Abstract
Objectives. To evaluate the multi-biomarker disease activity (MBDA) score as a predictor of radiographic progression and compare it with other risk factors among patients with established RA receiving non-biologic DMARDs.
Methods. For 163 patients with RA, we assessed 271 visits for MBDA score (scale of 1−100), clinical data and subsequent 1-year radiographic progression (change in Sharp−van der Heijde score [SHS]). Scatter plot and non-parametric quantile regression curves evaluated the relationship between the MBDA score and change in SHS. Changes in joint space narrowing and erosions were compared among MBDA categories with Wilcoxon rank-sum tests. The ability of the MBDA score to independently predict progression was determined by multivariate models and cross-classification of MBDA score with other risk factors. Generalized estimating equation methodology was used in model estimations to adjust for same-patient visits, always ≥1 year apart.
Results. Patient characteristics included 67% female, 66%/67% RF+/anti-CCP+; mean age 55 years, MBDA score 43 (moderate = 30−44); median disease duration 4.6 years, SHS 23. Radiographic progression was infrequent for low MBDA scores. Relative risk for progression increased continuously as the MBDA score increased, reaching 17.4 for change in SHS >5 with MBDA scores ≥60. Joint space narrowing and erosion progression were associated with MBDA score. MBDA score was associated with radiographic progression after adjustments for other risk factors. MBDA score significantly differentiated risk for progression when swollen joint count, CRP or DAS28–CRP was low, and among seropositive patients.
Conclusion. MBDA score enhanced the ability of conventional risk factors to predict radiographic progression in patients with established RA receiving non-biologic DMARDs.
Keywords: DMARD, multi-biomarker disease activity, non-biologic, prediction, radiographic progression, rheumatoid arthritis
Rheumatology key messages
Multi-biomarker disease activity score is correlated with radiographic progression for patients with established RA.
Progression risk in RA increases as multi-biomarker disease activity score increases, particularly within the high multi-biomarker disease activity range.
Multi-biomarker disease activity score improves progression prediction among RA patients with low clinical disease activity or CRP.
Introduction
The goals for treating RA are to minimize disease activity, damage to joints and disability. To achieve these goals, regular assessment of disease activity is important [1]. Current tools for assessing RA disease activity have shortcomings. Joint counts and global assessments are partially or entirely subjective and have been found to be poorly reproducible [2]. The objective blood tests, CRP and ESR, are in the normal range for large portions of patients with active RA and thus may be insensitive and unreliable indicators of joint inflammation [3–5]. MRI often detects subclinical synovitis in joints that are not swollen [6] and in patients who progress while in clinical remission [7]. However, MRI is time consuming and is not recommended for routine assessment of disease activity [8]. In view of these considerations, a convenient, objective measure of RA disease activity that is more sensitive and has a stronger association with risk of progressive joint damage than the current conventional tools may be helpful.
A multi-biomarker disease activity (MBDA) blood test has been developed as an objective tool for the management of patients with RA [9]. It combines the serum concentrations of 12 biomarkers, which include CRP, using a validated algorithm to produce a score that quantifies RA disease activity on a scale of 1–100 [10]. The MBDA score correlates with the DAS28 using CRP (DAS28–CRP) and other clinical measures of disease activity [10–12]. It reflects clinical response to therapy with non-biologic DMARDs and TNF inhibitors and has been validated in seropositive and seronegative RA patients [10].
An observational study from the Leiden Early Arthritis Clinic (EAC) demonstrated that, for patients with established RA who were receiving ongoing non-biologic DMARD therapy, the MBDA score was more strongly associated with subsequent radiographic progression than DAS28–CRP [13]. In addition, among patients who were in remission by DAS28–CRP, those with a high MBDA score (>44) progressed by >3 U of Sharp−van der Heijde (SHS) score more than twice as often as the overall DAS28–CRP remission group. This result indicates that the MBDA score provided additional information for predicting joint damage in a context where clinical measures detected little disease activity. An association between MBDA score and subsequent radiographic progression has also been found for patients from the SWEFOT and BeSt trials, which studied DMARD-naïve patients with active, early RA [14, 15].
The findings in the Leiden study left several questions that are relevant to the management of patients with established RA unanswered. Does the MBDA score independently add value for predicting progression when serologic status and other risk factors are taken into account? This feature may enhance prediction accuracy and aid therapeutic decision-making. Does the risk of progressive joint damage continue to increase as the MBDA score increases within the range of high MBDA scores? Is the MBDA score associated with progression of both joint erosions (JEs) and joint space narrowing? This study addresses these questions.
Methods
Patient population and sample size
The study population comprised 163 patients from the Leiden EAC cohort. Since 1993, the EAC at the Department of Rheumatology of Leiden University Medical Center has enrolled patients with <2 years of symptoms and evaluated them annually [16]. Leiden EAC patients are seen in a teaching hospital where fellows are tightly supervised and staff rheumatologists adhere to treatment protocols. Samples for this study were collected between 1995 and 2005. Median RA disease duration for the 163 patients was 4.6 years at the time of the first visit in this study, using the 1987 criteria of the ACR. A total of 271 visits were analysed, with 1, 2, 3 or 4 visits analysed for 65, 91, 4 and 3 patients, respectively. Multiple visits were always ≥1 year apart. Statistical methods were employed to take the multiple visits into account (see below). All patients in this study were receiving non-biologic DMARDs, predominantly SSZ, MTX or HCQ, alone or in combination, and <10% were receiving corticosteroids. None was receiving a biologic DMARD at the first study visit, and the frequency of anti-TNF use at follow-up was <5% [13]. Characteristics of the 163 patients in this observational study and criteria for inclusion of the 271 subject visits have been previously described in detail [13]. Written, informed consent was obtained for all patients, and the Leiden EAC cohort was approved by the local ethics committee for the Leiden University Medical Center. The analyses for this manuscript did not require separate ethical approval.
