Abstract
Background
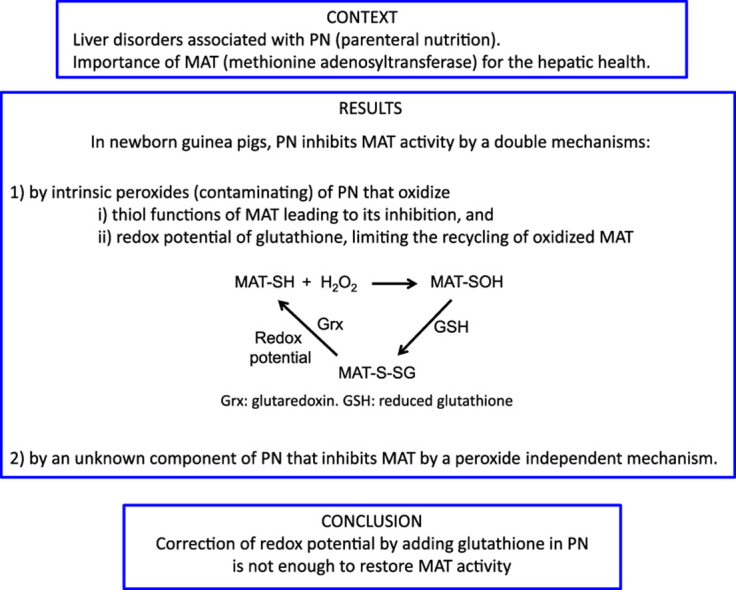
The oxidation of the methionine adenosyltransferase (MAT) by the combined impact of peroxides contaminating parenteral nutrition (PN) and oxidized redox potential of glutathione is suspected to explain its inhibition observed in animals. A modification of MAT activity is suspected to be at origin of the PN-associated liver disease as observed in newborns. We hypothesized that the correction of redox potential of glutathione by adding glutathione in PN protects the MAT activity.
Aim
To investigate whether the addition of glutathione to PN can reverse the inhibition of MAT observed in animal on PN.
Methods
Three days old guinea pigs received through a jugular vein catheter 2 series of solutions. First with methionine supplement, (1) Sham (no infusion); (2) PN: amino acids, dextrose, lipids and vitamins; (3) PN-GSSG: PN+10 μM GSSG. Second without methionine, (4) D: dextrose; (5) D+180 μM ascorbylperoxide; (6) D+350 μM H2O2. Four days later, liver was sampled for determination of redox potential of glutathione and MAT activity in the presence or absence of 1 mM DTT. Data were compared by ANOVA, p<0.05.
Results
MAT activity was 45±4% lower in animal infused with PN and 23±7% with peroxides generated in PN. The inhibition by peroxides was associated with oxidized redox potential and was reversible by DTT. Correction of redox potential (PN+GSSG) or DTT was without effect on the inhibition of MAT by PN. The slope of the linear relation between MAT activity and redox potential was two fold lower in animal infused with PN than in others groups.
Conclusion
The present study suggests that prevention of peroxide generation in PN and/or correction of the redox potential by adding glutathione in PN are not sufficient, at least in newborn guinea pigs, to restore normal MAT activity.
Keywords: Parenteral nutrition, Peroxide, Newborn, Methionine adenosyltransferase, Thiol oxidation, Redox potential of glutathione.
Graphical abstract
Highlights
-
•
Methionine adenosyltransferase (MAT) is essential for healthy liver.
-
•
Parenteral nutrition (PN) inhibits hepatic MAT.
-
•
The inhibition is caused by intrinsic peroxides and by unknown component of PN.
-
•
Adding glutathione in PN is not sufficient to prevent PN-associated liver diseases.
1. Introduction
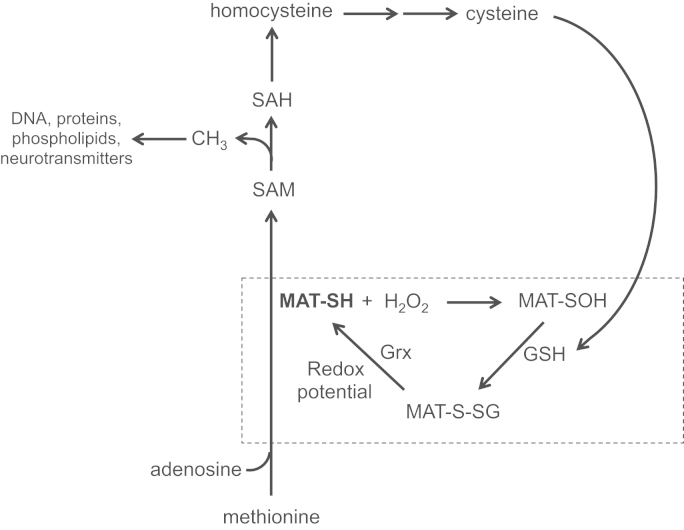
The intravenous nutritional support for individuals who have impaired or immature gastrointestinal tract such as extreme premature infants is essential for their development and health. However, several hepatic complications are associated with this mode of nutrition. In adults, parenteral nutrition (PN) induces hepatic steatosis [1], [2] whereas the intra-hepatic cholestasis is frequent in premature infants [3], [4]. Animal data suggest that peroxides, H2O2 and ascorbylperoxide (2,3-diketo-4-hydroxyperoxyl-5,6-dihydroxyhexanoic acid), that contaminating parenteral nutrition [5], [6] are involved in these disorders [7], [8]. Peroxides can lead to perturbation of the metabolism following oxidation of the redox-sensitive thiol functions of specific proteins (Fig. 1). Hence, the activity of the hepatic methionine adenosyltransferase (MAT) is inhibited by PN or infused H2O2 [9].
