Abstract
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder characterized by recurrent abdominal pain or discomfort associated with abnormal bowel habits. Diarrhea-predominant IBS (IBS-D) is a major subtype of IBS, the predominant manifestations of which are abdominal pain and diarrhea. The pathogenesis of IBS-D remained unknown until recently. The effects of psychosocial stress, central hypervigilance, neuroendocrine abnormality, disturbed gastrointestinal motility, mucosal immune activation, intestinal barrier dysfunction, visceral hypersensitivity (VH), altered gut flora, and genetic susceptibility may be involved in its development. Recently, increased attention has been placed on the neural-immune-endocrine network mechanism in IBS-D, especially the role of various neuroendocrine mediators. As a member of the neurotrophin family, nerve growth factor (NGF) has diverse biological effects, and participates in the pathogenesis of many diseases. Basic studies have demonstrated that NGF is associated with inflammatory- and stress-related VH, as well as stress-related intestinal barrier dysfunction. The aim of this study is to summarize recent literature and discuss the role of NGF in the pathophysiology of IBS-D, especially in VH and intestinal barrier dysfunction, as well as its potential as a therapeutic target in IBS-D.
Keywords: Nerve growth factor, Diarrhea-predominant irritable bowel syndrome, Pathophysiology, Intestinal barrier dysfunction, Visceral hypersensitivity
1. Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disease without organic abnormalitie characterized by recurrent abdominal pain or discomfort associated with abnormal bowel habits. The diagnosis is established primarily through symptoms according to the Rome III criteria of 2006 after exclusion of organic gastrointestinal diseases through endoscopy radioscopy and other biochemical tests. Epidemiological surveys show that the prevalence of IBS in China is between 1% and 16% (Zhang et al. 2014) while the prevalence in the western population is relatively high ranging from 10% to 18% (Choung and Locke. 2011). As a major subtype diarrhea-predominant IBS (IBS-D) accounts for 23.3%–65% of IBS cases (Yao et al. 2008; Shen et al.2011) and its predominant clinical manifestations are abdominal pain and diarrhea. The recurrent and distressing abdominal symptoms always accompanied by psychological disorders affect the patients’ quality of life to varying degrees.
The pathogenesis of IBS-D has not been completely understood until recently. The effects of psychosocial stress central hypervigilance neuroendocrine abnormality disturbed gastrointestinal motility mucosal immune activation intestinal barrier dysfunction visceral hypersensitivity (VH) altered gut flora as well as genetic susceptibility may be involved in its development (Camilleri 2012). A single factor cannot fully explain the pathogenesis and symptoms of IBS-D. These factors may have complex interactions and their positions may vary in the pathogenesis of the disorder in the individual IBS-D patient resulting in heterogeneity of IBS-D.
Recently, a neural-immune-endocrine network mechanism has gained increasing attention especially relating to the role of various neuroendocrine mediators such as serotonin (5-HT) substance P (SP) brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in IBS-D. This article will review the literature on the role of NGF in the pathophysiology of IBS-D especially in VH and intestinal barrier dysfunction.
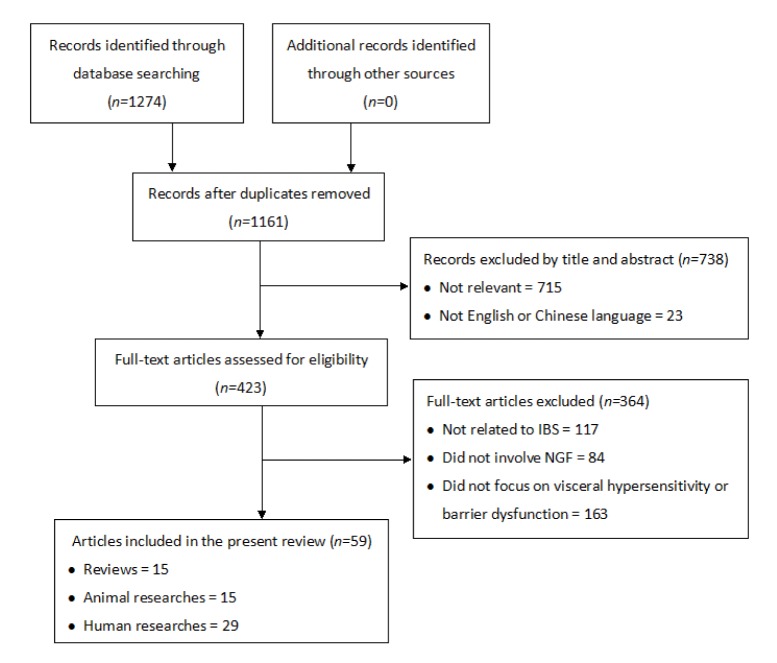
Publications related to IBS and NGF in both the English and Chinese languages were searched in PubMed EMBASE Web of Scienc China National Knowledge Infrastructure (CNKI). Weipu and Wanfang databases from their inception to May 2015 (Fig. 1). The initial literature search identified a total of 1274 citations. After removal of duplicates 1161 records were left; and among these 738 records were excluded based on their titles and abstracts. Full-text examinations further excluded 364 records because of irrelevance to the aim of the present study. Finally 59 eligible records were identified and included in this study consisting of 15 literature reviews 15 animal researches.
