Abstract
Background
The aim of the present study was to assess the efficacy of a ready-to-use injectable bone substitute for bone regeneration around dental implants placed into fresh extraction sockets.
Methods
Third and fourth mandibular premolars were extracted from 3 Beagle dogs and the interradicular septa were surgically reduced to induce a mesial bone defect. Thereafter, immediate placements of titanium implants were performed. On the left side of the jaw, mesial bone defects were filled with an injectable bone substitute (IBS), obtained by combining a polymer and a biphasic calcium phosphate ceramic. As a control, the right defects were left unfilled. After 3 months of healing, specimens were prepared for histological and histomorphometric evaluations.
Results
No post surgical complication was observed during the healing period. In all experimental conditions, histological observations revealed a lamellar bone formation in contact with the implant. Histomorphometric analysis showed that IBS triggers a significant (p<0.05) increase in term of thread numbers in contact with bone (TN), bone-to-implant contact (BIC) and peri-implant bone density (PBD), of about 8.6%, 11.0% and 14.7%, respectively. In addition, no significant difference was observed when TN, BIC and PBD in filled defects were compared to no-defect sites.
Conclusion
It is concluded that an injectable bone substitute composed of a polymeric carrier and calcium phosphate significantly increase bone regeneration around immediate implants.
Keywords: Alveolar Process; Animals; Dogs; Durapatite; Female; Injections; Methylcellulose; Osseointegration; Osteogenesis; Tooth Socket; Wound Healing; Biocompatible Materials; Bone Density; Bone Regeneration; Bone Substitutes; Calcium Phosphates; Ceramics; Dental Implantation, Endosseous; Dental Implants
INTRODUCTION
Delayed placement of dental implants after alveolar healing is a proven and aesthetic technique, but requires an extended time treatment before patients can enjoy the final result.1,2 Immediate implantation after tooth extraction is an attractive alternative that presents several advantages such as reduction of post-extraction resorption, optimal positioning of the implant and reduction of the time required for prosthetic rehabilitation.3,4 However, the discrepancy between the transversal diameter of the socket (conical) and of the implant (cylindrical) frequently generates a gap between the bony walls of the socket and the implant.5 In large bony defects, this void can be colonised by epithelial cells, which induces fibrointegration and then implant failure.6,7 Therefore, guided bone regeneration (GBR) alone or in association with bone replacement graft has been developed and currently gives satisfactory results to achieve implant osseointegration.7–9 Nevertheless, an early exposure of the barrier membrane may promote secondary infections and consequently delay the peri-implant bone regeneration.10,11 Rotated full thickness flaps were suggested as an alternative to GBR, but vestibular flaps were often a source of aesthetical problems and palatal flaps may provoke pain on exposed bone.12,13
Among the available materials used for pre-implant bone reconstruction, autologous bone is currently the gold-standard because it is a source of osseous matrix, cells, and growth modulating molecules.14 However, it requires the graft to be harvested at a distance from the operation site, which makes the initial operation more complicated. To overcome the autograft limits, many substitution biomaterials have been proposed. Materials of human and animal origin have the disadvantages of limited supply and potential risk of cross contamination.15,16 Consequently, synthetic products were developed.17 For example, biphasic calcium phosphate (BCP), an intimate mixture of hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP).18 BCP offers the potential for bone reconstruction since it has a chemical composition close to biologic bone apatites19 and has already proven its efficacy as a bone substitute material in many human clinical applications.20–24 More recently, the association of BCP granules to a polymeric gel has provided a ready-to-use injectable bone substitute material (IBS).25,26 Previous animal studies conducted in our laboratory have shown the biocompatibility and the osteoconductive property of IBS.27–29 We have also demonstrated in a preliminary study the IBS biofunctionality when used as socket filler in dogs.30
The aim of the present study was to assess the IBS influence on bone regeneration around titanium dental implants immediately placed after tooth extraction without using an osteopromotive membrane technique. A comparative histomorphometric study was performed on peri-implant bone defects. Defects were treated by IBS filling and the control defects were left unfilled to observe a physiological healing process.
MATERIALS AND METHODS
Injectable Bone Substitute
The biomaterial used in this study was a composite combining a polymer and a calcium phosphate mineral phase.31
Mineral phase
The ceramic composed of BCP granules with a 60/40 HA/β-TCP weight ratio was obtained by hydrolysis of a commercial dicalcium phosphate dihydrate. The resulting apatite powders were granulated and sifted to select only granules with a 40 to 80 μm diameter. After sintering at 1,150°C to form BCP, the granules were placed on a 40 μm sifter to eliminate the smallest particles.
Polymeric phase
As previously described,25 the associated polymer was a cellulose derivative (methyl-hydroxy-propyl-cellulose = MHPC) previously proposed as an efficient vehicle for the mineral phase.32 Briefly, a solution of 3% MPHC was prepared by dissolving raw dry MPHC powder§ in double distilled water with stirring for 48 hours.
Composite
The composite biomaterial, obtained by mixing a 3% MPHC solution with the 40 to 80 μm BCP granules in a 50/50 weight ratio, was placed in ready-to-use plastic injectors and sterilized by steam at 121°C for 20 minutes. This process preserves the gel consistency of the polymer and decreases viscosity only very slightly.30
Anim al experim ents
The three 4-year-old female adult Beagle dogs used were bred exclusively for biomedical studies and kept at the National Veterinary School of Nantes according to European Community guidelines for the care and the use of laboratory animals (DE 86/609/CEE). Gingival health was checked before experimentation, and teeth were scaled and polished under general anaesthesia. Antibiotic treatment with spiramycin and metronidazole|| was given for 5 days.
Surgical procedures
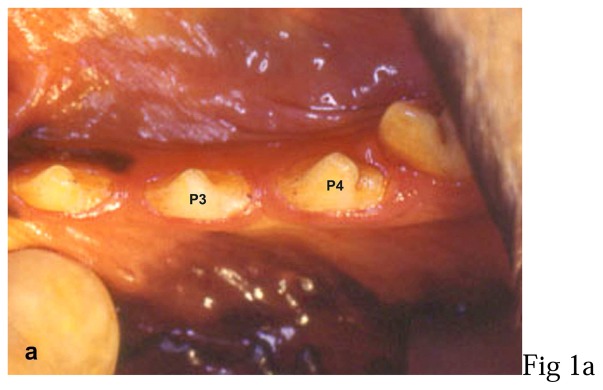
All surgical procedures were performed under general anaesthesia with intravenous sodium thiopental¶ (12.5 mg/kg) followed by volatile anaesthesia with halothane. During surgery, animals received 1 g of cephalosporin antibiotic# perfused intravenously. Implant placements were performed on right and left third and fourth mandibular premolars (fig. 1-a).