Clinical and serologic measures
Assessments for each visit included clinical evaluation and phlebotomy, to obtain blood for immediate testing and serum for frozen storage. Clinical measures based on 28-joint counts, patient global assessment and CRP have been previously described [13]. All CRP concentrations in these analyses were from the high-sensitivity measurement performed for the MBDA score. Anti-CCP antibodies and RF were measured in the laboratory of the Leiden University Medical Center, Leiden, NE.
Radiographic assessment
Anterior–posterior radiographs of hands and feet were obtained at each clinic visit and 1 year later, as previously described [13]. Erosions and joint space narrowing (JSN) were assessed by one experienced blinded reader to determine changes in erosions, JSN and total SHS. Changes were determined only for 1-year intervals. For patients who contributed two or more visits to the study, there was no overlap between different 1-year intervals of radiographic assessment.
The MBDA score
Archived, de-identified, frozen serum samples from each clinic visit were tested in the development laboratory of Crescendo Bioscience Inc. (South San Francisco, CA, USA). A multiplex, sandwich immunoassay (Meso Scale Discovery, Rockville, MD, USA) measured concentrations of the 12 MBDA biomarkers [vascular cell adhesion molecule-1, epidermal growth factor (EGF), VEGF-A, IL-6, TNF receptor type 1 (TNFR1), MMP-1, MMP-3, YKL-40, leptin, resistin, serum amyloid A and CRP], which were combined by a validated algorithm to generate an integer score on a scale of 1−100 [9, 10]. The immunoassay instrument and reagents are the same types as those used for the Vectra® DA test (Crescendo Bioscience, South San Francisco, CA, USA), and the algorithm is identical. Disease activity categories for the MBDA score, established prior to and independently of the analyses presented here, are: low (<30), moderate (30−44) and high (>44) [10]. The CRP measurement in the MBDA panel was used for all CRP analyses, including determination of DAS28–CRP.
Statistical analysis
To illustrate the correlation between MBDA score and radiographic progression based on continuous data, a scatter plot was constructed for MBDA score vs change in SHS over the following year for each of the 271 visits. Curves fitted by local linear quantile regression were generated for the 50th, 75th and 90th quantiles to delineate the trend for change in SHS across the spectrum of MBDA scores. These curves were designed to extend from the 5th to 95th percentile of MBDA scores to avoid misinterpretation at extreme values of MBDA score, where fitted curves are likely to have greater bias and variability due to edge effect. To explore the best threshold for predicting risk of radiographic progression, sensitivity and specificity for predicting change in SHS >3 or change in SHS >5 were determined for each MBDA score, and their sums (i.e. Youden’s index) were ranked from highest (corresponding to the best threshold) to lowest [17].
Association between the MBDA score and radiographic progression (change in SHS >3 or change in SHS >5) was also examined across six categories created by dividing the moderate MBDA category (range 30−44) into two subcategories and the high MBDA category (range >44) into three subcategories. MBDA subcategories were chosen to span similar absolute ranges of MBDA score for both moderate subcategories and the first two high subcategories, and to include ≥30 data points in all subcategories. Thresholds of three or five for change in SHS per year have been used previously for this and other cohorts [13, 14, 18]; change in SHS >5 per year is a definition of rapid radiographic progression [19]. Risks of change in SHS >3 or change in SHS >5 were determined for patients in each subcategory by logistic models using the method of generalized estimating equations (GEE) to adjust for inclusion of multiple visits [20]. The 95% CIs of the risks were constructed as Wald type CIs. Relative risk of change in SHS >3 or change in SHS >5 for patients in each MBDA subcategory was calculated relative to the low MBDA score category (<30). The score test, also known as the Lagrange multiplier test, was used to compare relative risk values to the reference value of 1 [21].
To understand the relationships between several risk factors (MBDA score, SJC28, DAS28–CRP, CRP, total SHS and serologic status) and radiographic progression (change in SHS >3 or change in SHS >5), univariate analyses were performed for all 271 visits using: (i) area under the receiver operating characteristic (AUROC) curve, with corresponding 95% CIs for AUROCs and P-values for differences between AUROCs for MBDA score and other continuous risk factors derived using bootstrap resampling [22, 23]; and (ii) logistic models with estimations by the GEE method. Effect size for each continuous variable in logistic models was reported as an odds ratio (OR), calculated as fold-change of the odds of radiographic progression per 1 U increase in the variable. OR for serologic status was calculated as the ratio of the odds of radiographic progression for seropositive patients (anti-CCP+ and/or RF+) vs that for seronegative patients (negative for anti-CCP and RF). To evaluate the independent contribution of each risk factor to radiographic progression (change in SHS >3 or change in SHS >5), multivariate analyses were performed using logistic models with estimations by the GEE method for 271 visits. When fitting the models for univariate and multivariate analyses, SJC28 and SHS were square-root transformed and CRP was logarithm base-10 transformed to better describe their underlying relationships with risk of radiographic progression. These transformations should be considered when interpreting the relationships between incremental change in SJC28, SHS or CRP and the fold change in odds for progression. P-values for testing the variable effects were determined by score test [21].
The ability of the MBDA score to enhance the predictive value of other risk factors for radiographic progression was explored by classifying the visits (N = 271) according to three categories of MBDA score (low, moderate or high) within the respective categories of SHS, serologic status, DAS28–CRP, CRP or SJC28, to determine percentages of patients with change in SHS >3 or change in SHS >5, using logistic models with GEE methods. When the GEE method failed to fit the model in a risk factor category (because no progressor was observed in ≥1 MBDA category or too few patients had >1 visit), only the first available visit for each patient in that category was analysed, with P-values calculated by Fisher’s exact test. Categories for DAS28–CRP were low (≤2.67), moderate (>2.67−4.09) and high (>4.09) [24]. Categories for SHS, CRP and SJC28 were chosen to reflect biologically meaningful distinctions within the distributions of values observed in this study.