Fig. 1.
Flow diagram of the study selection and exclusion process
2. What is NGF?
Neurotrophins (NTs) are a family of trophic factors that regulate the growth, function, plasticity, survival, and apoptosis of neurons, including NGF, BDNF, NT-3, and NT-4 (Bothwell, 2014). Secreted by various tissues and cells, such as innervated tissues, astrocytes, mast cells, and epithelial cells, NTs will enter nerve endings in a receptor-mediated manner, and are then backwards transported to the cell body of the neuron to modulate the synthesis of the related proteins. Recent studies have found that NTs and their receptors are widely distributed in the digestive tract, and exert important effects on the sensation, motility, and secretion of the digestive tract.
As the first member discovered in the NT family, NGF is a multimeric protein consisting of subunits α, β, and γ, of which β is the active subunit. NGF is distributed in various organs, such as the brain, ganglion, skeletal muscle, intestine, bladder, etc. NGF exerts its functions via two membrane receptors: a high-affinity receptor TrkA and a low-affinity receptor p75NTR (Wang and Wu, 2010), and the former is of particular importance. The main biological effects of NGF include promoting neuronal differentiation, stimulating the growth of dendrites and cell bodies, and affecting nerve fiber density. In addition, NGF plays a regulatory role in the function of non-nerve tissues, such as the activation, proliferation, and apoptosis of mast cells, lymphocytes, and epithelial cells. Under acute or chronic stress, tissue damage, inflammation, and other pathological conditions, increased NGF can change the local nerve fiber plasticity, resulting in hyperalgesia, as well as organ dysfunction (Lewin et al., 2014).
There is very limited clinical evidence on the relationship between NGF and IBS-D. However, basic studies have demonstrated that NGF is associated with inflammatory- and stress-related VH, as well as stress-related intestinal barrier dysfunction. Due to the existence of stress and low-grade mucosal inflammation in IBS-D, NGF may play a potential role in the pathophysiology of IBS-D, particularly in VH and intestinal barrier dysfunction. Detailed information of the individual studies exploring the role of NGF in IBS pathophysiology is presented in Table 1.
Table 1.
Key data from the various studies exploring the role of NGF in IBS pathophysiology
| Reference | Country | Study type | Employed techniques | Key findings |
| Willot et al., 2012 | Canada | Human | Immunohistochemistry, enzyme-linked immuno sorbent assay (ELISA) | Rectal mucosal NGF content was higher in IBS-D children than in control and correlated with mast cell (MC) number |
| Dothel et al., 2015 | Italy | Human | Immunohistochemistry, ELISA, primary cell culture, qRT-PCR | Higher nerve fiber density, neuronal sprouting, and mucosal NGF and TrkA levels in IBS patients; NGF antibodies inhibited the neurotrophic effect of mucosal supernatants from IBS patients |
| Barreau et al., 2004 | France | Animal | Immunohistochemistry, RT-PCR, gut permeability (GP) by 51Cr-EDTA | Maternal deprivation (MD) increased visceral sensitivity, GP, and colonic NGF expression in rats; NGF antibodies abolished these effects |
| van den Wijngaard, 2009 | The Netherlands | Animal | Immunohistochemistry, qRT-PCR, Western blotting | Acute stress induced colonic hypersensitivity and elevated colonic MC numbers in adult MD rats; MC stabilizer, NGF antibodies, or TRPV1 antagonist prevented the hypersensitivity |
| Yu et al., 2008 | China | Animal | Intraperitoneal NGF injection | NGF enhanced water avoidance (WA)-induced visceral hypersensitivity |
| Delafoy et al., 2003 | France | Animal | Intraperitoneal NGF injection | Both NGF and trinitrobenzene sulfonic acid (TNBS) induced colonic hypersensitivity via capsaicin-sensitive afferent fibers; NGF antibodies could reverse the effects of NGF and TNBS |
| Delafoy et al., 2006 | France | Animal | Drug intervention, colonic sensitivity assessment via barostat | Calcitonin gene-related peptide (CGRP) antagonist alleviated TNBS or NGF-induced colonic hypersensitivity; NGF antibodies failed to reverse CGRP-induced hypersensitivity |
| Barreau et al., 2007 | France | Animal | Drug intervention, GP by 51Cr-EDTA, immunohistochemistry, ELISA | MD rats showed increased GP and colonic NGF content; CRF acts on mast cells to stimulate NGF release and participates in the elevated GP observed in MD adult rats |
