D2 mRNA content [52]. Metabolic consequences of heart failure has been extensively studied by our group using the same experimental approaches [6,7,11,28]. We have investigated whether cellular hypothyroidism status might explain the energetic alterations of pathological hypertrophy or HF. The results of these studies are reported in Table 5 and compared to the results obtained in hypothyroid state.
Table 5.
Comparison between heart failure and hypothyroid energy metabolism.
| Sham rats$ | CHF rats$ | CT | HYPO | ||
|---|---|---|---|---|---|
| Mitochondrial respiration | Vmax (μmol O2.min−1.g−1dw) | 26.4 ± 1.5 | 20.9 ± 1.0 ** | 24.9 ± 0.9 | 19.3 ± 0.7 ** |
|
| |||||
| Mitochondrial enzyme activities (IU/g prot) | CS activity | 603 ± 18 | 378 ± 23 *** | 933 ± 48 | 777 ± 33 * |
| COX activity | 1516 ± 69 | 962 ± 82 *** | 1787 ± 165 | 1306 ± 130 * | |
|
| |||||
| Mitochondrial biogenesis : mRNA content (arbitrary unit) | PGC-1a | 2.69 ± 0.26 | 1.82 ± 0.14 ** | 0.8 ± 0.07 | 0.69 ± 0.04 |
| NRF1 | 0.77 ± 0.07 | 0.72 ± 0.04 | 0.25 ± 0.02 | 0.25 ± 0.03 | |
| NRF2 | 1.28 ± 0.11 | 0.87 ± 0.08 ** | 0.2 ± 0.02 | 0.2 ± 0.02 | |
| Tfam | 0.61 ± 0.04 | 0.41 ± 0.02 | 0.29 ± 0.03 | 0.27 ± 0.03 | |
|
| |||||
| Energy transfer (IU/g prot) | Total CK activity | 4276 ± 80 | 1966 ± 125 *** | 3742 ± 230 | 3367 ± 130 |
| M-CK activity | 2021 ± 137 | 1210 ± 61 ** | 2612 ± 227 | 2600 ± 170 | |
| mi-CK activity | 1303 ± 101 | 201 ± 38 *** | 743 ± 63 | 604 ± 42 | |
| AK activity | 1286 ± 202 | 978 ± 88 | 1209 ± 93 | 1477 ± 82 | |
|
| |||||
| Substrate utilization : enzyme activities (IU/g prot) | H-LDH | 2072 ± 76 | 1478 ± 63 *** | 1487 ± 51 | 1499 ± 59 |
| M-LDH | 883 ± 46 | 839 ± 56 | 934 ± 34 | 828 ± 36 * | |
| H-LDH/M-LDH | 2.35 ± 0.07 | 1.76 ± 0.12 ** | 1.59 ± 0.03 | 1.83 ± 0.1 | |
All values on sham and CHF rats come from Garnier et al J Physiol 2003, except for the energy transfer and the substrate utilization data, which come from De Sousa et al Circ Res 1999. Sham and CHF (congestive heart failure) rats data, originally expressed by g wet weight, were converted in mg of protein using a protein to wet weight ratio of 18%.
p<0.05,
p<0.01,
p<0.001 between sham and CHF rats, or between Eu and Hypothyroid rats. The mRNA content of Sham and CHF rats were normalized to β-actin transcription, while Eu and Hypothyroid rats mRNA are normalized to cyclophylin A.
Mitochondrial oxidative capacity
One of the main features of energetic alteration in HF is the decrease in oxidative capacity and mitochondrial protein content (Table 5, [51]). Cardiac oxidative capacities from complex I, II or IV were all found to be decreased in HYPO compared to CT rats. A similar decrease was observed when glycerol-3P, octanoate and pyruvate were all present. This was accompanied by the decrease of CS and COX activity. Thus our results confirm the influence of TH at the mitochondrial level to partly modulate cardiac energetic.