Fig. 1.
Interrelation between MAT activity, peroxides and glutathione. The active form of MAT (MAT-SH) is responsible for the transformation of methionine in cysteine in order to sustain the synthesis of glutathione (GSH). In a context of PN, when MAT is inhibited (MAT-SOH) by peroxides (H2O2) generated into PN, a vicious cycle occurs. The low activity of MAT induces a low synthesis of GSH that is essential for the MAT recovery. By a compromised generation of the methyl donor S-adenosylmethionine (SAM), several metabolisms (proteins, DNA, phospholipids, neurotransmitters, etc.) are altered. SAH: S-adenosylhomocysteine.
MAT is at the crossroads of several metabolic pathways (Fig. 1). For instance, MAT catalyzes the formation of S-adenosylmethionine, the main methyl donor of the organism [10], [11]. Perturbation in the generation of S-adenosylmethionine is frequently associated with hepatic disorders such as intrahepatic cholestasis [12], [13]. The activity of MAT is the first step in the transformation of methionine into cysteine of which the availability is a limiting step for glutathione synthesis [14]. Intracellular concentration of GSH affects the activity of glutathione peroxidase during detoxification of peroxides [17]. Thus, peroxides generated in the PN can induce a vicious cycle by inhibiting MAT that leads to a lower GSH [9], and therefore to a lower capacity to detoxify the infused peroxides.
The oxidation of thiol into sulfenic acid (MAT-SOH) by peroxide is reversible [15]. The mixed disulfide (MAT-SSG) formed following interaction of MAT-SOH with GSH is recycled into the native protein by the glutaredoxin using glutathione as electron donor. We expect that the inhibition of MAT by PN occurs by this mechanism. The influence of the redox potential in the regeneration of MAT-SH, from MAT-SSG, is explained by its participation in the Gibbs equation [16] explaining a better efficiency of glutaredoxin in a more reduced environment.
Recently we have reported that addition of glutathione into PN led to a more reduced status of redox potential in lungs of newborn guinea pigs [17]. We hypothesize that the addition of glutathione in PN will decrease the redox potential value (to a more reduced status) in the liver, and consequently, will improve the regeneration of MAT activity. Therefore, the objectives of the study were (1) to compare the redox potential values as well as the hepatic MAT activities in newborn guinea pigs receiving a PN enriched or not with glutathione, or intravenous solutions containing peroxides, (2) to assess that the inhibition is caused by oxidation of thiols by using dithiothreitol (DTT), and (3) to document the relation between the redox potential and the activity of MAT.
2. Methods
2.1. Animal model
At three days of life, Hartley guinea pigs (Charles River Laboratories, St-Constant, QC, Canada) were anaesthetized by using ketamine and xylazine in order to fix a jugular catheter (Lake Villa, IL, USA). The catheter was placed and externalized in the scapular region, and connected to the infusion system. The studied solutions were infused continuously through the catheter at rate of 22 ml/100 g body weight/d. The solutions were changed daily.
2.2. Experimental designs
We examined the stability of both GSH and GSSG in parenteral nutrition solution to decide which molecule should be used in further experiments. In plasma, γ-glutamyltranspeptidase uses GSSG and GSH with the same efficiency to enrich the tissues into cysteine (essential for the cellular synthesis of GSH) [17], [18]. 20 µM GSH or 10 µM GSSG (20 µM GSH equivalent) were added to PN (without lipid). After 1, 3, 5 and 24 h incubation at room temperature, samples were collected for the determination of total glutathione (GSH+GSSG) using a colorimetric method [19] as previously described [17].
Based on the report documenting that diets with different intakes in methionine influence the activity of MAT [20], two different protocols were used to assess the impact of PN or peroxides on the MAT activity and on redox potential. For the first protocol three groups of animals, in which methionine was included in the nutrition, were compared:
-
(1)
Sham: The catheter was closed and animals were fed the regular laboratory food for guinea pigs.