| Barreau et al., 2008 | France | Animal | Immunohistochemistry, ELISA, drug intervention | MD induced increased nerve fiber density and synaptogenesis, as well as mast cell hyperplasia and hypertrophy; NGF antibodies prevented these effects |
3. NGF and VH in IBS-D
VH is a clinical marker in a subset of IBS-D patients, and contributes to abdominal pain, bloating, urgency, and other symptoms. VH is defined as increased visceral sensitivity to mechanical, temperature, chemical, and other stimuli; a balloon distension test shows decreased rectal sensory thresholds compared with healthy individuals. It is a widely accepted fact that the mechanisms of VH involve multiple levels of the nervous system: sensory nerve endings, spinal afferent pathways, and the brain. In addition, neuroendocrine mediators, gut flora, and low-grade mucosal inflammation are also involved (Zhou and Li, 2014). An increasing number of clinical and basic studies have confirmed that NGF is closely related to VH.
3.1. Clinical studies on NGF and VH in IBS
Akbar et al. (2008) reported increased SP-positive and transient receptor potential vanilloid 1 (TRPV1)-positive nerve fibers in colonic mucosa of IBS patients, and a positive correlation between TRPV1 staining intensity and abdominal pain, speculating that in the low-grade inflamed mucosa of IBS, elevated NGF mediates TRPV1 up-regulation and leads to VH. However, this research lacks direct evidence of the NGF level in IBS. Willot et al. (2012) first determined NGF in rectal mucosa of IBS-D children by enzyme-linked immunosorbent assay (ELISA) testing, and observed elevated NGF content compared with healthy controls. Recently, Dothel et al. (2015) discovered increased density of neuronal-specific enolase (NSE)-positive and growth associated protein 43 (GAP43)-positive fibers in the colonic mucosa from IBS patients, indicating higher nerve fiber density and neuronal sprouting; mucosal NGF and TrkA levels were also increased in IBS patients; mucosal supernatants from these patients promoted neuritogenesis of human neuronal cell lines, which could be inhibited by anti-NGF antibodies. These results indicate that increased NGF acts on the intestinal sensory nerve endings, promotes their growth and synapse formation, and thus mediates VH. Limitations of these studies are that correlations between elevated NGF and visceral sensitivity indicators, as well as IBS symptoms, have not been analyzed. These issues deserve further investigation to clarify the role of NGF in the pathogenesis of IBS-D.
3.2. Mechanisms of NGF-mediated VH
In inflammatory diseases and animal models, by modifying neuronal plasticity, NGF promotes the perception and transduction of inflammatory pain, and mediates hypersensitivity of the related tissues and organs. For instance, colonic NGF and TrkA expression were increased in inflammatory bowel disease (IBD) patients (di Mola et al., 2000), and the NGF level in ulcerative colitis was positively correlated with the severity of the disease (Li et al., 2014). In rat models of chemically-induced cystitis, rats exhibited inflammatory bladder pain, skin mechanical hypersensitivity, and increased NGF content in the bladder mucosa; administration of TrkA antagonist before modeling prevented bladder and skin hyperalgesia, suggesting that coupled with TrkA, NGF transmits signals to the sensory neurons, activates pain pathway, and mediates hypersensitivity (Coelho et al., 2015).
Stress animal models present similar pathophysiologic abnormalities to that in IBS patients, such as VH and intestinal barrier dysfunction. Therefore, these experimental models are always used to explore the pathophysiology of IBS. NGF-mediated VH is not restricted to the inflammatory state, but also is involved in the stress state. Barreau et al. (2004) reported NGF to be an important mediator in neonatal maternal deprivation (MD)-induced VH. After experiencing MD for several weeks, the rats showed VH to rectal distention and elevated colonic NGF; treatment with anti-NGF antibodies during MD abolished MD-induced hypersensitivity. Another study showed that acute water avoidance (WA) stress induced hypersensitivity to colonic distention in adult MD rats, and also caused a slight increase in the colonic mast cell (MC) numbers, but not in non-MD rats; administration of MC stabilizer, anti-NGF antibody, or TRPV1 antagonist before WA prevented hypersensitivity (van den Wijngaard et al., 2009). Yu et al. (2008) showed that intraperitoneal injection of NGF enhanced WA-induced VH, through the up-regulation of TRPV1. This evidence suggests that NGF, MC, and TRPV1 are involved in stress-related VH, and the cascade sequence may be as follows: stress-MC-NGF-TRPV1-VH.