Decreased cardiac oxidative capacity in HF, is linked to a decrease in mitochondrial biogenesis through the PGC-1α transcription cascade [11]. Our first hypothesis was that hypothyroidism influences oxidative capacity by down-regulating mitochondrial biogenesis. The regulatory effect of thyroid hormone on mitochondrial transcription is partially exerted by a direct influence of the hormone on the mitochondrial transcription machinery [10,58] However, only a limited number of genes are known to be regulated by a TRE, suggesting an indirect regulation of gene expression. PGC-1a and its downstream target NRFs have been identified as likely intermediary factors controlling T3-induced mitochondrial biogenesis [55,57]. However, the present results show that hypothyroidism was not accompanied by a decrease in PGC-1α protein or mRNA content in the heart, or by a down-regulation of the PGC-1α transcription cascade (as mRNA content of NRFs, Tfam and their target genes) nor by a decrease in proteins of the mitochondrial complexes. This is consistent with the observation that glycolytic or aerobic metabolic enzymes appear less sensitive to TH than sarcoplasmic reticulum Ca2+-ATPase and myosin isoenzymes [26].
Impairment of mitochondrial function in hypothyroidism may have different origins. Other reports in the literature also point out a rather complex effect of thyroid hormone on cardiac mitochondrial biogenesis and function. TH modulates mitochondrial maturation in sheep through complex transcriptional and posttranscriptional mechanisms [27]. Increased COX activity without coordinated increase in COX subunit gene transcription has been described in hyperthyroidism [45]. Moreover, non genomic actions of TH on its target tissues and specifically in the heart have been described for a long time [5]. Interestingly, T2 can directly activate the COX by abolishing the allosteric inhibition of ATP [21], explaining the discrepancy between COX activity and COX expression under different thyroid states. These non genomic effects of thyroid hormone include direct activation of mitochondrial respiration and mitochondrial enzymes, although the mechanism involved are still debated [5,58]. Finally TH can also regulate the oxidative capacity by modulating the cardiolipin content. Cardiolipin is the main phospholipid of the mitochondrial inner membrane. It rises with increased metabolic rate and plays a key role in the activity of several inner membrane proteins including complex-I (for review see [40]). A decreased mitochondrial content of cardiolipin has been consistently observed in hypothyroidism and heart failure [3,29,32]. Moreover, key enzymes of the biosynthetic pathway of cardiolipin are sensitive to T3 [14,29]. This effect of thyroid hormone on the cardiolipin content could participate in the decreased function of the respiratory chain, without necessary changes in protein expression.
Thus hypothyroidism induces a unique pattern of mitochondrial changes including decreased mitochondrial function without coordinate changes in mitochondrial gene expression and transcription cascade. Alteration in mitochondrial function in hypothyroid state does not resemble that induced by heart failure (Table 5), suggesting potentially additive mechanisms involved in mitochondrial alterations in this disease.
Substrate utilization
Although not assessed on in situ mitochondria, it is well known that a switch from fatty acid to glucose utilization is observed in heart failure [16,48]. Cardiac mitochondria have a high capacity to utilize fatty acid and a reduced capacity for G3P utilization [34]. Decreased respiration rate under G3P and malate in the HYPO, is in accordance with the literature showing that mitochondrial G3P dehydrogenase mRNA expression is under the control of thyroid hormone [43] and with the decrease in total and mitochondrial MDH activities observed here and in previous studies [43,56]. While pyruvate-induced increase in respiration did not differ from control, mitochondria from HYPO utilize less octanoate than CT. This is consistent with the decreased mRNA content of MCAD observed here and with the known decrease in fatty acid flux of the whole myocardium [17]. By contrast, mCPT1 mRNA content was unchanged in hypothyroidism as previously shown during maturation in sheep [27]. Additionally no change in PPARα and PPARβ/δ expression pattern, which are involved in fatty acid oxidation [16], was observed, evidencing again a unique regulation of mitochondrial substrate utilization in hypothyroid state. Thus, as the decrease in fatty acid oxidation in HF seems to be mediated in part through a coordinated down-regulation of fatty acid oxidation enzymes among which MCAD and mCPT1 [36], hypothyroid state could be only partially involved in these effects.
