Abstract
It has long been recognized that energy metabolism is linked to the production of reactive oxygen species (ROS) and critical enzymes allied to metabolic pathways can be affected by redox reactions. This interplay between energy metabolism and ROS becomes most apparent during the aging process and in the onset and progression of many age-related diseases (i.e. diabetes, metabolic syndrome, atherosclerosis, neurodegenerative diseases). As such, the capacity to identify metabolic pathways involved in ROS formation, as well as specific targets and oxidative modifications is crucial to our understanding of the molecular basis of age-related diseases and for the design of novel therapeutic strategies.
Herein we review oxidant formation associated with the cell's energetic metabolism, key antioxidants involved in ROS detoxification, and the principal targets of oxidant species in metabolic routes and discuss their relevance in cell signaling and age-related diseases.
Abbreviations: ROS, reactive oxygen species; RNS, reactive nitrogen species; O2∙−, superoxide radical; H2O2, hydrogen peroxide; ∙NO, nitric oxide; ∙OH, hydroxyl radical; ETC, electron transport chain; O2, oxygen; Complex I, NADH–ubiquinone oxidoreductase; FMN, flavin mononucleotide; FeS, iron–sulfur; TCA, tricarboxylic acid; Q, ubiquinone; CoQH2, reduced coenzyme Q; RET, reverse electron transport; Complex III, ubiquinol–cytochrome c oxidoreductase; CoQ, coenzyme Q; CoQH∙, ubisemiquinone; SOD, superoxide dismutase; α-KGDH, alpha-ketoglutarate dehydrogenase; ONOO−, peroxynitrite anion; ONOOH, peroxynitrous acid; ∙NO2, nitrogen dioxide; CO3•−, carbonate radical; NOS, nitric oxide synthase; NOx, ∙NO-derived species; CLA, conjugated linoleic acid; NO2-OA, nitro-oleic acid; NO2-LA, nitro-linoleic acid; ACAD, Acyl-CoA dehydrogenase; ETF, electron transfer flavoprotein; ETF-QOR, electron transferring flavoprotein ubiquinone oxidoreductase; SCAD, short-chain acyl-CoA dehydrogenase; MCAD, medium-chain acyl-CoA dehydrogenase; LCAD, long-chain acyl-CoA dehydrogenase; VLCAD, very long-chain acyl-CoA dehydrogenase; NAFLD, nonalcoholic fatty liver disease; NOX, NADPH oxidase; ACOX, acyl-CoA oxidase; PPAR, peroxisome proliferator activated receptor; XOR, xanthine oxidoreductase; XDH, xanthine dehydrogenase; XO, xanthine oxidase; GAGs, glycosaminoglycan; Cu,ZnSOD, coper zinc superoxide dismutase; MnSOD, manganese superoxide dismutase; NF-κB, Nuclear Factor-Kappa B; SIRT, sirtuin; Prx, peroxiredoxin; GPx, glutathione peroxidase; GSH, glutathione; Trx, thioredoxin; AKS, serine/threonine kinase apoptosis signal-regulating kinase; GAP, glyceraldehyde 3-phosphate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; 6-PG, 6, phosphogluconate; PDHK, pyruvate dehydrogenase kinase; PDH, pyruvate dehydrogenase; SDH, succinate dehydrogenase; ATPase, ATP synthase; cyt c, cytochrome c; NO2, nitro group; PPP, pentose phosphate pathway; G6PD, glucose -6-phosphate dehydrogenase; PFKB, 6-phosphofructo-2-kinase/ fructose-2, 6-bisphosphatase
Keywords: Reactive oxygen species, Energy metabolism, Mitochondria, Reactive nitrogen species
Graphical abstract
Highlights
-
•
Energy metabolism is both a source and target of oxidant species.
-
•
Reactive oxygen species are formed in redox reactions in catabolic pathways.
-
•
Sensitive targets of oxidant species regulate the flux of metabolic pathways.
-
•
Metabolic pathways and antioxidant systems are regulated coordinately.
1. Introduction
Energy metabolism, the process of generating energy (ATP) from nutrients, comprises a series of reactions in which biomolecules are oxidized to simpler molecules and the energy released in these thermodynamically favorable processes is harnessed to phosphorylate ADP. Redox reactions that involve the transfer of electrons from reduced organic molecules, to acceptor molecules such as NAD+, NADP+ or oxygen, are key components of these pathways. Reactive oxygen species (ROS) such as superoxide anion radical (O2∙−) and hydrogen peroxide (H2O2) are products or byproducts of metabolic redox reactions. These species can participate in cell signaling events and their formation can affect the cell and tissue structure and function.
Neither O2∙− nor H2O2 are particularly toxic in vivo, since these species are not very reactive [1] and can be removed by a battery of antioxidant enzymes that catalyze their reduction or dismutation. However, the reaction of superoxide with the intercellular messenger nitric oxide (∙NO) leads to the formation of the reactive oxidant peroxynitrite and other oxidant species collectively known as reactive nitrogen species (RNS). In turn H2O2 interaction with metals such as iron, promotes the formation of the potent oxidants hydroxyl radical (∙OH) and oxo-metal complexes. These highly oxidant species are responsible of most of the oxidative damage observed in pathological conditions. Among the targets of reactive oxidant species, we find enzymes proteins and lipids from catabolic pathways involved in ATP synthesis, and whose inhibition may be involved in cell signaling events or organelle dysfunction.
In this review, we focus on oxidant formation associated with the cell's energetic metabolism, key antioxidants involved in ROS detoxification, and the principal targets of oxidant species in metabolic routes and discuss their relevance in cell signaling and age-related diseases.
2. Oxidant formation during mitochondrial ATP synthesis: tricarboxylic acid cycle and electron transport chain
Mitochondrial ROS and RNS production has been reported extensively in the literature [2], [3], [4], [5], [6], [7] and the electron transport chain (ETC) has been acknowledged for a long time as one of the main intracellular sites of O2∙− formation [8], [9]. In addition, modulation of oxidant formation by mitochondria can limit the initiation and progression of diseases whose pathogenesis involves mitochondrial dysfunction [10], [11], highlighting the relevance of these processes.
Mitochondria are primary sites of intracellular formation and reactions of ROS and RNS (Fig. 1). Depending on formation rates and steady-state levels, these short-lived reactive species contribute to signaling events and can mediate mitochondrial dysfunction in pathology through oxidative modifications of mitochondrial molecular components.
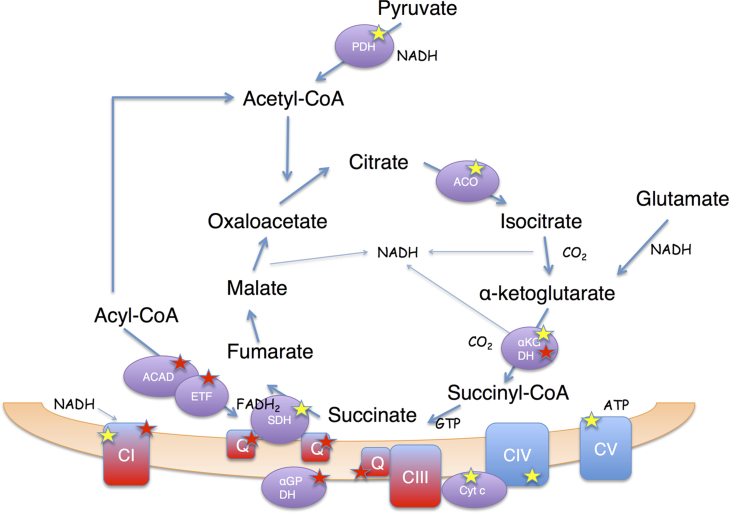
Fig. 1.
Oxidant formation and main oxidant targets during mitochondrial ATP synthesis. Main sites of oxidant formation in mitochondria (highlighted by a red star) include mitochondrial electron transport chain Complex I and Complex III; flavin dehydrogenases, α-ketoglutarate dehydrogenase (α-KGDH), α-glycerophosphate dehydrogenase (α-GPDH), acyl CoA dehydrogenase (ACAD) and electron transfer flavoprotein (ETF). The transfer of electrons to oxygen generates superoxide radical which leads to secondary oxidant formation (e.g., hydrogen peroxide, hydroxyl radical, peroxynitrite) by dismutation, reaction with metals or by reaction with nitric oxide respectively (see text for details). The main mitochondrial targets of the different oxidant species (highlighted by a yellow star) include aconitase (Aco), succinate dehydrogenase (SDH), α-KGDH, cytochrome c (Cyt c) and Complexes I, IV and IV of the respiratory chain (the mechanisms of inactivation and functional consequences are discussed in the text). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.1. Superoxide and hydrogen peroxide formation by complexes of the Electron Transport Chain and Tricarboxylic Acid Cycle
The addition of a single electron to oxygen (O2) leads to the formation of O2∙−. During catabolism O2∙− is formed mostly as a byproduct of the ETC in the mitochondria where, at physiological O2 levels, 0.1-2% of total O2 is converted to O2∙− [12]. Indeed, O2∙− is the principal ROS formed by the mitochondrial ETC. The level and rate of mitochondrial production of this radical depends on the tissue, the substrates metabolized, and the site of the mitochondrial electron transfer chain involved in its formation [13], [14]. Mitochondrial O2∙− is formed in the sites where monoelectronic reduction of O2 is thermodynamically and kinetically feasible (reviewed in [12], [15]). These sites are found in electron transport chain Complexes I and III.
Complex I (NADH–ubiquinone oxidoreductase), which constitutes the entry point for electrons from NADH, is a complex structure comprising 45 polypeptides, a flavin mononucleotide (FMN) cofactor and seven iron–sulfur (FeS) centers. Complex I produces O2∙− by two mechanisms. Firstly, when matrix NADH/NAD+ ratio is high electron transfer from the reduced FMN cofactor forms O2∙−. This mechanism involves the ubiquinone (Q) binding site of Complex I, therefore rotenone that blocks Q binding and maximizes FMN and FeS center reduction, increases H2O2 release from mitochondria [14]. Superoxide can also be formed during reverse electron transport (RET) from reduced coenzyme Q (CoQH2) to Complex I [15]. This mechanism, predominates at high transmembrane potentials when forward electron flux from CoQH2 to cytochrome c oxidase is hindered and electrons are forced from CoQH2 to Complex I [15], and is therefore inhibited by rotenone. RET is a relevant source of O2∙− in brain [14], particularly in neurodegenerative diseases such as Parkinson [16]. Recent reports, using a metabolomic approach, show RET is also responsible of much of O2∙− formation during ischemia/reperfusion [17] when succinate is accumulated during hypoxia and utilized during reperfusion leading to O2∙− formation by Complex I.
It is now known that a large proportion of the mitochondrial complexes are arranged in supramolecular assemblies called supercomplexes or respirosomes. In addition to conferring kinetic advantages through substrate channeling, supercomplexes are required for the stability and assembly of Complex I. Supercomplex formation also prevents excessive ROS formation from the respiratory chain, in fact disruption of supercomplex I-III increases ROS formation by Complex I [18], [19], [20].
Complex III (ubiquinol–cytochrome c oxidoreductase) accepts reducing equivalents formed in Complex I and II. Complex III contains 11 polypeptides, 3 hemes and a FeS center and interacts with coenzyme Q (CoQ) during the Q cycle. The reaction of O2 with ubisemiquinone (CoQH∙) leads to the formation of O2∙− in both sides of the inner mitochondrial membrane [21]. Therefore conditions which enhance the reduction and stabilization of the CoQH∙ will favor O2∙− formation by Complex III (e.g. electron transport inhibition with antimycin A) [12].
The anionic character of O2∙− limits its diffusion across membranes confining the reactions of this radical to the mitochondria. The main reactions of O2∙− in mitochondria include: (1) the spontaneous or superoxide dismutase (SOD)-catalyzed dismutation to H2O2 and O2 (k=2×109 M–1 s–1); (2) direct reaction with FeS centers leading to their disruption (e.g. mitochondrial aconitase FeS center k=107 M–1 s–1) [22] or (3) reaction with ∙NO to yield peroxynitrite (k=4.3-20×109 M–1 s–1) [23], [30], [31].
Hydrogen peroxide is a non-radical diffusible oxidant that becomes toxic to most cells types at concentrations over 10 µM [15], [24], [25]. It is a weak oxidant and reductant, and its reactivity with enzymes and proteins relies in its capacity to oxidize labile thiols and metal containing molecules [26], [27]. Oxidation of thiols in cysteine residues is responsible of most of H2O2 signaling inside the cell [26], [27] and reactions with metal centers are part of the catalytic mechanism of heme dependent peroxidases. However, the reactions with metal centers can also be cytotoxic mainly due to the formation of highly oxidative species such as ∙OH [28].
Although mitochondrial oxidant formation is primarily ascribed to the respiratory complexes, other mitochondrial enzymes in particular flavoenzymes, can also produce O2∙− and H2O2 as byproducts [14]. The Tricarboxylic Acid Cycle (TCA) enzyme α-ketoglutarate dehydrogenase (α-KGDH) and glycerophosphate dehydrogenase (α-GPDH) complexes are considered relevant sources of ROS in brain mitochondria, particularly under conditions of high mitochondrial membrane potential [13], [29].
2.2. Peroxynitrite formation in mitochondria
Peroxynitrite anion (ONOO–) is a strong oxidant formed in the fast reaction between the signaling radical ∙NO and O2∙– [30], [31]. Peroxynitrite anion is a rather stable molecule, able to react with Lewis acids, such as CO2 and electrophilic transition metal centers, yielding a variety of potent oxidants [32] such as nitrogen dioxide (∙NO2), carbonate radical (CO3∙–) [33] and oxo-metal complexes [34]. Peroxynitrous acid (pKa=6.8, ONOOH) in turn can undergo a proton-catalyzed homolysis to ∙NO2 and ∙OH in ~30% yields [35], [36], [37].
Nitric oxide is a small amphipathic molecule and modestly reactive radical that is freely permeable through cellular membranes [38], [39]. It is produced by the aerobic oxidation of l-arginine in a reaction catalyzed by nitric oxide synthases (NOS, i.e. constitutive endothelial and neuronal as well as inducible isoforms) [40] and can freely diffuse into the mitochondria. Besides, several reports support that a mitochondrial NOS can also contribute to mitochondrial ∙NO formation (reviewed in [41]).
The direct effects of ∙NO in mitochondria are mainly due to its capacity to compete with oxygen for the interaction with the binuclear (Cu+-Fe2+) center of cytochrome c oxidase, modulating O2 consumption, ATP and O2∙− formation (see below). However, increases in O2∙− concentrations such as those observed in pathological conditions will shift the reactivity of ∙NO towards O2∙−, because the reaction becomes kinetically favored, leading to peroxynitrite formation [6], [23]. Peroxynitrite-derived radical species and oxo-metal complexes are involved in oxidation, peroxidation and nitration reactions with mitochondrial components [32], [42], [43], [44], [45], [46], [47].
2.3. Lipid derived electrophiles formed in mitochondria
In the last years it has been recognized that lipid derived reactive species can be formed in mitochondria and their reactions with mitochondrial components result in mitochondrial dysfunction or have a physiological role modulating cell function [48], [49], [50].
Nitric oxide and ∙NO-derived species (NOx) lead to the formation of a wide variety of oxidized and nitrated products with biologically and physiologically relevant properties [51], [52], [53], [54]. Among these products, nitroalkenes have been characterized and quantified in plasma of healthy and hypercholesterolemic patients as well as in red blood cell membranes [55], [56]. The alkenyl nitro configuration of nitroalkenes is responsible of the electrophilic reactivity of the β-carbon adjacent to the nitro-bonded carbon. Nitroalkenes can participate in reversible Michael addition reactions with nucleophiles (i.e. cysteine or histidine residues in proteins) [57], [58] forming covalent, thiol reversible post-translational modifications that may impact on protein structure, function and subcellular distribution [58]. These modifications are now considered to transduce redox- and ∙NO-dependent cell signaling in a variety of pathways. The reported concentrations fluctuate from nanomolar [59] to low micromolar concentrations [55], all of them capable of exerting biological actions [60], [61]. For example, it has been reported that conjugated linoleic acid (CLA) is a preferential target of nitrating species in mitochondria leading to the formation of nitroalkenes [50], [62]. There are also reports that under ischemic preconditioning conditions, mitochondrial nitro-oleic (NO2-OA) and nitro-linoleic (NO2-LA) acids are formed reaching concentrations around 1 µM [50]. Nitroalkene formation can increase in pathological conditions such as ischemia, were ROS and RNS formation increase [50], [62]. Several mitochondrial targets for electrophiles can be found in mitochondria and the reactions of these compounds with respiratory chain components and uncoupling proteins have been postulated to modulate ATP and ROS production, O2 consumption, matrix metabolic enzymes, and apoptotic machinery and to exert cytoprotective actions in settings of mitochondrial dysfunction [50], [62].