-
(2)
PN: Animal were exclusively on intravenous solution containing 2% (w,v) amino acids (Primene, Baxter, Toronto, ON, Canada) 8,7% (w,v) dextrose, 1% (v,v) multivitamin preparation (Multi-12/K1 pediatrics, Sandoz, Boucherville, QC, Canada), 1.6% (w,v) lipid emulsion (Intralipid 20%, Fresenius Kabi, Mississauga, ON, Canada) and 1 U/ml heparin.
-
(3)
PN+10 µM GSSG: Animals were exclusively on PN containing 10 µM GSSG. This form of glutathione was choice to avoid interactions with other components of PN [17].
For the second protocol three other groups of animals were compared. In order to isolate the effect of peroxides, the only carbon source for energy was dextrose (no amino acids or lipids):
-
(4)
D: Animals were infused with a solution containing 8.7% (w,v) dextrose, 0.3% (w,v) NaCl and 1 U/ml heparin.
-
(5)
AscOOH: Animals were infused with D containing 180 µM ascorbylperoxide; a concentration inducing perturbation of hepatic lipid and glucose metabolism [8] as well as redox potential in liver [8] and in lung [21] of newborn guinea pigs.
-
(6)
H2O2: Animals were infused with D containing 350 µM H2O2; similar concentration of peroxides reported as contaminant in PN [21].
Four days later, at seven days of age, all animals were sacrificed. The liver samples were removed, processed and stored at −80 °C until biochemical determinations.
In accordance with the principles of the Canadian Council, the Institutional committee for good practice with animals in research of CHU Sainte-Justine approved the present protocol.
2.3. Determinations of redox potential of glutathione:
Briefly, as previously described [9], [21], 0.5 g of liver was mixed with 5 volumes of 5% (w/v) freshly prepared metaphosphoric acid and homogenized on ice during 20 s with Polytron (Biospec Products, Bartlesville, OK, USA). After centrifugation 3 min at 10,000 RPM, supernatants were isolated for glutathione determination and pellets were used for protein measurement. GSH and GSSG were separated by capillary electrophoresis (Beckman Coulter). Assuming a density of 1.0 for the liver, the redox potential was calculated (25 °C, pH 7) by using the Nernst equation.
2.4. Determination of MAT activity
The activity of MAT was quantified on the cytosolic fraction of liver as previously described [9]. Briefly, 300 μg of protein (measured by the Bradford essay with BSA as standard) were suspended in the buffer (75 mM Tris/HCL, 250 mM KCl, 9 mM MgCl2, pH 7.8) that contained substrates (5 mM methionine+5 mM [2,8-³H]ATP (1 Ci/mol)) for a final volume of 150 µl, and were incubated 30 min at 37 °C. To validate whether the inhibition of MAT was caused by oxidation of thiol functions of the protein, the buffer used for livers from Sham, PN and H2O2 groups contained or not, 1 mM dithiothreitol (DTT). Immediately after stopping the reaction by adding 3 ml of ice water, the total 3.15 ml were applied onto a 0.5 ml Dowex AG5OW column (BioRad laboratories). The column was treated with 20 ml water followed by 4 ml of 3 M NH4OH to displace the tritiated S-adenosylmethionine. The activity was calculated and expressed as nmol S-adenosylmethionine formed/min/mg protein.
2.5. Statistical analysis
Results were expressed as mean±s.e.m. and were orthogonally compared by ANOVA after verification of the homogeneity of the variances by the Bartlett’s Chi squared. Paired ANOVA was used to analyze the impact of DTT on the MAT activity. The Pearson’s correlations between MAT activity and redox potential value were reported. The significance of the difference was set at p<0.05.
3. Results
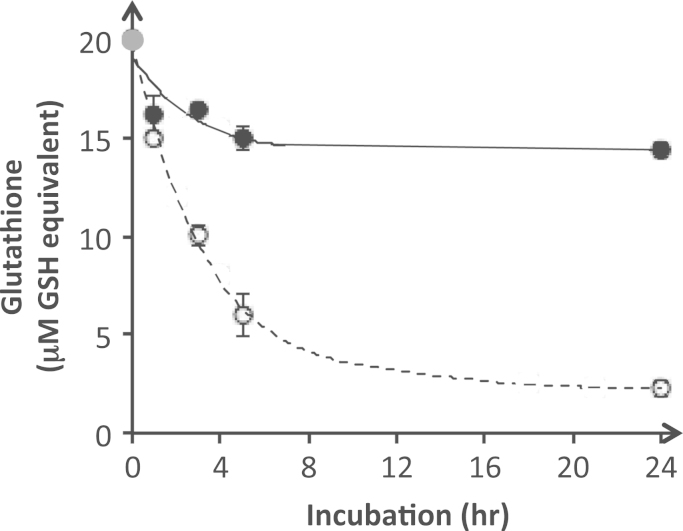
The stability of glutathione in PN (Fig. 2) differed according to its redox form. After 3 h incubation the concentration of GSH was 50±2% of the initial value whereas it remained 82±1% of GSSG. After 24 h, it was 11±2% of GSH and 72±2% of GSSG.
Fig. 2.
