Abstract
Objective. The aim of this analysis is to examine the longitudinal impact of symptoms of depression/anxiety on treatment response, long-term disease activity and physical disability in RA.
Methods. Secondary analysis of clinical trial data was performed. Data were collected at baseline and at 6-monthly intervals for 2 years. The EuroQoL (EQ-5DTM) indicated depression/anxiety symptom severity. Our primary outcomes of interest were (i) DAS-28 and (ii) physical disability measured via the HAQ. Secondary outcomes were: tender and swollen joint counts, patient global assessment, ESR and odds of reaching clinical remission. Multilevel models were used to assess the impact of baseline and persistent depression/anxiety on outcomes over 2 years.
Results. Data from 379 patients were included. After adjusting for covariates, baseline depression/anxiety symptoms were associated with increased DAS-28 outcomes and increased tender joint counts. Persistent depression/anxiety symptoms were associated with increased DAS-28 scores, HAQ scores, tender joint counts and patient global assessment of disease activity, and reduced odds of reaching clinical remission. Patients with symptoms of depression/anxiety at baseline also showed a 50% reduction in prednisolone treatment effect, in comparison with patients with no symptoms of depression/anxiety at baseline.
Conclusion. Baseline and persistent symptoms of depression/anxiety are associated with poorer health outcomes over time, as well as reduced treatment response. Mental health should be routinely measured both in clinical practice and in research, and managed alongside rheumatological disease to optimize health outcomes. Further research is required to examine whether treatment of mental disorders can improve rheumatological outcomes.
Keywords: depression, anxiety, rheumatoid arthritis (RA), longitudinal, disease activity, HAQ, pain, joint erosion, remission
Rheumatology key messages
Depression/anxiety symptoms are associated with increased long-term RA disease activity and physical disability.
Persistent depression/anxiety is associated with reduced odds of reaching RA remission at 2 years.
Baseline depression/anxiety is associated with 50% reduction in prednisolone treatment response in RA.
Introduction
Depression and anxiety are highly prevalent in RA, with a recent meta-analysis reporting a 16.8% point prevalence of depression, diagnosed via clinical interview [1]. This represents a substantially higher level of depression than that found in the general population, where estimates are typically 5.0% [2]. Additionally, 25.1% of RA outpatients screen positive for anxiety, according to the 7-item Generalized Anxiety Disorder (GAD-7 [3]) questionnaire [4], and 16.3% of RA patients screen positive for both anxiety and depression [4]. Not only are prevalence levels of depression and anxiety high, but an analysis of the Early Rheumatoid Arthritis Network sample found that 12% of RA patients report persistently high levels of distress throughout the first 10 years after diagnosis [5].
Depression and anxiety in RA have been shown to be associated with increased pain [6] and fatigue [7], reduced quality of life [8], and increased service use [9], disease activity [10] and disability [11]. Depression in RA can also increase the risk of premature mortality [12]. The mechanism for the association between mood and disease outcomes is uncertain and probably bidirectional [13]. There are several pathways through which psychological distress may impact health outcomes: distress can interfere with the stress response and produce glucocorticoid resistance [14–17]. Additionally, depression/anxiety in RA may also reduce adherence to medication [18] and inflate the reported severity of subjective symptoms such as pain, joint tenderness and physical dysfunction [19]. Furthermore, depression/anxiety also impact on physical behaviour and may result in reduced movement, a deconditioning of the body, loss of natural endorphins and increasing levels of pain [20].
There is systematic review evidence to suggest a downstream relationship between distress and disease outcomes, with depression increasing pain and disease activity, and decreasing short- and long-term treatment efficacy [21]. However the authors noted that the quality of the included research studies was low, with poor sampling and recruitment strategies, small sample sizes, inadequate adjustment for confounders and lack of information regarding randomization, measurement blinding and attrition rates. More research is needed to resolve these issues, which are of considerable clinical relevance because pharmacologic and psychological treatments could be used more widely to treat depression in RA if it is a substantial driver of disease activity.
This study aims to fill these gaps in the evidence by undertaking a secondary analysis of an existing randomized controlled trial in patients with early RA. We assessed three interrelated hypotheses: more symptoms of depression and anxiety at baseline status will predict increased DAS-28 and HAQ outcomes over a 2-year follow-up period; patients with persistent symptoms of depression and anxiety will show increased DAS-28 and HAQ outcomes over a 2-year follow-up period than will patients without such symptoms; and depression and anxiety symptoms at baseline will be associated with restricted treatment response to glucocorticoid treatment, measured via the HAQ and the DAS-28.
Patients and methods
Study design
This study comprised a secondary analysis of data collected as part of a factorial randomized controlled trial of glucocorticoids and combination DMARDs in patients with early RA: Combination Anti-Rheumatic Drugs in Early Rheumatoid Arthritis (CARDERA) [22]. This paper reports findings from a secondary data analysis only, and required no additional ethical approval.
Participants
Participants in the trial were 467 consecutively recruited patients from rheumatology outpatient clinics across 42 centres in England and Wales. The inclusion criteria for enrolment were: ability to give informed consent; aged over 18 years; and active RA (determined by the ACR criteria) for <2 years, with three of the following: ≥3 swollen joints; ≥6 tender joints; morning stiffness for ≥45 min; ESR of ≥28 mm/h. Patients were excluded according to the following criteria: other inflammatory arthropathies; current use of oral glucocorticoids; serious physical comorbidities; women with the possibility of pregnancy; contraindications for drug trials.