In addition, mitochondrial polyunsaturated fatty acids are susceptible to lipoperoxidation promoted by ∙OH. The lipid peroxidation products are α,β unsaturated aldehydes, such as 4-hydroxy-trans-2,3-nonenal and 4-oxo-trans-2,3-nonenal. These products covalently modify side chains of histidine, cysteine and lysine residues, producing free carbonyls attached to proteins. Increased protein carbonylation is observed in diet-induced obese mice adipose tissue, in obese human subcutaneous adipose tissue and in cultured adipocytes treated with TNF-α. In the latter, approximately one third of the mitochondrial proteins were carbonylated; in particular mitochondrial Complex I appears as a relevant target, leading to a decrease activity and increase ROS formation [63], [64].
3. Oxidant formation in fatty acid catabolism
3.1. Superoxide and hydrogen peroxide formation during mitochondrial fatty acid β-oxidation
Fatty acid metabolism is one of the principal sources of energy for skeletal and cardiac muscle and is a very active route in the liver. Mitochondrial β-oxidation is responsible for the degradation of short (<C8), medium (C8–C12) and long chain (C14–C20) fatty acids to acetyl-CoA and the energy released in this processes is used for ATP generation by oxidative phosphorylation [65]. Fatty acids are activated to acyl-CoA by acyl-CoA synthetases [66] in the outer mitochondrial membrane and β-oxidation, proceeds through 4 steps: (a) oxidation, (b) hydration, (c) a second oxidation and (d) thiolytic cleavage, releasing acetyl-CoA and an acyl-CoA two carbons shorter than the original molecule.
Acyl-CoA dehydrogenases (ACAD) are flavoproteins that catalyze the first step of mitochondrial β-oxidation, the oxidation of acyl-CoA to trans-2-enoyl-CoA leading to the reduction of the FAD prosthetic group in the active site of the enzyme which is then reoxidized by the electron transfer flavoprotein (ETF) [67]. Finally, electrons flow to ubiquinone through the electron transferring flavoprotein- ubiquinone oxidoreductase (ETF-QOR).
Five different enzymes of the ACAD family with different chain-length specificity catalyze the oxidation of acyl-CoA: short-chain acyl-CoA dehydrogenase (SCAD, <C8) medium chain acyl-CoA dehydrogenase (MCAD, C4–C12), long chain acyl-CoA dehydrogenase (LCAD, C8–C20), very long chain acyl-CoA dehydrogenases 1 and 2 (VLCAD1 and VLCAD2, C12–C24) [68], [69].
Mitochondrial β-oxidation of fatty acids is associated with an increase in O2∙− and H2O2 formation [14], [70], that is not only due to univalent oxygen reduction by the ETC [71]. In fact, VLCAD [72], [73] and ETF [74] appear as additional sources of O2∙− formation during fatty acid catabolism (Fig. 1, Fig. 3). ETF-QOR has also been suggested as a plausible site of O2∙− formation [75] in this setting but requires further confirmation.
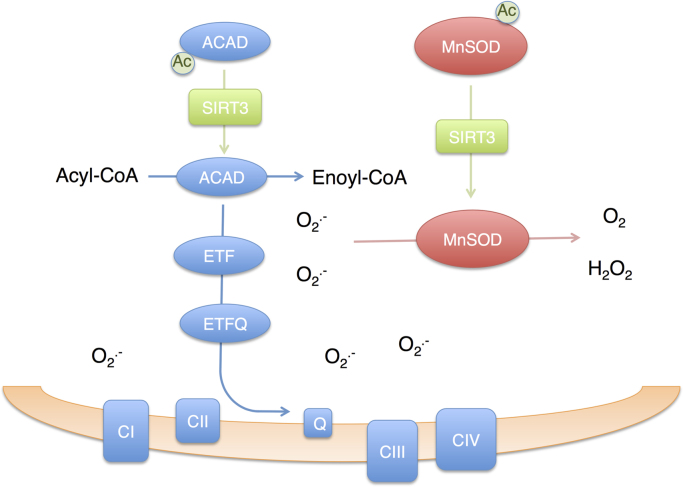
Fig. 3.
Sirtuin 3 coordinates fatty acid oxidation and superoxide detoxification to avoid oxidative stress. In fed conditions, acyl-CoA dehydrogenase (ACAD) and manganese superoxide dismutase (MnSOD) are acetylated and their activity is low. During starvation, Sirtuin 3 (SIRT3) levels increase. Upon deacetylation by SIRT3, ACAD activity increases promoting fatty acid β-oxidation in the mitochondria. An increase in fatty acid oxidation is accompanied by superoxide (O2∙−) formation by ACAD, electron transfer flavoprotein (ETF), electron transferring flavoprotein ubiquinone-oxidoreductase (ETF-QOR) and mitochondrial respiratory Complexes I and III. However, SIRT3 also catalyzes the deacetylation of MnSOD, increasing its activity, supporting the dismutation of O2∙− to O2 and hydrogen peroxide protecting from oxidative stress. Deacetylation of FOXO3 by SIRT3 leads to an increase in expression of MnSOD, mitochondrial peroxiredoxins and thioredoxin 2; and further increases mitochondrial antioxidant capacity (not shown in the figure).
Very long chain acyl-CoA dehydrogenase plays a relevant role in the catabolism of long-chain fatty acids in human tissues catalyzing the major part of palmitoyl-CoA dehydrogenation [76]. The levels of this enzyme are increased in the liver of mice under high fat diet and have been shown to contribute to the increase-in oxidative stress in the tissue in these conditions [73]. Further studies with recombinant purified VLCAD demonstrated that the enzyme possesses oxidase activity, and produces H2O2 as a byproduct of palmitoyl-CoA oxidation reactions [72]. Enzymatic activity of VLCAD is regulated by phosphorylation of Ser586 by protein kinase A (PKA) in fibroblasts and suppression of this posttranslational modification inhibits dehydrogenase enzyme activity and promotes ROS formation [76].
Studies with recombinant ETF have shown that this flavoprotein produces O2∙− and H2O2 upon reduction by its substrate MCAD [74]. As in most flavoproteins the reactivity of ETF with oxygen is dictated by the redox chemistry of the FAD cofactor that is heavily influenced by the protein environment and by solvent accessibility [77] The semiquinone radical (FADH∙) is responsible of the reduction of oxygen to O2∙− and H2O2. This radical specie is destabilized when ETF is tightly associated with its substrate MCAD and mutations impairing this association favor ROS formation [74].
Reactive oxygen species formation due to fatty acid oxidation might be involved in the pathogenesis of nonalcoholic fatty liver disease (NAFLD), the most common liver disorder worldwide. NAFLD includes several diseases characterized by hepatic steatosis, an excess of fat in the liver, in individuals who do not consume significant amounts of alcohol [78]. Hepatic steatosis can evolve to steatohepatitis (NASH) and ROS have been proposed to mediate hepatocyte damage and inflammation [79].
A role for fatty acid-derived ROS in cancer progression has been recently postulated as well [80]. In hypoxic conditions HIF-1 suppression of MCAD and LCAD expression decreases fatty acid oxidation and ROS formation, promoting cell proliferation of liver cancer cell lines. Analysis of human liver cancer samples showed that decreases in LCAD protein levels are associated with mortality [80], underscoring the relevance of fatty acid oxidation suppression in the progression of the disease.
Another setting were oxidant formation due to increased fatty acid catabolism might become relevant is cell senescence, a cellular state characterized by proliferation-arrest, activation of the DNA damage response, changes in morphology, increase in lysosomal enzymes (such as β-galactosidase) and increase in reactive oxygen species formation. Both NADPH oxidases (Nox1 and Nox4) [81], [82] and mitochondria [83] have been postulated as plausible sources of oxidants in oncogenic Ras-induced senescence. In a recent report, we observed a relevant increase in mitochondrial fatty acid β oxidation and fatty acid dependent O2 consumption, in human fibroblasts undergoing oncogenic Ras-induced senescence [84] that might be responsible for the increase in mitochondrial oxidant formation [83].
3.2. Peroxisomal hydrogen peroxide formation by acyl-CoA oxidases
Peroxisomes contain many enzymes involved in lipid metabolism that participate in the utilization of fatty acids as a source of energy. Peroxisomes are involved in the β-oxidation of long chain, very long chain fatty acids and branched fatty acids [65]. Fatty acids are activated to acyl-CoA by acyl-CoA synthetases [66] in the membrane of the peroxisome. Similar to mitochondrial oxidation, peroxisomal β-oxidation, proceeds through oxidation, hydration, a second oxidation and thiolytic cleavage reactions.
Acyl-CoA oxidases (ACOX) catalyze the first and rate limiting step in peroxisomal β-oxidation of fatty acids: The oxidation of acyl-CoA to trans-2-enoyl-CoA, where O2 is the electron acceptor of the reaction producing H2O2 [85].
In human liver peroxisomes, we can find acyl-CoA oxidases with different specificities, ACOX1, that oxidizes the CoA esters of straight chain fatty acids and prostaglandins and ACOX2, which oxidizes CoA esters of branched-chain fatty acids and bile acids [86]. Acyl-CoA oxidases contain a FAD prosthetic group non-covalently bound to the active site of the enzyme [87]. The flavin moiety of the cofactor is responsible of the two- electron reduction of O2 to H2O2 [68].
Acyl-CoA oxidase expression is regulated by peroxisome proliferator activated receptor alpha (PPARα) a ligand-activated transcription factor member of the nuclear-hormone receptor [88], abundant in liver, heart, brown adipose tissue, kidney, small and large intestine [89]. Upon activation by ligand binding PPARα promotes the transcription of several genes involved in fatty acid catabolism, including peroxisomal fatty acid β-oxidation enzymes, (e.g. ACOX1, bifunctional enzyme enoyl-CoA hydratase/L-3-hydroxyacyl-CoA dehydrogenase, and ketoacyl-CoA thiolase) [88], mitochondrial fatty acid β-oxidation enzymes, and microsomal ω-oxidation CYP4A enzymes (see [90] for a review on PPARα regulation and ligands). In mice, exposure to synthetic PPAR ligands, such as the hipolipidemic drug rosiglitazone, results in an increase in H2O2 production in the liver leading to DNA oxidation [91], [92], and has been proposed to play a role in the development of liver tumors induced by these non-genotoxic compounds [93].
4. Oxidant formation during purine catabolism
Xanthine oxidoreductase (XOR) is a molybdoflavin containing protein that catalyzes the rate-limiting and terminal steps of purine degradation in humans, the oxidation of hypoxanthine to xanthine and finally to uric acid [94]. Intracellularly, the primary reaction catalyzed by this enzyme is the reduction of NAD+ to NADH by the xanthine dehydrogenase (XDH) form. Upon the reversible oxidation of critical cysteine residues or limited proteolysis, XDH is converted to xanthine oxidase (XO), which utilizes O2 as the terminal electron acceptor [94]. This leads to the production of O2∙− and H2O2 rather than NADH [94], [95]. XDH also presents partial oxidase activity [94], [95], thus the conversion to XO is not always necessary for oxidant formation. Many reports in both animal models and clinical studies suggest a key role for XOR in pathophysiology, being inhibition of XOR beneficial in different vascular inflammatory processes [96], [97].
Circulating XO levels are elevated in several pathological states (e.g. obesity, sickle cell anemia, and heart failure) [96], [97], [98], [99], where reactive species formed by this enzyme as well as NOX and mitochondria contribute to the development of the disease [100]. In the endothelium, local concentrations of XO are increased by immobilization through the interactions of the enzyme with negatively charged glycosaminoglycan (GAGs) in the vessel walls [101]. Immobilization, alters the affinity of XO for xanthine at the molybdenum site and affects XO kinetics leading to an increase in the Km and Ki for substrates and products respectively, when compared to the soluble enzyme [101]. In healthy humans, plasma concentrations of xanthine plus hypoxanthine are in the low micromolar range (~2 µM); under these conditions immobilized XO has reduced enzymatic activity (Km~21 µM for xanthine) [101]. However, in pathological conditions plasma xanthine plus hypoxanthine concentrations can rise to 20–100 µM, a range sufficient to reach and exceed half-maximal activity for the immobilized enzyme. For example, purine concentrations are elevated under ischemic conditions where ATP catabolism is increased, enhancing the formation of ROS by XO in the vascular compartment [96], [102], [103]. The increase in O2∙− limits the bioavailability of ∙NO leading to the formation of peroxynitrite which potentiates oxidative inflammatory injury [32]. Reports in the literature show that treatment with the enzyme inhibitor allopurinol, increases ∙NO- dependent vasodilatation confirming the role for XO in vascular disease [96], [98], [104]. Overall, the increase in purine levels and the immobilization-induced increase in XO stability and activity, support that XO bound to vascular cell GAGs could be an important source of ROS in the vascular compartment, affecting nitric oxide- and/or redox-dependent cell signaling reactions [96], [98], [104].
5. Key antioxidant /detoxifying enzymes
Reactive oxygen and nitrogen species produced by the mitochondria are implicated in the development of different metabolic diseases [105], [106], [107], [108], [109], [110], [111]. Herein we detail the main antioxidants involved in the detoxification of reactive species (Fig. 2). Special attention has been given to mitochondrial antioxidant enzymes, since these play a key role in protecting the cell from most of the oxidants formed in energy producing catabolic routes.
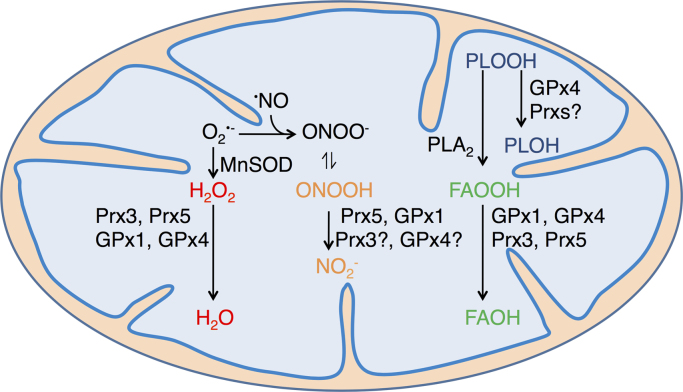
Fig. 2.
Mitochondrial antioxidant systems. MnSOD catalyzes the dismutation of O2∙–, leading to oxygen plus hydrogen peroxide (H2O2) formation. The latter is reduced to water by thiol- as well as selenol-dependent peroxidases, namely Prx3, Prx5, GPx1 and GPx4, and in cardiomyocytes, also by catalase. According to kinetic predictions, Prx3 is expected to be responsible for >90% of mitochondrial H2O2 decomposition. Alternatively, O2∙– can react with nitric oxide (∙NO) forming peroxynitrite anion (ONOO–). Its conjugated acid, peroxynitrous acid (ONOOH, pKa=6.8) is rapidly reduced to nitrite (NO2-) by GPx1 and Prx5, and probably by Prx3 and GPx4, although the peroxynitrite reductase activity of the Prx3 and GPx4 is yet to be demonstrated. GPx4 catalyzes the reduction of phospholipid hydroperoxides (PLOOH) to the corresponding alcohols (PLOH). It is not clear whether Prxs can also catalyze PLOOH reduction efficiently. PLOOH are hydrolyzed by phospholipase A2 (PLA2) releasing fatty acid hydroperoxides (FAOOH), which are in turn reduced to fatty acid alcohols (FAOH) by mitochondrial GPxs as well as Prx3 [267]. Although the ability of Prx5 to reduce FAOOH has not been investigated so far, the enzyme has been reported to reduce other organic hydroperoxides [148]. The activity of mitochondrial GPxs and Prxs is sustained by the glutathione/glutathione reductase and/or the thioredoxin 2/thioredoxin reductase 2 systems, both relying on NADPH as final electron donor.