Moreover, although the ratio of the cardiac isoform (H-LDH), which is associated with increased lactate utilization, versus the muscle (M-LDH), which is associated with lactate formation, was decreased in heart failure [6], it was not affected by hypothyroidism (Table 5).
Energy transfer
Activity and function of cytosolic and mitochondrial isoforms of creatine kinase, that are involved in the fine regulation of energy transfer in cardiac cells, are all profoundly affected in heart failure (Table 5, [30]). At present, the upstream events inducing these changes are completely unknown [7]. Hypothyroid state did not affect cytosolic or mitochondrial creatine kinase isoenzymes activity or expression, or adenylate kinase activity, another phosphotransfer enzyme [7]. Interestingly, hypothyroid state decreased the sensitivity of mitochondria to ADP and creatine as well as mi-CK efficacy. This effect could rather be due to hypothyroid-induced decrease in cardiolipin content [29,32] because cardiolipin is known to be important for mi-CK binding [41]. In general, thyroid state cannot explain the dramatic decrease in creatine kinase expression and function in heart failure (Table 5).
Signaling pathways
An important way by which genomic and non-genomic effects of T3 occur in heart muscle could be by modulating transcriptional or post-transcriptional activity of other signaling pathways. For example, it has been shown that some effects of TH could be induced via mitogen-activated protein kinases (MAPK) and vice versa [5,23]. Chronic activation of p38 MAPK alters oxidative phosphorylation complexes in the heart [53]. However, hypothyroidism rather induced a decreased phosphorylation of p38 MAPK, suggesting a post-translational regulation, making the implication of p38 MAPK in the respiratory effects of low T3 observed here rather unlikely.
AMP-activated protein kinase (AMPK) signaling pathway plays an important role in controlling energy homeostasis and fatty acid or glucose utilization in the heart [15,37]. AMPK can also affect energy metabolism through changes in gene expression. In contrary to skeletal muscle [33], we did not observe any change in the protein content of AMPKa1 or a2, but the basal phosphorylation state of AMPK was clearly decreased in hypothyroid state showing again the complex tissue-specificity of T3 effects. It is tempting to speculate that activation of AMPK phosphorylation by T3 may counteract the catabolic effects of thyroid hormone.
Calcineurin activation is a key player in hypertrophy and heart failure that has been involved in mitochondrial gene expression [38]. Hypothyroidism had no influence on calcineurin expression. Calcineurin activity is also modulated by calcineurin interacting proteins, among which modulatory calcineurin-interacting proteins (MCIPs). In mouse heart, expression of MCIP1 is induced by calcineurin activation, whereas MCIP2 expression is increased by thyroid hormone [59] suggesting a cross-talk between calcineurin and thyroid hormone pathways. In the present study, the mRNA expression of MCIP1 but not MCIP2 was decreased in the HYPO, suggesting that calcineurin activation could be under the control of T3 in rat heart. Interestingly, it was suggested that TH signaling pathway may play a role in differentiating physiological and pathological hypertrophy [24]. The present data open the interesting possibility that T3 controls the hypertrophic gene program at least in part through calcineurin activation.
In summary, thyroid hormone signaling in heart is characterized by pleiotropic functional changes, a unique mitochondrial expression pattern, and ability to interfere and mobilize many other signaling pathways. Table 5 underlines that the cardiac energetic fingerprint of hypothyroidism is unique compared to heart failure. Thus, cellular hypothyroidism can hardly account for the specific energetic alterations of HF or even pathological hypertrophy, but it may be an additional deleterious factor.