Interventions
Detailed summaries of the intervention content are provided in the original trial article [22]. In short, patients were randomly allocated to receive one of four treatment options: (i) MTX (commencing 7.5 mg weekly, increasing incrementally to 15 mg/week target dose); (ii) MTX and ciclosporin (started 3 months after MTX, commencing 100 mg/day, increasing incrementally to 3 mg/kg daily target dose); (iii) MTX and prednisolone (started alongside MTX, commencing 60 mg/day, reduced to 7.5 mg at 6 weeks, 7.5 mg daily between 6 and 28 weeks, and stopped by 34 weeks); and (iv) MTX and ciclosporin and prednisolone. Of the 467 randomized patients, 88 were lost to follow-up and 132 discontinued the intervention, spread relatively evenly across treatment groups.
The primary outcome of the original trial was the development of new erosions in X-rays of hands and feet; however, several secondary outcomes were also collected at baseline and at 6-monthly intervals for the duration of the 2-year trial. Secondary outcomes in the original trial included function, quality-of-life, disease activity and adverse events.
Symptoms of depression/anxiety
Rheumatology trials do not measure mood systematically using diagnostic interviews, or typically even with validated symptom severity scales such as the Patient Health Questionnaire (PHQ)-9 [23]. We therefore relied upon patients’ responses to the depression/anxiety item of the EuroQoL (EQ-5DTM; [24]). The EQ-5DTM asks patients to respond to five health domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) according to how they are feeling today. The depression/anxiety domain provides three answer options: I am not anxious or depressed; I am moderately anxious or depressed; or I am extremely anxious or depressed. Patients’ responses to this domain of the EQ-5DTM at baseline were used to categorize patients into one of three baseline mood categories: no depression/anxiety; moderate depression/anxiety; extreme depression/anxiety. Although not a validated method of identifying mood disorders, there is evidence to suggest good sensitivity of similar one-item measures. Meta-analysis evidence from the National Institute for Health and Care Excellence (NICE) suggests that one-item mood screening questionnaires have 84% sensitivity and 65% specificity [25]. We can be fairly certain that we have identified patients with depression/anxiety; however, there may also be patients with subthreshold psychological distress included in our groups. Therefore, rather than indicating the presence of a diagnosed mood disorder, we have used responses to this tool to quantify levels of general psychological distress in participants that, for the purposes of this study, we refer to as depression/anxiety symptoms.
The persistence of depression/anxiety symptoms throughout the course of the trial was also established using the responses to the EQ-5DTM. To examine the impact of persistent depression/anxiety symptoms on physical health outcomes, patients were categorized into four mutually exclusive groups, according to their EQ-5DTM scores across all measurement time points: never reporting depression/anxiety symptoms; reporting depression/anxiety symptoms <50% of the time; reporting depression/anxiety symptoms >50% of the time; reporting depression/anxiety symptoms at every time point.
Outcomes
Our primary outcomes are DAS-28 and HAQ [26]. The DAS-28 is the gold standard for measuring RA disease activity: it is recommended by all major RA guidelines; it is easily calculated, and widely accepted as a high-quality indicator of the value of treatment [27]. The HAQ [26] is a self-reported measure, recommended by clinical guidelines to measure functional ability [27]. Both the DAS-28 and HAQ are used widely, both as outcomes in clinical trials and in clinical practice, and were therefore selected as primary outcomes for the current analysis [27, 28]. Secondary outcomes of interest are the components of the DAS-28: tender joint count (TJC), swollen joint count (SJC), ESR and patient global assessment (PGA). We also examined the relationship between depression/anxiety symptoms and the odds of reaching clinical remission at the study end point. This was assessed through establishing the number of patients scoring <2.6 on the DAS-28 at 2-year follow-up [29]. These were included in the current analysis as secondary outcomes, and were all measured at 6-, 12-, 18- and 24-months following baseline assessment.
Data analysis
Multilevel models were used to examine hypotheses 1 and 2, pooling across the CARDERA 2-year treatment arms. Multilevel modelling is designed to handle hierarchically nested data, allowing for missing outcome data, and accounting for variation both between participants and within patients over time. These models were estimated using Stata (version 11.0), which provides parameters in the form of unstandardized maximum likelihood estimates (b coefficients), which estimate magnitude and direction of changes in outcome variables associated with differences in predictor variables (in this case, depression/anxiety). All models included random intercepts to account for variation in the outcome variable across individuals. Assessment time was included as a dummy coded variable, with a random time slope to allow for variation in change in the outcome over time across individuals. Alongside the b coefficients, confidence intervals and P-values, we also calculated standardized mean difference effect sizes, to provide an estimate of the standardized effect size of depression/anxiety status on physical health outcomes.
Multilevel linear models for hypotheses 1 and 2 were created in two stages for continuous outcome variables at the post-intervention assessments. First, unadjusted models including only depression/anxiety status and time were estimated; second, adjusted models were estimated that additionally included age, gender, disease duration, time, baseline level of physical health, the type of treatment received and RF status. Additionally, in models of DAS-28 outcomes, HAQ scores were adjusted for; in models of HAQ outcomes, DAS-28 scores were adjusted for. Additional logistic regression models, adjusting for the same variables, were created to assess whether baseline depression/anxiety and persistent depression/anxiety predicted clinical remission at 2 years.