Figure 1. Photographs of the surgical procedures.
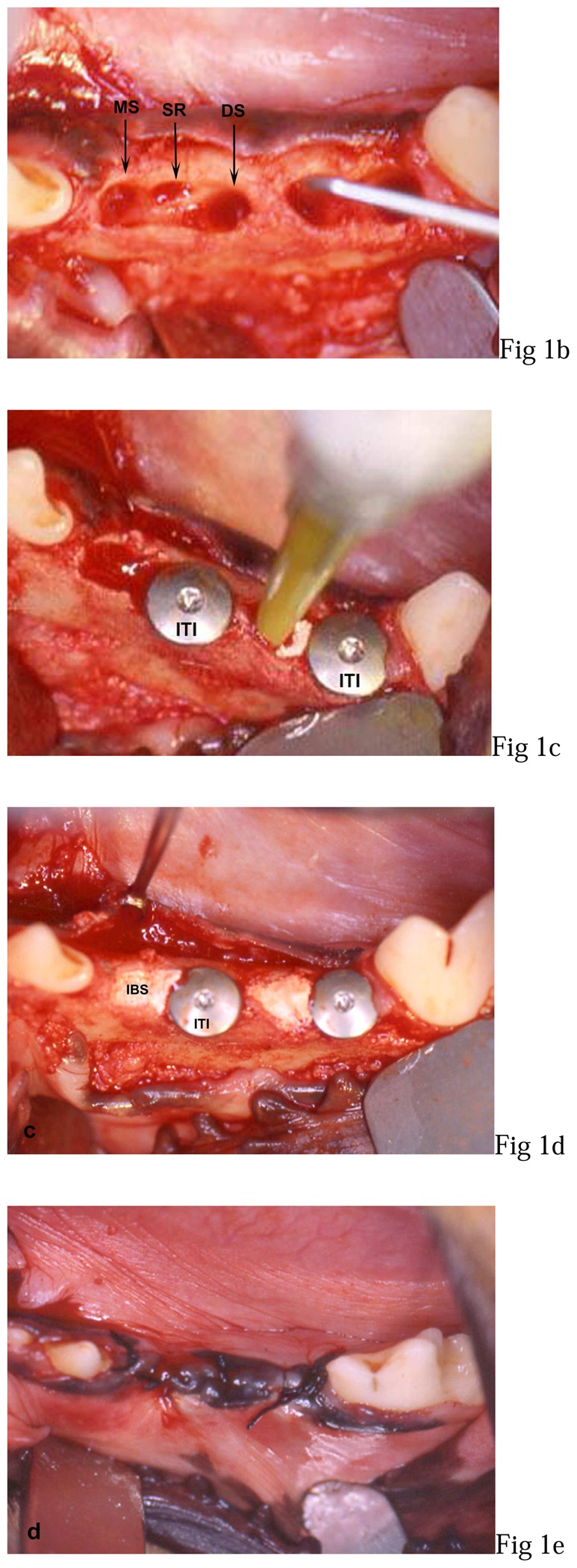
a: third (P3) and fourth (P4) left mandibular premolar (preoperative view),
b: mesial socket after extraction (MS), interadicular septum resection (SR), distal socket preparation (DS) in P3 and mesial bone defect (MS + SR) probing in P4,
c: implants (ITI) placed in distal socket of P3 before filling with IBS and IBS injection in mesial bone defect of P4,
d: 2 implants placed in distal sockets and non-overpacked fill with IBS,
e: overlapping flap sutures.
Gingival incisions were performed mesially from the first premolar and distally to the molar teeth. Then, full-thickness buccal and lingual flaps were raised. After vertical interradicular section, each root was carefully elavated and then gently extracted.
Distal sockets were then drilled following the basic surgical principles governing the placement of ITI dental implants.33 Thereafter, the interradicular septa were resected with a mini-rongeur Friedman to create a mesial bone defect adjacent to the mesial socket on a 6 mm height, 4 mm in the bucco-lingual direction and 5 mm in the mesio-distal direction (fig. 1-b). After a complete alveolar cleaning, solid cylindrical screw implants** were manually inserted in the implant beds (fig. 1-c). Specifications of ITI implants included a 3.3 mm diameter, an 8 mm length and a titanium plasma spray (TPS) coating. The primary stability was assessed and implants were covered with 1.5 mm closure screws. On the left side, mesial sockets were filled with IBS injection from the bottom to the top of the defect (fig. 1-c) in direct contact with the implant (fig. 1-d). On the right side, implants were placed following the same procedure but without IBS. The muccoperiosteal flaps were then sutured with an interrupted non-absorbent suture†† (fig. 1-e).
Antibiotic treatment by intramuscular injection of cephalosporin (15 mg/kg, b.i.d.) was continued for 48 hours after surgery. Animals were checked daily and fed with a soft diet. Sutures were removed under short general anaesthesia 3 weeks later (day 21), and a normal diet was then given. The animals were sacrificed 3 months after implantation (day 91) by intravenous injection of overdosed sodium pentobarbital‡‡.
Sample preparation
Bone segments were immediately dissected from animals with a diamond disk and fixed in a 4% paraformaldehyde solution buffered with phosphate buffered saline (PBS; pH 7.2). Each sample was then dehydrated by successive immersion in graded ethanol for 24 hours at 4°C (80°, 95°, 100°) and acetone (1 day). Thereafter, specimen were infiltrated with a glycolmethylmetacrylate resin (GMMA) at −20°C for 8 days (repeated twice). Finally, samples were embedded in GMMA (4 days, 4 °C).
Histological evaluation
Both filled and unfilled sites were histologically evaluated with light§§ and scanning electron|||| microscopy (SEM). For each sample, 10 μm thick sections were cut with a diamond microtome saw¶¶ along to the long axis of implants in a mesiodistal direction and then stained with Movat pentachrome for light microscope observation. Then, three 500 μm sections were isolated from each sample and both surfaces were prepared by sputtering with gold-palladium## for SEM observations at 20 kV.
Histo morphometric studies
For quantitative evaluation of bone regeneration, 500 μm sections prepared as described above were observed with SEM apparatus coupled to a semi-automatic image analyser***. With image analysis, three parameters were measured in mesial bone defects and distal sites:
The number of threads in contact with bone in relation to the total number of threads (TN),
-
The Bone-to-Implant Contact (BIC) with a percentage ratio studied at two different heights:
the endosseous part of the implant (EH)
a 3 mm cervical height limited to the bone defects (DH),
-
The Peri-implant-Bone-Density (PBD) inside DH on 3 zones at a 0.5 mm thickness:
zone a : from the implant surface to 0.5 mm,
zone b : from 0.51 to 1 mm,
zone c : from 1.1 to 1.5 mm.