5.1. Superoxide detoxification by MnSOD
Superoxide detoxification is mediated mainly by SODs, a family of enzymes that catalyze the dismutation of two molecules of O2∙− to H2O2 and O2. In mammals we can find three forms of the enzyme, cytosolic copper zinc superoxide dismutase (Cu,ZnSOD or SOD1) mitochondrial manganese superoxide dismutase (MnSOD or SOD2) and extracellular Cu,ZnSOD (SOD3) [112]. In this review, we will focus on MnSOD due to its key role detoxifying O2∙− formed in the mitochondria during aerobic catabolism of biomolecules.
Mammalian MnSOD is a tetrameric enzyme with a redox active manganese ion Mn2+/Mn3+ in the active site [113]. The metal ion participates in the catalysis mechanism where O2∙− disproportionate to oxygen and H2O2 in a ping- pong mechanism were O2∙− first reduces de manganese ion and then a second O2∙− molecule re-oxidizes the metal ion [114], [115]. At high O2∙− concentrations (>5 fold enzyme concentration), kinetics become more complicated and an inhibited complex between the reduced enzyme and O2∙− is formed, (see review [112] for a full discussion).
MnSOD activity is regulated at multiple levels. Firstly gene expression is controlled at the transcriptional level by several transcription factors including Nuclear Factor-Kappa B (NF-κB), Sp1, AP-1, AP-2, CCAAT-Enhancer-Binding Proteins (C/EBP), forehead transcription factor FOXO3a and p53 and at the epigenetic level [116], [117], [118]. Its activity depends as well on the correct import to mitochondria, the insertion of the manganese ion in the active site and on several posttranslational modifications, including nitration, phosphorylation and acetylation [119].
Nitration of highly conserved Tyr34 has been detected both in vitro and in vivo under pathological conditions where oxidative stress is implicated. This post translational modification of a residue in the active site inhibits MnSOD activity [120], [121], [122], [123], apparently by electrostatically and sterically hindering the access of O2∙− to the metal center [124]. Tyr34 nitration is mediated by peroxynitrite, that reacts with the manganese ion in the active site in a fast reaction [120].
Phosphorylation of Ser106 results in an increase enzymatic activity and protein stability of human MnSOD. Cyclin B1/Cyclin dependent kinase 1 and Cyclin D1/Cyclin dependent kinase 4 have been shown to phosphorylate the enzyme in response to genotoxic stress [125], [126]. Acetylation of MnSOD on conserved residues Lys122, Lys68, Lys53 and Lys89 has been observed in vivo and is reported to decrease enzyme activity [127], [128], [129]. The mechanism by which acetylation interferes with catalysis has not been elucidated, but it has been proposed that acetylation of Lys122 alters the positive electric charge at the entrance to the active site of the enzyme interfering with the electrostatic attraction of the O2∙− anion, resulting in lower catalytic rates [130].
Of all the mechanisms involved in the regulation of MnSOD, acetylation is clearly one of the most important linking enzyme activity, and thus O2∙− and H2O2 levels, with energy metabolism. Enzymes responsible of MnSOD acetylation have not been unequivocally identified yet. GCN5L1 ((general control of amino acid synthesis 5)-like 1) acetyltransferase and chemical acetylation appear as possible candidates [131], [132]. On the other hand, the mitochondrial Sirtuin 3 (SIRT3), a mitochondrial NAD+ dependent protein deacetylase has been clearly identified as the enzyme responsible for MnSOD deacetylation [127], [128], [129] (Fig. 3).
5.2. Peroxides and peroxynitrite detoxification by mitochondrial peroxidases.
In mitochondria, H2O2, organic hydroperoxides and peroxynitrite are reduced by peroxidases from different families. These include (a) cysteine-dependent peroxidases of the peroxiredoxin (Prx) family (Prx3 and Prx5); (b) selenocysteine-dependent glutathione peroxidases (GPx), GPx1 and GPx4; (c) heme-dependent peroxidases, particularly catalase. Other heme-dependent peroxidases that participate in hydroperoxide reduction in the mitochondria but can also propagate damage due to compound I – dependent reactivity with mitochondrial components (e.g. peroxidase activity of alternative conformations of cyt c3+) are discussed briefly below [133], [134].
5.2.1. Peroxiredoxins
Prx are cysteine-dependent peroxidases that catalyze the reduction of different peroxides playing key roles in peroxide detoxification as well as in redox signaling [135], [136], [137], [138]. Among them Prx3, that is exclusively located in mitochondria, has been estimated to reduce ~90% of H2O2 produced in the compartment, due to its high reactivity (k=2×107 M–1 s–1) and abundance (~60 µM) [139]. Recent evidence suggest that Prx3 not only controls the intramitochondrial levels of H2O2 but also plays a key regulatory role in the efflux of H2O2 to the cytosol [140].
Prx3 is probably also involved in peroxynitrite detoxification, as inferred from its protective role in an animal model of glutamate-mediated exicitoxicity [141], although kinetic data on peroxynitrite reduction by Prx3 is still lacking. Tyrosine nitration of Prx3 has been identified in mitochondria from diabetic mice, yet the site of nitration and the functional consequences of this modification remain to be established [142].
Prx3 is highly up regulated during adipogenesis, and its expression decreases in white adipose tissue of obese mice and humans. Prx3 KO mice are characterized by an increased susceptibility to oxidative stress [143], [144], [145]. Prx3 KO mice are glucose intolerant and insulin resistant, have higher body weight, increased white fat content, hypertrophied adipocytes and adipogenic gene expression compared to WT mice. When lipid metabolism gene expression is evaluated, adypocytes from Prx3 KO mice showed increased gene expression of fatty acid synthase (FAS), lipoprotein lipase and hormone sensitive lipase and decreased mRNA levels of carnitine palmitoyl transferase 1α, involved in fatty acid oxidation. Moreover, Prx3 KO adypocytes show changes in the expression of proteins involved in oxidative phosphorylation, mitochondrial biogenesis, fatty acid metabolism, MnSOD and show an increase in oxidative stress markers, as well as decreased mitochondrial potential [146]. The mechanism by which Prx3 suppression leads to these profound metabolic alterations, particularly in the adipocyte, is still to be elucidated.
Mitochondrial Prx5, the other Prx present in human mitochondria, is produced from a long alternative splicing form of Prx5 that contains a mitochondrial targeting sequence that is cleaved once translated to the mitochondria [147]. It is considered to be a preferential mitochondrial target for peroxynitrite, due to rapid reactivity (k=7×107 M–1 s–1 at pH 7.8, 25 °C) and concentrations (~1–10 µM) [148], [149], [150]. It probably has a minor role in reducing H2O2 compared with Prx3, since it has ~100-fold lower reactivity with this oxidant and lower protein concentration. Nevertheless, overexpression of mitochondrial Prx5 was shown to protect from H2O2-mediated mitochondrial DNA damage [151]. Recent data related to 1-methyl-4-phenylpyridinium (MPP)+-induced cell death suggested that mitochondrial Prx5 regulates mitochondrial apoptotic pathways in a process that could involve oxidative regulation of endoplasmic reticulum Ca2+ release [152], [153].
5.2.2. Glutathione peroxidases
Glutathione peroxidases comprise another family of cysteine- or selenocysteine- dependent peroxidases, that participate in the detoxification of different peroxides, redox signaling and control of many cellular processes [154]. Human mitochondria express two selenocysteine-dependent glutathione peroxidases, namely GPx1 and GPx4, which are expressed in other cellular compartments as well. GPx1 rapidly reduces H2O2, peroxynitrite and free fatty acid hydroperoxides at the expense of glutathione (GSH) [155]. However, its lower concentration (1-2 µM) compared with the Prxs argues against a primary role of GPx1 in H2O2 or peroxynitrite reduction in mitochondria under most physiological conditions [139], [150]. Gpx1 knockout mice did not have decreased lifespan, but showed increased incidence of cataracts, hypertension, as well as sensitivity to ischemia-reperfusion injury and to oxidative stress [156]. Surprisingly, GPx1 over-expressing mice show a complex diabetogenic phenotype, and although the cause is not clear, it probably involves the disruption of the oxidative inactivation of protein tyrosine phosphatases required for insulin-signaling [157], [158]. GPx1 overexpression also indicated that the enzyme participates in redox-dependent cellular responses promoting mitochondrial dysfunction, decreasing mitochondrial potential and ATP production [159]. In turn, GPx4, phospholipid hydroperoxide glutathione peroxidase, catalyzes the reduction of lipid hydroperoxides, which cannot be reduced by most of the other peroxidases [160]. Its deletion leads to death in embryos and even in adult mice [161], [162]. However, heterozygous mice have a mild increase in lifespan and reduced cancer incidence [163]. GPx4 is considered to protect mitochondria, preventing the loss of membrane potential under oxidative stress conditions [164]. Glutathione peroxidases, and particularly Gpx4, inhibit the production of lipid mediators by different mechanisms, including the inhibition of different lipoxygenases [165], [166].
5.2.3. Catalase
Catalase is an antioxidant enzyme that catalyzes de dismutation of H2O2 to O2 and water (H2O) [167]. In most tissues its expressed in peroxisomes, but it is also present in the mitochondria of mammalian cardiomyocytes [168]. To note, catalase content and activity in cardiomyocyte mitochondria significantly increase during high fat feeding [169], probably reflecting increased mitochondrial H2O2 production from fat metabolism. The lifespan of mice overexpressing catalase in the mitochondria is increased [170], and the enzyme is considered to prevent mitochondrial dysfunction and resistance to insulin [171].
5.3. Mitochondrial thioredoxin/thioredoxin reductase and glutaredoxin systems
Thioredoxin 2 (Trx) is the main reducing substrate for mitochondrial peroxiredoxins. It is reduced by thioredoxin reductase 2 at NADPH expense. Besides, both thioredoxin 1 (cytosolic) and thioredoxin 2 (mitochondrial) form complexes with the redox-sensitive serine/threonine kinase apoptosis signal-regulating kinase 1 (AKS1). Under oxidative stress conditions, Trx is oxidized and dissociates from the Trx-ASK1 complex, leading to ASK1 activation and induction of mitochondrial-dependent apoptotic cell death [172]. In plants, the activity of two TCA enzymes, namely succinate dehydrogenase and fumarase, as well as the associated cytosolic enzyme, ATP citrate lyase, are regulated by Trx [173].
Mitochondrial glutaredoxin 2 plus GSH can also act as reducing substrates for Prx3 [174]. Furthermore, monothiol glutaredoxins have been proposed to play a crucial role in iron-sulfur protein biogenesis mediating the transfer of 2Fe2S clusters from scaffold proteins to target apo proteins [175].
6. Key oxidant targets in metabolic routes
6.1. Glyceraldehyde- 3-phosphate dehydrogenase
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a tetramer consisting of four identical catalytically active subunits, is a key glycolytic enzyme that reversibly catalyzes the oxidation and phosphorylation of glyceraldehyde-3-phosphate (GAP). The catalytic reaction involves the formation of a hemithioacetal between GAP and the catalytic Cys149, thus rendering GAPDH highly sensitive to inactivation by oxidants [58], [176], [177] and lipid oxidation products [58], [178].
It has been shown that GAPDH can be inhibited by ∙NO through the formation of a S-nitrosothiol intermediate. Besides ∙NO can induce translocation of GAPDH to specific cellular microenvironments influencing its role in intermediary metabolism and apoptotic signaling [179], [180], [181]. GAPDH is sensitive to peroxynitrite and H2O2 that react with Cys149 inducing enzyme inhibition through sulfenic acid formation [176], [182], [183] (Fig. 4). In a recent report, the molecular basis of the high sensitivity of the catalytic cysteine residue to H2O2 was unraveled, evidencing the existence of a proton shuttling pathway that stabilizes the transition state favoring sulfenic acid formation and hydroxyl anion departure [177]. The impact of these reactions on glucose metabolism is discussed below.
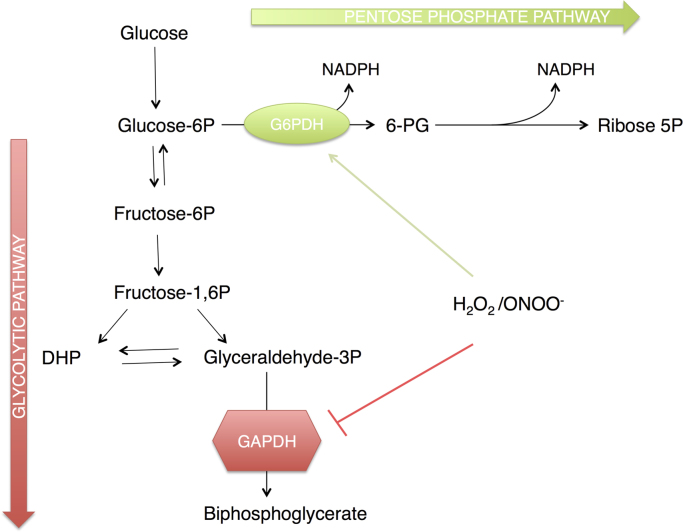
Fig. 4.
Oxidant regulation of glucose metabolism. Following uptake by glucose transporters, glucose is phosphorylated by hexokinase to Glucose-6-phosphate (Glucose-6P). Glucose-6P can enter the Glycolytic pathway to generate pyruvate, ATP and NADH or it can enter the Pentose Phosphate Pathway yielding NADPH and Ribose-5-phosphate (Ribose-5P). The level of reactive oxygen and nitrogen species, such as hydrogen peroxide (H2O2) and peroxynitrous acid (ONOOH) influences the fate of glucose. Reactive species inhibit glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and increase the activity of glucose-6-phosphate dehydrogenase (G6PDH); shifting glucose from an energy producing pathway to NADPH formation for antioxidant systems.
Finally, GAPDH Cys149 and other nucleophilic Cys and His residues can be modified by nitroalkenes, inactivating the enzyme [58]. Some reports show that besides from its known role as an intermediary metabolism enzyme, GAPDH can participate in cell signaling, DNA repair, transcriptional regulation of genes, membrane fusion, tubulin bundling, and apoptotic signaling [182], [183], [184]. Importantly, nitroalkylation of GAPDH promotes its migration to cell membranes and might be responsible for changes in the subcellular distribution and function of GAPDH in certain situations [58].
6.2. Tricarboxylic acid cycle enzymes
The TCA cycle, also known as citric acid cycle or Krebs cycle, is a hub in metabolism where degradative pathways converge and precursors for anabolic pathways are synthesized. In the TCA cycle, acetyl-CoA formed during the catabolism of carbohydrates, aminoacids and fatty acids is oxidized to carbon dioxide. The energy released in these oxidative reactions is conserved in reduced electron carriers, NADH and FADH2, which are utilized to generate ATP by oxidative phosphorylation in the inner mitochondrial membrane. The intermediates of the cycle are also used in biosynthetic reactions of glucose, aminoacids, lipids, heme, and other biomolecules. The TCA cycle is tightly regulated in coordination with other mitochondrial pathways. Two enzymes from the Krebs cycle appear as the main targets of mitochondrial oxidants: aconitase and α-ketoglutarate dehydrogenase (Fig. 1).
Mitochondrial aconitase catalyzes the reversible isomerization of citrate to isocitrate via cis-aconitate. Aconitase contains a non-redox [4Fe–4S] prosthetic group in which one of the iron ions (Feα) is not ligated to a protein residue and thus can bind to hydroxyl groups of substrates or water [185]. Superoxide, peroxynitrite and CO3∙– rapidly react with the FeS cluster, yielding a catalytically inactive [3Fe–4S]-protein [186], [187]. The aconitase iron–sulfur cluster has a net oxidation state of +2; the local positive charge of the Fe is exposed attracting mitochondrial anionic oxidants. Superoxide reaction with aconitase iron–sulfur cluster is very selective and fast (k~107 M–1 s–1) and leads to the release of the Feα reactivation of mitochondrial aconitase is achieved by reincorporation of Fe2+ and is favored by reductants such as GSH. The ratio between [4Fe-4S]-aconitase and [3Fe–4S]-aconitase has been used to determine the steady-state concentration of O2∙− in cells [22], [186]. Hydrogen peroxide inactivates aconitase by the same mechanism as O2∙−, but with lower efficiency [188]. In addition, ∙NO interacts directly and reversibly with aconitase FeS cluster, while sustained ∙NO production slowly promotes an irreversible disassembly of the FeS cluster [187], [189].