Administration of exogenous NGF to normal tissues induces hypersensitivity similar to that in inflammation and stress. Rukwied et al. (2010) injected 1 μg NGF into a normal human forearm and induced local temperature and mechanical hypersensitivity. Delafoy et al. (2003; 2006) showed that intraperitoneal injection of NGF lowered colonic distention thresholds in rats, which could be reversed by anti-NGF antibodies or a calcitonin gene-related peptide (CGRP) antagonist; injection of CGRP also induced similar hypersensitivity, while anti-NGF antibodies failed to reverse it, indicating that CGRP is a critical element downstream in NGF signaling; if capsaicin-sensitive C-fiber afferents were damaged during the neonatal period, then NGF-induced colonic hypersensitivity was abolished.
Taken together, the possible mechanisms of NGF-mediated VH are that NGF causes neurotrophic effects on sensory nerve endings, promoting the proliferation of SP-, CGRP-, and TRPV1-positive nociceptive fibers; moreover, NGF modulates the activation state of nociceptor proteins such as TRPV1 to sensitize the nerve endings (Bonnington and McNaughton, 2003; Zhuang et al., 2004; Zhu and Oxford, 2007); NGF is also backwards transported to the cell body in the dorsal root ganglia (DRG), increasing the TRPV1 expression and translocation to the peripheral terminals (Stein et al., 2006). All these lead to hypersensitivity of the sensory nerve endings, increased release of pain-related peptides (such as CGRP, SP) after stimulation, and subsequent hyperalgesia.
4. NGF and intestinal barrier dysfunction in IBS-D
Intact intestinal barrier defense relies on three components: pre-epithelium, epithelium, and post-epithelium (Hao and Duan, 2010). The pre-epithelium component includes the mucus and microbiota, together with chemical substances such as cytokines, inflammatory mediators, and antimicrobial peptides. The epithelium plays a critical role in the intestinal barrier function, and primarily consists of intestinal epithelial cells and intercellular tight junctions (TJs). The post-epithelium component includes immunocytes in lamina propria, blood vessels, and the enteric nervous system (ENS) (Keita and Söderholm, 2010; Bischoff et al., 2014). It is now an accepted fact that intestinal barrier dysfunction plays a pathogenic role in IBS-D, which manifests itself as increased mucosal permeability, ultrastructural alteration of the TJ, an abnormal level of TJ-related proteins and other associated blood markers.
4.1. Evidence of intestinal barrier dysfunction in IBS-D
Several studies observed increased intestinal permeability in IBS patients via the oral multi-sugar test (Mujagic et al., 2014; Shulman et al., 2014). A study on IBS-D patients performed by Liu et al. (2014) showed elevated urinary levels of I-FABP, I-BABP, and L-FABP, which are regarded as clear markers of intestinal barrier function. Lee et al. (2013) confirmed increased intestinal permeability in IBS-D using an Ussing chamber. Martínez et al. (2013) observed abnormalities in the expression, phosphorylation, and distribution of TJ-related proteins (such as claudin and occludin), as well as disrupted ultrastructure of the intercellular apical junction complex (AJC) in the jejunal mucosa of IBS-D patients, revealing the underlying molecular and structural basis of intestinal barrier dysfunction in IBS-D.
4.2. Mechanisms of NGF-mediated barrier dysfunction
The neonatal MD model is usually used to mimic the intestinal barrier dysfunction in IBS patients. Barreau et al. (2004; 2007) reported that NGF was a key factor in MD-induced intestinal barrier dysfunction. In that study, MD rats presented increased intestinal permeability measured by 51Cr-EDTA excretion, as well as elevated MC numbers and NGF expression; MD rats administered anti-NGF antibodies showed normal permeability; the intraperitoneal injection of NGF induced similar changes in permeability and the MC numbers in non-MD rats. Possible explanation is that stress promotes intestinal NGF expression. Increased NGF acts on MCs and promotes their proliferation, activation, and release of mediators (Barreau et al., 2008). Various MC-derived mediators (such as tryptase, histamine, prostaglandins, interleukin (IL)-4, IL-10, and tumor necrosis factor (TNF)-α) combine with specific membrane receptors on epithelial cells, and modify the expression and location of TJ proteins, resulting in increased intestinal permeability. NGF can not only indirectly affect permeability via MC, but also act on intestinal epithelial cells via its receptor TrkA, and directly change permeability. Due to increased intestinal permeability, exposure of submucosal tissue to antigens such as intraluminal pathogens occurs more frequently, overstimulating the local immune system. Various immune and inflammatory mediators activate intestinal receptors and cause disturbances in sensation, motility, and secretion in the digestive tract, contributing to the symptoms of IBS-D (Piche et al., 2009; Zhou et al., 2009).