To assess hypothesis 3, a multiple regression model was created, examining the interaction between baseline depression/anxiety status and the receipt of steroidal treatment on physical health outcomes over the initial 6 months of treatment. Follow-up responses were limited to the first 6 months of treatment due to the step-down treatment regime utilized [22] and in order to examine the short-term treatment response that is appropriate for this medication type. The regression model for hypothesis 3 was adjusted for age, gender, disease duration, baseline physical health and RF status.
Results
Participant characteristics
Of the original 467 participants randomized in the CARDERA trial, 379 (81.2%) completed the trial and were included in this secondary analysis. Table 1 shows the baseline demographic, clinical and psychological variables for participants.
Table 1.
Baseline demographic, clinical and psychological variables
| Variable | Total sample, n = 379 | No baseline depression/anxiety, n = 166 | Moderate baseline depression/anxiety, n = 180 | Extreme baseline depression/anxiety, n = 24 |
|---|---|---|---|---|
| Age, mean (s.d.) | 54.1 (12.3) | 54.1 (12.3) | 54.7 (12.4) | 49.1 (10.5) |
| Female,a % | 68.3 | 62.7 | 70.6 | 83.3* |
| Disease duration, mean (s.d.),b months | 3.9 (5.4) | 3.8 (5.2) | 4.1 (5.5) | 1.5 (1.9)* |
| Ethnicity,c n (%) | ||||
| Afro–Caribbean | 6 (1.6) | 1 (0.6) | 4 (2.2)* | 1 (4.2) |
| Asian | 6 (1.6) | 1 (0.6) | 3 (1.7) | 2 (8.3) |
| White-British | 364 (96.0) | 164 (98.8) | 172 (95.6) | 20 (83.3) |
| White-European | 2 (0.5) | 0 (0.0) | 0 (0.0) | 1 (4.2) |
| Other | 1 (0.3) | 0 (0.0) | 1 (0.6) | 0 (0.0) |
| RF positive, n (%) | 254 (67.0) | 114 (68.7) | 117 (65.0) | 18 (75.0) |
| Larsen score, median (IQR) | 7.0 (3.0–17.0) | 7.0 (2.5–17.0) | 7.0 (3.0–16.0) | 6.5 (1.5–17.5) |
| DAS28, mean (s.d.)d | 5.7 (1.3) | 5.3 (1.3) | 6.0 (1.1)*** | 6.5 (1.3)*** |
| HAQ, mean (s.d.)e | 1.6 (0.7) | 1.3 (0.7) | 1.7 (0.6)*** | 2.0 (0.6)*** |
| TJC, mean (s.d.)f | 11.4 (7.6) | 9.9 (7.2) | 12.2 (7.5)** | 15.5 (8.6)** |
| SJC, mean (s.d.)g | 9.7 (6.2) | 8.4 (5.6) | 10.8 (6.4)*** | 11.7 (7.4)* |
| ESR, mean (s.d.) | 41.4 (29.5) | 38.4 (28.7) | 44.5 (30.3) | 40.3 (28.7) |
| PGA, mean (s.d.)h | 55.2 (26.3) | 45.8 (25.4) | 60.3 (24.6)*** | 77.3 (22.5)*** |
aExtreme baseline depression/anxiety has a significantly higher percentage female than no baseline depression/anxiety. bExtreme baseline depression/anxiety has a significantly shorter disease duration than no baseline depression/anxiety. cModerate baseline depression/anxiety has significantly more Afro–Caribbean patients than no baseline depression/anxiety. dModerate and extreme baseline depression/anxiety has a significantly higher baseline DAS28 than no baseline depression/anxiety. eModerate and extreme baseline depression/anxiety has a significantly higher baseline HAQ than no baseline depression/anxiety. fModerate and extreme baseline depression/anxiety has a significantly higher baseline TJC than no baseline depression/anxiety. gModerate and extreme baseline depression/anxiety has a significantly higher baseline SJC than no baseline depression/anxiety. hModerate and extreme baseline depression/anxiety has a significantly higher baseline PGA than no baseline depression/anxiety. *Significant between-group difference P < 0.05. **Significant between-group difference P < 0.01. ***Significant between-group difference P < 0.001. SF-36: Short-Form 36-item health survey.
The relationship between baseline depression/anxiety and health outcomes
Primary outcomes analysis
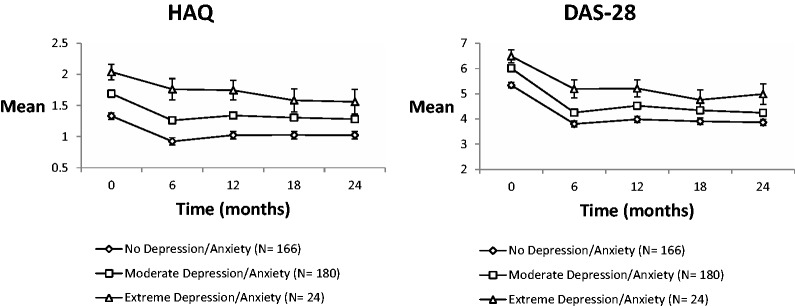
Fig. 1 shows the changes in observed DAS-28 and HAQ scores over time. These results are also summarized in Table 2. Before adjusting for covariates, in comparison with patients with no symptoms of depression/anxiety at baseline, patients with moderate depression/anxiety reported significantly increased scores over time. Patients with extreme baseline depression/anxiety also reported significantly increased HAQ scores over time. After adjusting for age, gender, disease duration, time, treatment type, baseline HAQ and DAS-28 scores, RF status and disease activity, there were no longer between-group differences in HAQ outcomes over time (Table 2). After adjusting for age, gender, disease duration, time, treatment type, baseline level of disease activity and HAQ scores, patients with extreme baseline depression/anxiety showed significantly increased levels of follow-up DAS-28 in comparison with patients with no depression/anxiety at baseline.