Stability of GSH and GSSG in PN. PN contained 2% (w,v) amino acids, 8,7% (w,v) dextrose, 1% (v,v) multivitamin preparation and 1 U/ml heparin. The gray circle represents the initial concentration of GSH and GSSG added in PN. The concentration of total glutathione (GSH+GSSG, expressed in GSH equivalent) decreased in function of time. The drop was greater with GSH (open circle) than with GSSG (dark circle). Mean±s.e.m. (some s.e.m. are smaller than the symbol), n=3.
The initial body weights (108±1.7 g, n=43) as well as the relative liver weights (3.5±0.1 g/100 g body weight) were similar between groups.
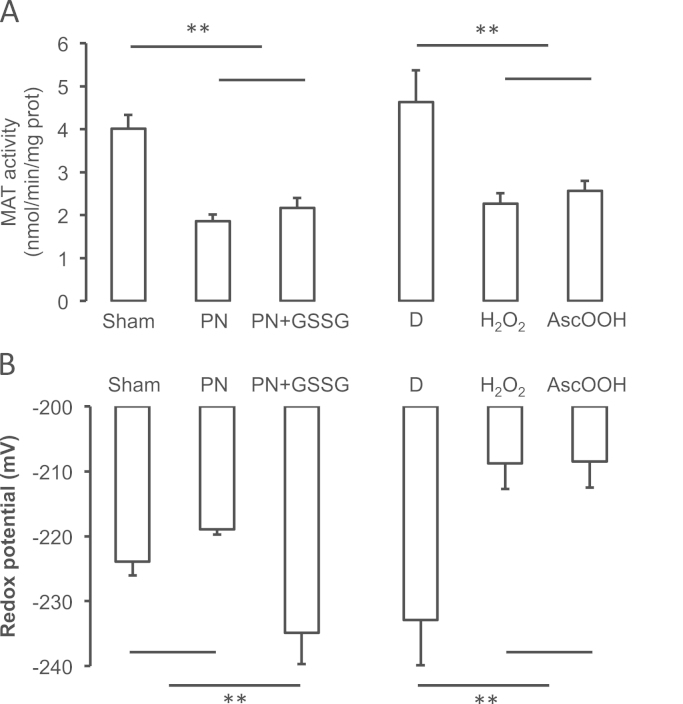
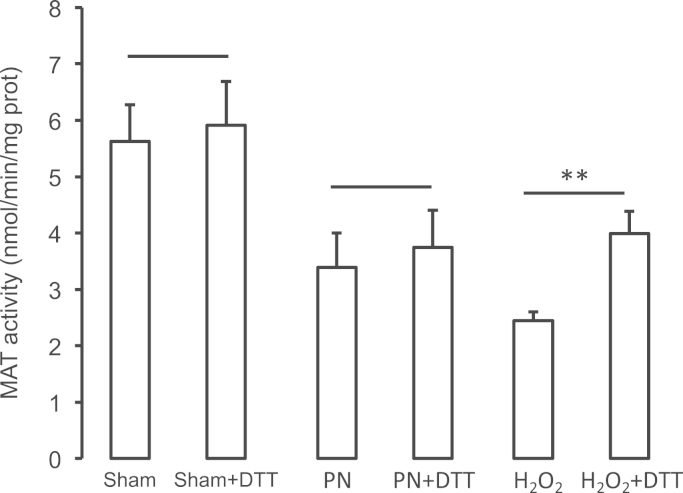
Compared to the Sham group, MAT activity (Fig. 3A) was lower (p<0.01) in groups that received PN or PN+GSSG. There was no difference (F(1,17)=1.1) between PN and PN+GSSG groups. Compared to the Control group (D), the MAT activity was lower (p<0.01) in groups that received H2O2 or ascorbylperoxide. There was no difference (F(1,18)=0.3) between H2O2 or ascorbylperoxide groups. The presence of DTT (Fig. 4) in assay has increased the activity of MAT only in the H2O2 group. DTT was without effect in the Sham (F(1,14)=1.4) and PN (F(1,14)=2.2) groups.
Fig. 3.
Impact of intravenous infusion of isolated peroxides or of the presence or absence of glutathione in PN on the activity of methionine adenosyltransferase (MAT) and on the redox potential of glutathione in liver. Sham: animals with closed catheter and enterally fed with chow; PN: animals exclusively on parenteral nutrition; PN+GSSG: 10 μM GSSG added to PN; D: animals infused with a solution of dextrose; H2O2: D+350 µM H2O2; AscOOH: D+180 μM ascorbylperoxide. Two sets of statistical analyses were used according of the presence of methionine into their nutrition (Sham, PN, PN+GSSG). Panel A: The MAT activities were lower (p<0.01) in groups PN and PN+GSSG compared to the Sham group and in groups H2O2 and AscOOH compared to the Control group (D). Panel B: The redox potential of glutathione was more reduced (lower) (p<0.01) in PN+GSSG group than in Sham and PN and in D group compared to H2O2 and AscOOH groups. Mean±s.e.m., n=4–9/group. ** p<0.01.