As mentioned above, evidence of numerous basic researches has demonstrated the critical role of NGF in stress-related intestinal barrier dysfunction. Stress is involved in the pathogenesis of IBS-D, and is a trigger of IBS symptoms. Thus, it can be assumed that NGF participates in intestinal barrier dysfunction in IBS-D patients, which remains to be confirmed by further studies.
5. NGF-MC-nerve interaction in IBS-D pathophysiology
Intestinal MC is regarded as the key component of the neural-immune-endocrine network mechanism in IBS-D, and also the main source of NGF in the intestinal mucosa (Leon et al., 1994; Levi-Montalcini et al., 1996; Skaper et al., 2001). MCs are always adjacent to small-diameter unmyelinated nerve fibers that are primarily peptidergic fibers containing neuropeptides such as SP and CGRP, and can respond to noxious stimuli and induce pain (Park et al., 2003; Barbara et al., 2004). Moreover, these fibers express TrkA and receive regulation of NGF (Snider and McMahon, 1998). These spacial and structural features suggest functional interaction of the NGF-MC-nerve.
In the pathogenesis of IBS-D (Fig. 2), psychophysiological stress (such as adverse life events, catching cold, and intestinal infection) activates the hypothalamic-pituitary-adrenal (HPA) axis (Mayer, 2000; Chang et al., 2009; Kennedy et al., 2014). The central and intestinal corticotropin releasing factor (CRF) increases and activates intestinal MCs (Barreau et al., 2007; Overman et al., 2012). Activated MCs degranulate and release mediators such as NGF and tryptase. These mediators act on intestinal epithelial cells, and influence the expression and localization of TJ proteins, resulting in increased intestinal permeability and intestinal barrier dysfunction (Ferrier et al., 2003; Cenac et al., 2007; Coëffier et al., 2010). In addition, the ENS, especially the VIPergic submucosal nerve fibers, has been demonstrated to regulate the expression of TJ-related proteins (such as ZO-1) and affect the intestinal barrier homeostasis. Thus, in addition to the MC-related mechanism, NGF may modulate the barrier function via ENS and its neuropeptides (such as vasoactive intestinal peptide (VIP)) (Neunlist et al., 2003).
Fig. 2.
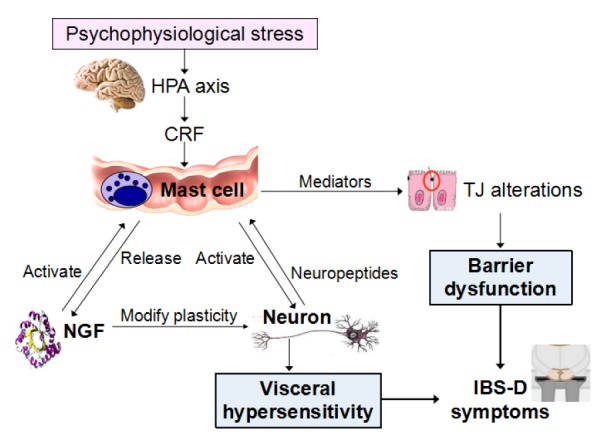
NGF-MC-nerve interaction in IBS-D pathophysiology
With respect to VH, MC-derived mediators such as tryptase, NGF, histamine, 5-HT, and prostaglandins activate the intestinal sensory nerve endings (Barbara et al., 2007; Ohashi et al., 2008). Moreover, as a neurotrophic factor, NGF modifies local neuronal plasticity, leading to changes in the distribution of sensory nerve endings, expression and location of nociceptor proteins (such as TRPV1), and the release of neuropeptides (such as SP, CGRP, VIP). All these factors cause hypersensitivity of the sensory nerve endings to noxious and even physiological stimuli, which forms the foundation of VH.
In addition, MC-derived NGF can act on the MC in a paracrine or autocrine manner. Likewise, sensory nerve endings, activated by MC mediators, can release neuropeptides to affect the MC. All these in turn will promote MC activation and NGF production, forming the positive feedback loop.
Intestinal barrier dysfunction and VH caused by NGF-MC-nerve interaction constitute the pathophysiological basis of IBS-D symptoms, such as abdominal pain and diarrhea.