Fig. 1.
Estimated unadjusted mean outcomes by baseline depression/anxiety (with standard error bars) for DAS-28/ HAQ
Table 2.
Post-treatment mean differences and standardized mean differences by baseline level of depression/anxiety symptoms
| Model | Primary outcomes |
Secondary outcomes- DAS-28 components |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAQ |
DAS-28 |
SJC |
ESR |
PGA |
TJC |
|||||||||||||
| Post-treatment mean differences (s.e.) | Standardized mean differences | P-value | Post-treatment mean differences (s.e.) | Standardized mean differences | P-value | Post-treatment mean differences (s.e.) | Standardized mean differences | P-value | Post-treatment mean differences (s.e.) | Standardized mean differences | P-value | Post-treatment mean differences (s.e.) | Standardized mean differences | P-value | Post-treatment mean differences (s.e.) | Standardized mean differences | P-value | |
| Unadjusted | ||||||||||||||||||
| No depression/anxiety | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||
| Moderate depression/anxiety | 0.31 (0.07) | 0.44 | <0.001 | 0.47 (0.15) | 0.34 | <0.01 | 0.07 (0.08) | 0.10 | 0.33 | 0.06 (0.09) | 0.08 | 0.50 | 8.00 (2.24) | 0.37 | <0.001 | 0.16 (0.09) | 0.18 | 0.10 |
| Extreme baseline depression/anxiety | 0.72 (0.15) | 1.01 | <0.001 | 1.20 (0.30) | 0.86 | <0.001 | 0.13 (0.15) | 0.19 | 0.38 | 0.18 (0.17) | 0.23 | 0.31 | 18.81 (4.56) | 0.87 | <0.001 | 0.61 (0.18) | 0.70 | <0.001 |
| Adjusteda | ||||||||||||||||||
| No depression/anxiety | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||||
| Moderate depression/anxiety | 0.04 (0.06) | 0.06 | 0.45 | 0.10 (0.14) | 0.07 | 0.49 | −0.01 (0.08) | −0.01 | 0.95 | −0.04 (0.07) | −0.05 | 0.57 | 2.81 (2.19) | 0.13 | 0.20 | 0.05 (0.09) | 0.06 | 0.59 |
| Extreme baseline depression/anxiety | 0.21 (0.12) | 0.30 | 0.08 | 0.59 (0.29) | 0.42 | 0.04 | 0.06 (0.15) | 0.09 | 0.68 | 0.07 (0.15) | 0.09 | 0.64 | 8.72 (4.54) | 0.40 | 0.06 | 0.38 (0.17) | 0.44 | 0.02 |
aPrimary outcomes model adjusted for age, gender, disease duration, time, baseline HAQ and DAS, treatment type and RF status. Secondary outcomes model adjusted for age, gender, disease duration, time, baseline SJC/ESR/PGA/or TJC, treatment type and RF status. Bold indicates statistically significant associations. SJC: swollen joint count; PGA: patient global assessment; TJC: tender joint count; ESR: erythrocyte sedimentation rate.
Secondary outcomes analysis
In comparison with patients with no depression/anxiety at baseline, patients with moderate depression/anxiety at baseline reported significantly increased PGA, and patients with extreme depression/anxiety reported significantly increased PGA and TJC (Table 2). The relationship between baseline depression/anxiety and long-term TJC outcomes was sustained after adjusting for all covariates. The relationships between depression/anxiety and SJC and ESR outcomes did not reach statistical significance, and effect sizes were small.
At 2-year follow-up, 80 (21.1%) patients reached clinical remission, achieving DAS-28 scores of <2.6. Patients reporting moderate depression/anxiety had reduced odds of reaching clinical remission in comparison with patients with no depression/anxiety at baseline (OR = 0.50, standard error [s.e.] = 0.14, P = 0.02, 95% CI: 0.29, 0.88). Patients with extreme depression/anxiety symptoms at baseline had reduced odds of reaching clinical remission at 2-year follow-up in comparison with patients with no depression/anxiety symptoms at baseline; however, this comparison was non-significant (OR = 0.77, s.e. = 0.43, P = 0.64, 95% CI: 0.25, 2.33). This may be due to the small number of patients with extreme symptoms at baseline (n = 24), reducing the power to find a significant effect and resulting in an imprecise estimate.
The relationship between persistent depression/ anxiety and health outcomes
Primary outcomes analysis
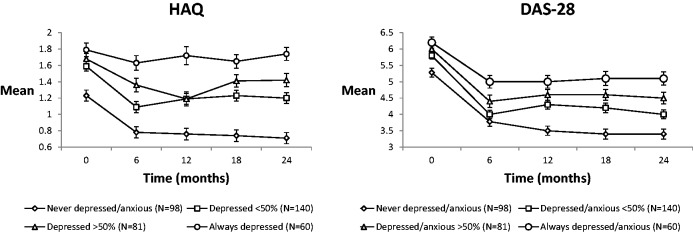
Fig. 2 shows the changes in observed DAS-28 and HAQ scores over time according to the persistence of depression/anxiety symptoms. Before adjusting for covariates, increasing persistence of depression/anxiety symptoms was associated with increased HAQ and DAS-28 severity over time (Table 3). These significant relationships were sustained after adjusting for age, gender, disease duration, time, baseline physical health, treatment type, RF status and HAQ/DAS-28. In comparison with patients who were never depressed/anxious, patients with depression/anxiety symptoms <50% of the, >50% of the time, or at all time points reported significantly increased HAQ scores at follow-up. In comparison with patients who were never depressed/anxious, patients with depression/anxiety symptoms <50% of the time, >50% of the time or at all time points reported significantly increased DAS-28 scores at follow-up.