X- ray micro-analysis
Sample sections (500 μm thick) were polished in order to eliminate gold-palladium and were sputter coated with carbon†††. Then, a quantitative X-ray micro-analysis was performed with Energy Dispersive System (EDS)‡‡‡. Calcium (Ca), phosphate (P), magnesium (Mg), and sodium (Na) values were measured at four different locations with regard to bone defects (IBS filled and unfilled), alveolar bone (in implant proximity) and basal bone.
Statistical analysis
Results were expressed as mean (± SEM). Comparative study of means of the 16 filled sites, the 18 unfilled sites and the 34 distal sites were performed using one way analysis of variance (ANOVA) followed by a post-hoc test (Fischer’s projected least significant differences) with a statistical significance at p<0.05.
RESULTS
Clinical results
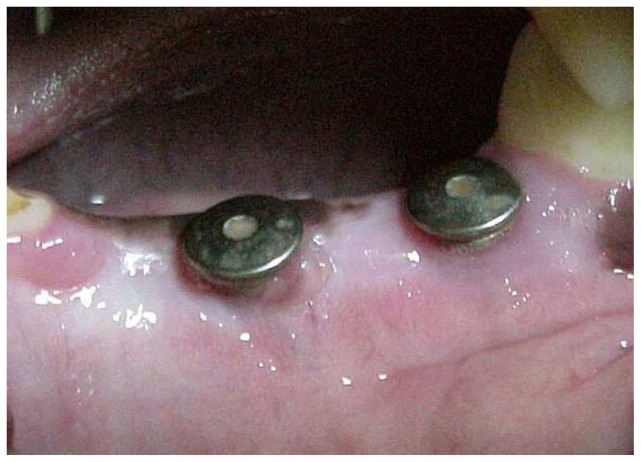
No post surgical complication or infection was observed during the healing period. Sutures were removed after three weeks. After one month, the closure screws initially covered by muccoperiosteal flaps were exposed with excellent peri-implant soft tissue conditions (fig. 2). At the time of euthanasia, all implants seemed to be clinically osseointegrated and none presented with mobility.
Figure 2.
Photograph one month after surgery. Note the soft tissues healing around closure screws emergence.
Histological results
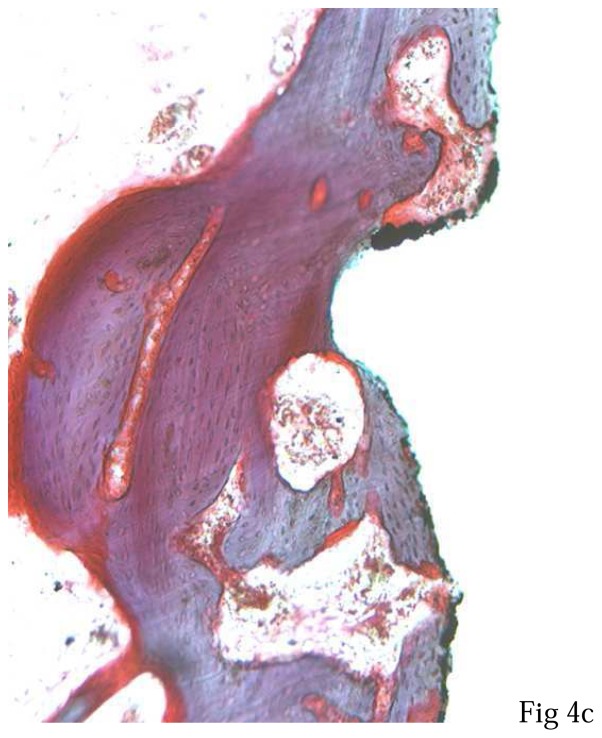
Light microscopy observations (magnification x 50) showed homogeneous bone healing in all tested conditions (fig. 3). A bone-to-implant contact was observed on all the distal threads and on most mesial threads in filled defects. An exposure of the first or the second thread was observed in five unfilled defects. Higher magnification (x200) confirmed the close contacts between lamellar bone and implant TPS surface (fig. 4). As previously described34, in the filled defects (fig. 4a), BCP granules were not visible and bone tissue showed the same histological characteristics observed in the distal sites (fig. 4b). For all implants, junctional epithelium was closely adapted to the machined smooth neck of the implant.
Figure 3.
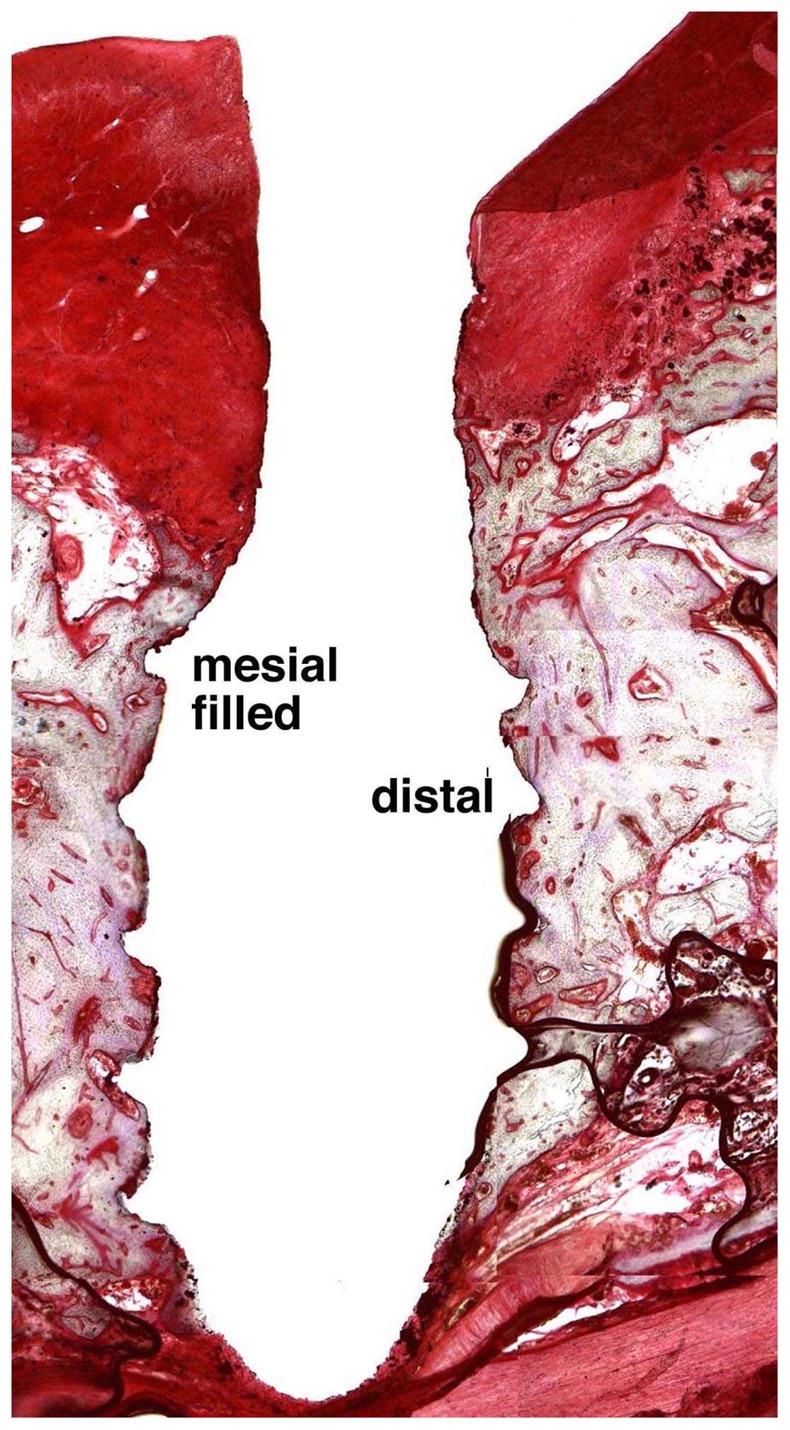
Light microscopy observation of an axial section of implant after three months of healing with IBS. Note the homogeneous bone healing around implant.