Acknowledgments
We thank Dr. Rodolphe Fischmeister for continuous support, Antoine Pilon for thyroid hormone determination, Valérie Domergue-Dupont and the IFR 141 animal care center for their help with animal treatments. Yoni Athea is supported by the « Fondation pour la Recherche Médicale ». Renée Ventura-Clapier is supported by the « Centre National de la Recherche Scientifique ». This work was supported by grant from the European Union Contract n°LSHM-CT-2005-018833/EUGeneHeart.
REFERENCE LIST
- 1.Bahi L, Garnier A, Fortin D, Serrurier B, Veksler V, Bigard AX, Ventura-Clapier R. Differential effects of thyroid hormones on energy metabolism of rat slow- and fast-twitch muscles. J Cell Physiol. 2005;203:589–598. doi: 10.1002/jcp.20273. [DOI] [PubMed] [Google Scholar]
- 2.Bergmeyer HU. Methods in Enzymatic Analysis. 3. III. Weinheim: Verlag Chemie; 1983. Oxidoreductases, transferases; pp. 163–175. [Google Scholar]
- 3.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2006;292:C33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 4.d’Amati G, di Gioia CR, Mentuccia D, Pistilli D, Proietti-Pannunzi L, Miraldi F, Gallo P, Celi FS. Increased expression of thyroid hormone receptor isoforms in end-stage human congestive heart failure. J Clin Endocrinol Metab. 2001;86:2080–2084. doi: 10.1210/jcem.86.5.7456. [DOI] [PubMed] [Google Scholar]
- 5.Davis PJ, Davis FB. Nongenomic actions of thyroid hormone on the heart. Thyroid. 2002;12:459–466. doi: 10.1089/105072502760143827. [DOI] [PubMed] [Google Scholar]
- 6.De Sousa E, Lechene P, Fortin D, N’Guessan B, Belmadani S, Bigard X, Veksler V, Ventura-Clapier R. Cardiac and skeletal muscle energy metabolism in heart failure: beneficial effects of voluntary activity. Cardiovasc Res. 2002;56:260–268. doi: 10.1016/s0008-6363(02)00540-0. [DOI] [PubMed] [Google Scholar]
- 7.De Sousa E, Veksler V, Minajeva A, Kaasik A, Mateo P, Mayoux E, Hoerter J, Bigard X, Serrurier B, Ventura-Clapier R. Subcellular creatine kinase alterations - Implications in heart failure. Circ Res. 1999;85:68–76. doi: 10.1161/01.res.85.1.68. [DOI] [PubMed] [Google Scholar]
- 8.Dzeja PP, Redfield MM, Burnett JC, Terzic A. Failing energetics in failing hearts. Curr Cardiol Rep. 2000;2:212–217. doi: 10.1007/s11886-000-0071-9. [DOI] [PubMed] [Google Scholar]
- 9.Emdin M, Passino C, Prontera C, Iervasi A, Ripoli A, Masini S, Zucchelli GC, Clerico A. Cardiac natriuretic hormones, neuro-hormones, thyroid hormones and cytokines in normal subjects and patients with heart failure. Clin Chem Lab Med. 2004;42:627–636. doi: 10.1515/CCLM.2004.108. [DOI] [PubMed] [Google Scholar]
- 10.Enriquez JA, Fernandez-Silva P, Garrido-Perez N, Lopez-Perez MJ, Perez-Martos A, Montoya J. Direct regulation of mitochondrial RNA synthesis by thyroid hormone. Mol Cell Biol. 1999;19:657–70. doi: 10.1128/mcb.19.1.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenthal MJ, Weiss HR, Marin-Garcia J. Bioenergetic remodeling of heart mitochondria by thyroid hormone. Mol Cell Biochem. 2004;265:97–106. doi: 10.1023/b:mcbi.0000044321.17680.a2. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton MA, Stevenson LW, Luu M, Walden JA. Altered thyroid hormone metabolism in advanced heart failure. J Am Coll Cardiol. 1990;16:91–5. doi: 10.1016/0735-1097(90)90462-x. [DOI] [PubMed] [Google Scholar]