Association between the three categories of MBDA score and progression of SHS, JEs or JSN was assessed descriptively, with cumulative probability plots to show the distributions of change in SHS, change in JE, or change in JSN for all 271 visits and for all first visits of the 163 patients. The Wilcoxon rank-sum test was used for pairwise comparisons between the three MBDA score categories for change in SHS, change in JE or change in JSN for all visits (N = 271) and for all first visits (N = 163). Changes were determined for 1-year intervals only.
All P-values were from two-sided tests; P-values < 0.05 were considered significant; no adjustments of P-values for multiple testing were implemented. All analyses were performed using R 2.15.1 (www.r-project.org) and SAS 9.4.
Results
Patient characteristics
Of the 163 patients included in this study, 67% were female; the median disease duration was 4.6 years, and disease activity was generally moderate, with median values of 3.3 for DAS28–CRP and 0.8 mg/dl for CRP, and a mean value of 43 for the MBDA score at the first study visit (Table 1). The median SHS was 23. All patients were receiving non-biologic DMARDs without biologic DMARDs at the first study visit. Anti-TNF biologic frequency during follow-up was <5% [13].
Table 1.
Patient characteristics
| Age, mean (s.d.), years | 55 (14) |
| Female, % | 67 |
| RF+, % | 66 |
| anti-CCP+, % | 67 |
| TJC28, 0−28, median (IQR) | 2 (0–7) |
| SJC28, 0−28, median (IQR) | 1 (0–4) |
| Patient global, 0−100, median (IQR) | 33 (12–50) |
| CRP, median (IQR), mg/dl | 0.8 (0.3–1.7) |
| MBDA score, mean (s.d.) | 43 (15) |
| DAS28–CRP, median (IQR) | 3.3 (2.3–4.3) |
| Erosion score, median (IQR) | 14 (6–31) |
| JSN score, median (IQR) | 8 (2–19) |
| SHS, median (IQR) | 23 (11–47) |
N = 163 patients. All data are for first visits. For RF status n = 161; for anti-CCP status n = 162. IQR: interquartile range; JSN: joint space narrowing; MBDA: multi-biomarker disease activity; N/A: not applicable; SHS: Sharp–van der Heijde score; SJC28: swollen joint count for 28 joints; TJC28: tender joint count for 28 joints.
Relationship between the MBDA score and radiographic progression
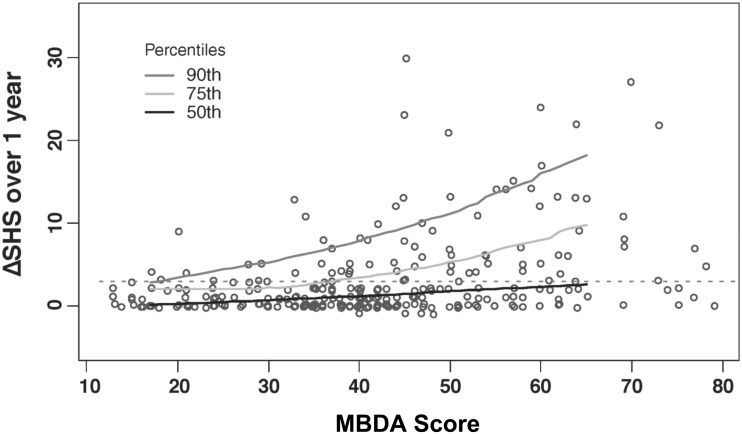
The change in SHS over the following year was >3 for 26% and >5 for 17% of all visits evaluated. The mean change in SHS was 3.0 U. A scatter plot (Fig. 1) showed that radiographic progression was infrequent when MBDA scores were low (<30) and more frequent as MBDA scores increased. Quantile regression curves indicated an upswing in the trend towards more frequent and severe radiographic progression at approximately the point at which MBDA scores entered the high range (>44). This observation was confirmed by an analysis of sensitivity and specificity showing that, for this cohort, the best thresholds for the MBDA score to differentiate radiographic progressors from non-progressors were MBDA score >43 (for change in SHS >3) and MBDA score >44 (for change in SHS>5) (supplementary Table S1, available at Rheumatology Online).
Fig. 1.
Radiographic progression over 1 year by MBDA score at start of the year
For each of 271 clinic visits of patients with established RA, the MBDA score was measured, and radiographs of hands and feet were obtained then and 1 year later to determine change (Δ) in total Sharp−van der Heijde score (SHS). Each pairing of MBDA score and ΔSHS is represented by an open circle. Quantile regression curves demarcate 90th, 75th and 50th percentiles of ΔSHS across the 5–95% range of observed MBDA scores. The horizontal dashed line demarcates ΔSHS = 3. MBDA: multi-biomarker disease activity.