(Movat pentachrome, original magnification x 50).
Figure 4. Light microscopy observations of the first cervical thread of implants after three months of healing. Note different bone regenerations at the implant contact.
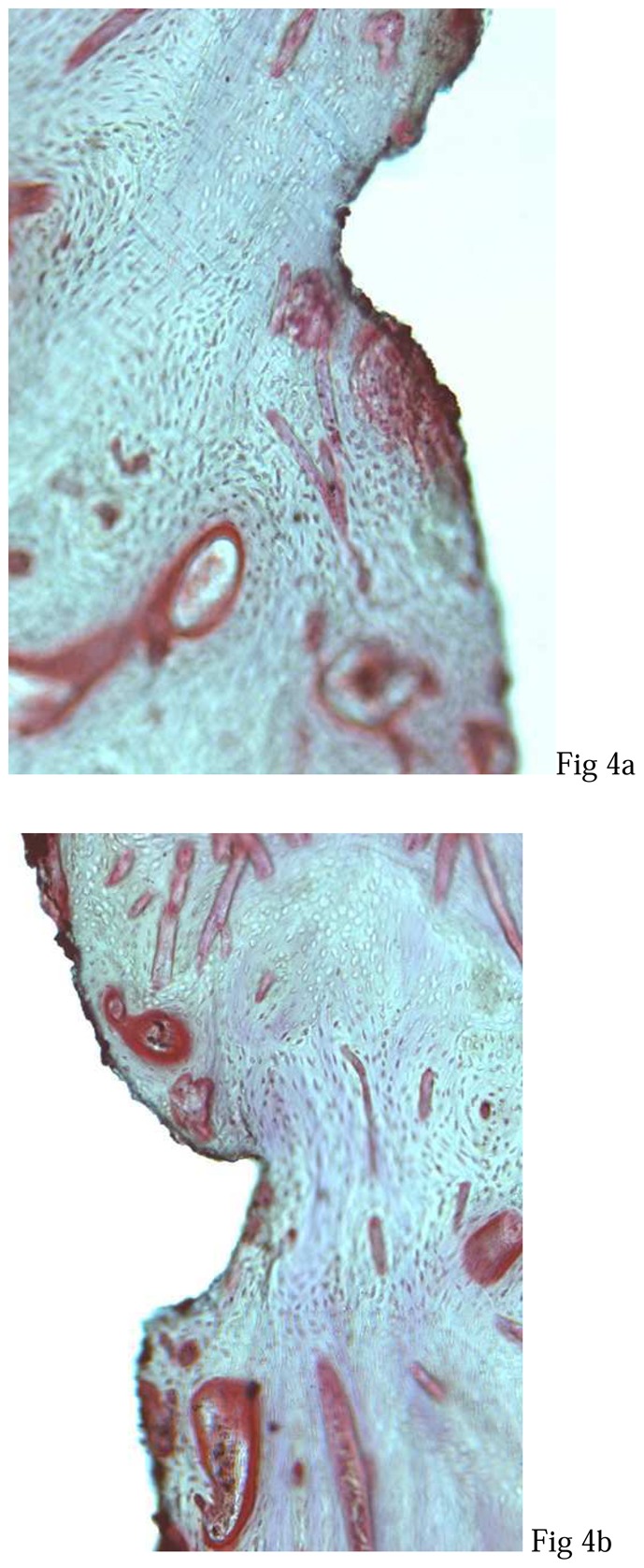
a: mesial defect filled by IBS,
b: distal site without defect,
c: mesial defect unfilled by IBS.
(Movat pentachrome, original magnification x 200).
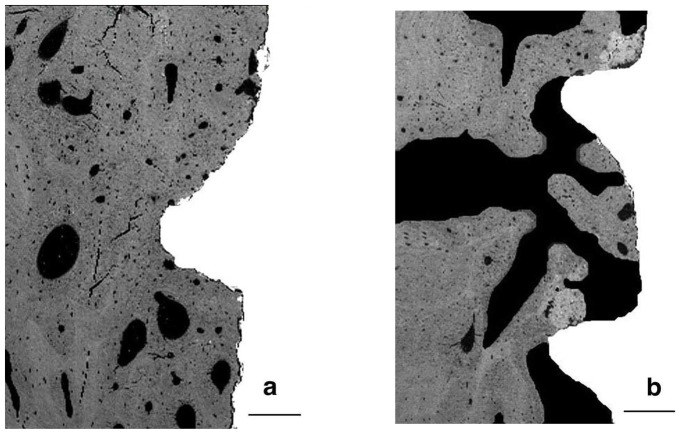
SEM showed new bone formation more homogenous and dense in the filled sites (fig. 5a) as compare to the unfilled sites (fig. 5b). Large unmineralised areas were observed in absence of IBS (fig. 6b), whereas bone tissue occupied a large extent of the filled sites (fig. 6a).