- 14.Hatch GM. Regulation of cardiolipin biosynthesis in the heart. Mol Cell Biochem. 1996;159:139–148. doi: 10.1007/BF00420916. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins TA, Dyck JR, Lopaschuk GD. AMP-activated protein kinase regulation of fatty acid oxidation in the ischaemic heart. Biochem Soc Trans. 2003;31:207–212. doi: 10.1042/bst0310207. [DOI] [PubMed] [Google Scholar]
- 16.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–578. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- 17.Hyyti OM, Ning XH, Buroker NE, Ge M, Portman MA. Thyroid hormone controls myocardial substrate metabolism through nuclear receptor-mediated and rapid posttranscriptional mechanisms. Am J Physiol Endocrinol Metab. 2006;290:E372–E379. doi: 10.1152/ajpendo.00288.2005. [DOI] [PubMed] [Google Scholar]
- 18.Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, L’Abbate A, Donato L. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation. 2003;107:708–713. doi: 10.1161/01.cir.0000048124.64204.3f. [DOI] [PubMed] [Google Scholar]
- 19.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 20.Irrcher I, Adhihetty PJ, Sheehan T, Joseph AM, Hood DA. PPARgamma coactivator-1alpha expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol Cell Physiol. 2003;284:C1669–C1677. doi: 10.1152/ajpcell.00409.2002. [DOI] [PubMed] [Google Scholar]
- 21.Kadenbach B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 2003;1604:77–94. doi: 10.1016/s0005-2728(03)00027-6. [DOI] [PubMed] [Google Scholar]
- 22.Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704–28. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- 23.Kinugawa K, Jeong MY, Bristow MR, Long CS. Thyroid hormone induces cardiac myocyte hypertrophy in a thyroid hormone receptor alpha1-specific manner that requires TAK1 and p38 mitogen-activated protein kinase. Mol Endocrinol. 2005;19:1618–1628. doi: 10.1210/me.2004-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinugawa K, Yonekura K, Ribeiro RC, Eto Y, Aoyagi T, Baxter JD, Camacho SA, Bristow MR, Long CS, Simpson PC. Regulation of thyroid hormone receptor isoforms in physiological and pathological cardiac hypertrophy. Circ Res. 2001;89:591–598. doi: 10.1161/hh1901.096706. [DOI] [PubMed] [Google Scholar]
- 25.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 26.Ling E, O’Brien PJ, Salerno T, Ianuzzo CD. Effects of different thyroid treatments on the biochemical characteristics of rabbit myocardium. Can J Cardiol. 1988;4:301–306. [PubMed] [Google Scholar]
- 27.McClure TD, Young ME, Taegtmeyer H, Ning XH, Buroker NE, Lopez-Guisa J, Portman MA. Thyroid hormone interacts with PPARalpha and PGC-1 during mitochondrial maturation in sheep heart. Am J Physiol Heart Circ Physiol. 2005;289:H2258–H2264. doi: 10.1152/ajpheart.00473.2005. [DOI] [PubMed] [Google Scholar]
- 28.Momken I, Kahapip J, Bahi L, Badoual T, Hittinger L, Ventura-Clapier R, Veksler V. Does angiotensin-converting enzyme inhibition improve the energetic status of cardiac and skeletal muscles in heart failure induced by aortic stenosis in rats? J Mol Cell Cardiol. 2003;35:399–407. doi: 10.1016/s0022-2828(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 29.Mutter T, Dolinsky VW, Ma BJ, Taylor WA, Hatch GM. Thyroxine regulation of monolysocardiolipin acyltransferase activity in rat heart. Biochem J. 2000;346:403–406. [PMC free article] [PubMed] [Google Scholar]