Fig. 2.
Estimated unadjusted mean outcomes by depression/anxiety persistence (with standard error bars) for DAS-28/ HAQ
Table 3.
Post-treatment mean differences (b) and standardized mean differences (d) by persistence of depression/anxiety symptoms
| Model | Primary outcomes |
Secondary outcomes-DAS-28 components |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAQ |
DAS-28 |
SJC |
ESR |
PGA |
TJC |
|||||||||||||
| Post-treatment mean differences (SE) | Standardized mean differences | P-value | Post-treatment mean differences (SE) | Standardized mean differences | P-value | Post-treatment mean differences (SE) | Standardized mean differences | P-value | Post-treatment mean differences (SE) | Standardized mean differences | P-value | Post-treatment mean differences (SE) | Standardized mean differences | P-value | Post-treatment mean differences (SE) | Standardized mean differences | P-value | |
| Unadjusted | ||||||||||||||||||
| Never depressed/anxious | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Depressed/anxious <50% | 0.40 (0.09) | 0.56 | <0.001 | 0.68 (0.17) | 0.49 | <0.001 | 0.17 (0.10) | 0.24 | 0.08 | 0.04 (0.10) | 0.05 | 0.73 | 10.20 (2.50) | 0.47 | <0.001 | 0.33 (0.12) | 0.38 | <0.01 |
| Depressed/anxious >50% | 0.67 (0.10) | 0.94 | <0.001 | 1.14 (0.19) | 0.81 | <0.001 | 0.15 (0.11) | 0.21 | 0.15 | 0.15 (0.12) | 0.19 | 0.21 | 19.32 (2.85) | 0.89 | <0.001 | 0.49 (0.13) | 0.56 | <0.001 |
| Always depressed/anxious | 0.92 (0.11) | 1.30 | <0.001 | 1.68 (0.21) | 1.20 | <0.001 | 0.20 (0.11) | 0.29 | 0.07 | 0.18 (0.13) | 0.23 | 0.17 | 30.92 (3.11) | 1.43 | <0.001 | 0.74 (0.14) | 0.85 | <0.001 |
| Adjusteda | ||||||||||||||||||
| Never depressed/anxious | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Depressed/anxious <50% | 0.17 (0.07) | 0.24 | <0.01 | 0.39 (0.16) | 0.28 | 0.02 | 0.17 (0.10) | 0.70 | 0.07 | −0.07 (0.09) | −0.09 | 0.43 | 6.59 (2.43) | 0.30 | <0.01 | 0.28 (0.11) | 0.32 | <0.01 |
| Depressed/anxious >50% | 0.37 (0.08) | 0.52 | <0.001 | 0.75 (0.18) | 0.54 | <0.001 | 0.17 (0.11) | 0.70 | 0.11 | 0.08 (0.10) | 0.10 | 0.42 | 14.18 (2.84) | 0.66 | <0.001 | 0.32 (0.12) | 0.37 | <0.01 |
| Always depressed/anxious | 0.53 (0.08) | 0.73 | <0.001 | 1.20 (0.20) | 0.86 | <0.001 | 0.21 (0.11) | 0.30 | 0.07 | 0.12 (0.11) | 0.15 | 0.25 | 24.04 (3.13) | 1.11 | <0.001 | 0.52 (0.13) | 0.60 | <0.001 |
aPrimary outcomes model adjusted for age, gender, disease duration, time, baseline HAQ and DAS, treatment type and RF status. Secondary outcomes model adjusted for age, gender, disease duration, time, baseline SJC/ESR/PGA/or TJC, treatment type and RF status. Bold indicates statistically significant associations. SJC: swollen joint count; PGA: patient global assessment. TJC: tender joint count; ESR: erythrocyte sedimentation rate.
Secondary outcomes analysis
After adjusting for covariates, in comparison with patients who were never depressed/anxious, patients depressed/anxious <50% of the time, >50% of the time or at all time points reported significantly increased TJC at follow-up. In comparison with patients who were never depressed/anxious, patients depressed/anxious <50% of the, >50% of the time or at all time points reported significantly increased PGA at follow-up. ESR scores were not associated with depression/anxiety persistence, and the relationship between persistent depression/anxiety and SJC outcomes showed moderate effect sizes, although did not reach statistical significance (Table 3).
Logistic regression analyses revealed that after controlling for all clinical and demographic variables, in comparison with patients never reporting depression/anxiety, patients reported symptoms of depression/anxiety at <50% of the time points did not have significantly smaller odds of reaching clinical remission (OR = 0.58, s.e. = 0.19, P = 0.09). However, both patients reporting symptoms of depression/anxiety at >50% of the time points (OR = 0.38, s.e. = 0.17, P < 0.05) and patients reporting depression/anxiety at every time point (OR = 0.10, s.e. = 0.08, P < 0.01) showed significantly smaller odds of reaching clinical remission when compared with patients with no symptoms of depression throughout. Adjustment for baseline depression status in these models did not significantly alter our findings (data not shown).