Fig. 4.
Impact of DTT on the activity of methionine adenosyltransferase (MAT). Sham: animals with closed catheter and enterally fed with chow; PN: animals exclusively on parenteral nutrition; H2O2: animals infused with a solution of dextrose containing 350 µM H2O2. The presence of 1 mM DTT in the assay has improved (p<0.01) the activity of MAT only in the H2O2 group. Mean±s.e.m., n=4–7/group. ** p<0.01.
The redox potential of glutathione (Fig. 3B) was lower (p<0.01) in PN+GSSG group compared to the Sham group and PN groups. There was no difference (F(1,15)=0.4) between Sham and PN groups. Compared to the Control group (D), redox potential of glutathione was higher (p<0.01) in groups that received H2O2 or ascorbylperoxide. There was no difference (F(1,15)=0.01) between H2O2 and ascorbylperoxide groups.
The GSH values (Table 1) were higher (p<0.01) in PN+GSSG group compared to the Sham group and PN groups. There was no difference (F(1,15)=0.5) between Sham and PN groups. Compared to the control group (D), GSH levels were lower (p<0.01) in groups that received H2O2 or ascorbylperoxide. There was no difference (F(1,15)=0.06) between H2O2 or ascorbylperoxide groups. Levels of GSSG (Table 1) did not statistically differ between groups (F(1,15)<3.1).
Table 1.
Hepatic GSH and GSSG values as a function of treatments.
| Sham | PN | PN+GSSG | D | H2O2 | AscOOH | |
|---|---|---|---|---|---|---|
| GSH (nmol/mg prot) | 45±2 | 34±4 | 76±14* | 79±18† | 21±1 | 23±3 |
| GSSG (nmol/mg prot) | 0.58±0.07 | 0.71±0.12 | 0.58±0.07 | 0.67±0.10 | 0.44±0.11 | 0.5±0.03 |
Sham: animals with closed catheter and enterally fed with chow; PN: animals exclusively on parenteral nutrition; PN+GSSG: 10 µM GSSG added to PN; D: animals infused with a solution of dextrose; H2O2: D+350 µM H2O2; AscOOH: D+180 µM ascorbylperoxide. Two sets of statistical analyses were used according of the presence of methionine into their nutrition (Sham, PN, PN+GSSG). (1) There was no statistical difference in GSH between Sham and PN, both are different from PN+GSSG (*p<0.01). (2) There was no statistical difference in GSH between H2O2 and AscOOH, both are different from D (†p<0.01). The GSSG levels did not differ between groups. Mean±s.e.m., n=4–7/group.
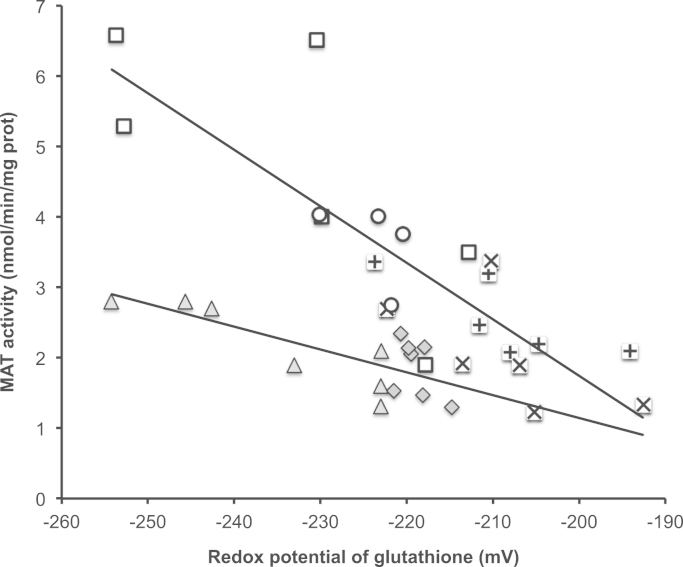
The influence of the redox potential on MAT activity (Fig. 5) was linear and significant (r2=0.70, p<0.01) among the groups Sham, D, H2O2 and ascorbylperoxide (AscOOH) (−0.080 U mV−1 x–14.3 U; U=nmol/min/mg prot). The relation was also significant (r2=0.56, p<0.01) for the groups PN and PN+GSSG (−0.032 U mV−1 x–5.3 U). However, the slopes were statistically different (p<0.01, respectively (IC95): −0.080 (−0.105 to −0.056) and −0.032 (−0.050 to −0.014) U mV−1).
Fig. 5.
Activity of hepatic methionine adenosyltransferase (MAT) in function of the redox potential of glutathione. The relation between MAT activity and the redox potential was statistically different (p<0.01) according to that the animals were infused with PN (±GSSG) or not. Without PN, the redox potential explained 70% of the variation in MAT activity (r2=0.70, p<0.01). With PN, the redox potential explained 56% of the variation in MAT activity (r2=0.56, p<0.01). However, with PN groups, the slope of the linear relationship was half (p<0.01) that observed with animals without PN. Open circles: Sham; open squares: D; x: H2O2; +: AscOOH; gray diamonds: PN; gray triangles: PN+GSSG.