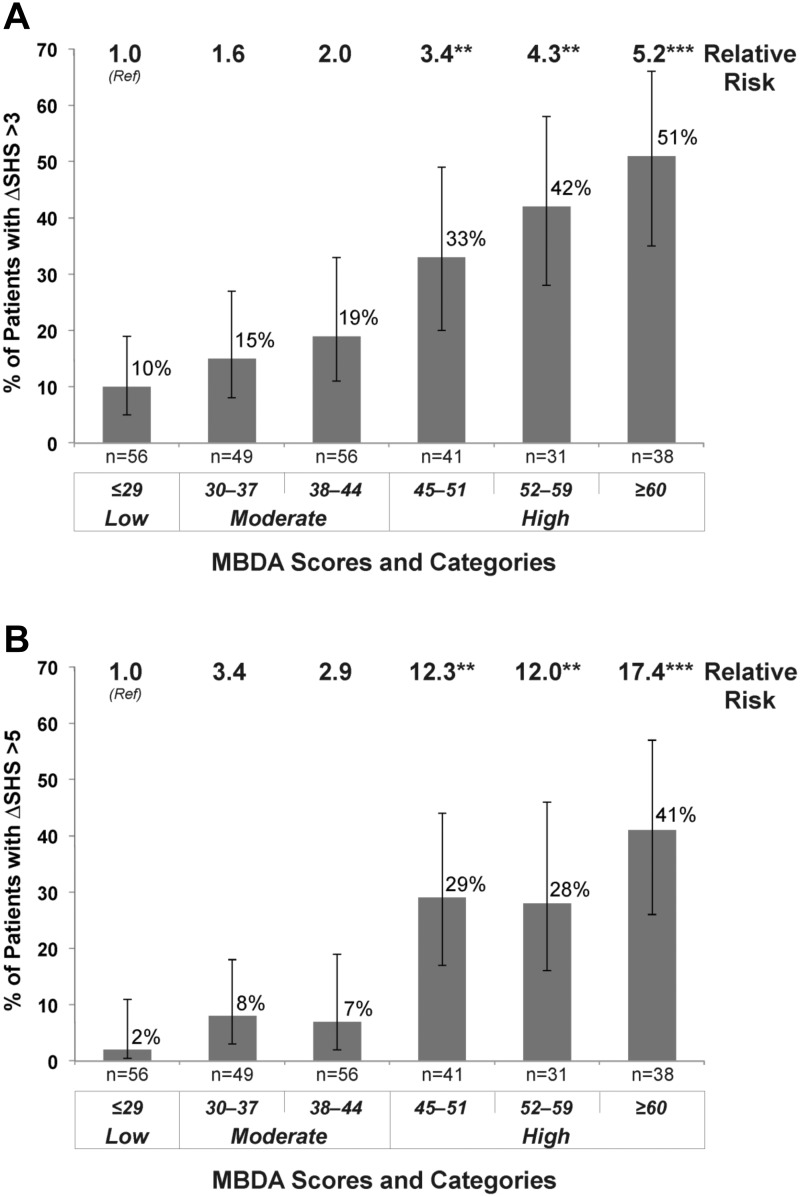
The frequency and severity of radiographic progression increased further as MBDA scores became higher within the high range (Figs 1 and 2). For MBDA scores ≥60, the estimated rates for change in SHS >3 or >5 were 51% and 41%, respectively, with relative risks of 5.2 and 17.4, compared with the low MBDA score category (<30) (Fig. 2). When erosions and JSN were considered separately, similar associations with the MBDA score were observed, with progression in erosions and JSN being significantly greater following visits with high MBDA scores vs visits with low or moderate MBDA scores (see cumulative probability plots in supplementary Fig. S1 and supplementary Table S2, available at Rheumatology Online).
Fig. 2.
Frequency and relative risk of radiographic progression by category and subcategory of MBDA score
Estimates (and 95% confidence intervals) of percentages of patients with progression and P-values for RR were derived from logistic models with a generalized estimating equations method using 271 visits from 163 patients, with progression defined as change (Δ) in total Sharp−van der Heijde score (SHS) >3 (A) or ΔSHS >5 (B). RRs were derived by using the low MBDA category as the reference group (Ref). **P < 0.01, ***P < 0.001 for testing the null hypothesis RR = 1. The number of visits evaluated (n) is indicated for each MBDA score range. RR: relative risk; MBDA: multi-biomarker disease activity.
Univariate and multivariate analysis of variables associated with radiographic progression
Univariate and multivariate analyses were performed to assess the association with radiographic progression (change in SHS >3 or change in SHS >5) for the MBDA score and several established risk factors for radiographic progression (pre-existing joint damage [SHS], serologic status, SJC28, CRP and DAS28–CRP). In univariate analyses, each variable was associated with radiographic progression, with the MBDA score and pre-existing joint damage (SHS) having the strongest associations among the continuous variables, in terms of AUROC and test statistic (supplementary Table S3, available at Rheumatology Online). Multivariate analyses demonstrated that the MBDA score had the most significant association with radiographic progression (P = 0.002 for change in SHS >3, P = 0.005 for change in SHS >5), after adjustment for other factors (Table 2).
Table 2.
Multivariate analysis of risk factors for radiographic progression
| Variables | Model for change in SHS >3 |
Model for change in SHS >5 |
||||
|---|---|---|---|---|---|---|
| Score test statistic | P-value | Score test statistic | P-value | |||
| Continuous type | ||||||
| MBDA score | 10.1 | 0.002 | 7.8 | 0.005 | ||
| SJC28a | 2.1 | 0.144 | 1.4 | 0.237 | ||
| DAS28–CRP | 0.0 | 0.947 | 0.0 | 0.852 | ||
| CRPb | 3.1 | 0.079 | 1.3 | 0.264 | ||
| SHSa | 5.7 | 0.017 | 2.1 | 0.145 | ||
| Categorical type | ||||||
| Seropositive | 3.5 | 0.060 | 1.6 | 0.208 | ||
aSquare-root transformed. bLog10 transformed. n = 270 visits (serologic status was missing for one patient). P-values indicate the level of significance of the effect of the specified variable on risk for radiographic progression after accounting for the other explanatory variables by logistic models with a GEE method. Seropositive was defined as positive for anti-CCP antibodies and/or RF. GEE: generalized estimating equations; MBDA: multi-biomarker disease activity; SHS: Sharp–van der Heijde score; SJC28: swollen joint count for 28 joints.