6. Conclusions and prospects
IBS has always been considered a functional gastrointestinal disease, without the clear evidence of any organic gastrointestinal diseases. However, it is a general rule in most diseases that there are always structural or organic abnormalities underlying the functional alterations. Many efforts have been made to seek the underlying culprits of IBS symptoms, but the pathophysiology of IBS-D is still not entirely understood, leading to a lack of specific and effective therapy.
The neural-immune-endocrine network mechanism has become a hot spot for research at present. NGF, as an important neuroendocrine mediator, presents diverse biological and pathological effects. Its relationship with visceral sensitivity and the intestinal barrier function, as well as mobility, gut flora, mental condition, and the genetic background of IBS-D patients, deserves further investigation, in order to elucidate the pathophysiology of IBS-D.
In disease management, NGF-targeted therapy has shown encouraging prospects in several illnesses. For instance, with the clarification of the role NGF plays in chronic inflammatory pain, researchers evaluated the efficacy of anti-NGF therapy in chronic pain associated with osteoarthritis (Sanga et al., 2013; Tiseo et al., 2014), interstitial cystitis (Evans et al., 2011), diabetic neuropathy (Bramson et al., 2015), chronic pelvic pain syndrome (Nickel et al., 2012), and chronic low back pain (Gimbel et al., 2014), and the results were optimistic. Given that chronic abdominal pain is one of the main manifestations of IBS-D, it is worth further investigation as to whether anti-NGF therapy could show similar optimistic results in IBS-related abdominal pain, as well as other symptoms. In the future, NGF may be a promising therapeutic target in IBS-D.
Footnotes
Project supported by the National Ministry of Science and Technology “Twelfth Five-Year” Supporting Project (No. 2014BAI08B02), China
Compliance with ethics guidelines: Xiao-juan XU, Liang LIU, and Shu-kun YAO declare that they have no conflict of interest.
This paper does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Akbar A, Yiangou Y, Facer P, et al. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57(7):923–929. doi: 10.1136/gut.2007.138982. (Available from: http://dx.doi.org/10.1136/gut.2007.138982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbara G, Stanghellini V, de Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702. doi: 10.1053/j.gastro.2003.11.055. (Available from: http://dx.doi.org/10.1053/j.gastro.2003.11.055) [DOI] [PubMed] [Google Scholar]
- 3.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132(1):26–37. doi: 10.1053/j.gastro.2006.11.039. (Available from: http://dx.doi.org/10.1053/j.gastro.2006.11.039) [DOI] [PubMed] [Google Scholar]
- 4.Barreau F, Cartier C, Ferrier L, et al. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. 2004;127(2):524–534. doi: 10.1053/j.gastro.2004.05.019. (Available from: http://dx.doi.org/10.1053/j.gastro.2004.05.019) [DOI] [PubMed] [Google Scholar]
- 5.Barreau F, Cartier C, Leveque M, et al. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol. 2007;580(1):347–356. doi: 10.1113/jphysiol.2006.120907. (Available from: http://dx.doi.org/10.1113/jphysiol.2006.120907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreau F, Salvador-Cartier C, Houdeau E, et al. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut. 2008;57(5):582–590. doi: 10.1136/gut.2007.126680. (Available from: http://dx.doi.org/10.1136/gut.2007.126680) [DOI] [PubMed] [Google Scholar]
- 7.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14(1):189. doi: 10.1186/s12876-014-0189-7. (Available from: http://dx.doi.org/10.1186/s12876-014-0189-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551(2):433–446. doi: 10.1113/jphysiol.2003.039990. (Available from: http://dx.doi.org/10.1113/jphysiol.2003.039990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bothwell M. NGF, BDNF, NT3, and NT4. In: Lewin GR, Carter BD, editors. Neurotrophic Factors. Berlin Heidelberg: Springer; 2014. pp. 3–15. (Available from: http://dx.doi.org/10.1007/978-3-642-45106-5_1) [Google Scholar]
- 10.Bramson C, Herrmann DN, Carey W, et al. Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain. Pain Med. 2015;16(6):1163–1176. doi: 10.1111/pme.12677. (Available from: http://dx.doi.org/10.1111/pme.12677) [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367(17):1626–1635. doi: 10.1056/NEJMra1207068. (Available from: http://dx.doi.org/10.1056/NEJMra1207068) [DOI] [PubMed] [Google Scholar]