Figure 5. Scanning electron microscopy observations of axial sections of implants after three months of healing.
a: mesial filled defect (left) and distal site (right). Note the presence of an homogeneous bone ingrowth in the filled defect when compared with distal site,
b: unfilled mesial defect (left) and distal site (right). Note the presence of decreased bone regeneration in mesial unfilled defect when compared with distal site.
(bar = 1 mm).
Figure 6. Scanning electron microscopy observations of the cervical threads of implants after three months of healing.
a: filled defect. Note the close contact between bone and TPS surface
b: unfilled defect. Note the presence of large unmineralised areas
(bar = 0.2 mm).
Quantitative evaluation
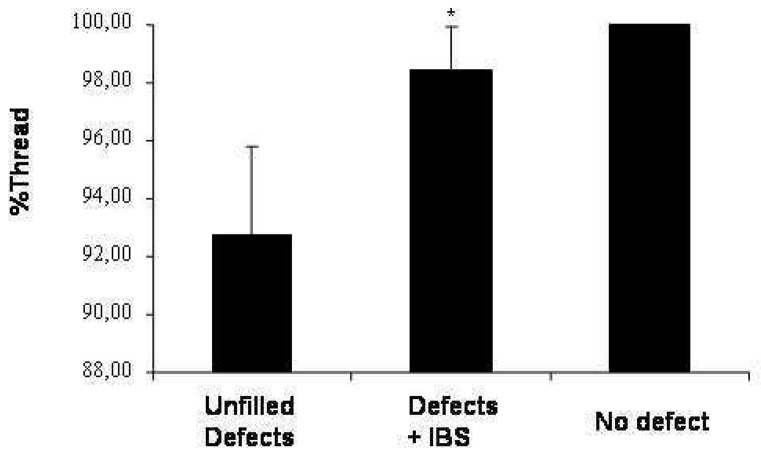
For all distal sites, threads were always in contact with bone surface. On the contrary, TN was always lower in mesial bone defects. However, in mesial bone defects, TN remained always significantly (p<0.01) higher in the presence of IBS as compare to the unfilled defect (fig. 7) (98.44% ±1.52 and 92.78% ±2.98 respectively).
Figure 7.
Threads of implants in contact with bone after three months of defect healing either in sites filled (defects + IBS) or unfilled (unfilled defects) with IBS. As a control, distal sites were left without bone defects (no-defect). Results are expressed in percentage (± SEM) of threads not in contact with bone.
* : p < 0.01 compared with the unfilled defects.
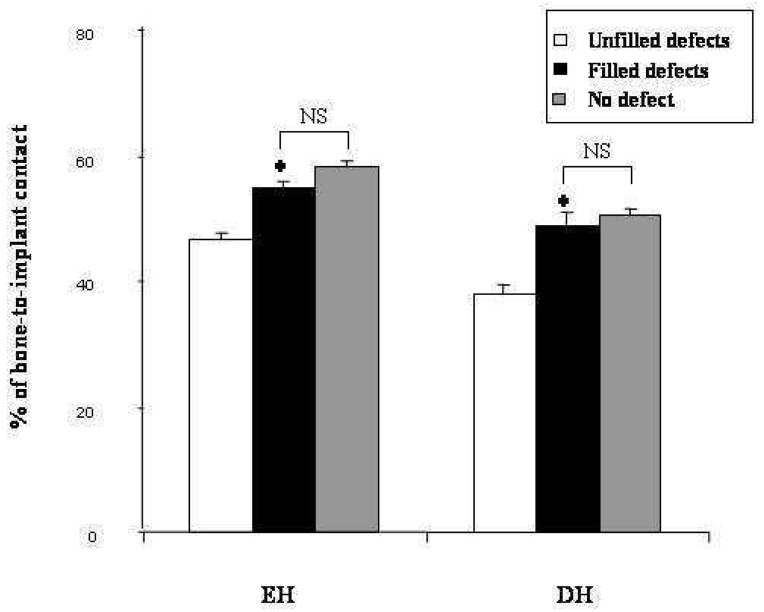
For EH, BIC in filled defects showed a significant (p<0.01) increase when compared with the unfilled defects (54.54% ±1.29 and 46.67% ±1.08 respectively). In addition, a significant augmentation of BIC in DH was observed in filled sites (48.86% ±1.97 vs 37.60% ±1.58 in unfilled defects). Interestingly, BIC was not significantly different between the filled mesial sites and the distal sites (fig. 8).
Figure 8.
Bone-to-implant contact in endo-osseous height of implants (EH) and in bone defect height (DH) after three months of defect healing either in sites filled (filled defects) or unfilled (unfilled defects) with IBS. As a control, distal sites were left without bone defects (no-defect). Results are expressed in height percentage (± SEM).
*: p < 0.01 compared with the unfilled defects.
NS: no significant
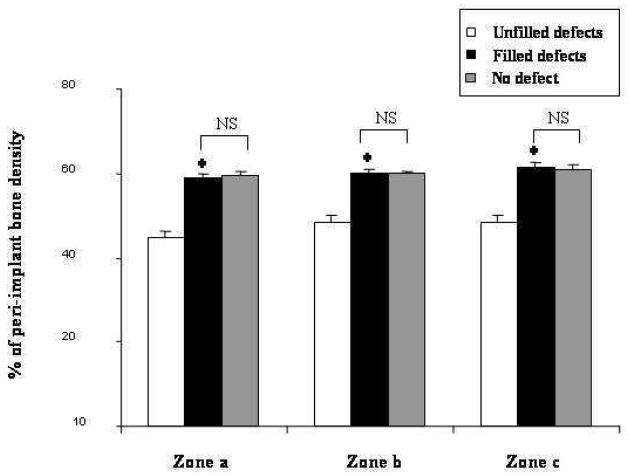
In all sites, PBD was always significantly lower for the zone close to the implant (zone a) as compared to the zones located at a distance (zones b and c). No significant difference was observed in zone b and zone c. Whatever the studied zone, minor PBD was obtained in unfilled defects. IBS induced a significant (p<0.01) increase of PBD in zone a, b and c: 58.68% ±2.32 vs 44.5% ±1.76; 60.1% ±1.48 vs 48.46% ±1.51 and 61.71% ±0.85 vs 48.21% ±1.68 respectively. Furthermore, there was no significant difference between PBD values in mesial filled sites and those of distal sites (fig. 9).
Fig. 9.
Peri-implant bone density of 3 various zones after three months of defect healing either in sites filled (filled defects) or unfilled (unfilled defects) with IBS. As a control, distal sites were left without bone defects (no-defect). As described in materials and methods, zone a was from TPS surface to 0.5 mm, zone b, from 0.51 to 1 mm, zone c, from 1.1 to 1.5 mm. Results are expressed as percentage (± SEM) of total surface in each zone.