MBDA score for predicting progression within categories of serologic status or SHS
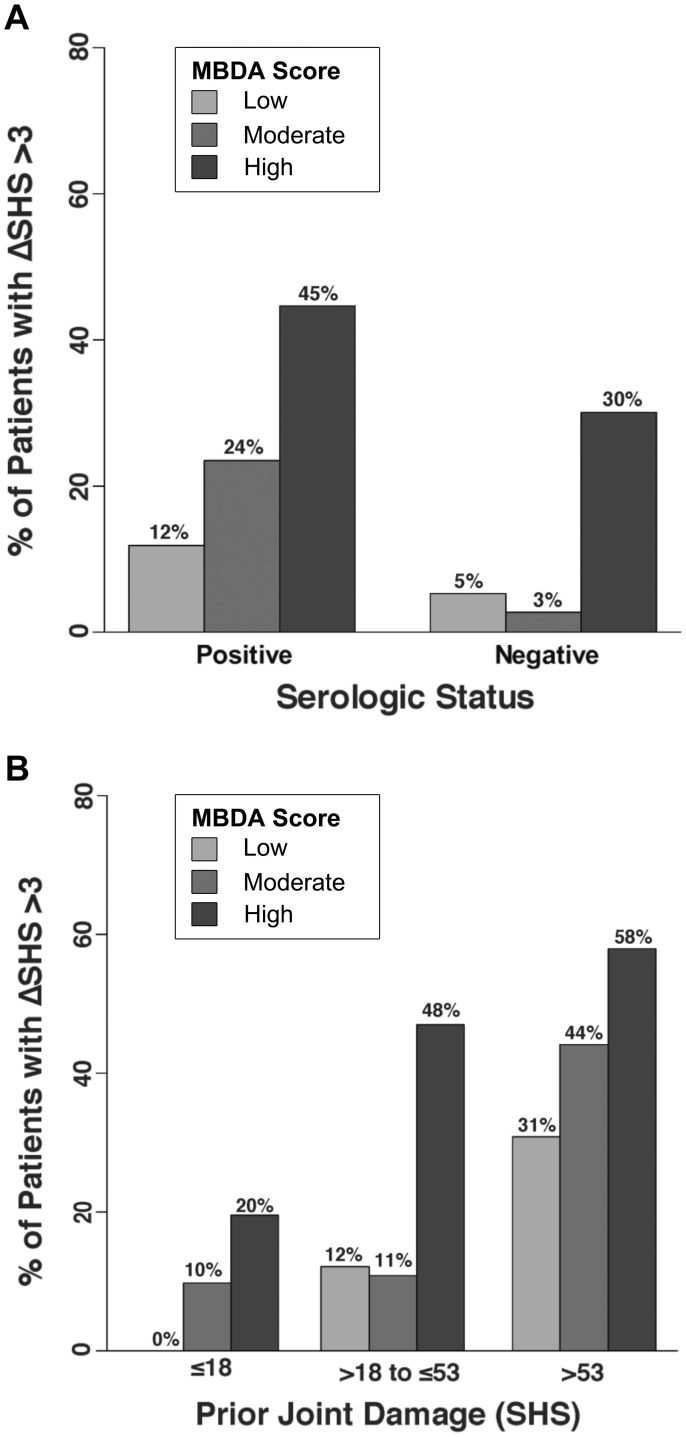
Radiographic progression (change in SHS >3) was more frequent among seropositive than seronegative patients (31% vs 10%, P = 0.001), and with increasing levels of prior joint damage (11, 25, 50%, P < 0.001, for SHS values ≤18, >18–53 and >53, respectively). Within the seropositive group, progression was significantly less frequent when MBDA scores were low (12% for analysis of 38 visits), intermediate when they were moderate (24% for 72 visits) and greatest when they were high (45% for 90 visits). Similarly, a trend was observed within the seronegative group (5, 3 and 30% for analyses of 18, 32 and 20 visits, respectively) (Fig. 3A and supplementary Tables S4 and S5, available at Rheumatology Online). The MBDA score was also associated with progression within strata of prior joint damage (SHS), significantly so within the middle SHS stratum (12, 11 and 48% for analyses of 25, 36 and 40 visits with low, moderate or high MBDA score, respectively) and with numeric trends observed in the lowest and highest SHS strata (Fig. 3B and supplementary Tables S4 and S5, available at Rheumatology Online). Analyses using change in SHS >5 as the threshold for progression yielded similar results, with the result for seronegative patients showing statistical significance (supplementary Table S6, available at Rheumatology Online).
Fig. 3.
Radiographic progression by MBDA score category and serologic status or level of prior joint damage
Frequency of change (Δ) in total Sharp−van der Heijde score (SHS) >3 over the following year was determined for visits (N = 271) subgrouped by MBDA score [low (<30), moderate (30−44) or high (>44)] within groupings by serologic status (A) or amount of prior joint damage (B). Seronegative: negative for both RF and anti-CCP antibody tests; all other patients were considered seropositive. Categories of prior joint damage: SHS ≤18, SHS of 18−53 and SHS >53. Sample sizes and P-values appear in supplementary Tables S4 and S5, respectively, available at Rheumatology Online. MBDA: multi-biomarker disease activity.