- 12.Cenac N, Andrews CN, Holzhausen M, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117(3):636–647. doi: 10.1172/JCI29255. (Available from: http://dx.doi.org/10.1172/JCI29255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang L, Sundaresh S, Elliott J, et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21(2):149–159. doi: 10.1111/j.1365-2982.2008.01171.x. (Available from: http://dx.doi.org/10.1111/j.1365-2982.2008.01171.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choung RK, Locke GR. Epidemiology of IBS. Gastroenterol Clin N Am. 2011;40(1):1–10. doi: 10.1016/j.gtc.2010.12.006. (Available from: http://dx.doi.org/10.1016/j.gtc.2010.12.006) [DOI] [PubMed] [Google Scholar]
- 15.Coëffier M, Gloro R, Boukhettala N, et al. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am J Gastroenterol. 2010;105(5):1181–1188. doi: 10.1038/ajg.2009.700. (Available from: http://dx.doi.org/10.1038/ajg.2009.700) [DOI] [PubMed] [Google Scholar]
- 16.Coelho A, Wolf-Johnston AS, Shinde S, et al. Urinary bladder inflammation induces changes in urothelial nerve growth factor and vanilloid receptor 1. Br J Pharmacol. 2015;172(7):1691–1699. doi: 10.1111/bph.12958. (Available from: http://dx.doi.org/10.1111/bph.12958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delafoy L, Raymond F, Doherty AM, et al. Role of nerve growth factor in the trinitrobenzene sulfonic acid-induced colonic hypersensitivity. Pain. 2003;105(3):489–497. doi: 10.1016/S0304-3959(03)00266-5. (Available from: http://dx.doi.org/10.1016/S0304-3959(03)00266-5) [DOI] [PubMed] [Google Scholar]
- 18.Delafoy L, Gelot A, Ardid D, et al. Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut. 2006;55(7):940–945. doi: 10.1136/gut.2005.064063. (Available from: http://dx.doi.org/10.1136/gut.2005.064063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.di Mola FF, Friess H, Zhu ZW, et al. Nerve growth factor and Trk high affinity receptor (TrkA) gene expression in inflammatory bowel disease. Gut. 2000;46(5):670–678. doi: 10.1136/gut.46.5.670. (Available from: http://dx.doi.org/10.1136/gut.46.5.670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dothel G, Barbaro MR, Boudin H, et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148(5):1002–1011. doi: 10.1053/j.gastro.2015.01.042. (Available from: http://dx.doi.org/10.1053/j.gastro.2015.01.042) [DOI] [PubMed] [Google Scholar]
- 21.Evans RJ, Moldwin RM, Cossons N, et al. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol. 2011;185(5):1716–1721. doi: 10.1016/j.juro.2010.12.088. (Available from: http://dx.doi.org/10.1016/j.juro.2010.12.088) [DOI] [PubMed] [Google Scholar]
- 22.Ferrier L, Mazelin L, Cenac N, et al. Stress-induced disruption of colonic epithelial barrier: role of interferon-γ and myosin light chain kinase in mice. Gastroenterology. 2003;125(3):795–804. doi: 10.1016/s0016-5085(03)01057-6. (Available from: http://dx.doi.org/10.1016/S0016-5085(03)01057-6) [DOI] [PubMed] [Google Scholar]
- 23.Gimbel JS, Kivitz AJ, Bramson C, et al. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain. 2014;155(9):1793–1801. doi: 10.1016/j.pain.2014.06.004. (Available from: http://dx.doi.org/10.1016/j.pain.2014.06.004) [DOI] [PubMed] [Google Scholar]
- 24.Hao JX, Duan LP. Progress in the relationship of intestinal mucosal barrier function and irritable bowel syndrome. Chin J Dig. 2010;30(11):861-863(in Chinese):861–863 (in Chinese). (Available from: http://dx.doi.org/10.3760/cma.j.issn.0254-1432.2010.11.026) [Google Scholar]
- 25.Keita AV, Söderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil. 2010;22(7):718–733. doi: 10.1111/j.1365-2982.2010.01498.x. (Available from: http://dx.doi.org/10.1111/j.1365-2982.2010.01498.x) [DOI] [PubMed] [Google Scholar]
- 26.Kennedy PJ, Cryan JF, Quigley EM, et al. A sustained hypothalamic-pituitary-adrenal axis response to acute psychosocial stress in irritable bowel syndrome. Psychol Med. 2014;44(14):3123–3134. doi: 10.1017/S003329171400052X. (Available from: http://dx.doi.org/10.1017/S003329171400052X) [DOI] [PubMed] [Google Scholar]