- 30.Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC, Allen PD. Creatine kinase system in failing and non failing human myocardium. Circulation. 1996;94:1894–1901. doi: 10.1161/01.cir.94.8.1894. [DOI] [PubMed] [Google Scholar]
- 31.Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 32.Paradies G, Ruggiero FM, Dinoi P, Petrosillo G, Quagliariello E. Decreased Cytochrome Oxidase Activity and Changes in Phospholipids in Heart Mitochondria from Hypothyroid Rats. Arch Biochem Biophys. 1993;307:91–95. doi: 10.1006/abbi.1993.1565. [DOI] [PubMed] [Google Scholar]
- 33.Park SH, Paulsen SR, Gammon SR, Mustard KJ, Hardie DG, Winder WW. Effects of thyroid state on AMP-activated protein kinase and acetyl-CoA carboxylase expression in muscle. J Appl Physiol. 2002;93:2081–2088. doi: 10.1152/japplphysiol.00504.2002. [DOI] [PubMed] [Google Scholar]
- 34.Ponsot E, Zoll J, N’Guessan B, Ribera F, Lampert E, Richard R, Veksler V, Ventura-Clapier R, Mettauer B. Quantitative and qualitative mitochondrial adaptations of substrates utilizations in rat cardiac and skeletal muscles. J Cell Physiol. 2005;203:479–486. doi: 10.1002/jcp.20245. [DOI] [PubMed] [Google Scholar]
- 35.Quigley AF, Kapsa RM, Esmore D, Hale G, Byrne E. Mitochondrial respiratory chain activity in idiopathic dilated cardiomyopathy. J Card Fail. 2000;6:47–55. doi: 10.1016/s1071-9164(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 36.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 37.Sambandam N, Lopaschuk GD. AMP-activated protein kinase (AMPK) control of fatty acid and glucose metabolism in the ischemic heart. Prog Lipid Res. 2003;42:238–256. doi: 10.1016/s0163-7827(02)00065-6. [DOI] [PubMed] [Google Scholar]
- 38.Schaeffer PJ, Wende AR, Magee CJ, Neilson JR, Leone TC, Chen F, Kelly DP. Calcineurin and calcium/calmodulin-dependent protein kinase activate distinct metabolic gene regulatory programs in cardiac muscle. J Biol Chem. 2004;279:39593–603. doi: 10.1074/jbc.M403649200. [DOI] [PubMed] [Google Scholar]
- 39.Schipke JD. Cardiac efficiency. Basic Res Cardiol. 1994;89:207–240. doi: 10.1007/BF00795615. [DOI] [PubMed] [Google Scholar]
- 40.Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 41.Schlattner U, Eder M, Dolder M, Khuchua ZA, Strauss AW, Wallimann T. Divergent enzyme kinetics and structural properties of the two human mitochondrial creatine kinase isoenzymes. Biol Chem. 2000;381:1063–1070. doi: 10.1515/BC.2000.131. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt-Ott UM, Ascheim DD. Thyroid hormone and heart failure. Curr Heart Fail Rep. 2006;3:114–119. doi: 10.1007/s11897-006-0010-1. [DOI] [PubMed] [Google Scholar]
- 43.Scholz TD, TenEyck CJ, Schutte BC. Thyroid hormone regulation of the NADH shuttles in liver and cardiac mitochondria. J Mol Cell Cardiol. 2000;32:1–10. doi: 10.1006/jmcc.1999.1047. [DOI] [PubMed] [Google Scholar]
- 44.Sharov VG, Goussev A, Lesch M, Goldstein S, Sabbah HN. Abnormal mitochondrial function in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol. 1998;30:1757–1762. doi: 10.1006/jmcc.1998.0739. [DOI] [PubMed] [Google Scholar]
- 45.Sheehan TE, Kumar PA, Hood DA. Tissue-specific regulation of cytochrome c oxidase subunit expression by thyroid hormone. Am J Physiol Endocrinol Metab. 2004;286:E968–F974. doi: 10.1152/ajpendo.00478.2003. [DOI] [PubMed] [Google Scholar]