MBDA score for predicting risk of progression when disease activity is low according to other measures of disease activity
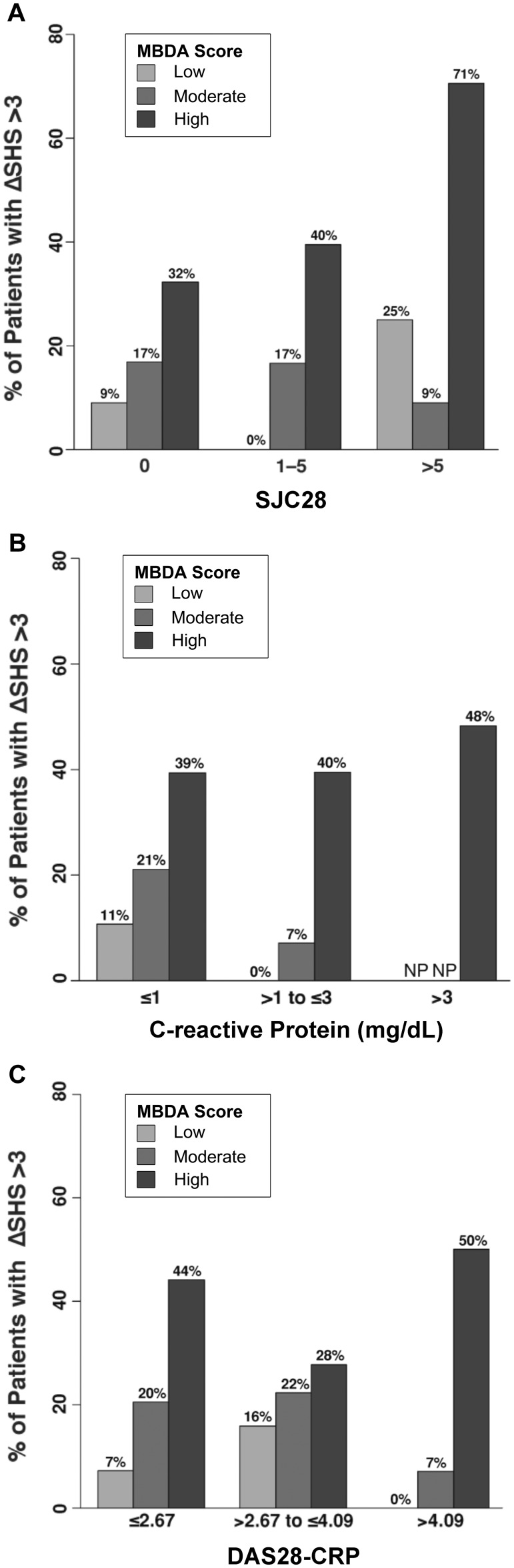
The MBDA score was frequently discordant when SJC28, CRP or DAS28–CRP was low (supplementary Table S4, available at Rheumatology Online). For example, the MBDA score was high (>44) in 44 (30%) of 146 visits with SJC28 = 0, 20 (13%) of 158 visits with CRP ≤1.0 mg/dl, and 25 (22%) of 113 visits with low DAS28–CRP (≤2.67). Within these three low disease activity categories, radiographic progression (change in SHS >3) was most frequent when MBDA scores were high and least frequent when they were low (32% vs 9% for low SJC28; 39% vs 11% for low CRP; and 44% vs 7% for low DAS28–CRP) (Fig. 4), with the differences across low, moderate and high MBDA categories being statistically significant (supplementary Table S5, available at Rheumatology Online). Similar trends were also observed in the other categories for SJC28, CRP and DAS28–CRP, although sample size was limited in some (e.g. only four patients with >5 swollen joints had a low MBDA score, and no patients with CRP >3.0 mg/dl had a low or moderate MBDA score) (Fig. 4 and supplementary Table S4, available at Rheumatology Online). Analyses using change in SHS >5 as the threshold for radiographic progression yielded similar results (supplementary Table S6, available at Rheumatology Online).
Fig. 4.
Radiographic progression by categories of MBDA score and conventional measures of disease activity
Frequency of change (Δ) in total Sharp−van der Heijde score (SHS) >3 over the following year was determined for visits (N = 271) subgrouped by MBDA score [low (<30), moderate (30−44) or high (>44)] within groupings of low, moderate or high based on swollen joint count (SJC28) (A), CRP (B) or DAS28–CRP (C). Sample sizes and P-values appear in supplementary Tables S4 and S5, respectively, available at Rheumatology Online. NP: no patients in that subgroup; MBDA: multi-biomarker disease activity.
Discussion
This observational study of patients with established RA who were receiving ongoing non-biologic DMARD therapy demonstrated that the MBDA score was an independent predictor of risk for radiographic progression. Low MBDA scores were associated with infrequent radiographic progression over the following year, and high MBDA scores were associated with increased frequency and severity of radiographic progression. This association was observed for both radiographic JSN and JEs, a finding that has not been reported previously in any population, and it was observed even when other assessment tools, such as serologic status, SJC28, CRP or DAS28–CRP, were negative or low. Moreover, within the high range of MBDA scores (>44), risk for joint damage increased further as the MBDA score increased.
The most direct demonstration of the relationship between the MBDA score and joint damage progression came from the scatter plot (Fig. 1), which showed that when the MBDA score was low, progression was infrequent, and when it occurred, it was rarely rapid (change in SHS >5). By contrast, although progression was not observed for all patients with high MBDA scores (>44), the optimal threshold for predicting progression in this study was ∼44, and risk of progression continued to increase within the high MBDA range. For MBDA scores ≥60, which represented 14% of all measurements, the relative risk was 5.2 for change in SHS >3 and 17.4 for change in SHS >5, compared with the low MBDA range. Thus, progression risk was found to exist on a continuum, from little risk when MBDA scores were low, to increasing risk as scores entered the high range, and substantial risk for the highest MBDA scores. This finding implies that reducing the MBDA score of a patient receiving non-biologic DMARD therapy may reduce the risk of radiographic progression, especially for scores in the high MBDA range. Longitudinal data are needed to address this question directly.
Multivariate analysis extended our findings by demonstrating that the MBDA score remained significantly associated with subsequent joint damage after adjusting for several established risk factors for joint damage. The interaction between the MBDA score and other risk factors was further explored by cross-classification analyses. For conventional measures of disease activity (SJC28, CRP or DAS28–CRP), the most striking finding was that a high MBDA score was significantly associated with more frequent progression, even when the clinical measures or the acute phase reactant, CRP, indicated low disease activity. This finding suggests that such patients may have subclinical synovitis [7]. Conversely, for patients with high DAS28–CRP, risk of progression was low when the MBDA score was low or moderate, suggesting that their signs or symptoms may have a non-inflammatory component [25, 26]. These findings elucidate the clinical implications of discordances between MBDA scores and conventional measures. Discordances with the MBDA score have been previously reported in patients with early RA [11, 12, 14, 15]. Further study of patients with such discordances is needed.