Symptoms of depression/anxiety and anxiety at baseline are associated with reduced response to prednisolone
Regression models adjusted for age, gender, disease duration, baseline HAQ for DAS outcomes and baseline DAS for HAQ outcomes, RF status and DAS-28/HAQ were created to assess the interaction between baseline depression/anxiety and treatment type on change in HAQ and DAS-28 outcomes for the first 6 months of treatment.
A significant interaction between mood status at baseline and receipt of prednisolone was found for HAQ outcomes: in comparison with patients with no low mood at baseline, patients with moderate symptoms of depression/anxiety taking prednisolone reported significantly higher follow-up HAQ scores (b coefficient = 0.25, s.e. = 0.12, standardized mean differences = 0.14, P = 0.05). The effect of steroidal treatment on HAQ outcomes was −0.48 for patients without depression/anxiety; however, this decreased to −0.23 for patients with some depression/anxiety. This represents a 48.4% (95% CI: 9.0, 87.8) reduction in treatment effect for HAQ in patients with moderate depression/anxiety at baseline.
No significant interaction was found between extreme symptoms of depression/anxiety at baseline and HAQ outcomes and receipt of prednisolone (b coefficient = 0.19, s.e. = 0.26, standardized mean differences = 0.04, P = 0.45). However, the effect of steroidal treatment on HAQ outcomes was −0.48 for patients without depression/anxiety at baseline, decreasing to −0.29 for patients with extreme depression/anxiety. This represents a 59.7% (95% CI: −0.42, 1.61) reduction in treatment effect for HAQ in patients with extreme depression/anxiety at baseline, which although reflecting a clinically meaningful reduction in size of effect, may have failed to reach statistical significance due to the small proportion of patients with extreme symptoms at baseline.
No significant interaction between mood status at baseline and receipt of prednisolone was found for DAS-28 outcomes. Patients with moderate depression/anxiety at baseline who received prednisolone had somewhat increased DAS-28 outcomes in comparison with patients with no depression/anxiety at baseline receiving prednisolone (b coefficient = 0.52, s.e. = 0.30, standardized mean differences = 0.14, P = 0.08). Patients with extreme baseline depression/anxiety symptoms receiving prednisolone did not report markedly different DAS28 outcomes at 6 months in comparison with patients with no depression/anxiety symptoms at baseline receiving prednisolone (b coefficient = 0.06, s.e. = 0.62, standardized mean differences = 0.01, P = 0.93).
No significant interaction was found between mood status at baseline and receipt of ciclosporin. Neither patients with moderate symptoms of depression/anxiety (b coefficient = −0.11, s.e. = 0.13, standardized mean differences = −0.06, P = 0.41) nor extreme depression/anxiety symptoms (b coefficient = −0.09, s.e. = 0.26, standardized mean differences = −0.02, P = 0.74) showed a significant reduction in HAQ scores in response to ciclosporin treatment, in comparison with patients with no symptoms at baseline. Similarly, neither patients with moderate symptoms of depression/anxiety (b coefficient = −0.15, s.e. = 0.31, standardized mean differences = −0.04, P = 0.64) nor extreme depression/anxiety symptoms (b coefficient = −0.43, s.e. = 0.64, standardized mean differences = −0.05, P = 0.50) showed a significant reduction in DAS scores in response to ciclosporin treatment, in comparison with patients with no symptoms at baseline.
Discussion
Our results suggest that baseline depression/anxiety status predicts both disease activity and disability across a 2-year follow-up period, and may reduce response to prednisolone treatment. Examination of the components of the DAS reveal that the subjective elements of TJC and PGA are those most consistently associated with mental health, although strong effect sizes were found for SJC outcomes in relation to persistent depression/anxiety symptoms. These results support previous systematic review findings that depression/anxiety influences long-term disease activity. Rathbun et al.’s [21] systematic review concludes that longitudinal assessments of the relationships between depression/anxiety and downstream physical health outcomes are limited by small sample sizes, poor recruitment strategies, a lack of control of confounding variables and a lack of information regarding randomization, measurement blinding and attrition levels. The strength of this paper is that it directly addresses these limitations: we had a larger sample size than all but one of the studies included in Rathbun et al.’s [21] systematic review, recruited consecutively from outpatient clinics from multiple centres across the UK; we adequately controlled for many potential confounders, including demographic and clinical variables; and full information about the randomization process and attrition levels are available in the original RCT paper [22]. We were also able to assess the impact of depression/anxiety on a range of both subjectively and objectively reported outcomes.
Our results suggest that symptoms of depression/anxiety may reduce the body’s ability to respond to glucocorticoid treatment. This association was significant for only one outcome, the HAQ measure of physical disability, although moderate effect sizes were found for several other outcomes, including DAS, TJC, PGA and ESR. Our failure to find this relationship in patients taking ciclosporin tentatively supports the evidence suggesting that depression/anxiety may induce a state of glucocorticoid resistance [14–17], and reduce response to steroidal treatments specifically.
More consistent relationships were found between depression/anxiety and subjectively measured components of the DAS-28: TJC and PGA. However, we found large and near-significant effect sizes when assessing the relationship between persistence of depression/anxiety over time and SJC. The adjustment made for baseline physical health in the analyses and the time points at which predictor and outcome variables were measured would imply the depression/anxiety status preceded the worsened physical health outcomes; however, we cannot claim the significant associations reported here to be causal relationships. Further research using time-lagged analysis or randomized controlled trials would allow confirmation of the direction of causality.