4. Discussion
The main finding of the study is that, at least in newborn guinea pig, the mechanism of inhibition of the hepatic methionine adenosyltransferase (MAT) by parenteral nutrition (PN) is not explained solely by the classical and reversible oxidation of its thiol functions by the peroxides present in the nutritive solution. Therefore, the prevention of peroxides formation in PN or improving the redox potential value in liver by adding glutathione would not be enough to eliminate hepatic metabolic complications associated with this mode of nutrition.
The results confirm the induction of a lower activity of MAT [9] and a higher (more oxidized) redox potential of glutathione [9], [21] in animals infused with PN or with peroxides that contaminate the nutritive solution. A classical way of inhibition of MAT is the reversible oxidation of its redox sensitive thiol functions by peroxides (Fig. 1). It is through this mechanism that the H2O2 inhibits MAT, since the activity is recovered by using DTT. The failure to obtain a rescue with DTT in tissues from PN group suggests that the inhibition is caused by a different way than oxidation of thiols by peroxide.
The metabolic importance of the activity of MAT led us to investigate a way to reverse its inhibition. Previously, we have added glutathione in PN to correct the low level of glutathione that is observed both in newborn animals [22] and in premature infants [23]. The rational was that (1) the hepatic transformation of methionine into cysteine by MAT is low in premature infants [24] and in individuals on PN [9], (2) the availability of cysteine being a limiting step for the glutathione synthesis, the liver produces and releases a lower quantity of glutathione in blood stream [25], (3) the presence of glutathione in PN allows to reach a higher plasma concentration of glutathione [17], (4) cellular γ-glutamyltranspeptidase uses GSH as well as GSSG present in plasma to enrich cells in cysteine for a de novo synthesis of GSH. Thus the addition of GSSG in PN has prevented the oxidation of redox potential in lung of animal on PN [17]. With the same strategy, we wanted to improve the hepatic redox potential in order to improve the capacity of the liver to recycle the oxidized MAT (Fig. 1). However, the results shown that even with an enhanced GSH level as well as a more reduced redox potential in animals fed with PN+GSSG, the activity of MAT remained inhibited. Yet, the value of the redox potential influences the MAT activity (Fig. 5). Nevertheless, there was a strong difference according if animals received or not PN. 70% (r2=0.70) of the activity of MAT was explained by the redox potential in animals without PN. Data from all groups but PN (Sham, D, H2O2, ascorbylperoxide) were aligned on the same correlation, where the activity increases by one unit (nmol/min/mg prot) at each 12.5 mV reduction in the redox potential. 56% (r2=0.56) of the activity of MAT was explained by the redox potential in animals on PN. However, here, the efficiency of redox potential on MAT is lower; a reduction of 33 mV in redox potential is required to observe a one-unit increase in MAT activity. This 2.5 fold magnitude of change required in redox to obtain a same impact on MAT activity underlines that the inhibition observed with PN is from another kind in addition to oxidative. Of course, the relation between MAT and the redox potential is bi-directional; the activity of MAT has also an influence on glutathione synthesis. Nevertheless, the difference between slopes remains and supports the presence of a different mechanism of inhibition between peroxides and PN.
In liver, MAT activity is regulated by the oxidative state of their numerous thiols (10 per subunit [26]) that are present in active site (cysteinyl residue 121(11)) or are involved in oligomerization of the enzyme [11]. MAT I is a homo tetramer whereas MAT III is a homo dimer [26]. MAT I has a greater affinity for methionine (Km about 30 μM in rat liver [27]) than MAT III [11], [27] (Km about 200 μM [27]). At physiological concentration of methionine (60 μM), the activity of MAT I is 10 fold greater than MAT III [26]. The global activity of MAT as measured here is dependent of the proportion of MAT I and III. Their oligomerization involves several thiol functions [11], [26]. Sanchez-Perez et al. [26] reported that, in vitro, the oligomerization of MAT differs according to the presence of DTT (10 mM) or a mixture of GSH (10 mM) and GSSG (1 mM). DTT favors the formation of MAT III whereas GSH/GSSG favors a stable mixture of MAT I and III. Thus, with the glutathione system, we expect to have a greater global activity of MAT. The ratio GSH/GSSG is known to regulate the MAT activity [26], [28]. The calculated redox potential of glutathione used by Sanchez-Perez et al. is −210 mV, a value close to that observed in our animals infused with H2O2, ascorbylperoxide or PN (Fig. 3). The redox potential of the medium containing DTT is certainly more reduced (approximately −330 mV). Fig. 5 shows that the activity of MAT increased in function of reduction of redox environment, whereas data from Sanchez-Perez et al. [26] suggest the opposite, a greater activity (MAT I+III) in a more oxidized redox environment (−210 mV) compared to the lowest activity (mainly MAT I) in the most reduced redox environment (−330 mV) obtained with DTT. This discrepancy suggests that the inhibition observed with PN, or with peroxides, does not occur at the level of oligomerization.