* : p < 0.01 compared with the unfilled defects.
NS: no significant
The atomic composition of the analysed samples in EDS showed no significant difference with respect to the studied locations. All the measurements in the implant peripheries were very close to basal bone values. Calcium and phosphate values were respectively of 23.29% ±1.95 and 13.81% ±0.96 in filled bone defects. Magnesium and sodium represented less than 1% of the total elements. Ca/P ratio showed very little variation (from 1.66 ±0.03 for basal bone to 1.70 ±0.07 in distal sites). In the filled and unfilled defects, this ratio was similar (1.68 ±0.04 with IBS and 1.68 ±0.06 without IBS).
DISCUSSION
To our knowledge, this study demonstrates for the first time that the use of an injectable bone substitute, composed of a calcium phosphate ceramic and a polymeric carrier, promotes bone regeneration around dental implants immediately placed into fresh extractions sockets.
Cortical and trabecular bone grafts are the materials of choice for pre-implant surgery. Among the biomaterials proposed as an alternative to bone autografts, calcium phosphate (CaP) ceramics such as hydroxyapatite (HA), beta-tricalcium phosphate (β-TCP) and the HA/β-TCP association [termed biphasic calcium phosphate (BCP)] have been used successfully because their chemical composition is closely related to that of bone mineral.19 Within the past few years, a proposed ready-to-use injectable bone substitute (IBS) has been developed. IBS is an association of BCP granules with a cellulosic hydrogel.25 Our laboratory has previously demonstrated the osteoconductive potential of this innovative biomaterial in an alveolar bone site.30,35
In contrast to the delayed implantation protocol, immediate implant placement presents numerous advantages including prevention of alveolar bone resorption and reduction of rehabilitation time.3,4 Nevertheless, the success rate of both methods remains similar when primary stability of implants is associated with an osteopromotive technique.36,37 To favour bone healing in cases where there are wide gaps between implant surfaces and sockets walls, guided bone regeneration (GBR), alone or in association with bone substitute materials, has been investigated with satisfactory but variable results.5,10 In an attempt to propose an alternative to occlusive barrier membranes, the present work investigates the efficiency of IBS to promote bone regeneration around solid-screw implants placed immediately after tooth extraction.
Immediate implantation in dogs has displayed variable failure rates.10,38 The present study has shown no implant mobility and no implant failure. This clinical success rate is comparable to that obtained with delayed implants placed after a 3 month healing period.39 Several hypotheses may explain these encouraging results: atraumatic extraction with respect to alveolar walls; no bone heating during the defect creation thanks to the manual rongeur; implant primary stability through preservation of vestibular and lingual processes; postoperative soft diet to reduce mastication forces and daily postoperative observation to prevent gingival inflammation.
To strengthen our clinical data, we conducted histological examinations of peri-implant tissues regeneration. SEM and light microscopic observations showed mucosa, osteoid and mineralised lamellar bone, but no residual BCP particles despite high magnification, as previously reported.34 Furthermore, EDS demonstrated that newly-formed bone presents the same Ca and P values as well as Ca/P ratio with regards to basal bone. Thus, IBS confirmed his osteoconductive potential previously demonstrated.30,35 Nevertheless, the lack of information during intermediary intervals did not permit evaluation of the biomaterial transformation kinetics, which however seemed to be compatible with healing periods currently recommended for immediate implantation.5,40
Numerous unmineralised areas were observed in unfilled defects witch suggest a delay in bone healing. However, partial bone regeneration was observed in these sites. In filled sites, bone regeneration showed the same histological characteristics as compared to no-defect sites. Interestingly, the absence of fibrous interposition in filled defects strongly suggest that IBS may represent an occlusive barrier against epithelial cell colonisation.
The main objective of this work was to obtain reliable quantitative assessment of the different parameters of bone regeneration: the number of osseointegrated threads (TN), the rate of bone-to-implant contact (BIC), and the peri-implant bone density (PBD). Among the numerous available tools, we focused on semi-automatic image analysis because it permits quick and accurate analysis of a large amount of information.41,42 Previous histomorphometrical studies, dedicated to implant osseointegration in animals, have mostly been restricted to BIC evaluation.43,44 In order to further evaluate the bone regeneration process beyond the bone/implant interface, image analysis was also conducted on PBD at various distances from the implant surface.
A centripetal bone regeneration from the alveolar walls is even observed in unfilled sites because experimental defects were not critical-size defects.30 However, all quantitative data remains lower in unfilled defects. In addition, TN, BIC and PBD in filled defects demonstrated no significant difference as compared to the no-defect sites. Since the quantitative results in IBS-treated sites are higher or equal to the values reported with immediate implantation in dog,10,44–47 IBS appears to satisfy immediate implantation requirements.
CONCLUSION
This study demonstrates that our injectable bone substitute (IBS), a composite biomaterial, promotes bone regeneration around implants immediately placed after tooth extraction. Therefore, it seems conceivable that IBS could be an alternative to autologous grafts or GBR alone or in association with bone substitute biomaterial. However, these promising results are to be confirmed by further biomechanical studies after implant loading, pull out tests and by evaluation of reverse torque. IBS could be further propose for clinical applications such as socket filling for ridge preservation, pre-implant reconstruction of osseous deficiencies or sinus floor elevations.
Acknowledgments
The authors gratefully acknowledge Dr J.M. Bouler for providing us with calcium phosphate material and for his helpful contribution to EDS analysis.
The authors wish to thank the Straumann AG Institute (Waldenburg, CH) for providing ITI dental implants.
The authors wish to thank Drs Julie Kazimiroff and Racquel Z LeGeros (Department of Biomaterials and Biomimetics, New York University College of Dentistry) and Ms Karine Sinander for their editorial assistance.