The limitations of the current study are as follows. First, the method of measuring depression/anxiety is suboptimal. The EQ-5DTM is a generic measure of quality of life, and its one-item assessment of depression/anxiety is a far from ideal method of identifying depression/anxiety status [24]. Rather, responses to this item may reflect a more general level of psychological distress, and the overlap between symptoms of depression and the somatic symptoms of RA may result in overestimating the number of patients with depression/anxiety. Despite this, the proportion of patients indicating a constant state of depression/anxiety in the current research are similar to prevalence estimates of depression/anxiety in RA, and the percentage of patients reporting extreme depression/anxiety at baseline are substantially lower than those prevalence estimates [1, 3]. Therefore, although not a validated measure of depression/anxiety in RA, the number of patients identified via the EQ-5DTM may reflect an underestimation of the number of patients who would be classified as having probable depression/anxiety when measured with a more robust measurement tool, resulting in a reduction in effect size. Thus, the fact that we still found significant associations despite this indicates that further research is needed to replicate these findings using a validated measure for identifying depression/anxiety symptoms.
An additional concern is the representativeness of the sample for the wider RA population. People from a low socioeconomic status (SES) are less likely to participate in medical research, resulting in research samples that tend to represent higher SES groups [30]. Low SES is associated with increased susceptibility to RA [31] and depression [32], and with worse disease activity, physical health and quality of life in RA [33]. Patients’ SES was not measured as part of this study, making it impossible to establish the extent to which our results reflect patients from low SES groups, who are more at risk of experiencing poor health outcomes in RA. SES was not measured in the original clinical trial; therefore, we were unable to examine or control for it in our analysis. Future research would benefit from taking into account the SES levels present in the sample, controlling for this in any analysis, and attempting to replicate these results in a clinical rather than research sample.
Conclusion
These findings suggest that both baseline and persistent symptoms of depression/anxiety predict several subjective and objective rheumatological outcome measures, after controlling for pertinent demographic and disease characteristics. These findings have several implications. First, mental health should be routinely measured in rheumatological randomized controlled trials (RCTs) and cohorts. Regardless of direction of causality, the association between rheumatological outcomes and depression/anxiety is strong, particularly for patient-reported outcomes such as PGA and TJC. Whatever the underlying mechanisms, we suggest that measuring depression/anxiety is an easily collected and significant psychomarker of rheumatological outcome.
Second, mental health status should be measured both before commencing treatment and throughout the course of treatment. There is substantial evidence that depression/anxiety is highly treatable in patients with co-existing physical disease [34, 35], and even if treating depression/anxiety has no impact on rheumatological outcome, we suggest that given the high frequency of depression/anxiety in this context, identification and treatment of depression/anxiety might well be considered part of routine care, in line with the NICE guidelines [28].
Third, research is required to determine whether treating depression/anxiety leads to improved rheumatological outcome, given that DAS-28 scores are used to make crucial treatment decisions and the relationships we report between increased baseline depression/anxiety and persistent depression/anxiety and increased disease activity suggest that the detection and management of depression/anxiety in clinical environments may be necessary for effective disease management [3]. Such an approach has been used with success in diabetes and coronary heart disease [36]. Our results suggest the need for an RCT examining whether the treatment of depression/anxiety in RA impacts physical health outcomes.
Acknowledgements
F.M. and M.H. receive salary support from the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley National Health Service (NHS) Foundation Trust and King’s College London. The paper presents independent research funded by the NIHR as one of its Programme Grants For Applied Research (Grant Reference Number: RP-PG-0610-10066; Programme Title: Treatment Intensities and Targets in Rheumatoid Arthritis Therapy: Integrating Patients’ And Clinicians’ Views – The TITRATE Programme). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. We also acknowledge support from the NIHR Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London. The CARDERA trial was funded by the Medical Research Council.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: D.L.S. receives support from the National Institute for Health Research through a Programme Grant For Applied Research (RP-PG-0610-10066) on Treatment Intensities and Targets in Rheumatoid Arthritis Therapy: Integrating Patients’ And Clinicians’ Views – The TITRATE Programme. All other authors have declared no conflicts of interest.