The presence of an extremely O2∙−-sensitive enzyme within the mitochondrial matrix led to the speculation that mitochondrial aconitase may provide a redox control mechanism of the TCA cycle, the ETC and O2∙− production by mitochondria [190]. With a metabolic control approach using isolated mammalian mitochondria our laboratory showed that, inhibition of aconitase could impact on mitochondrial metabolism by diminishing the entry of reducing equivalents to the ETC, decreasing mitochondrial membrane potential and the diminishing the rate of O2∙− and H2O2 production [191].
Besides from the oxidation and disruption of the FeS cluster of aconitase, other redox post-translational modifications of this enzyme have been found. Proteomic analysis of mitochondria from animal models of sepsis, diabetes and aging revealed aconitase inactivation and nitration in vivo [142], [192], [193], [194], [195]. Additionally, glutathionylation, carbonylation and S-nitrosylation have been detected in mitochondrial aconitase [196], [197] but have not been shown to affect the catalytical activity of the enzyme and their biological significance is still unclear.
Further, aconitase activity seems to be regulated by phosphorylation. In particular, phosphorylated aconitase was found in skeletal muscle of human and in hearts from type 1 diabetic rats [198], [199]. Exercising accompanied aconitase dephosphorylation and augmented its catalytic activity towards isocitrate formation. While protein kinase C phosphorylation of mitochondrial aconitase in diabetic rat hearts was associated with an increase in its reverse activity, leading to citrate formation [200]. This mechanism together with redox inhibition of aconitase activity could inhibit the TCA cycle leading to the accumulation of upstream metabolites, decreased electron transport and lower O2∙− production by the ETC.
Aconitase activity increases upon acetylation, which increases the enzyme’s maximal velocity (Vmax). SIRT3 was shown to deacetylate several lysines in aconitase, reversing the activation of the enzyme. Aconitase acetylation has been observed in vitro and in vivo in the hearts of mice fed a high-fat diet and may play a role sensing nutrient availability and regulating fatty acid oxidation [201].
Modulation of the TCA cycle by aconitase can operate as part of a coordinated mitochondrial redox regulation network. Using proteomic techniques, several thiol containing mitochondrial proteins that react with low physiological levels of H2O2 were identified [202]. Among them, pyruvate dehydrogenase kinase 2 (PDHK2), a major regulator of pyruvate dehydrogenase complex (PDH), can be inactivated by oxidation of Cys residues 45 and 392 [202], [203]. PDHK2 inactivation results in a decrease in PDH phosphorylation and an increase in PDH activity that subsequently increases acetyl-CoA formation [203]. Therefore, in the face of oxidative stress, formation of acetyl-CoA would increase but aconitase inhibition would decrease TCA flux, shifting the balance from carbohydrate catabolism and to fatty acid synthesis.
Alpha-ketoglutarate dehydrogenase is a TCA cycle enzyme that catalyzes the non-equilibrium reaction converting α-ketoglutarate, coenzyme A and NAD+ to succinyl-CoA, NADH and CO2. α-KGDH is a complex enzyme consisting of multiple copies of three subunits, a thiamine pyrophosphate-dependent dehydrogenase (E1), dihydrolipoamide succinyltransferase (E2) and a flavoprotein, dihydrolipoamide dehydrogenase (E3) [204], [205]. α-Ketoglutarate dehydrogenase is considered to be both a target and a source of oxidant species [204]. As a target, α-KGDH was found to be sensitive to peroxynitrite and H2O2-dependent inactivation [206], [207], [208], [209]. While the intimate mechanism by which H2O2 inactivates α-KGDH is unresolved, it probably involves the oxidation of sulfhydryl groups present in one or more of the three subunits. In fact, a recent report indicates that H2O2 can inactivate dihydrolipoamide dehydrogenase both in α-KGDH and PDH [210]. Peroxynitrite in turn promotes specific tyrosine nitration, which results in enzyme inactivation [209]. Inactivation of α-KGDH has been reported in several neurodegenerative disorders. [204], [205]. α-Ketoglutarate dehydrogenase is a primary control site of the TCA cycle and is regulated by the ATP/ADP ratio, the NADH/NAD+ ratio, substrate availability and calcium concentration in mitochondria [211], [212]. Therefore impaired α-KGDH activity due to oxidative stress will limit TCA cycle activity (i.e. NADH production).
A study with brain synaptosomes showed that aconitase was the principal enzyme inhibited at low H2O2 concentrations. In this condition, with aconitase blocked, the Krebs cycle could be fueled by glutamate and therefore NADH generation was not impaired. However, at higher H2O2 concentrations (≥ 100 µM) inhibition of α-KGDH limited NADH availability for respiration [208]. Therefore, under conditions where pyruvate or acetyl-CoA are the main substrate of the TCA cycle, oxidant-mediated inhibition of aconitase would affect NADH formation. Yet, in conditions or tissues where glutamate could fuel the TCA cycle, NADH production would be maintained until mitochondrial oxidants inhibited α-KGDH activity, and only then would oxidant stress severely impair mitochondrial bioenergetics.
Interestingly, aconitase and α-KGDH are associated with the mitochondrial nucleoid and may be involved in mitochondrial DNA stabilization [213], [214], [215]. The signals that modulate these interactions are to still to be determined but probably involve oxidative modifications of the proteins.
6.3. Succinate dehydrogenase
Former reports from our group showed that physiologically relevant concentrations of peroxynitrite inactivate mitochondrial ETC through preferential inactivation of succinate dehydrogenase (SDH), leading to a decrease in O2 consumption [6], [216].
6.4. ATP synthase and creatine kinase
Peroxynitrite inactivates mitochondrial ATP synthase (ATPase; Complex V) and results in reduced ATP synthesis in mitochondria [216], [217]. Peroxynitrite inactivation of the enzyme is accompanied of tyrosine nitration [216], [217]. Critical Tyr residues are found in ATP synthase, but it remains to be determined if nitration of these residues is responsible of Complex V inactivation. Creatine kinase is also inhibited by peroxynitrite in a fast reaction involving thiol oxidation (k=8.85×105 M−1 s−1) [218].
6.5. Cytochrome c
Cytochrome c (cyt c) is a peripheral membrane protein of the inner mitochondrial membrane with a covalently attached heme group.
In mitochondria, cyt c has different activities depending of its interaction with the mitochondrial inner membrane. Almost 85% of cyt c is loosely bound to the membrane while the remaining is tightly bound through its interactions with cardiolipin [219], [220]. While the loosely bound cyt c participates in electron transport, membrane-bound cyt c has peroxidase activity and can reduce H2O2 formed in the inter-membrane space preventing its diffusion to the cytosol [219], [220], [221], [222], [223], [224]. However, cyt c peroxidase activity can also lead to the formation of hydroxy and hydroperoxy derivatives of the mitochondrial lipid cardiolipin, a critical event in the release of cyt c and induction of apoptosis [219], [220].
Interaction of cyt c with anionic phospholipids and/or reactions with oxidants, such as peroxynitrite, increases the peroxidase activity of cyt c [219], [220], [224]. In fact, biologically relevant concentrations of peroxynitrite have been shown to promote cyt c release from intact mitochondria [219], [224], [225], [226]. It has been proposed that ∙NO may regulate the peroxidase activity of cyt c through reduction of highly oxidized states of the heme [224], [227].
Peroxynitrite reaction with cyt c results in tyrosine nitration [225]. This posttranslational modification affects cyt c redox properties impairing its electron transport ability in the mitochondrial respiratory chain. Indeed, basal respiration cannot be recovered when cyt c-depleted mitochondria are reconstituted with peroxynitrite- treated cyt c [224], [226].
6.6. Cytochrome c oxidase
The most sensitive target of ∙NO in mitochondria is the heme group in cytochrome c oxidase. The ∙NO- cytochrome c oxidase interaction is involved in the maintenance of O2 gradients within tissues, through the modulation of actively respiring mitochondria. [228], [229], [230]. High levels of ∙NO can affect ATP synthesis and can promote the generation of O2∙− by the ETC [228], [229], [230]. In contrast to ∙NO, peroxynitrite is unable to inhibit cytochrome c oxidase, since Complex IV is highly resistant to oxidative damage [230], [231], [232].
6.7. Fatty acids
Mitochondria are the place where most of the oxidation of fatty acids (β-oxidation) to obtain energy occurs. As explained above, increased ROS and RNS levels in mitochondria can lead to the formation of nitro-fatty acids [48], [62]. The presence of the nitro group (NO2) affects the catabolism of the fatty acid. A study of the metabolic fate of NO2-OA (NO2-18:1 Δ9) injected to mice showed the desaturation of the double bond where the NO2 was bonded was followed by the formation of β-oxidation products. β-oxidation products of 16, 14 and 12 carbons containing the NO2 group were detected suggesting that β-oxidation stops at the carbon with the attached NO2 group [49], [233].
7. Peroxisome proliferator-activated receptor-γ (PPAR-γ)
Peroxisome Proliferator-Activated Receptor-γ (PPAR-γ) is a member of the nuclear hormone receptor superfamily of ligand-activated transcription factors that regulate genes involved mainly in lipid and glucose metabolism [234], [235]. Different ligands activate the receptor including antidiabetic thiazolidinedione (TZD) drugs or fatty acids derived products such as eicosanoids or 15D-PGJ2, [234], [235]. It is now well demonstrated that nitroalkenes are potent activators of PPAR-γ. For example, NO2-LA and NO2-OA exert many of their cell signaling actions via ligation and activation of PPARγ [55], [236]. Activated PPARγ modulates the expression of metabolic and cellular differentiation genes (e.g. genes related to lipid trafficking, cell proliferation and inflammatory signaling in the vasculature) and regulates inflammatory responses [237], [238], [239]. Importantly, nitroalkenes exert their actions at lower doses (low micromolar concentrations) than other endogenous ligands for PPARγ [236]. The biological relevance of the regulation of PPARγ by nitroalkenes was established when NO2-OA was administrated to ob/ob mice and was seen to modulate insulin and glucose levels without inducing adverse side effects [234].
8. Remarkable examples of interplay between oxidant species and energy metabolism
8.1. Sirtuin 3 coordinates catabolic reactions with antioxidant systems in mitochondria
Sirtuin 3 (SIRT3) is a NAD dependent deacetylase of protein lysine residues. Its levels increase during fasting and caloric restriction and mediate the switch from glucose to fatty acid utilization as a source of energy [240], by catalyzing the deacetylation and activation of LCAD [241]. Increased fatty acid oxidation would be coupled to an increase in O2∙− formation, yet SIRT3 also promotes the activation of MnSOD by deacetylation [127], [128], [129] probably preventing the accumulation of O2∙− and thus oxidative stress in this setting (Fig. 3). SIRT3 also catalyzes the deacetylation and activation of Isocitrate dehydrogenase 2 [242], a TCA cycle enzyme that maintains the NADPH pool required by GSH reductase, further protecting the cell from oxidative damage. Finally, SIRT3 has been demonstrated to catalyze the deacetylation of the transcriptional regulator FOXO3, leading to FOXO3 stabilization. Consequently, there is an increased expression of not only MnSOD but also other mitochondrial antioxidant systems under its control, including Prx3 and thioredoxin 2 [243].
8.2. Shifting glucose to the pentose phosphate pathway for oxidant detoxification
Glucose enters the cell through glucose transporters and, is then phosphorylated to glucose-6-phosphate. Glucose-6-phosphate is a substrate of the glycolytic pathway, that produces ATP, NADH and pyruvate, and of the pentose phosphate pathway (PPP) rendering ribose-5-phosphate and NADPH. The ability of the cell to efficiently channel these metabolites into different pathways depending on its needs is key to survival [244]. In the face of oxidative stress, glucose utilization must be shifted from the glycolytic pathway to the PPP to produce NADPH, in order to maintain the antioxidants glutathione and thioredoxin in reduced states [245], [246].
An important sensor of oxidant formation, that appears to play a role shifting glucose utilization to the PPP, is GAPDH. As mentioned above, GAPDH is a glycolytic enzyme, sensitive to oxidants, since the cysteine residue in the active site of the enzyme is oxidized by H2O2 and peroxynitrite with higher reaction rates than most thiols other than peroxidatic thiols [176], [247], [248]. This critical cysteine oxidation allows yeast to reroute glucose from glycolysis to the PPP favoring NADPH formation, and cell survival in oxidative stress conditions [177] (Fig. 4). The glycolytic enzyme pyruvate kinase M2 is sensitive to oxidants as well. Oxidation of Cys358 influences enzyme activity and seems to contribute to glucose rerouting to the PPP [249].
While GAPDH is inhibited by oxidants glucose -6-phosphate dehydrogenase (G6PD), the enzyme that catalyzes the first rate-limiting step in the PPP, is activated by hydrogen peroxide [250] and by peroxynitrite [251]. Activation of G6PD up regulates the PPP and increases NAPDH levels and the ability of cells to regenerate GSH from GSSG [251]. Thus, an increase in oxidant formation is accompanied of activation and inhibition of key regulatory enzymes that result in a shift from ATP to NADPH formation, increasing the cells antioxidant capacities (Fig. 4).
A recent metabolomic study shows that rerouting of metabolites to the PPP is in fact activated immediately after exposure to H2O2 and precedes the accumulation of glycolytic intermediates due to GAPDH inhibition; instead, they propose that NADPH allosteric regulation of G6PD plays a key role in this early response to oxidative stress [246].
Herrero-Mendez and colleagues present another striking example of metabolic reprogramming showing that bioenergetics and antioxidant status of neurons is controlled by continuous degradation of enzyme 6‐phosphofructo‐2‐kinase/ fructose‐2, 6‐bisphosphatase‐3 (PFKB3) by E3 ubiquitin ligase, anaphase‐promoting complex/cyclosome (APC/C)–Cdh1 [252]. PFKB3 catalyzes the synthesis of fructose‐2,6‐bisphosphate (F2,6P2), a potent activator of 6‐phosphofructo‐1‐kinase the principal regulator of the glycolytic pathway. In neurons, continuous degradation of PFKB3 in the proteasome assures a low flux of glucose through the glycolytic pathway allowing the neurons to use glucose in the PPP. Interfering with PFKB3 degradation or overexpression of PFK3B led to oxidative stress and apoptotic cell death [252]. In fact neuron exicitoxicity cell death promoted by activation of N-methyl-D-aspartate subtype of glutamate receptors (NMDAR), appears to involve not only an increase nitric oxide production by nitric oxide synthase 1 (NOS1) but also a stabilization of PFKB3, that results in GSH oxidation triggering oxidative damage and cell death [253].
9. Concluding remarks and future directions
Reactive oxygen species are continuously being formed during the catabolism of biomolecules. Superoxide and H2O2 are produced during electron transfer reactions in several metabolic pathways and can lead to the formation of highly oxidizing species such as peroxynitrite and lipid derived electrophiles. Reactive species are detoxified by a battery of antioxidant enzymes, but react as well with several cellular components. In this review, we have tried to identify key targets of oxidant species in metabolic routes and discussed the impact of these reactions on overall metabolic flux. We have also reviewed examples of coordinate regulation between antioxidant and energy producing pathways that help to avoid oxidative stress. In this setting reactive species, appear to play a role as signaling molecules capable of regulating metabolic flux in response to changes in the redox status of the cell.
Among future directions of research in the field, we find the role of the mitochondria quality control systems in maintaining bioenergetic efficiency and controlling oxidant formation by the ETC. Autophagy is the main process involved in mitochondrial turnover and removal of damaged mitochondria and impaired autophagy is implicated in many pathological conditions, including neurological disorders, inflammatory bowel disease, diabetes, aging, and cancer [254]. Defects in components of the mitochondrial autophagy (mitophagy) machinery, and impaired mitophagy result in an increase in mitochondrial ROS formation [255], [256], yet sites of oxidant formation have not been identified. Moreover, mitochondrial ROS appear to play signaling roles, promoting autophagy during cell starvation or metabolic stress [257], [258], evidencing a complex regulatory axis still to be fully characterized. Fusion and fission processes governing mitochondrial morphology and function [259] are affected by ROS levels in several cell and animal models; and mounting evidence suggests that cellular and mitochondrial redox homeostasis are linked to mitochondrial dynamics (recently revised in [260]). These events require further characterization at the molecular level and appear as a promising area of research.