The regulatory function of redox potential of glutathione on MAT activity observed in the present study supports the fact that the inhibitions are obtained following the oxidation of a thiol function, probably on the C121 at the active site as suggested by Pajares et al. [11]. However, in animal infused with PN, the inhibition cannot be explained only by oxidation of this thiol function, since DTT was without effect. Other molecules must be involved. 4-Hydroxynonenal, from the lipid peroxidation of cellular membranes or from the lipid moiety of the PN, is a possible candidate. It is a well known that this aldehyde has a strong reactivity with several amino acids such as histidine, lysine and cysteine [29]. These kinds of involved reactions (Schiff-base formation or Michael addition) are irreversible and led to inactivation of several proteins [30].
5. Conclusion
The present report suggests that prevention of peroxide generation in PN and/or correction of the redox potential by adding glutathione in PN [17] are not sufficient, at least in newborn guinea pigs, to restore normal MAT activity. Further studies should be undertaken to identify all chemical players, in addition of peroxides, present in PN that influence the activity of the various isoforms of MAT. The knowledge of the molecules and pathways implied is the first step in the prevention of deleterious effects of PN on hepatic metabolism.
Contributors' statement
Wesam Elremaly: Ms. Elremaly has contributed to the experimental design, the biochemical determinations, the analysis and interpretation, writing the manuscript, and has approved the final manuscript as submitted.
Ibrahim Mohamed: Dr. Mohamed made a critical revision of the manuscript with important intellectual content and he has approved the final manuscript as submitted.
Thérèse Rouleau: Ms. Rouleau did the animal surgery (catheter in jugular vein), coordinated and supervised the biochemical determinations, critically reviewed the manuscript, and has approved the final manuscript as submitted.
Jean-Claude Lavoie: Dr. Lavoie did the study conception, the statistical analysis. He has contributed to the interpretation of the data and to the writing of the manuscript and has approved the final manuscript as submitted.
Acknowledgments
This work was supported by Grant from the Canadian Institutes of Health Research (MOP-115035). W.E. holds a scholarship from the Front de Recherche en Santé du Québec (FRQS).
References
- 1.Cavicchi M., Beau P., Crenn P., Degott C., Messing B. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann. Intern Med. 2000;132:525–532. doi: 10.7326/0003-4819-132-7-200004040-00003. [DOI] [PubMed] [Google Scholar]
- 2.Sax H., Bower R. Hepatic complications of total parenteral nutrition. J. Parenter. Enter. Nutr. 1988;12:615–618. doi: 10.1177/0148607188012006615. [DOI] [PubMed] [Google Scholar]
- 3.Calkins K.L., Venick R.S., Devaskar S.U. Complications associated with parenteral nutrition in the neonate. Clin. Perinatol. 2014;41:331–345. doi: 10.1016/j.clp.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauriti G., Zani A., Aufieri R., Cananzi M., Chiesa P.L., Eaton S., Pierro A. Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: a systematic review. J. Parenter. Enter. Nutr. 2014;38:70–85. doi: 10.1177/0148607113496280. [DOI] [PubMed] [Google Scholar]
- 5.Lavoie J.C., Bélanger S., Spalinger M., Chessex P. Admixture of a multivitamin preparation to parenteral nutrition: the major contributor to in vitro generation of peroxides. Pediatrics. 1997;99:e6. doi: 10.1542/peds.99.3.e6. [DOI] [PubMed] [Google Scholar]
- 6.Chessex P., Lavoie J.C., Rouleau T., Brochu P., St-Louis P., Lévy E., Alvarez F. Photooxidation of parenteral multivitamins induces hepatic steatosis in a neonatal guinea pig model of intravenous nutrition. Pediatr. Res. 2002;52:958–963. doi: 10.1203/00006450-200212000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Lavoie J.C., Chessex P., Rouleau T., Migneault D., Comte B. Light-induced byproducts of vitamin C in multivitamin solutions. Clin. Chem. 2004;50:135–140. doi: 10.1373/clinchem.2003.025338. [DOI] [PubMed] [Google Scholar]
- 8.Maghdessian R., Côté F., Rouleau T., Ouadda A.B.D., Levy É., Lavoie J.C. Ascorbylperoxide contaminating parenteral nutrition perturbs the lipid metabolism in newborn guinea pig. J. Pharmacol. Exp. Ther. 2010;334:278–284. doi: 10.1124/jpet.110.166223. [DOI] [PubMed] [Google Scholar]