Footnotes
E4M PREMIUM EP, Colorcon, Bougival, France
Stomorgyl, Rhône-Mérieux, Lyon, France
Nesdonal, Rhône-Mérieux
Cefaloject, Bristol Laboratories, Paris, France
ITI Esthetic plus, Straumann AG Institute, Waldenburg, Switzerland
Silk black braided, PRED, Paris, France
Doléthal, Vétoquinol laboratory, Lure, France
Axioplan 2, Zeiss, Jena, Germany
JSM 6300, Jeol, Tokyo, Japan
MS 1600, Leitz, Westlar, Germany
AE1230, EMScope, Ashford, UK
Quantimeter 500, Leica, Cambridge, UK
JEE-4B, Jeol
EXL II, Oxford instruments, London, UK
References
- 1.Albrektsson T, Dahl E, Enbom L, et al. Osseointegrated oral implants. A Swedish multicenter study of 8139 consecutively inserted Nobelpharma implants. J Periodontol. 1988;59:287–296. doi: 10.1902/jop.1988.59.5.287. [DOI] [PubMed] [Google Scholar]
- 2.Branemark PI, Zarb GA, Albrektsson T. Tissue-integrated prostheses. Osseointegration in clinical dentistry. Chicago: Quintessence; 1985. p. 352. [Google Scholar]
- 3.Block MS, Kent JN. Placement of endosseous implants into tooth extraction sites. J Oral Maxillofac Surg. 1991;49:1269–1276. doi: 10.1016/0278-2391(91)90302-3. [DOI] [PubMed] [Google Scholar]
- 4.Yukna RA. Placement of hydroxyapatite-coated implants into fresh or recent extraction sites. Dent Clin North Am. 1992;36:97–115. [PubMed] [Google Scholar]
- 5.Huys LW. Replacement therapy and the immediate post-extraction dental implant. Implant Dent. 2001;10:93–102. doi: 10.1097/00008505-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Paolantonio M, Dolci M, Scarano A, et al. Immediate implantation in fresh extraction sockets. A controlled clinical and histological study in man. J Periodontol. 2001;72:1560–1571. doi: 10.1902/jop.2001.72.11.1560. [DOI] [PubMed] [Google Scholar]
- 7.Nemcovsky CE, Moses O, Artzi Z, Gelernter I. Clinical coverage of dehiscence defects in immediate implant procedures: three surgical modalities to achieve primary soft tissue closure. Int J Oral Maxillofac Implants. 2000;15:843–852. [PubMed] [Google Scholar]
- 8.Becker W, Lynch SE, Lekholm U, et al. A comparison of e-PTFE membranes alone or in combination with platelet-derived growth factors and insulin-like growth factor-I or demineralized freeze-dried bone in promoting bone formation around immediate extraction socket implants. J Periodontol. 1992;63:929–940. doi: 10.1902/jop.1992.63.11.929. [DOI] [PubMed] [Google Scholar]
- 9.Gher ME, Quintero G, Assad D, Monaco E, Richardson AC. Bone grafting and guided bone regeneration for immediate dental implants in humans. J Periodontol. 1994;65:881–891. doi: 10.1902/jop.1994.65.9.881. [DOI] [PubMed] [Google Scholar]
- 10.Alliot B, Piotrowski B, Marin P, Zahedi S, Brunel G. Regeneration procedures in immediate transmucosal implants: an animal study. Int J Oral Maxillofac Implants. 1999;14:841–848. [PubMed] [Google Scholar]
- 11.Simion M, Baldoni M, Rossi P, Zaffe D. A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period. Int J Periodontics Restorative Dent. 1994;14:166–180. [PubMed] [Google Scholar]
- 12.Rosenquist B, Grenthe B. Immediate placement of implants into extraction sockets: implant survival. Int J Oral Maxillofac Implants. 1996;11:205–209. [PubMed] [Google Scholar]
- 13.Nemcovsky CE, Artzi Z, Moses O, Gelernter I. Healing of marginal defects at implants placed in fresh extraction sockets or after 4–6 weeks of healing. A comparative study. Clin Oral Implants Res. 2002;13:410–419. doi: 10.1034/j.1600-0501.2002.130410.x. [DOI] [PubMed] [Google Scholar]
- 14.Misch CE, Dietsh F. Bone-grafting materials in implant dentistry. Implant Dent. 1993;2:158–167. doi: 10.1097/00008505-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Pasquier G, Hardouin P, Fontaine C, Migaud H, Duquennoy A. Different methods of bone filling in orthopedic surgery (in French) Rev Rhum Mal Osteoartic. 1992;59:821–828. [PubMed] [Google Scholar]
- 16.Zerbib R, Ouhayoun JP, Freyss G. Bone augmentation in implant surgery (in French) J Parodontol. 1991;10:177–188. [PubMed] [Google Scholar]
- 17.Damien CJ, Parsons JR. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991;2:187–208. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 18.Bouler JM, LeGeros RZ, Daculsi G. Biphasic calcium phosphates: influence of three synthesis parameters on the HA/β-TCP ratio. J Biomed Mater Res. 2000;51:680–684. doi: 10.1002/1097-4636(20000915)51:4<680::aid-jbm16>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 19.LeGeros RZ. Material for bone repair, augmentation and implants coating. In: Niwa P, editor. Biomechanics in orthopedics. Tokyo: Springler-Verlag; 1991. pp. 147–174. [Google Scholar]
- 20.Nery EB, Lee KK, Czajkowski S, et al. A Veterans Administration Cooperative Study of biphasic calcium phosphate ceramic in periodontal osseous defects. J Periodontol. 1990;61:737–744. doi: 10.1902/jop.1990.61.12.737. [DOI] [PubMed] [Google Scholar]
- 21.Nery EB, LeGeros RZ, Lynch KL, Lee K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J Periodontol. 1992;63:729–735. doi: 10.1902/jop.1992.63.9.729. [DOI] [PubMed] [Google Scholar]
- 22.Nery EB, Eslami A, Van SR. Biphasic calcium phosphate ceramic combined with fibrillar collagen with and without citric acid conditioning in the treatment of periodontal osseous defects. J Periodontol. 1990;61:166–172. doi: 10.1902/jop.1990.61.3.166. [DOI] [PubMed] [Google Scholar]
- 23.Piattelli A, Scarano A, Mangano C. Clinical and histologic aspects of biphasic calcium phosphate ceramic (BCP) used in connection with implant placement. Biomaterials. 1996;17:1767–1770. doi: 10.1016/0142-9612(95)00342-8. [DOI] [PubMed] [Google Scholar]
- 24.Daculsi G, Passuti N, Martin S, Deudon C, LeGeros RZ, Raher S. Macroporous calcium phosphate ceramic for long bone surgery in humans and dogs. Clinical and histological study. J Biomed Mater Res. 1990;24:379–396. doi: 10.1002/jbm.820240309. [DOI] [PubMed] [Google Scholar]