Reactions of oxidants with master regulators of cellular energy state, such as AMPK, mTOR and Akt, are probably essential in encompassing metabolic pathways and redox status. Although ROS are known to impact in the signaling pathways governed by these kinases [261], [262], [263], [264], few of the reactions have been fully characterized (e.g. AMPK oxidation and activation by H2O2 [265]), and are still unexplored. Biochemical identification of oxidative posttranslational modifications in target residues, and kinetic constants for the reactions of cell energy sensors with different oxidant species, will surely contribute to the advancement of the field.
Sources of funding
This work was supported by Grants from Fondo Clemente Estable-ANII (FCE_6353) and CSIC grupos I+D 2014 (536) to AT; Fondo Clemente Estable-ANII (FCE_6381) and CSIC I+D 2012 (ID 47) to CQ; CSIC grupos I+D 2014 (767) to MT and LC; and Espacio Interdisciplinario – Centros, UDELAR 2015 to CQ, MT, LC and AT.
Disclosures
The authors declare that they have no conflicts of interest with the contents of this article.
Contributor Information
Celia Quijano, Email: celiq@fmed.edu.uy.
Andrés Trostchansky, Email: trocha@fmed.edu.uy.
References
- 1.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brookes P.S., Yoon Y., Robotham J.L., Anders M.W., Sheu S.S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287(4):C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 3.Davidson S.M., Duchen M.R. Endothelial mitochondria: contributing to vascular function and disease. Circ. Res. 2007;100(8):1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez J., Ballinger S.W., Darley-Usmar V.M., Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ. Res. 2006;99(9):924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- 5.Koeck T., Fu X., Hazen S.L., Crabb J.W., Stuehr D.J., Aulak K.S. Rapid and selective oxygen-regulated protein tyrosine denitration and nitration in mitochondria. J. Biol. Chem. 2004;279(26):27257–27262. doi: 10.1074/jbc.M401586200. [DOI] [PubMed] [Google Scholar]
- 6.Radi R., Cassina A., Hodara R., Quijano C., Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic. Biol. Med. 2002;33(11):1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 7.Vasquez-Vivar J., Kalyanaraman B., Kennedy M.C. Mitochondrial aconitase is a source of hydroxyl radical. An electron spin resonance investigation. J. Biol. Chem. 2000;275(19):14064–14069. doi: 10.1074/jbc.275.19.14064. [DOI] [PubMed] [Google Scholar]
- 8.Boveris A., Chance B. The mitochondrial production of hydrogen peroxide. Biochem. J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dionisi O., Galeotti T., Terranova T., Azzi A. Superoxide radicals and hydrogen peroxide formation in mitochondria from normal and neoplastic tissues. Biochim. Biophys. Acta. 1975;403(2):292–300. doi: 10.1016/0005-2744(75)90059-5. [DOI] [PubMed] [Google Scholar]
- 10.Dai D.F., Santana L.F., Vermulst M., Tomazela D.M., Emond M.J., MacCoss M.J. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119(21):2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz E., Anter E., Keaney J.F., Jr. Oxidative stress, antioxidants, and endothelial function. Curr. Med Chem. 2004;11(9):1093–1104. doi: 10.2174/0929867043365369. [DOI] [PubMed] [Google Scholar]
- 12.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552(2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowaltowski A.J., de Souza-Pinto N.C., Castilho R.F., Vercesi A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009;47(4):333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Tahara E.B., Navarete F.D., Kowaltowski A.J. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic. Biol. Med. 2009;46(9):1283–1297. doi: 10.1016/j.freeradbiomed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panov A., Dikalov S., Shalbuyeva N., Taylor G., Sherer T., Greenamyre J.T. Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J. Biol. Chem. 2005;280(51):42026–42035. doi: 10.1074/jbc.M508628200. [DOI] [PubMed] [Google Scholar]
- 17.Chouchani E.T., Pell V.R., Gaude E., Aksentijevic D., Sundier S.Y., Robb E.L. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genova M.L., Lenaz G. A critical appraisal of the role of respiratory supercomplexes in mitochondria. Biol. Chem. 2013;394(5):631–639. doi: 10.1515/hsz-2012-0317. [DOI] [PubMed] [Google Scholar]
- 19.Genova M.L., Lenaz G. The interplay between respiratory supercomplexes and ROS in aging. Antioxid. Redox Signal. 2015;23(3):208–238. doi: 10.1089/ars.2014.6214. [DOI] [PubMed] [Google Scholar]
- 20.Maranzana E., Barbero G., Falasca A.I., Lenaz G., Genova M.L. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid. Redox Signal. 2013;19(13):1469–1480. doi: 10.1089/ars.2012.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han D., Williams E., Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 2001;353(2):411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner P.R., Raineri I., Epstein L.B., White C.W. Superoxide radical and iron modulate aconitase activity in mammalian cells. J. Biol. Chem. 1995;270(22):13399–13405. doi: 10.1074/jbc.270.22.13399. [DOI] [PubMed] [Google Scholar]
- 23.Castro L., Demicheli V., Tortora V., Radi R. Mitochondrial protein tyrosine nitration. Free Radic. Res. 2011;45(1):37–52. doi: 10.3109/10715762.2010.516254. [DOI] [PubMed] [Google Scholar]
- 24.Riemer J., Bulleid N., Herrmann J.M. Disulfide formation in the ER and mitochondria: two solutions to a common process. Science. 2009;324(5932):1284–1287. doi: 10.1126/science.1170653. [DOI] [PubMed] [Google Scholar]
- 25.Janssen-Heininger Y.M., Mossman B.T., Heintz N.H., Forman H.J., Kalyanaraman B., Finkel T. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic. Biol. Med. 2008;45(1):1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ardanaz N., Pagano P.J. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp. Biol. Med.(Maywood) 2006;231(3):237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- 27.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc. Res. 2005;68(1):26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Jang S., Imlay J.A. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J. Biol. Chem. 2007;282(2):929–937. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adam-Vizi V., Tretter L. The role of mitochondrial dehydrogenases in the generation of oxidative stress. Neurochem. Int. 2013;62(5):757–763. doi: 10.1016/j.neuint.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Czapski G., Goldstein S. The reaction of NO– with O2∙– and HO2∙–: a pulse radiolysis study. Free Radic. Biol. Med. 1995;19(4):505–510. doi: 10.1016/0891-5849(95)00034-u. [DOI] [PubMed] [Google Scholar]
- 31.Nauser T., Koppenol W.H. The rate constant of the reaction of superoxide with nitrogen monoxide: approaching the diffusion limit. J. Phys. Chem. A. 2002;106:4084–4086. [Google Scholar]
- 32.Radi R., Denicola A., Alvarez B., Ferrer G., Rubbo H. The biological chemistry of peroxynitrite. In: Ignarro L.J., editor. Nitric Oxide Biology and Pathobiology. Academic Press; San Diego: 2000. pp. 57–82. [Google Scholar]
- 33.Denicola A., Freeman B.A., Trujillo M., Radi R. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch. Biochem. Biophys. 1996;333(1):49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 34.Ferrer-Sueta G., Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem. Biol. 2009;4(3):161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- 35.Beckman J.S., Beckman T.W., Chen J., Marshall P.A., Freeman B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U. S. A. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Augusto O., Gatti R.M., Radi R. Spin-trapping studies of peroxynitrite decomposition and of 3- morpholinosydnonimine N-ethylcarbamide autooxidation: direct evidence for metal-independent formation of free radical intermediates. Arch. Biochem. Biophys. 1994;310(1):118–125. doi: 10.1006/abbi.1994.1147. [DOI] [PubMed] [Google Scholar]
- 37.Merenyi G., Lind J., Goldstein S., Czapski G. Mechanism and termochemistry of peroxynitrite decomposition in water. J. Phys. Chem. A. 1999;103:5685–5691. [Google Scholar]
- 38.Shaw A.W., Vosper A.J. Solubility of nitric oxide in aqueous and nonaqueous solvents. J. Chem. Soc. Faraday Trans. 1977;8:1239–1244. [Google Scholar]
- 39.Zacharia I.G., Deen W.M. Diffusivity and solubility of nitric oxide in water and saline. Ann. Biomed. Eng. 2005;33(2):21–222. doi: 10.1007/s10439-005-8980-9. [DOI] [PubMed] [Google Scholar]
- 40.Alderton W.K., Cooper C.E., Knowles R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaobornyj T., Ghafourifar P. Strategic localization of heart mitochondrial NOS: a review of the evidence. Am. J. Physiol. Heart Circ. Physiol. 2012;303(11):H1283–H1293. doi: 10.1152/ajpheart.00674.2011. [DOI] [PubMed] [Google Scholar]
- 42.Botti H., Trostchansky A., Batthyany C., Rubbo H. Reactivity of peroxynitrite and nitric oxide with LDL. IUBMB Life. 2005;57(6):407–412. doi: 10.1080/15216540500137701. [DOI] [PubMed] [Google Scholar]
- 43.Botti H., Trujillo M., Batthyany C., Rubbo H., Ferrer-Sueta G., Radi R. Homolytic pathways drive peroxynitrite-dependent Trolox C oxidation. Chem. Res. Toxicol. 2004;17(10):1377–1384. doi: 10.1021/tx034269i. [DOI] [PubMed] [Google Scholar]
- 44.Daiber A., Bachschmid M. Enzyme inhibition by peroxynitrite-mediated tyrosine nitration and thiol oxidation. Curr. Enzym. Inhib. 2007;3(2):103–117. [Google Scholar]
- 45.d’Ischia M. Nitrosation and nitration of bioactive molecules: toward the basis of disease and its prevention. C. R. Chim. 2005;8(5):797–806. [Google Scholar]
- 46.Radi R., Beckman J.S., Bush K.M., Freeman B.A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991;266(7):4244–4250. [PubMed] [Google Scholar]
- 47.Rubbo H., Radi R., Trujillo M., Telleri R., Kalyanaraman B., Barnes S. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 1994;269(42):26066–26075. [PubMed] [Google Scholar]
- 48.Koenitzer J.R., Freeman B.A. Redox signaling in inflammation: interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Ann. N. Y. Acad. Sci. 2010;1203:45–52. doi: 10.1111/j.1749-6632.2010.05559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schopfer F.J., Batthyany C., Baker P.R., Bonacci G., Cole M.P., Rudolph V. Detection and quantification of protein adduction by electrophilic fatty acids: mitochondrial generation of fatty acid nitroalkene derivatives. Free Radic. Biol. Med. 2009;46(9):1250–1259. doi: 10.1016/j.freeradbiomed.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nadtochiy S.M., Baker P.R., Freeman B.A., Brookes P.S. Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc. Res. 2009;82(2):333–340. doi: 10.1093/cvr/cvn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubbo H., Batthyány C., Freeman B.A., Radi R., Denicola A. Nitric oxide diffusion across low density lipoprotein and inhibition of lipid oxidation-dependent chemiluminescence. Nitric oxide. 1998;2(Suppl. 2):S117. [Google Scholar]
- 52.Rubbo H., Freeman B.A. Nitric oxide regulation of lipid oxidation reactions: formation and analysis of nitrogen-containing oxidized lipid derivatives. Methods Enzym. 1996;269:385–394. doi: 10.1016/s0076-6879(96)69039-9. [DOI] [PubMed] [Google Scholar]
- 53.Rubbo H., O'Donnell V. Nitric oxide, peroxynitrite and lipoxygenase in atherogenesis: mechanistic insights. Toxicology. 2005;208(2):305–317. doi: 10.1016/j.tox.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Rubbo H., Radi R. Protein and lipid nitration: role in redox signaling and injury. Biochim. Biophys. Acta. 2008;1780(11):1318–1324. doi: 10.1016/j.bbagen.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 55.Baker P.R., Lin Y., Schopfer F.J., Woodcock S.R., Groeger A.L., Batthyany C. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J. Biol. Chem. 2005;280(51):42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baker P.R., Schopfer F.J., Sweeney S. Freeman BA. Red cell membrane and plasma linoleic acid nitration products: synthesis, clinical identification, and quantitation. Proc. Natl. Acad. Sci. U. S. A. 2004;101(32):11577–11582. doi: 10.1073/pnas.0402587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alexander R.L., Bates D.J., Wright M.W., King S.B., Morrow C.S. Modulation of nitrated lipid signaling by multidrug resistance protein 1 (MRP1): glutathione conjugation and MRP1-mediated efflux inhibit nitrolinoleic acid-induced, PPARgamma-dependent transcription activation. Biochemistry. 2006;45(25):7889–7896. doi: 10.1021/bi0605639. [DOI] [PubMed] [Google Scholar]
- 58.Batthyany C., Schopfer F.J., Baker P.R., Duran R., Baker L.M., Huang Y. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J. Biol. Chem. 2006;281(29):20450–20463. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsikas D., Zoerner A.A., Mitschke A., Gutzki F.M. Nitro-fatty acids occur in human plasma in the picomolar range: a targeted nitro-lipidomics GC–MS/MS study. Lipids. 2009;44(9):855–865. doi: 10.1007/s11745-009-3332-4. [DOI] [PubMed] [Google Scholar]
- 60.Coles B., Bloodsworth A., Clark S.R., Lewis M.J., Cross A.R., Freeman B.A. Nitrolinoleate inhibits superoxide generation, degranulation, and integrin expression by human neutrophils: novel antiinflammatory properties of nitric oxide-derived reactive species in vascular cells. Circ. Res. 2002;91(5):375–381. doi: 10.1161/01.res.0000032114.68919.ef. [DOI] [PubMed] [Google Scholar]
- 61.Trostchansky A., Souza J.M., Ferreira A., Ferrari M., Blanco F., Trujillo M. Synthesis, isomer characterization, and anti-inflammatory properties of nitroarachidonate. Biochemistry. 2007;46(15):4645–4653. doi: 10.1021/bi602652j. [DOI] [PubMed] [Google Scholar]
- 62.Bonacci G., Baker P.R., Salvatore S.R., Shores D., Khoo N.K., Koenitzer J.R. Conjugated linoleic acid is a preferential substrate for fatty acid nitration. J. Biol. Chem. 2012;287(53):44071–44082. doi: 10.1074/jbc.M112.401356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Curtis J.M., Hahn W.S., Stone M.D., Inda J.J., Droullard D.J., Kuzmicic J.P. Protein carbonylation and adipocyte mitochondrial function. J. Biol. Chem. 2012;287(39):32967–32980. doi: 10.1074/jbc.M112.400663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frohnert B.I., Bernlohr D.A. Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Adv. Nutr. 2013;4(2):157–163. doi: 10.3945/an.112.003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reddy J.K., Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu. Rev. Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki H., Kawarabayasi Y., Kondo J., Abe T., Nishikawa K., Kimura S. Structure and regulation of rat long-chain acyl-CoA synthetase. J. Biol. Chem. 1990;265(15):8681–8685. [PubMed] [Google Scholar]
- 67.Thorpe C., Kim J.J. Structure and mechanism of action of the acyl-CoA dehydrogenases. FASEB J. 1995;9(9):718–725. doi: 10.1096/fasebj.9.9.7601336. [DOI] [PubMed] [Google Scholar]
- 68.Kim J.J., Miura R. Acyl-CoA dehydrogenases and acyl-CoA oxidases. Structural basis for mechanistic similarities and differences. Eur. J. Biochem. 2004;271(3):483–493. doi: 10.1046/j.1432-1033.2003.03948.x. [DOI] [PubMed] [Google Scholar]