The multivariate analyses also indicated that the MBDA score was associated with joint damage progression independently of two non–disease activity risk factors— serologic status and the amount of pre-existing joint damage. Cross-classification analysis demonstrated that the MBDA score significantly discriminated risk for progression among seropositive patients, with a similar trend observed for seronegative patients; this was also observed in patients with intermediate levels of prior joint damage (SHS >18–53), with similar trends observed for patients with lower or higher levels of prior joint damage. The results of our cross-classification analyses are consistent with studies that evaluated matrixed combinations of conventional risk factors for patients with new onset RA [19, 27, 28] and established RA [29], and with an analysis of MBDA scores in patients with early RA [14].
Our findings imply that the MBDA score may be a useful addition to conventional clinical, serologic and radiographic measures to identify patients who are or are not at risk of radiographic progression during non-biologic DMARD therapy. Second, they support the prior, clinically based validation of the MBDA score [10] by providing radiographic data indicating that the MBDA score is reflective of pathological inflammation in RA.
Our findings are relevant to clinical trials of RA therapies because new drugs have sometimes failed to demonstrate inhibition of structural damage, due to limited damage progression in the MTX comparator arm [30, 31]. Our analyses suggest that a high MBDA score may be useful as an inclusion criterion to improve the selection of patients at risk of radiographic progression. This consideration may be especially applicable for patients who would be excluded from trials due to low CRP (e.g. ≤1 mg/dl) but could be eligible if the MBDA score is high, because in both this study and a post hoc analysis of patients with early RA from the SWEFOT study [14], patients with a high MBDA score and CRP ≤1 mg/dl demonstrated a similar frequency of progression to that of patients with a high MBDA score and CRP >1 mg/dl. The MBDA score may also have the potential to screen out patients who have relatively inactive disease despite elevated DAS28-CRP, thereby avoiding misclassifications that can reduce treatment effect when comparing active drug and placebo.
A strength of this study is that the cohort had a wide range of clinical disease activities and MBDA scores while receiving DMARD treatment for established RA. This distribution allowed discordances between the MBDA score and conventional measures to be analysed at both ends of their respective ranges. MBDA scores and radiographs have also been analysed for patients from SWEFOT and BeSt, trials of DMARD-naïve patients with active, early RA [14, 15]. Those analyses included proportionately fewer patients with a low MBDA score than our analysis. Nonetheless, risk of progression was also found to increase within the high MBDA category for patients from SWEFOT [14], and all three studies showed that the MBDA score is independently associated with radiographic progression.
A limitation of this study is that it retrospectively analysed data from patients who were initially evaluated when biologic use was not as widespread as now. However, these patients are relevant to current practice because prolonged treatment with non-biologic DMARDs despite inadequate response is still commonplace [32]. Sample size was limiting for some subset analyses, but key findings were statistically significant and similar trends were found overall. Data on smoking status and obesity in this cohort were not available for evaluation as risk factors for radiographic progression [33, 34].
In summary, this observational study is the first to show, for patients with established RA receiving non-biologic DMARD therapy, that the MBDA score is a predictor of future joint damage that is independent of and stronger than several established risk factors, including serologic status, SJC28, CRP and DAS28–CRP. We found that low MBDA scores were associated with infrequent joint damage progression, and high MBDA scores were associated with more frequent and more severe progression. These associations were also observed separately for radiographic JEs and JSN. Moreover, risk of radiographic progression was found to continue increasing as the MBDA score increased within the high MBDA category. These findings suggest that the MBDA score may be able to complement conventional tools for assessing RA patients during DMARD therapy and to help determine which patients need treatment intensification to prevent progressive joint damage.
Supplementary Material
Acknowledgements
The authors thank Arbor Communications for assistance preparing figures and tables and for editorial assistance. C.C. Hwang is thanked for his careful review of the manuscript. The work of A.H.M. vdH.-vM. is supported by the Netherlands Organization for Health Research and Development.
Funding: Crescendo Bioscience funded the generation of biomarker data and statistical analysis. Crescendo Bioscience provided funds to the Leiden University Medical Center for the retrieval, aliquoting, labelling and shipping of study samples to the laboratory facility of Crescendo Bioscience, in South San Francisco, CA, USA. No other funding was received for the study.