- 46.Shonk CE, Boxer GE. Enzyme patterns in human tissues. I. Methods for the determination of glycolytic enzymes. Cancer Res. 1964;24:709–721. [PubMed] [Google Scholar]
- 47.Short KR, Nygren J, Barazzoni R, Levine J, Nair KS. T(3) increases mitochondrial ATP production in oxidative muscle despite increased expression of UCP2 and -3. Am J Physiol Endocrinol Metab. 2001;280:E761–E769. doi: 10.1152/ajpendo.2001.280.5.E761. [DOI] [PubMed] [Google Scholar]
- 48.van Bilsen M, Smeets PJ, Gilde AJ, van der Vusse GJ. Metabolic remodelling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res. 2004;61:218–226. doi: 10.1016/j.cardiores.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Veksler VI, Kuznetsov AV, Anflous K, Mateo P, van Deursen J, Wieringa B, Ventura-Clapier R. Muscle creatine kinase-deficient mice.2. Cardiac and skeletal muscles exhibit tissue-specific adaptation of the mitochondrial function. J Biol Chem. 1995;270:19921–19929. doi: 10.1074/jbc.270.34.19921. [DOI] [PubMed] [Google Scholar]
- 50.Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta. 1987;892:191–196. doi: 10.1016/0005-2728(87)90174-5. [DOI] [PubMed] [Google Scholar]
- 51.Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol. 2004;555:1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner MS, Morimoto R, Dora JM, Benneman A, Pavan R, Maia AL. Hypothyroidism induces type 2 iodothyronine deiodinase expression in mouse heart and testis. J Mol Endocrinol. 2003;31:541–50. doi: 10.1677/jme.0.0310541. [DOI] [PubMed] [Google Scholar]
- 53.Wall JA, Wei J, Ly M, Belmont P, Martindale JJ, Tran D, Sun J, Chen WJ, Yu W, Oeller P, Briggs S, Gustafsson AB, Sayen MR, Gottlieb RA, Glembotski CC. Alterations in oxidative phosphorylation complex proteins in the hearts of transgenic mice that overexpress the p38 MAP kinase activator, MAP kinase kinase 6. Am J Physiol Heart Circ Physiol. 2006;291:H2462–H272. doi: 10.1152/ajpheart.01311.2005. [DOI] [PubMed] [Google Scholar]
- 54.Wassen FW, Schiel AE, Kuiper GG, Kaptein E, Bakker O, Visser TJ, Simonides WS. Induction of thyroid hormone-degrading deiodinase in cardiac hypertrophy and failure. Endocrinology. 2002;143:2812–2815. doi: 10.1210/endo.143.7.8985. [DOI] [PubMed] [Google Scholar]
- 55.Weitzel JM, Iwen KAH, Seitz HJ. Regulation of mitochondrial biogenesis by thyroid hormone. Exp Physiol. 2003;88:121–128. doi: 10.1113/eph8802506. [DOI] [PubMed] [Google Scholar]
- 56.Weitzel JM, Kutz S, Radtke C, Grott S, Seitz HJ. Hormonal regulation of multiple promoters of the rat mitochondrial glycerol-3-phosphate dehydrogenase gene: identification of a complex hormone-response element in the ubiquitous promoter B. Eur J Biochem. 2001;268:4095–4103. doi: 10.1046/j.1432-1327.2001.02332.x. [DOI] [PubMed] [Google Scholar]
- 57.Weitzel JM, Radtke C, Seitz HJ. Two thyroid hormone-mediated gene expression patterns in vivo identified by cDNA expression arrays in rat. Nucleic Acids Res. 2001;29:5148–5155. doi: 10.1093/nar/29.24.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wrutniak-Cabello C, Casas F, Cabello G. Thyroid hormone action in mitochondria. J Mol Endocrinol. 2001;26:67–77. doi: 10.1677/jme.0.0260067. [DOI] [PubMed] [Google Scholar]
- 59.Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000;87:E61–E68. doi: 10.1161/01.res.87.12.e61. [DOI] [PubMed] [Google Scholar]