- 69.Eaton S., Bartlett K., Pourfarzam M. Mammalian mitochondrial beta-oxidation. Biochem. J. 1996;320(2):345–357. doi: 10.1042/bj3200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.St-Pierre J., Buckingham J.A., Roebuck S.J., Brand M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002;277(47):44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 71.Seifert E.L., Estey C., Xuan J.Y., Harper M.E. Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J. Biol. Chem. 2010;285(8):5748–5758. doi: 10.1074/jbc.M109.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kakimoto P.A., Tamaki F.K., Cardoso A.R., Marana S.R., Kowaltowski A.J. H2O2 release from the very long chain acyl-CoA dehydrogenase. Redox Biol. 2015;4:375–380. doi: 10.1016/j.redox.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cardoso A.R., Kakimoto P.A., Kowaltowski A.J. Diet-sensitive sources of reactive oxygen species in liver mitochondria: role of very long chain acyl-CoA dehydrogenases. PLoS One. 2013;8(10):e77088. doi: 10.1371/journal.pone.0077088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodrigues J.V., Gomes C.M. Mechanism of superoxide and hydrogen peroxide generation by human electron-transfer flavoprotein and pathological variants. Free Radic. Biol. Med. 2012;53(1):12–19. doi: 10.1016/j.freeradbiomed.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 75.Pierre J., Buckingham J.A., Roebuck S.J., Brand M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002;277(47):44784–44790. doi: 10.1074/jbc.M207217200. St. [DOI] [PubMed] [Google Scholar]
- 76.Aoyama T., Souri M., Ushikubo S., Kamijo T., Yamaguchi S., Kelley R.I. Purification of human very-long-chain acyl-coenzyme A dehydrogenase and characterization of its deficiency in seven patients. J. Clin. Invest. 1995;95(6):2465–2473. doi: 10.1172/JCI117947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim H., Haluzik M., Gavrilova O., Yakar S., Portas J., Sun H. Thiazolidinediones improve insulin sensitivity in adipose tissue and reduce the hyperlipidaemia without affecting the hyperglycaemia in a transgenic model of type 2 diabetes. Diabetologia. 2004;47(12):2215–2225. doi: 10.1007/s00125-004-1581-6. [DOI] [PubMed] [Google Scholar]
- 78.Musso G., Gambino R., Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD) Prog. Lipid Res. 2009;48(1):1–26. doi: 10.1016/j.plipres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 79.Wei Y., Sowers J.R., Clark S.E., Li W., Ferrario C.M., Stump C.S. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-{kappa}B activation via NADPH oxidase. Am. J. Physiol. Endocrinol. Metab. 2008;294(2):E345–E351. doi: 10.1152/ajpendo.00456.2007. [DOI] [PubMed] [Google Scholar]
- 80.Huang K.Y., Chen Y.Y., Fang Y.K., Cheng W.H., Cheng C.C., Chen Y.C. Adaptive responses to glucose restriction enhance cell survival, antioxidant capability, and autophagy of the protozoan parasite Trichomonas vaginalis. Biochim. Biophys. Acta. 2014;1840(1):53–64. doi: 10.1016/j.bbagen.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 81.Weyemi U., Lagente-Chevallier O., Boufraqech M., Prenois F., Courtin F., Caillou B. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene. 2012;31(9):1117–1129. doi: 10.1038/onc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kodama R., Kato M., Furuta S., Ueno S., Zhang Y., Matsuno K. ROS-generating oxidases Nox1 and Nox4 contribute to oncogenic Ras-induced premature senescence. Genes Cells. 2013;18(1):32–41. doi: 10.1111/gtc.12015. [DOI] [PubMed] [Google Scholar]
- 83.Moiseeva O., Bourdeau V., Roux A., Deschenes-Simard X., Ferbeyre G. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol. Cell. Biol. 2009;29(16):4495–4507. doi: 10.1128/MCB.01868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quijano C., Cao L., Fergusson M.M., Romero H., Liu J., Gutkind S. Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle. 2012;11(7):1383–1392. doi: 10.4161/cc.19800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Osumi T., Hashimoto T. Acyl-CoA oxidase of rat liver: a new enzyme for fatty acid oxidation. Biochem. Biophys. Res. Commun. 1978;83(2):479–485. doi: 10.1016/0006-291x(78)91015-x. [DOI] [PubMed] [Google Scholar]
- 86.Vanhove G.F., Van Veldhoven P.P., Fransen M., Denis S., Eyssen H.J., Wanders R.J. The CoA esters of 2-methyl-branched chain fatty acids and of the bile acid intermediates di- and trihydroxycoprostanic acids are oxidized by one single peroxisomal branched chain acyl-CoA oxidase in human liver and kidney. J. Biol. Chem. 1993;268(14):10335–10344. [PubMed] [Google Scholar]
- 87.Nakajima Y., Miyahara I., Hirotsu K., Nishina Y., Shiga K., Setoyama C. Three-dimensional structure of the flavoenzyme acyl-CoA oxidase-II from rat liver, the peroxisomal counterpart of mitochondrial acyl-CoA dehydrogenase. J. Biochem. 2002;131(3):365–374. doi: 10.1093/oxfordjournals.jbchem.a003111. [DOI] [PubMed] [Google Scholar]
- 88.Lee S.S.T., Pineau T., Drago J., Lee E.J., Owens J.W., Kroetz D.L. Targer disruption of the α isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell Biol. 1995;15(6):3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Escher P., Braissant O., Basu-Modak S., Michalik L., Wahli W., Desvergne B. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology. 2001;142(10):4195–4202. doi: 10.1210/endo.142.10.8458. [DOI] [PubMed] [Google Scholar]
- 90.Pyper S.R., Viswakarma N., Yu S., Reddy J.K. PPARalpha: energy combustion, hypolipidemia, inflammation and cancer. Nucl. Recept. Signal. 2010;8:e002. doi: 10.1621/nrs.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kasai H., Okada Y., Nishimura S., Rao M.S., Reddy J.K. Formation of 8-hydroxydeoxyguanosine in liver DNA of rats following long-term exposure to a peroxisome proliferator. Cancer Res. 1989;49(10):2603–2605. [PubMed] [Google Scholar]
- 92.Fahl W.E., Lalwani N.D., Watanabe T., Goel S.K., Reddy J.K. DNA damage related to increased hydrogen peroxide generation by hypolipidemic drug-induced liver peroxisomes. Proc. Natl. Acad. Sci. U. S. A. 1984;81(24):7827–7830. doi: 10.1073/pnas.81.24.7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yeldandi A.V., Rao M.S., Reddy J.K. Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutat. Res. 2000;448(2):159–177. doi: 10.1016/s0027-5107(99)00234-1. [DOI] [PubMed] [Google Scholar]
- 94.Kelley E.E., Khoo N.K., Hundley N.J., Malik U.Z., Freeman B.A., Tarpey M.M. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic. Biol. Med. 2010;48(4):493–498. doi: 10.1016/j.freeradbiomed.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Malik U.Z., Hundley N.J., Romero G., Radi R., Freeman B.A., Tarpey M.M. Febuxostat inhibition of endothelial-bound XO: implications for targeting vascular ROS production. Free Radic. Biol. Med. 2011;51(1):179–184. doi: 10.1016/j.freeradbiomed.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.George J., Struthers A. The role of urate and xanthine oxidase in vascular oxidative stress: future directions. Ther. Clin. Risk Manag. 2009;5:799–803. doi: 10.2147/tcrm.s5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu X., Hu X., Lu Z., Zhang P., Zhao L., Wessale J.L. Xanthine oxidase inhibition with febuxostat attenuates systolic overload-induced left ventricular hypertrophy and dysfunction in mice. J. Card. Fail. 2008 11;14(9):746–753. doi: 10.1016/j.cardfail.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao L., Roche B.M., Wessale J.L., Kijtawornrat A., Lolly J.L., Shemanski D. Chronic xanthine oxidase inhibition following myocardial infarction in rabbits: Effects of early versus delayed treatment. Life Sci. 2008;82(9–10):495–502. doi: 10.1016/j.lfs.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 99.Aslan M., Ryan T.M., Adler B., Townes T.M., Parks D.A., Thompson J.A. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc. Natl. Acad. Sci. U. S. A. 2001;98(26):15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sohn H.Y., Krotz F., Gloe T., Keller M., Theisen K., Klauss V. Differential regulation of xanthine and NAD(P)H oxidase by hypoxia in human umbilical vein endothelial cells. Role of nitric oxide and adenosine. Cardiovasc. Res. 2003;58(3):638–646. doi: 10.1016/s0008-6363(03)00262-1. [DOI] [PubMed] [Google Scholar]
- 101.Kelley E.E., Trostchansky A., Rubbo H., Freeman B.A., Radi R., Tarpey M.M. Binding of xanthine oxidase to glycosaminoglycans limits inhibition by oxypurinol. J. Biol. Chem. 2004;279(36):37231–37234. doi: 10.1074/jbc.M402077200. [DOI] [PubMed] [Google Scholar]
- 102.Lee S.J., Terkeltaub R.A. New developments in clinically relevant mechanisms and treatment of hyperuricemia. Curr. Rheumatol. Rep. 2006;8(3):224–230. doi: 10.1007/s11926-996-0029-z. [DOI] [PubMed] [Google Scholar]
- 103.Hou M., Hu Q., Chen Y., Zhao L., Zhang J., Bache R.J.M. Acute effects of febuxostat, a nonpurine selective inhibitor of xanthine oxidase, in pacing induced heart failure. J. Cardiovasc. Pharmacol. 2006 11;48(5):255–263. doi: 10.1097/01.fjc.0000249961.61451.da. [DOI] [PubMed] [Google Scholar]
- 104.Teramoto S., Kume H., Yamaguchi Y., Yamamoto H., Hanaoka Y., Ishii M. Improvement of endothelial function with allopurinol may occur in selected patients with OSA: effect of age and sex. Eur. Respir. J. 2007;29(1):216–217. doi: 10.1183/09031936.00104806. [DOI] [PubMed] [Google Scholar]
- 105.de Cavanagh E.M., Inserra F., Ferder M., Ferder L. From mitochondria to disease: role of the renin–angiotensin system. Am. J. Nephrol. 2007;27(6):545–553. doi: 10.1159/000107757. [DOI] [PubMed] [Google Scholar]
- 106.Federico A., Cardaioli E., Da Pozzo P., Formichi P., Gallus G.N., Radi E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012;322(1-2):254–262. doi: 10.1016/j.jns.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 107.Newsholme P., Gaudel C., Krause M. Mitochondria and diabetes. An intriguing pathogenetic role. Adv. Exp. Med. Biol. 2012;942:235–247. doi: 10.1007/978-94-007-2869-1_10. [DOI] [PubMed] [Google Scholar]
- 108.Nicolson G.L. Metabolic syndrome and mitochondrisal function: molecular replacement and antioxidant supplements to prevent membrane peroxidation and restore mitochondrial function. J. Cell. Biochem. 2007;100(6):1352–1369. doi: 10.1002/jcb.21247. [DOI] [PubMed] [Google Scholar]
- 109.Sas K., Robotka H., Toldi J., Vecsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol. Sci. 2007;257(1–2):221–239. doi: 10.1016/j.jns.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 110.Taylor C.T. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem. J. 2008;409(1):19–26. doi: 10.1042/BJ20071249. [DOI] [PubMed] [Google Scholar]
- 111.Verdejo H.E., del Campo A., Troncoso R., Gutierrez T., Toro B., Quiroga C. Mitochondria, myocardial remodeling, and cardiovascular disease. Curr. Hypertens. Rep. 2012;14(6):532–539. doi: 10.1007/s11906-012-0305-4. [DOI] [PubMed] [Google Scholar]
- 112.Sheng Y., Abreu I.A., Cabelli D.E., Maroney M.J., Miller A.F., Teixeira M. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014;114(7):3854–3918. doi: 10.1021/cr4005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Borgstahl G.E., Parge H.E., Hickey M.J., Beyer W.F., Jr., Hallewell R.A., Tainer J.A. The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles. Cell. 1992;71(1):107–118. doi: 10.1016/0092-8674(92)90270-m. [DOI] [PubMed] [Google Scholar]
- 114.Hsu J.L., Hsieh Y., Tu C., O'Connor D., Nick H.S., Silverman D.N. Catalytic properties of human manganese superoxide dismutase. J. Biol. Chem. 1996;271(30):17687–17691. doi: 10.1074/jbc.271.30.17687. [DOI] [PubMed] [Google Scholar]
- 115.Bull C., Niederhoffer E.C., Yoshida T., Fee J.A. kinetic studies of superoxide dismutases: properties of the manganese-containing protein from Thermus thermophilus. J. Am. Chem. Soc. 1991;113:4069–4076. [Google Scholar]
- 116.Miao L., St, Clair D.K. Regulation of superoxide dismutase genes: implications in disease. Free Radic. Biol. Med. 2009;47(4):344–356. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Drane P., Bravard A., Bouvard V., May E. Reciprocal down-regulation of p53 and SOD2 gene expression-implication in p53 mediated apoptosis. Oncogene. 2001;20(4):430–439. doi: 10.1038/sj.onc.1204101. [DOI] [PubMed] [Google Scholar]
- 118.Kops G.J., Dansen T.B., Polderman P.E., Saarloos I., Wirtz K.W., Coffer P.J. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419(6904):316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 119.Candas D., Li J.J. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid. Redox Signal. 2014;20(10):1599–1617. doi: 10.1089/ars.2013.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Quijano C., Hernandez-Saavedra D., Castro L., McCord J.M., Freeman B.A., Radi R. Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J. Biol. Chem. 2001;276(15):11631–11638. doi: 10.1074/jbc.M009429200. [DOI] [PubMed] [Google Scholar]
- 121.MacMillan-Crow L.A., Crow J.P., Kerby J.D., Beckman J.S., Thompson J.A. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc. Natl. Acad. Sci. U. S. A. 1996;93(21):11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pittman K.M., MacMillan-Crow L.A., Peters B.P., Allen J.B. Nitration of manganese superoxide dismutase during ocular inflammation. Exp. Eye Res. 2002;74(4):463–471. doi: 10.1006/exer.2002.1141. [DOI] [PubMed] [Google Scholar]
- 123.Redondo-Horcajo M., Romero N., Martinez-Acedo P., Martinez-Ruiz A., Quijano C., Lourenco C.F. Cyclosporine A-induced nitration of tyrosine 34 MnSOD in endothelial cells: role of mitochondrial superoxide. Cardiovasc. Res. 2010;87(2):356–365. doi: 10.1093/cvr/cvq028. [DOI] [PubMed] [Google Scholar]
- 124.Moreno D.M., Marti M.A., De Biase P.M., Estrin D.A., Demicheli V., Radi R. Exploring the molecular basis of human manganese superoxide dismutase inactivation mediated by tyrosine 34 nitration. Arch. Biochem. Biophys. 2011;507(2):304–309. doi: 10.1016/j.abb.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 125.Candas D., Fan M., Nantajit D., Vaughan A.T., Murley J.S., Woloschak G.E. CyclinB1/Cdk1 phosphorylates mitochondrial antioxidant MnSOD in cell adaptive response to radiation stress. J. Mol. Cell. Biol. 2013;5(3):166–175. doi: 10.1093/jmcb/mjs062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jin C., Qin L., Shi Y., Candas D., Fan M., Lu C.L. CDK4-mediated MnSOD activation and mitochondrial homeostasis in radioadaptive protection. Free Radic. Biol. Med. 2015;81:77–87. doi: 10.1016/j.freeradbiomed.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tao R., Coleman M.C., Pennington J.D., Ozden O., Park S.H., Jiang H. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell. 2010;40(6):893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen Y., Zhang J., Lin Y., Lei Q., Guan K.L., Zhao S. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011;12(6):534–541. doi: 10.1038/embor.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qiu X., Brown K., Hirschey M.D., Verdin E., Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell. Metab. 2010;12(6):662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 130.Zhu Y., Park S.H., Ozden O., Kim H.S., Jiang H., Vassilopoulos A. Exploring the electrostatic repulsion model in the role of Sirt3 in directing MnSOD acetylation status and enzymatic activity. Free Radic. Biol. Med. 2012;53(4):828–833. doi: 10.1016/j.freeradbiomed.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Scott I., Webster B.R., Li J.H., Sack M.N. Identification of a molecular component of the mitochondrial acetyltransferase programme: a novel role for GCN5L1. Biochem. J. 2012;443(3):655–661. doi: 10.1042/BJ20120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wagner G.R., Payne R.M. Widespread and enzyme-independent Nepsilon-acetylation and Nepsilon-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J. Biol. Chem. 2013;288(40):29036–29045. doi: 10.1074/jbc.M113.486753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kapralov A.A., Kurnikov I.V., Vlasova, Belikova N.A., Tyurin V.A., Basova L.V. The hierarchy of structural transitions induced in cytochrome c by anionic phospholipids determines its peroxidase activation and selective peroxidation during apoptosis in cells. Biochemistry. 2007;46(49):14232–14244. doi: 10.1021/bi701237b. [DOI] [PubMed] [Google Scholar]