- 25.Weiss P, Gauthier O, Bouler JM, Grimandi G, Daculsi G. Injectable bone substitute using a hydrophilic polymer. Bone. 1999;25:67S–70S. doi: 10.1016/s8756-3282(99)00146-5. [DOI] [PubMed] [Google Scholar]
- 26.Bourges X, Schmitt M, Amouriq Y, Daculsi G, Legeay G, Weiss P. Interaction between hydroxypropylmethylcellulose and biphasic calcium phosphate after steam sterilisation: capillary gas chromatography studies. J Biomater Sci Polym Ed. 2001;12:573–579. doi: 10.1163/156856201316883412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gauthier O, Bouler JM, Weiss P, Bosco J, Aguado E, Daculsi G. Short-term effects of mineral particle sizes on cellular degradation activity after implantation of injectable calcium phosphate biomaterials and the consequences for bone substitution. Bone. 1999;25:71S–74S. doi: 10.1016/s8756-3282(99)00137-4. [DOI] [PubMed] [Google Scholar]
- 28.Gauthier O, Bouler JM, Weiss P, Bosco J, Daculsi G, Aguado E. Kinetic study of bone ingrowth and ceramic resorption associated with the implantation of different injectable calcium-phosphate bone substitutes. J Biomed Mater Res. 1999;47:28–35. doi: 10.1002/(sici)1097-4636(199910)47:1<28::aid-jbm4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 29.Dupraz A, Delecrin J, Moreau A, Pilet P, Passuti N. Long-term bone response to particulate injectable ceramic. J Biomed Mater Res. 1998;42:368–375. doi: 10.1002/(sici)1097-4636(19981205)42:3<368::aid-jbm4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 30.Gauthier O, Boix D, Grimandi G, et al. A new injectable calcium phosphate biomaterial for immediate bone filling of extraction socket: a preliminary study in dogs. J Periodontol. 1998;70:359–367. doi: 10.1902/jop.1999.70.4.375. [DOI] [PubMed] [Google Scholar]
- 31.Daculsi G, Weiss P, Delecrin J, Grimandi G, Passuti N. CNRS Patent WO 95/21634: Biomaterial composition - preparation proceeding (licence: Biomatlante, MBCP gelTM) France: Feb 8, 1994. [Google Scholar]
- 32.Grimandi G, Weiss P, Millot F, Daculsi G. In vitro evaluation of a new injectable calcium phosphate material. J Biomed Mater Res. 1998;39:660–666. doi: 10.1002/(sici)1097-4636(19980315)39:4<660::aid-jbm22>3.0.co;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buser D, von Arx T, ten Bruggenkate C, Weingart D. Basic surgical principles with ITI implants. Clin Oral Implants Res. 2000;11(Suppl 1):59–68. doi: 10.1034/j.1600-0501.2000.011s1059.x. [DOI] [PubMed] [Google Scholar]
- 34.Bodic F, Amouriq F, Gauthier O, et al. Computed tomography assessment of alveolar filling with an injectable bone substitute. J Mater Sci Mater Med. 2002;13:953–958. doi: 10.1023/a:1019808613046. [DOI] [PubMed] [Google Scholar]
- 35.Amouriq Y. Thesis. Nantes: Odontologie of Nantes; 2001. Contribution à l’étude d’un substitut osseux phosphocalcique injectable, évaluation en site alvéolaire, intérêt préprothétique; p. 209. [Google Scholar]
- 36.Barber HD, Seckinger RJ, Silverstein K, Abughazaleh K. Comparison of soft tissue healing and osseointegration of IMZ implants placed in one-stage and two-stage techniques: a pilot study. Implant Dent. 1996;5:11–14. doi: 10.1097/00008505-199600510-00002. [DOI] [PubMed] [Google Scholar]
- 37.Becker W, Becker BE, Ricci A, et al. A prospective multicenter clinical trial comparing one- and two-stage titanium screw-shaped fixtures with one-stage plasma-sprayed solid-screw fixtures. Clin Implant Dent Relat Res. 2000;2:159–165. doi: 10.1111/j.1708-8208.2000.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 38.Levy D, Deporter DA, Pilliar RM, Watson PA, Valiquette N. Initial healing in the dog of submerged versus non-submerged porous-coated endosseous dental implants. Clin Oral Implants Res. 1996;7:101–110. doi: 10.1034/j.1600-0501.1996.070203.x. [DOI] [PubMed] [Google Scholar]
- 39.Cochran DL, Nummikoski PV, Higginbottom FL, Hermann JS, Makins SR, Buser D. Evaluation of an endosseous titanium implant with a sandblasted and acid-etched surface in the canine mandible: radiographic results. Clin Oral Implants Res. 1996;7:240–252. doi: 10.1034/j.1600-0501.1996.070306.x. [DOI] [PubMed] [Google Scholar]
- 40.Cosci F, Cosci B. A 7-year retrospective study of 423 immediate implants. Compend Contin Educ Dent. 1997;18:940–946. [PubMed] [Google Scholar]
- 41.Gauthier O, Bouler JM, Aguado E, Pilet P, Daculsi G. Macroporous biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials. 1998;19:133–139. doi: 10.1016/s0142-9612(97)00180-4. [DOI] [PubMed] [Google Scholar]
- 42.Nefussi JR, Ollivier A, Oboeuf M, Forest N. Rapid nodule evaluation computer-aided image analysis procedure for bone nodule quantification. Bone. 1997;20:5–16. doi: 10.1016/s8756-3282(96)00319-5. [DOI] [PubMed] [Google Scholar]
- 43.Barzilay I, Graser GN, Iranpour B, Natiella JR, Proskin HM. Immediate implantation of pure titanium implants into extraction sockets of Macaca fascicularis. Part II: Histologic observations. Int J Oral Maxillofac Implants. 1996;11:489–497. [PubMed] [Google Scholar]
- 44.Ettinger RL, Spivey JD, Han DH, Koorbusch GF. Measurement of the interface between bone and immediate endosseous implants: a pilot study in dogs. Int J Oral Maxillofac Implants. 1993;8:420–427. [PubMed] [Google Scholar]
- 45.Gottlander M, Albrektsson T, Carlsson LV. A histomorphometric study of unthreaded hydroxyapatite-coated and titanium-coated implants in rabbit bone. Int J Oral Maxillofac Implants. 1992;7:485–490. [PubMed] [Google Scholar]
- 46.Gotfredsen K, Nimb L, Buser D, Hjorting-Hansen E. Evaluation of guided bone generation around implants placed into fresh extraction sockets: an experimental study in dogs. J Oral Maxillofac Surg. 1993;51:879–884. doi: 10.1016/s0278-2391(10)80108-9. [DOI] [PubMed] [Google Scholar]
- 47.Karabuda C, Sandalli P, Yalcin S, Steflik DE, Parr GR. Histologic and histomorphometric comparison of immediately placed hydroxyapatite-coated and titanium plasma-sprayed implants: a pilot study in dogs. Int J Oral Maxillofac Implants. 1999;14:510–515. [PubMed] [Google Scholar]