References
- 1.Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology 2013;52:2136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waraich P, Goldner EM, Somers JM, Hsu L. Prevalence and incidence studies of mood disorders: a systematic review of the literature. Can J Psychiatry 2004;49:124–38. [DOI] [PubMed] [Google Scholar]
- 3.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. [DOI] [PubMed] [Google Scholar]
- 4.Rayner L, Matcham F, Hutton J, et al. Embedding integrated mental health assessment and management in general hospital settings: feasibility, acceptability and the prevalence of common mental disorder. Gen Hosp Psychiatry 2014;36:318–24. [DOI] [PubMed] [Google Scholar]
- 5.Norton S, Sacker A, Young A, Done J. Distinct psychological distress trajectories in rheumatoid arthritis: findings from an inception cohort. J Psychosom Res 2011;71:290–5. [DOI] [PubMed] [Google Scholar]
- 6.Kojima M, Kojima T, Suzuki S, et al. Depression, inflammation, and pain in patients with rheumatoid arthritis. Arthritis Care Res 2009;61:1018–24. [DOI] [PubMed] [Google Scholar]
- 7.Matcham F, Ali S, Hotopf M, Chalder T. Psychological correlates of fatigue in rheumatoid arthritis: A systematic review. Clin Psychol Rev 2015;39:16–29. [DOI] [PubMed] [Google Scholar]
- 8.Mikuls T, Saag K, Criswell L, Merlino L, Cerhan JR. Health related quality of life in women with elderly onset rheumatoid arthritis. J Rheumatol 2003;30:952–7. [PubMed] [Google Scholar]
- 9.Joyce AT, Smith P, Khandker R, Melin JM, Singh A. Hidden cost of rheumatoid arthritis (RA): estimating cost of comorbid cardiovascular disease and depression among patients with RA. J Rheumatol 2009;36:743–52. [DOI] [PubMed] [Google Scholar]
- 10.Overman CL, Bossema ER, van Middendorp H, et al. The prospective association between psychological distress and disease activity in rheumatoid arthritis: a multilevel regression analysis. Ann Rheum Dis 2012;71:192–7. [DOI] [PubMed] [Google Scholar]
- 11.Karpouzas G, Dolatabadi S, Moran R, et al. Correlates and predictors of disability in vulnerable US Hispanics with rheumatoid arthritis. Arthritis Care Res 2012;64:1274–81. [DOI] [PubMed] [Google Scholar]
- 12.Ang DC, Choi H, Kroenke K, Wolfe F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol 2005;32:1013–9. [PubMed] [Google Scholar]
- 13.Sheehy C, Murphy E, Barry M. Depression in rheumatoid arthritis – underscoring the problem. Rheumatology 2006;45:1325–7. [DOI] [PubMed] [Google Scholar]
- 14.McAllister-Williams RH, Ferrier IN, Young AH. Mood and neuropsychological function in depression: the role of corticosteroids and serotonin. Psychol Med 1998;28:573–84. [DOI] [PubMed] [Google Scholar]
- 15.Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression: relations to the neurobiology of stress. New Eng J Med 1988;319:348–53. [DOI] [PubMed] [Google Scholar]
- 16.Barnes PJ, Adcock IM. How do corticosteroids work in asthma? Ann Intern Med 2003;139:359–70. [DOI] [PubMed] [Google Scholar]
- 17.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. The Lancet 2009;373:1905–17. [DOI] [PubMed] [Google Scholar]
- 18.DiMatteo M, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101–7. [DOI] [PubMed] [Google Scholar]
- 19.Baumeister H, Balke K, Härter M. Psychiatric and somatic comorbidities are negatively associated with quality of life in physically ill patients. J Clin Epidemiol 2005;58:1090–100. [DOI] [PubMed] [Google Scholar]
- 20.Fichna J, Janecka A, Costentin J, Do Rego JC. The endomorphin system and its evolving neurophysiological role. Pharmacol Rev 2007;59:88–123. [DOI] [PubMed] [Google Scholar]
- 21.Rathburn AM, Reed GW, Harrold LR. The temporal relationship between depression and rheumatoid arthritis disease activity, treatment persistence and response: a systematic review. Rheumatology 2013;52:1785–94. [DOI] [PubMed] [Google Scholar]
- 22.Choy EHS, Smith CM, Farewell V, et al. Factorial randomised controlled trial of glucocorticoids and combination disease modifying drugs in early rheumatoid arthritis. Ann Rheum Dis 2008;67:656–63. [DOI] [PubMed] [Google Scholar]
- 23.Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994;272:1749–56. [PubMed] [Google Scholar]
- 24.Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Rheumatology 1997;36:551–9. [DOI] [PubMed] [Google Scholar]
- 25.National Collaborating Centre for Mental Health. Depression in adults with a chronic physical health problem: treatment and management. NICE Clinical Guidance 91 London: National Institute for Health and Clinical Excellence, 2009.
- 26.Fries JF, Spitz P, Kraines GR, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Health and Care Excellence (NICE). Rheumatoid arthritis: the management of rheumatoid arthritis in adults. http://publications.nice.org.uk/rheumatoid-arthritis-cg79. Accessed 2 July 2013.
- 28.Kalyoncu U, Dougados M, Daures J-P, Gossec L. Reporting of patient-reported outcomes in recent trials in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis 2009;68:183–90. [DOI] [PubMed] [Google Scholar]
- 29.Fransen J, van Riel PL. DAS remission cut points. Clin Exp Rheumatol 2006;24:S29–32. [PubMed] [Google Scholar]
- 30.Farmer DF, Jackson SA, Camacho F, Hall MA. Attitudes of African American and low socioeconomic status white women towards medical research. J Health Care Poor Underserved 2007;18:85–99. [DOI] [PubMed] [Google Scholar]
- 31.Bengtsson C, Nordmark B, Klareskog L, Lundberg I, Alfredsson L. Socioeconomic status and the risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis 2005;64:1588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miech RA, Caspi A, Moffitt TE, Wright BRE, Silva PA. Low socioeconomic status and mental disorders: a longitudinal study of selection and causation during young adulthood. Am J Sociol 1999;104:1096–131. [Google Scholar]
- 33.Jacobi CE, Mol GD, Boshuizen HC, et al. Impact of socioeconomic status on the course of rheumatoid arthritis and on related use of health care services. Arth Care Res 2003;49:567–73. [DOI] [PubMed] [Google Scholar]
- 34.Rayner L, Price A, Evans A, et al. Antidepressants for depression in physically ill people. Cochrane DB Syst Rev 2010;(3):CD007503. [DOI] [PubMed] [Google Scholar]
- 35.Matcham F, Rayner L, Hutton J, et al. Self-help interventions for symptoms of depression, anxiety and psychological distress in patients with physical illness: a systematic review and meta-analysis. Clin Psychol Rev 2014;34:141–57. [DOI] [PubMed] [Google Scholar]
- 36.Katon WJ, Lin EHB, Von Korff M, et al. Collaborative care for patients with depression in chronic illnesses. New Eng J Med 2010;363:2611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]