Disclosure statement: W.L. and E.H.S. are employees of Crescendo Bioscience, a wholly owned subsidiary of Myriad Genetics, Inc., and both are shareholders of Myriad Genetics, Inc. T.W.J.H. serves as a consultant for Crescendo. The other author has declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying anti-rheumatic drugs. Ann Rheum Dis 2010;69:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pincus T. Advantages and limitations of quantitative measures to assess rheumatoid arthritis. Joint counts, radiographs, laboratory tests and patient questionnaires. Bull NYU Hosp Joint Dis 2006;64:32–9. [PubMed] [Google Scholar]
- 3.Sokka T, Pincus T. Erythrocyte sedimentation rate, C-reactive protein, or rheumatoid factor are normal at presentation in 35%–45% of patients with rheumatoid arthritis seen between 1980 and 2004: analyses from Finland and the United States. J Rheumatol 2009;36:1387–90. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe F. The many myths of erythrocyte sedimentation rate and C-reactive protein. J Rheumatol 2009;36:1568–9. [DOI] [PubMed] [Google Scholar]
- 5.Kay J, Morgacheva O, Messing SP, et al. Clinical disease activity and acute phase reactant levels are discordant among patients with active rheumatoid arthritis: acute phase reactant levels contribute separately to predicting outcome at one year. Arthritis Res Ther 2014;16:R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krabben A, Stomp W, Huizinga TWJ, et al. Concordance between inflammation at physical examination and on MRI in patients with early arthritis. Ann Rheum Dis 2015;74:506–12. [DOI] [PubMed] [Google Scholar]
- 7.Brown AK, Conaghan PG, Karim Z, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum 2008;58:2958–67. [DOI] [PubMed] [Google Scholar]
- 8.Yazdany J, Schmajuk G, Robbins M, et al. Choosing wisely: the American College of Rheumatology’s top 5 list of things physicians and patients should question. Arthritis Rheum 2013;65:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centola M, Cavet G, Shen Y, et al. Development of a multi-biomarker disease activity test for rheumatoid arthritis. PLoS ONE 2013;8:e60635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis JR, van der Helm-van Mil AH, Knevel R, et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res 2012;64:1794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakker MF, Cavet G, Jacobs JWG, et al. Performance of a multi-biomarker score measuring rheumatoid arthritis disease activity in the CAMERA tight control study. Ann Rheum Dis 2012;71:1692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirata S, Dirven L, Shen Y, et al. A multi-biomarker score measures rheumatoid arthritis disease activity in the BeSt study. Rheumatology 2013;52:1202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Helm-van Mil AH, Knevel R, Cavet G, Huizinga TW, Haney DJ. An evaluation of molecular and clinical remission in rheumatoid arthritis by assessing radiographic progression. Rheumatology 2013;52:839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hambardzumyan K, Bolce R, Saevarsdottir S, et al. Pretreatment multi-biomarker disease activity score and radiographic progression in early RA: results from the SWEFOT trial. Ann Rheum Dis 2015;74:1102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markusse I, Dirven L, van den Broek M, et al. A multibiomarker disease activity score for rheumatoid arthritis predicts radiographic joint damage in the BeSt study. J Rheumatology 2014;41:2114–9. [DOI] [PubMed] [Google Scholar]
- 16.De Rooy DP, van der Linden MP, Knevel R, Huizinga TW, van der Helm-van Mil AH. Predicting arthritis outcomes − what can be learned from the Leiden Early Arthritis Clinic? Rheumatology 2011;50:93–100. [DOI] [PubMed] [Google Scholar]
- 17.Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 18.Klarenbeek NB, Koevoets R, van der Heijde DMFM, et al. Association with joint damage and physical functioning of nine composite indices and the 2011 ACR/EULAR remission criteria in rheumatoid arthritis. Ann Rheum Dis 2011;70:1815–21. [DOI] [PubMed] [Google Scholar]
- 19.Vastesaeger N, Xu S, Aletaha D, St Clair EW, Smolen JS. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology 2009;48:1114–21. [DOI] [PubMed] [Google Scholar]
- 20.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 2003;157:364–75. [DOI] [PubMed] [Google Scholar]
- 21.Boos DD. On generalized score tests. Am Stat 1992;46:327–33. [Google Scholar]
- 22.Efron B. Better bootstrap confidence intervals. J Am Stat Assoc 1987;82:171–85. [Google Scholar]
- 23.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoue E, Yamanaka H, Hara M, Tomatsu T, Kamatani N. Comparison of Disease Activity Score (DAS)28- erythrocyte sedimentation rate and DAS28- C-reactive protein threshold values. Ann Rheum Dis 2007;66:407–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranzolin A, Brenol JCT, Bredmeier M, et al. Association of concomitant fibromyalgia with worse disease activity score in 28 joints, Health Assessment Questionnaire, and Short Form 36 scores in patients with rheumatoid arthritis. Arthritis Care Res 2009;61:794–800. [DOI] [PubMed] [Google Scholar]
- 26.Lee YC, Frits ML, Iannaccone CK, et al. Subgrouping patients with rheumatoid arthritis based on pain, fatigue, inflammation, and psychosocial factors. Arthritis Rheumatol 2014;66:2006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Visser K, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. A matrix risk model for the prediction of rapid radiographic progression in patients with rheumatoid arthritis receiving different dynamic treatment strategies: post hoc analysis from the BeSt study. Ann Rheum Dis 2010;69:1333–7. [DOI] [PubMed] [Google Scholar]
- 28.Fautrel B, Granger B, Combe B, et al. Matrix to predict rapid radiographic progression of early rheumatoid arthritis patients from the community treated with methotrexate or leflunomide: results from the ESPOIR cohort. Arthritis Res Ther 2012;14:R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lillegraven S, Paynter N, Prince FHM, et al. The performance of matrix-based risk models for rapid radiographic progression in BRASS, a cohort of patients with established rheumatoid arthritis. Arthritis Care Res 2013;65:526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emery P, Fleischmann R, van der Heijde D, et al. The effect of golimumab on radiographic progression in rheumatoid arthritis: results of the GO-BEFORE and GO-FORWARD randomized controlled studies. Arthritis Rheum 2011;63:1200–10. [DOI] [PubMed] [Google Scholar]
- 31.van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 32.Harrold LR, Harrington JT, Curtis JR, et al. Prescribing practices in a US cohort of rheumatoid arthritis patients before and after publication of the American College of Rheumatology treatment recommendations. Arthritis Rheum 2012;64:630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finkch A, Dehler S, Costenbader KH, Gabay C; Swiss Clinical Quality Management project for RA. Cigarette smoking and radiographic progression in rheumatoid arthritis. Ann Rheum Dis 2007;66:1066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker JF, George M, Baker DG, et al. Associations between body mass, radiographic joint damage, adipokines and risk factors for bone loss in rheumatoid arthritis. Rheumatology 2011;50:2100–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.