- 9.Elremaly W., Rouleau T., Lavoie J.C. Inhibition of hepatic methionine adenosyltransferase by peroxides contaminating parenteral nutrition leads to a lower level of glutathione in newborn Guinea pigs. Free Radic. Biol. Med. 2012;53:2250–2255. doi: 10.1016/j.freeradbiomed.2012.10.541. [DOI] [PubMed] [Google Scholar]
- 10.Markham G.D., Pajares M.A. Structure–function relationships in methionine adenosyltransferases. Cell. Mol. Life Sci. 2009;66:636–648. doi: 10.1007/s00018-008-8516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pajares M.A., Álvarez L., Pérez-Sala D. How are mammalian methionine adenosyltransferases regulated in the liver? A focus on redox stress. FEBS Lett. 2013;587:1711–1716. doi: 10.1016/j.febslet.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 12.Almasio P., Bortolini M., Pagliaro L., Coltorti M. Role of S-adenosyl-l-methionine in the treatment of intrahepatic cholestasis. Drugs. 1990;40:111–123. doi: 10.2165/00003495-199000403-00011. [DOI] [PubMed] [Google Scholar]
- 13.Coltorti M., Bortolini M., Di Padova C. A review of the studies on the clinical use of S-adenosylmethionine (SAMe) for the symptomatic treatment of intrahepatic cholestasis. Methods Find. Exp. Clin. Pharmacol. 1989;12:69–78. [PubMed] [Google Scholar]
- 14.Lu S.C. Glutathione synthesis. Biochim. Biophys. Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandes N., Schmitt S., Jakob U. Thiol-based redox switches in eukaryotic proteins. Antioxid. Redox Signal. 2009;11:997–1014. doi: 10.1089/ars.2008.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 17.Elremaly W., Mohamed I., Rouleau T., Lavoie J.C. Adding glutathione to parenteral nutrition prevents alveolar loss in newborn guinea pig. Free Radic. Biol. Med. 2015;87:274–281. doi: 10.1016/j.freeradbiomed.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 18.Lavoie J.C., Chessex P. Development of glutathione synthesis and γ-glutamyltranspeptidase activities in tissues from newborn infants. Free Radic. Biol. Med. 1998;24:994–1001. doi: 10.1016/s0891-5849(97)00384-5. [DOI] [PubMed] [Google Scholar]
- 19.Griffith O.W. Determination of glutathione and glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 20.Martionez-Chantar M.L., Latasa M.U., Varela-Rey M., Lu S.C., Garcia-Trevijano E.R., Mato J.M., Avila M.A. l-Methionine availability regulates expression of the methionine adenosyltransferase 2A gene in human hepatocarcinoma cells. Role of S-adenosylmethionine. J. Biol. Chem. 2003;278:19885–19890. doi: 10.1074/jbc.M211554200. [DOI] [PubMed] [Google Scholar]
- 21.Elremaly W., Mohamed I., Mialet-Marty T., Rouleau T., Lavoie J.C. Ascorbylperoxide from parenteral nutrition induces an increase of redox potential of glutathione and loss of alveoli in newborn guinea pig lungs. Redox Biol. 2014;2:725–731. doi: 10.1016/j.redox.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chessex P., Lavoie J.C., Laborie S., Vallée J. Survival of guinea pig pups in hyperoxia is improved by enhanced nutritional substrate availability for glutathione production. Pediatr. Res. 1999;46:305–310. doi: 10.1203/00006450-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Lavoie J.C., Chessex P. Gender and maturation affect glutathione status in human neonatal tissues. Free Radic. Biol. Med. 1997;23:648–657. doi: 10.1016/s0891-5849(97)00011-7. [DOI] [PubMed] [Google Scholar]
- 24.Vina J., Vento M., Garcia-Sala F., Puertes I., Gasco E., Sastre J., Asensi M., Pallardo F. l-cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. Am. J. Clin. Nutr. 1995;61:1067–1069. doi: 10.1093/ajcn/61.4.1067. [DOI] [PubMed] [Google Scholar]
- 25.Wu G., Fang Y.-Z., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez-Pérez G.F., Ma Gasset, Calvete J.J., Pajares M.A. Role of an intrasubunit disulfide in the association state of the cytosolic homo-oligomer methionine adenosyltransferase. J. Biol. Chem. 2003;278:7285–7293. doi: 10.1074/jbc.M210177200. [DOI] [PubMed] [Google Scholar]
- 27.Sufrin J.R., Dunn D.A., Marshall G.R. Steric mapping of the l-methionine binding site of ATP: l-methionine S-adenosyltransferase. Mol. Pharmacol. 1981;19:307–313. [PubMed] [Google Scholar]
- 28.Martinez-Chantar M.L., Pajares M.A. Role of thioltransferases on the modulation of rat liver S-adenosylmethionine synthetase activity by glutathione. FEBS Lett. 1996;397:293–297. doi: 10.1016/s0014-5793(96)01201-x. [DOI] [PubMed] [Google Scholar]
- 29.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog. Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 30.Dalleau S., Baradat M., Guéraud F., Huc L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013;20:1615–1630. doi: 10.1038/cdd.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]