- 134.Abriata L.A., Cassina A., Tortora V., Marin M., Souza J.M., Castro L. Nitration of solvent-exposed tyrosine 74 on cytochrome c triggers heme iron-methionine 80 bond disruption. Nuclear magnetic resonance and optical spectroscopy studies. J. Biol. Chem. 2009;284(1):17–26. doi: 10.1074/jbc.M807203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brigelius-Flohe R., Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 2011;15(8):2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Poole L.B., Hall A., Nelson K.J. Overview of peroxiredoxins in oxidant defense and redox regulation. Curr. Protoc. Toxicol. 2011:9. doi: 10.1002/0471140856.tx0709s49. (Chapter 7:Unit 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Winterbourn C.C., Hampton M.B. Redox biology: signaling via a peroxiredoxin sensor. Nat. Chem. Biol. 2015;11(1):5–6. doi: 10.1038/nchembio.1722. Jan. [DOI] [PubMed] [Google Scholar]
- 138.Rhee S.G., Woo H.A. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H(2)O(2), and protein chaperones. Antioxid. Redox Signal. 2011;15(3):781–794. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- 139.Cox A.G., Winterbourn C.C., Hampton M.B. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem. J. 2010;425(2):313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- 140.Kil I.S., Ryu K.W., Lee S.K., Kim J.Y., Chu S.Y., Kim J.H. Circadian oscillation of sulfiredoxin in the mitochondria. Mol. Cell. 2015;59(4):651–663. doi: 10.1016/j.molcel.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 141.Hattori F., Murayama N., Noshita T., Oikawa S. Mitochondrial peroxiredoxin-3 protects hippocampal neurons from excitotoxic injury in vivo. J. Neurochem. 2003;86(4):860–868. doi: 10.1046/j.1471-4159.2003.01918.x. Aug. [DOI] [PubMed] [Google Scholar]
- 142.Turko I.V., Li L., Aulak K.S., Stuehr D.J., Chang J.Y., Murad F. Protein tyrosine nitration in the mitochondria from diabetic mouse heart. Implications to dysfunctional mitochondria in diabetes. J. Biol. Chem. 2003;278(36):33972–33977. doi: 10.1074/jbc.M303734200. [DOI] [PubMed] [Google Scholar]
- 143.Li L., Shoji W., Takano H., Nishimura N., Aoki Y., Takahashi R. Increased susceptibility of MER5 (peroxiredoxin III) knockout mice to LPS-induced oxidative stress. Biochem. Biophys. Res. Commun. 2007;355(3):715–721. doi: 10.1016/j.bbrc.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 144.Li L., Shoji W., Oshima H., Obinata M., Fukumoto M., Kanno N. Crucial role of peroxiredoxin III in placental antioxidant defense of mice. FEBS Lett. 2008;582(16):2431–2434. doi: 10.1016/j.febslet.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 145.Li L., Kaifu T., Obinata M., Takai T. Peroxiredoxin III-deficiency sensitizes macrophages to oxidative stress. J. Biochem. 2009;145(4):425–427. doi: 10.1093/jb/mvp011. [DOI] [PubMed] [Google Scholar]
- 146.Huh J.Y., Kim Y., Jeong J., Park J., Kim I., Huh K.H. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Antioxid. Redox Signal. 2012;16(3):229–243. doi: 10.1089/ars.2010.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Knoops B., Goemaere J., Van der Eecken V., Declercq J.P. Peroxiredoxin 5: structure, mechanism, and function of the mammalian atypical 2-Cys peroxiredoxin. Antioxid. Redox Signal. 2011;15(3):817–829. doi: 10.1089/ars.2010.3584. [DOI] [PubMed] [Google Scholar]
- 148.Trujillo M., Clippe A., Manta B., Ferrer-Sueta G., Smeets A., Declercq J.P. Pre-steady state kinetic characterization of human peroxiredoxin 5: taking advantage of Trp84 fluorescence increase upon oxidation. Arch. Biochem. Biophys. 2007;467(1):95–106. doi: 10.1016/j.abb.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 149.Dubuisson M., Vander Stricht D., Clippe A., Etienne F., Nauser T., Kissner R. Human peroxiredoxin 5 is a peroxynitrite reductase. FEBS Lett. 2004;571(1–3):161–165. doi: 10.1016/j.febslet.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 150.Trujillo M., Ferrer-Sueta G., Radi R. Peroxynitrite detoxification and its biologic implications. Antioxid. Redox Signal. 2008;10(9):1607–1620. doi: 10.1089/ars.2008.2060. [DOI] [PubMed] [Google Scholar]
- 151.Banmeyer I., Marchand C., Clippe A., Knoops B. Human mitochondrial peroxiredoxin 5 protects from mitochondrial DNA damages induced by hydrogen peroxide. FEBS Lett. 2005;579(11):2327–2333. doi: 10.1016/j.febslet.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 152.Davey G.P., Bolanos J.P. Peroxiredoxin 5 links mitochondrial redox signalling with calcium dynamics: impact on Parkinson disease. J. Neurochem. 2013;125(3):332–333. doi: 10.1111/jnc.12171. [DOI] [PubMed] [Google Scholar]
- 153.De Simoni S., Linard D., Hermans E., Knoops B., Goemaere J. Mitochondrial peroxiredoxin-5 as potential modulator of mitochondria-ER crosstalk in MPP+-induced cell death. J. Neurochem. 2013;125(3):473–485. doi: 10.1111/jnc.12117. [DOI] [PubMed] [Google Scholar]
- 154.Brigelius-Flohe R., Flohe L. Is there a role of glutathione peroxidases in signaling and differentiation? Biofactors. 2003;17(1–4):93–102. doi: 10.1002/biof.5520170110. [DOI] [PubMed] [Google Scholar]
- 155.Toppo S., Flohe L., Ursini F., Vanin S., Maiorino M. Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme. Biochim. Biophys. Acta. 2009;1790(11):1486–1500. doi: 10.1016/j.bbagen.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 156.Hamilton R.T., Walsh M.E., Van Remmen H. Mouse Models of Oxidative Stress Indicate a Role for Modulating Healthy. Aging J. Clin. Exp. Pathol. 2012;(Suppl 4) doi: 10.4172/2161-0681.S4-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lei X.G., Wang X. Glutathione peroxidase 1 and diabetes. In: Hatfield D.L., Berry M.J., Gladyshev V.N., editors. Selenium its Molecular Biology and Role in Human Health. third ed. Springer; New York: 2012. pp. 261–270. [Google Scholar]
- 158.Flohe L. The impact of thiol peroxidases on redox regulation. Free Radic. Res. 2015:1–17. [PubMed] [Google Scholar]
- 159.Handy D.E., Lubos E., Yang Y., Galbraith J.D., Kelly N., Zhang Y.Y. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J. Biol. Chem. 2009;284(18):11913–11921. doi: 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Maiorino M., Bosello V., Cozza G., Roveri A., Toppo S., Ursini F. Glutathione peroxidase-4. In: Hatfield D.L., BM, Gladyshev V.N., editors. Selenium its Molecular Biology and Role in Human Health. third ed. Springer; New York: 2012. pp. 181–195. [Google Scholar]
- 161.Yant L.J., Ran Q., Rao L., Van Remmen H., Shibatani T., Belter J.G. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 2003;34(4):496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 162.Yoo S.E., Chen L., Na R., Liu Y., Rios C., Van Remmen H. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic. Biol. Med. 2012;52(9):1820–1827. doi: 10.1016/j.freeradbiomed.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ran Q., Liang H., Ikeno Y., Qi W., Prolla T.A., Roberts L.J., 2nd Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62(9):932–942. doi: 10.1093/gerona/62.9.932. [DOI] [PubMed] [Google Scholar]
- 164.Liang H., Van Remmen H., Frohlich V., Lechleiter J., Richardson A., Ran Q. Gpx4 protects mitochondrial ATP generation against oxidative damage. Biochem. Biophys. Res. Commun. 2007;356(4):893–898. doi: 10.1016/j.bbrc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 165.Schneider M., Wortmann M., Mandal P.K., Arpornchayanon W., Jannasch K., Alves F. Absence of glutathione peroxidase 4 affects tumor angiogenesis through increased 12/15-lipoxygenase activity. Neoplasia. 2010;12(3):254–263. doi: 10.1593/neo.91782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Villette S., Kyle J.A., Brown K.M., Pickard K., Milne J.S., Nicol F. A novel single nucleotide polymorphism in the 3' untranslated region of human glutathione peroxidase 4 influences lipoxygenase metabolism. Blood Cells Mol. Dis. 2002;29(2):174–178. doi: 10.1006/bcmd.2002.0556. [DOI] [PubMed] [Google Scholar]
- 167.Kirkman H.N., Gaetani G.F. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem. Sci. 2007;32(1):44–50. doi: 10.1016/j.tibs.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 168.Radi R., Turrens J.F., Chang L.Y., Bush K.M., Crapo J.D., Freeman B.A. Detection of catalase in rat heart mitochondria. J. Biol. Chem. 1991;266(32):22028–22034. [PubMed] [Google Scholar]
- 169.Rindler P.M., Plafker S.M., Szweda L.I., Kinter M. High dietary fat selectively increases catalase expression within cardiac mitochondria. J. Biol. Chem. 2013;288(3):1979–1990. doi: 10.1074/jbc.M112.412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schriner S.E., Linford N.J., Martin G.M., Treuting P., Ogburn C.E., Emond M. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308(5730):1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 171.Lee H.Y., Choi C.S., Birkenfeld A.L., Alves T.C., Jornayvaz F.R., Jurczak M.J. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell. Metab. 2010;12(6):668–674. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hatai T., Matsuzawa A., Inoshita S., Mochida Y., Kuroda T., Sakamaki K. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J. Biol. Chem. 2000;275(34):26576–26581. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- 173.Daloso D.M., Muller K., Obata T., Florian A., Tohge T., Bottcher A. Thioredoxin, a master regulator of the tricarboxylic acid cycle in plant mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2015;112(11):E1392–E1400. doi: 10.1073/pnas.1424840112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hanschmann E.M., Lonn M.E., Schutte L.D., Funke M., Godoy J.R., Eitner S. Both thioredoxin 2 and glutaredoxin 2 contribute to the reduction of the mitochondrial 2-Cys peroxiredoxin Prx3. J. Biol. Chem. 2010;285(52):40699–40705. doi: 10.1074/jbc.M110.185827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lill R., Dutkiewicz R., Freibert S.A., Heidenreich T., Mascarenhas J., Netz D.J. The role of mitochondria and the CIA machinery in the maturation of cytosolic and nuclear iron-sulfur proteins. Eur. J. Cell. Biol. 2015;94(7-9):280–291. doi: 10.1016/j.ejcb.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 176.Souza J.M., Radi R. Glyceraldehyde-3-phosphate dehydrogenase inactivation by peroxynitrite. Arch. Biochem. Biophys. 1998;360(2):187–194. doi: 10.1006/abbi.1998.0932. [DOI] [PubMed] [Google Scholar]
- 177.Peralta D., Bronowska A.K., Morgan B., Doka E., Van Laer K., Nagy P. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat. Chem. Biol. 2015;11(2):156–163. doi: 10.1038/nchembio.1720. [DOI] [PubMed] [Google Scholar]
- 178.Ishii T., Tatsuda E., Kumazawa S., Nakayama T., Uchida K. Molecular basis of enzyme inactivation by an endogenous electrophile 4-hydroxy-2-nonenal: identification of modification sites in glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 2003;42(12):3474–3480. doi: 10.1021/bi027172o. [DOI] [PubMed] [Google Scholar]
- 179.Kornberg M.D., Sen N., Hara M.R., Juluri K.R., Nguyen J.V., Snowman A.M. GAPDH mediates nitrosylation of nuclear proteins. Nat. Cell Biol. 2010;12(11):1094–1100. doi: 10.1038/ncb2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hara M.R., Cascio M.B., Sawa A. GAPDH as a sensor of NO stress. Biochim. Biophys. Acta. 2006;1762(5):502–509. doi: 10.1016/j.bbadis.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 181.Hara M.R., Agrawal N., Kim S.F., Cascio M.B., Fujimuro M., Ozeki Y. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell. Biol. 2005;7(7):665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 182.Hildebrandt T., Knuesting J., Berndt C., Morgan B., Scheibe R. Cytosolic thiol switches regulating basic cellular functions: GAPDH as an information hub? Biol. Chem. 2015;396(5):523–537. doi: 10.1515/hsz-2014-0295. [DOI] [PubMed] [Google Scholar]
- 183.Hwang N.R., Yim S.H., Kim Y.M., Jeong J., Song E.J., Lee Y. Oxidative modifications of glyceraldehyde-3-phosphate dehydrogenase play a key role in its multiple cellular functions. Biochem. J. 2009;423(2):253–264. doi: 10.1042/BJ20090854. [DOI] [PubMed] [Google Scholar]
- 184.Landino L.M., Hagedorn T.D., Kennett K.L. Evidence for thiol/disulfide exchange reactions between tubulin and glyceraldehyde-3-phosphate dehydrogenase. Cytoskeleton (Hoboken) 2014;71(12):707–718. doi: 10.1002/cm.21204. [DOI] [PubMed] [Google Scholar]
- 185.Beinert H., Kennedy M.C. 19th Sir Hans Krebs lecture. Engineering of protein bound iron–sulfur clusters. A tool for the study of protein and cluster chemistry and mechanism of iron-sulfur enzymes. Eur. J. Biochem. 1989;186(1–2):5–15. doi: 10.1111/j.1432-1033.1989.tb15170.x. [DOI] [PubMed] [Google Scholar]
- 186.Gardner P.R. Aconitase: sensitive target and measure of superoxide. Methods Enzym. 2002;349:9–23. doi: 10.1016/s0076-6879(02)49317-2. [DOI] [PubMed] [Google Scholar]
- 187.Tortora V., Quijano C., Freeman B., Radi R., Castro L. Mitochondrial aconitase reaction with nitric oxide, S-nitrosoglutathione, and peroxynitrite: Mechanisms and relative contributions to aconitase inactivation. Free Radic. Biol. Med. 2007;42(7):1075–1088. doi: 10.1016/j.freeradbiomed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 188.Bulteau A.L., Ikeda-Saito M., Szweda L.I. Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry. 2003;42(50):14846–14855. doi: 10.1021/bi0353979. [DOI] [PubMed] [Google Scholar]
- 189.Castro L., Rodriguez M., Radi R. Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J. Biol. Chem. 1994;269(47):29409–29415. [PubMed] [Google Scholar]
- 190.Armstrong J.S., Whiteman M., Yang H., Jones D.P. The redox regulation of intermediary metabolism by a superoxide-aconitase rheostat. Bioessays. 2004;26(8):894–900. doi: 10.1002/bies.20071. [DOI] [PubMed] [Google Scholar]
- 191.Scandroglio F., Tortora V., Radi R., Castro L. Metabolic control analysis of mitochondrial aconitase: influence over respiration and mitochondrial superoxide and hydrogen peroxide production. Free Radic. Res. 2014;48(6):684–693. doi: 10.3109/10715762.2014.900175. [DOI] [PubMed] [Google Scholar]
- 192.Aulak K.S., Koeck T., Crabb J.W., Stuehr D.J. Dynamics of protein nitration in cells and mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2004;286(1):H30–H38. doi: 10.1152/ajpheart.00743.2003. [DOI] [PubMed] [Google Scholar]
- 193.Aulak K.S., Miyagi M., Yan L., West K.A., Massillon D., Crabb J.W. Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proc. Natl. Acad. Sci. U. S. A. 2001;98(21):12056–12061. doi: 10.1073/pnas.221269198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Casoni F., Basso M., Massignan T., Gianazza E., Cheroni C., Salmona M. Protein nitration in a mouse model of familial amyotrophic lateral sclerosis: possible multifunctional role in the pathogenesis. J. Biol. Chem. 2005;280(16):16295–16304. doi: 10.1074/jbc.M413111200. [DOI] [PubMed] [Google Scholar]
- 195.Kanski J., Behring A., Pelling J., Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am. J. Physiol. Heart Circ. Physiol. 2005;288(1):H371–H381. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- 196.Liu Q., Simpson D.C., Gronert S. Carbonylation of mitochondrial aconitase with 4-hydroxy-2-(E)-nonenal: localization and relative reactivity of addition site. Biochim. Biophys. Acta. 2013;1834(6):1144–1154. doi: 10.1016/j.bbapap.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Han D., Canali R., Garcia J., Aguilera R., Gallaher T.K., Cadenas E. Sites and mechanisms of aconitase inactivation by peroxynitrite: modulation by citrate and glutathione. Biochemistry. 2005;44(36):11986–11996. doi: 10.1021/bi0509393. [DOI] [PubMed] [Google Scholar]
- 198.Zhang S.J., Sandstrom M.E., Lanner J.T., Thorell A., Westerblad H., Katz A. Activation of aconitase in mouse fast-twitch skeletal muscle during contraction-mediated oxidative stress. Am. J. Physiol. Cell Physiol. 2007;293(3):C1154–C1159. doi: 10.1152/ajpcell.00110.2007. [DOI] [PubMed] [Google Scholar]
- 199.Lin G., Brownsey R.W., MacLeod K.M. Regulation of mitochondrial aconitase by phosphorylation in diabetic rat heart. Cell. Mol. Life Sci. 2009;66(5):919–932. doi: 10.1007/s00018-009-8696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chen V., Ianuzzo C.D., Fong B.C., Spitzer J.J. The effects of acute and chronic diabetes on myocardial metabolism in rats. Diabetes. 1984;33(11):1078–1084. doi: 10.2337/diab.33.11.1078. [DOI] [PubMed] [Google Scholar]
- 201.Fernandes J., Weddle A., Kinter C.S., Humphries K.M., Mather T., Szweda L.I. Lysine acetylation activates mitochondrial aconitase in the heart. Biochemistry. 2015;54(25):4008–4018. doi: 10.1021/acs.biochem.5b00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hurd T.R., Prime T.A., Harbour M.E., Lilley K.S., Murphy M.P. Detection of reactive oxygen species-sensitive thiol proteins by redox difference gel electrophoresis: implications for mitochondrial redox signaling. J. Biol. Chem. 2007;282(30):22040–22051. doi: 10.1074/jbc.M703591200. [DOI] [PubMed] [Google Scholar]
- 203.Hurd T.R., Collins Y., Abakumova I., Chouchani E.T., Baranowski B., Fearnley I.M. Inactivation of pyruvate dehydrogenase kinase 2 by mitochondrial reactive oxygen species. J. Biol. Chem. 2012;287(42):35153–35160. doi: 10.1074/jbc.M112.400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tretter L., Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360(1464):2335–2345. doi: 10.1098/rstb.2005.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Starkov A.A., Fiskum G., Chinopoulos C., Lorenzo B.J., Browne S.E., Patel M.S. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J. Neurosci. 2004;24(36):7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tretter L., Adam-Vizi V. Inhibition of alpha-ketoglutarate dehydrogenase due to H2O2-induced oxidative stress in nerve terminals. Ann. N. Y. Acad. Sci. 1999;893:412–416. doi: 10.1111/j.1749-6632.1999.tb07867.x. [DOI] [PubMed] [Google Scholar]
- 207.Andersson U., Leighton B., Young M.E., Blomstrand E., Newsholme E.A. Inactivation of aconitase and oxoglutarate dehydrogenase in skeletal muscle in vitro by superoxide anions and/or nitric oxide. Biochem. Biophys. Res. Commun. 1998;249(2):512–516. doi: 10.1006/bbrc.1998.9171. [DOI] [PubMed] [Google Scholar]
- 208.Tretter L., Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J. Neurosci. 2000;20(24):8972–8979. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shi Q., Xu H., Yu H., Zhang N., Ye Y., Estevez A.G. Inactivation and reactivation of the mitochondrial alpha-ketoglutarate dehydrogenase complex. J. Biol. Chem. 2011;286(20):17640–17648. doi: 10.1074/jbc.M110.203018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yan L.J., Sumien N., Thangthaeng N., Forster M.J. Reversible inactivation of dihydrolipoamide dehydrogenase by mitochondrial hydrogen peroxide. Free Radic. Res. 2013;47(2):123–133. doi: 10.3109/10715762.2012.752078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hansford R.G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev. Physiol. Biochem. Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- 212.Lai J.C., Cooper A.J. Brain alpha-ketoglutarate dehydrogenase complex: kinetic properties, regional distribution, and effects of inhibitors. J. Neurochem. 1986;47(5):1376–1386. doi: 10.1111/j.1471-4159.1986.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 213.Chen X.J., Wang X., Butow R.A. Yeast aconitase binds and provides metabolically coupled protection to mitochondrial DNA. Proc. Natl. Acad. Sci. U. S. A. 2007;104(34):13738–13743. doi: 10.1073/pnas.0703078104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen X.J., Wang X., Kaufman B.A., Butow R.A. Aconitase couples metabolic regulation to mitochondrial DNA maintenance. Science. 2005;307(5710):714–717. doi: 10.1126/science.1106391. [DOI] [PubMed] [Google Scholar]
- 215.Kaufman B.A., Newman S.M., Hallberg R.L., Slaughter C.A., Perlman P.S., Butow R.A. In organello formaldehyde crosslinking of proteins to mtDNA: identification of bifunctional proteins. Proc. Natl. Acad. Sci. U. S. A. 2000;97(14):7772–7777. doi: 10.1073/pnas.140063197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Radi R., Rodriguez M., Castro L., Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch. Biochem. Biophys. 1994;308(1):89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 217.Lokuta A.J., Maertz N.A., Meethal S.V., Potter K.T., Kamp T.J., Valdivia H.H. Increased nitration of sarcoplasmic reticulum Ca2+-ATPase in human heart failure. Circulation. 2005;111(8):988–995. doi: 10.1161/01.CIR.0000156461.81529.D7. [DOI] [PubMed] [Google Scholar]
- 218.Konorev E.A., Hogg N., Kalyanaraman B. Rapid and irreversible inhibition of creatine kinase by peroxynitrite. FEBS Lett. 1998;427(2):171–174. doi: 10.1016/s0014-5793(98)00413-x. [DOI] [PubMed] [Google Scholar]
- 219.Ott M., Robertson J.D., Gogvadze V., Zhivotovsky B., Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. U. S. A. 2002;99(3):1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.McMillin J.B., Dowhan W. Cardiolipin and apoptosis. Biochim. Biophys. Acta. 2002;1585(2–3):97–107. doi: 10.1016/s1388-1981(02)00329-3. [DOI] [PubMed] [Google Scholar]
- 221.Borisenko G.G., Kapralov A.A., Tyurin V.A., Maeda A., Stoyanovsky D.A., Kagan V.E. Molecular design of new inhibitors of peroxidase activity of cytochrome c/cardiolipin complexes: fluorescent oxadiazole-derivatized cardiolipin. Biochemistry. 2008;47(51):13699–13710. doi: 10.1021/bi801507s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Belikova N.A., Vladimirov Y.A., Osipov A.N., Kapralov A.A., Tyurin V.A., Potapovich M.V. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45(15):4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kagan V.E., Tyurin V.A., Jiang J., Tyurina Y.Y., Ritov V.B., Amoscato A.A. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005;1(4):223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 224.Cassina A.M., Hodara R., Souza J.M., Thomson L., Castro L., Ischiropoulos H. Cytochrome c nitration by peroxynitrite. J. Biol. Chem. 2000;275(28):21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 225.Lassus P., Opitz-Araya X., Lazebnik Y. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science. 2002;297(5585):1352–1354. doi: 10.1126/science.1074721. [DOI] [PubMed] [Google Scholar]
- 226.Batthyany C., Souza J.M., Duran R., Cassina A., Cervenansky C., Radi R. Time course and site(s) of cytochrome c tyrosine nitration by peroxynitrite. Biochemistry. 2005;44(22):8038–8046. doi: 10.1021/bi0474620. [DOI] [PubMed] [Google Scholar]
- 227.Hortelano S., Alvarez A.M., Bosca L. Nitric oxide induces tyrosine nitration and release of cytochrome c preceding an increase of mitochondrial transmembrane potential in macrophages. FASEB J. 1999;13(15):2311–2317. doi: 10.1096/fasebj.13.15.2311. [DOI] [PubMed] [Google Scholar]
- 228.Palacios-Callender M., Hollis V., Frakich N., Mateo J., Moncada S. Cytochrome c oxidase maintains mitochondrial respiration during partial inhibition by nitric oxide. J. Cell Sci. 2007;120(1):160–165. doi: 10.1242/jcs.03308. [DOI] [PubMed] [Google Scholar]
- 229.Brown G.C. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim. Biophys. Acta. 2001;1504(1):46–57. doi: 10.1016/s0005-2728(00)00238-3. [DOI] [PubMed] [Google Scholar]
- 230.Cooper C.E., Davies N.A. Effects of nitric oxide and peroxynitrite on the cytochrome oxidase K(m) for oxygen: implications for mitochondrial pathology. Biochim. Biophys. Acta. 2000;1459(2–3):390–396. doi: 10.1016/s0005-2728(00)00176-6. [DOI] [PubMed] [Google Scholar]
- 231.Lizasoain I., Moro M.A., Knowles R.G., Darley-Usmar V., Moncada S. Nitric oxide and peroxynitrite exert distinct effects on mitochondrial respiration which are differentially blocked by glutathione or glucose. Biochem. J. 1996;314(3):877–880. doi: 10.1042/bj3140877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sharpe M.A., Cooper C.E. Interaction of peroxynitrite with mitochondrial cytochrome oxidase. Catalytic production of nitric oxide and irreversible inhibition of enzyme activity. J. Biol. Chem. 1998;273(47):30961–30972. doi: 10.1074/jbc.273.47.30961. [DOI] [PubMed] [Google Scholar]
- 233.Rudolph V., Schopfer F.J., Khoo N.K., Rudolph T.K., Cole M.P., Woodcock S.R. Nitro-fatty acid metabolome: saturation, desaturation, {beta}-oxidation, and protein adduction. J. Biol. Chem. 2009;284(3):1461–1473. doi: 10.1074/jbc.M802298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Schopfer F.J., Cole M.P., Groeger A.L., Chen C.S., Khoo N.K., Woodcock S.R. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J. Biol. Chem. 2010;285(16):12321–12333. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Villacorta L., Schopfer F.J., Zhang J., Freeman B.A., Chen Y.E. PPARgamma and its ligands: therapeutic implications in cardiovascular disease. Clin. Sci. (Lond.) 2009;116(3):205–218. doi: 10.1042/CS20080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Schopfer F.J., Lin Y., Baker P.R., Cui T., Garcia-Barrio M., Zhang J. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc. Natl. Acad. Sci. U. S. A. 2005;102(7):2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Marx N., Duez H., Fruchart J.C., Staels B. Peroxisome proliferator-activated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ. Res. 2004;94(9):1168–1178. doi: 10.1161/01.RES.0000127122.22685.0A. [DOI] [PubMed] [Google Scholar]
- 238.Marx N. PPARgamma and vascular inflammation: adding another piece to the puzzle. Circ. Res. 2002;91(5):373–374. doi: 10.1161/01.res.0000033472.61704.ce. [DOI] [PubMed] [Google Scholar]
- 239.Marx N., Kehrle B., Kohlhammer K., Grub M., Koenig W., Hombach V. PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis. Circ. Res. 2002;90(6):703–710. doi: 10.1161/01.res.0000014225.20727.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Newman J.C., He W., Verdin E. Mitochondrial protein acylation and intermediary metabolism: regulation by sirtuins and implications for metabolic disease. J. Biol. Chem. 2012;287(51):42436–42443. doi: 10.1074/jbc.R112.404863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hirschey M.D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D.B. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Someya S., Yu W., Hallows W.C., Xu J., Vann J.M., Leeuwenburgh C. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143(5):802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tseng A.H., Wu L.H., Shieh S.S., Wang D.L. SIRT3 interactions with FOXO3 acetylation, phosphorylation and ubiquitinylation mediate endothelial cell responses to hypoxia. Biochem. J. 2014;464(1):157–168. doi: 10.1042/BJ20140213. [DOI] [PubMed] [Google Scholar]
- 244.Buchakjian M.R., Kornbluth S. The engine driving the ship: metabolic steering of cell proliferation and death. Nat. Rev. Mol. Cell. Biol. 2010;11(10):715–727. doi: 10.1038/nrm2972. [DOI] [PubMed] [Google Scholar]
- 245.Ralser M., Wamelink M.M., Kowald A., Gerisch B., Heeren G., Struys E.A. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J. Biol. 2007;6(4):10. doi: 10.1186/jbiol61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kuehne A., Emmert H., Soehle J., Winnefeld M., Fischer F., Wenck H. Acute activation of oxidative pentose phosphate pathway as first-line response to oxidative stress in human skin cells. Mol. Cell. 2015;59(3):359–371. doi: 10.1016/j.molcel.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 247.Marinho H.S., Real C., Cyrne L., Soares H., Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Little C., O'Brien P.J. Mechanism of peroxide-inactivation of the sulphydryl enzyme glyceraldehyde-3-phosphate dehydrogenase. Eur. J. Biochem. 1969;10(3):533–538. doi: 10.1111/j.1432-1033.1969.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 249.Anastasiou D., Poulogiannis G., Asara J.M., Boxer M.B., Jiang J.K., Shen M. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334(6060):1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ursini M.V., Parrella A., Rosa G., Salzano S., Martini G. Enhanced expression of glucose-6-phosphate dehydrogenase in human cells sustaining oxidative stress. Biochem. J. 1997;323(3):801–806. doi: 10.1042/bj3230801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Garcia-Nogales P., Almeida A., Bolanos J.P. Peroxynitrite protects neurons against nitric oxide-mediated apoptosis. A key role for glucose-6-phosphate dehydrogenase activity in neuroprotection. J. Biol. Chem. 2003;278(2):864–874. doi: 10.1074/jbc.M206835200. [DOI] [PubMed] [Google Scholar]
- 252.Herrero-Mendez A., Almeida A., Fernandez E., Maestre C., Moncada S., Bolanos J.P. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat. Cell. Biol. 2009;11(6):747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 253.Rodriguez-Rodriguez P., Fernandez E., Almeida A., Bolanos J.P. Excitotoxic stimulus stabilizes PFKFB3 causing pentose-phosphate pathway to glycolysis switch and neurodegeneration. Cell. Death Differ. 2012;19(10):1582–1589. doi: 10.1038/cdd.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kongara S., Karantza V. The interplay between autophagy and ROS in tumorigenesis. Front. Oncol. 2012;2:171. doi: 10.3389/fonc.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wu J.J., Quijano C., Chen E., Liu H., Cao L., Fergusson M.M. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY) 2009;1(4):425–437. doi: 10.18632/aging.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mathew R., Karp C.M., Beaudoin B., Vuong N., Chen G., Chen H.Y. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137(6):1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Li L., Chen Y., Gibson S.B. Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell. Signal. 2013;25(1):50–65. doi: 10.1016/j.cellsig.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 258.Bensaad K., Cheung E.C., Vousden K.H. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 2009;28(19):3015–3026. doi: 10.1038/emboj.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Labbe K., Murley A., Nunnari J. Determinants and functions of mitochondrial behavior. Annu. Rev. Cell. Dev. Biol. 2014;30:357–391. doi: 10.1146/annurev-cellbio-101011-155756. [DOI] [PubMed] [Google Scholar]
- 260.Willems P.H., Rossignol R., Dieteren C.E., Murphy M.P., Koopman W.J. Redox homeostasis and mitochondrial dynamics. Cell Metab. 2015;22(2):207–218. doi: 10.1016/j.cmet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 261.Hart P.C., Mao M., de Abreu A.L., Ansenberger-Fricano K., Ekoue D.N., Ganini D. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat. Commun. 2015;6:6053. doi: 10.1038/ncomms7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mungai P.T., Waypa G.B., Jairaman A., Prakriya M., Dokic D., Ball M.K. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol. Cell. Biol. 2011;31(17):3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang X., McCullough K.D., Franke T.F., Holbrook N.J. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J. Biol. Chem. 2000;275(19):14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- 264.Cao J., Xu D., Wang D., Wu R., Zhang L., Zhu H. ROS-driven Akt dephosphorylation at Ser-473 is involved in 4-HPR-mediated apoptosis in NB4 cells. Free Radic. Biol. Med. 2009;47(5):536–547. doi: 10.1016/j.freeradbiomed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 265.Zmijewski J.W., Banerjee S., Bae H., Friggeri A., Lazarowski E.R., Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J. Biol. Chem. 2010;285(43):33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cordray P., Doyle K., Edes K., Moos P.J., Fitzpatrick F.A. Oxidation of 2-Cys-peroxiredoxins by arachidonic acid peroxide metabolites of lipoxygenases and cyclooxygenase-2. J. Biol. Chem. 2007;282(45):32623–32629. doi: 10.1074/jbc.M704369200. [DOI] [PubMed] [Google Scholar]