- 27.Lee H, Park JH, Park DI, et al. Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. J Neurogastroenterol Motil. 2013;19(2):244–250. doi: 10.5056/jnm.2013.19.2.244. (Available from: http://dx.doi.org/10.5056/jnm.2013.19.2.244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leon A, Buriani A, Dal Toso R, et al. Mast cells synthesize, store, and release nerve growth factor. PNAS. 1994;91(9):3739–3743. doi: 10.1073/pnas.91.9.3739. (Available from: http://dx.doi.org/10.1073/pnas.91.9.3739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19(11):514–520. doi: 10.1016/S0166-2236(96)10058-8. (Available from: http://dx.doi.org/10.1016/S0166-2236(96)10058-8) [DOI] [PubMed] [Google Scholar]
- 30.Lewin GR, Lechner SG, Smith ES. Nerve growth factor and nociception: from experimental embryology to new analgesic therapy. In: Lewin GR, Carter BD, editors. Neurotrophic Factors. Springer Berlin Heidelberg; 2014. pp. 251–282. (Available from: http://dx.doi.org/10.1007/978-3-642-45106-5_10) [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Xu CP, Yang M, et al. Expression and significance of nerve growth factor in ulcerative colitis. Chin J Gastroenterol. 2014;19(10):593-595(in Chinese):593–595 (in Chinese). (Available from: http://dx.doi.org/10.3969/j.issn.1008-7125.2014.10.004) [Google Scholar]
- 32.Liu L, Liu BN, Chen S, et al. Visceral and somatic hypersensitivity, autonomic cardiovascular dysfunction and low-grade inflammation in a subset of irritable bowel syndrome patients. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2014;15(10):907–914. doi: 10.1631/jzus.B1400143. (Available from: http://dx.doi.org/10.1631/jzus.B1400143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez C, Lobo B, Pigrau M, et al. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62(8):1160–1168. doi: 10.1136/gutjnl-2012-302093. (Available from: http://dx.doi.org/10.1136/gutjnl-2012-302093) [DOI] [PubMed] [Google Scholar]
- 34.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47(6):861–869. doi: 10.1136/gut.47.6.861. (Available from: http://dx.doi.org/10.1136/gut.47.6.861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mujagic Z, Ludidi S, Keszthelyi D, et al. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment Pharmacol Ther. 2014;40(3):288–397. doi: 10.1111/apt.12829. (Available from: http://dx.doi.org/10.1111/apt.12829) [DOI] [PubMed] [Google Scholar]
- 36.Neunlist M, Toumi F, Oreschkova T, et al. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol. 2003;285(5):G1028–G1036. doi: 10.1152/ajpgi.00066.2003. (Available from: http://dx.doi.org/10.1152/ajpgi.00066.2003) [DOI] [PubMed] [Google Scholar]
- 37.Nickel JC, Atkinson G, Krieger JN, et al. Preliminary assessment of safety and efficacy in proof-of-concept, randomized clinical trial of tanezumab for chronic prostatitis/chronic pelvic pain syndrome. Urology. 2012;80(5):1105–1110. doi: 10.1016/j.urology.2012.07.035. (Available from: http://dx.doi.org/10.1016/j.urology.2012.07.035) [DOI] [PubMed] [Google Scholar]
- 38.Ohashi K, Sato Y, Kawai M, et al. Abolishment of TNBS-induced visceral hypersensitivity in mast cell deficient rats. Life Sci. 2008;82(7-8):419–423. doi: 10.1016/j.lfs.2007.11.027. (Available from: http://dx.doi.org/10.1016/j.lfs.2007.11.027) [DOI] [PubMed] [Google Scholar]
- 39.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS ONE. 2012;7(6):e39935. doi: 10.1371/journal.pone.0039935. (Available from: http://dx.doi.org/10.1371/journal.pone.0039935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park CH, Joo YE, Choi SK. Activated mast cell infiltrate in close proximity to enteric nerves in diarrhea-predominant irritable bowel syndrome. J Korean Med Sci. 2003;18(2):204–210. doi: 10.3346/jkms.2003.18.2.204. (Available from: http://dx.doi.org/10.3346/jkms.2003.18.2.204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58(2):196–201. doi: 10.1136/gut.2007.140806. (Available from: http://dx.doi.org/10.1136/gut.2007.140806) [DOI] [PubMed] [Google Scholar]
- 42.Rukwied R, Mayer A, Kluschina O, et al. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity inhuman skin. Pain. 2010;148(3):407–413. doi: 10.1016/j.pain.2009.11.022. (Available from: http://dx.doi.org/10.1016/j.pain.2009.11.022) [DOI] [PubMed] [Google Scholar]
- 43.Sanga P, Katz N, Polverejan E, et al. Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain. 2013;154(10):1910–1919. doi: 10.1016/j.pain.2013.05.051. (Available from: http://dx.doi.org/10.1016/j.pain.2013.05.051) [DOI] [PubMed] [Google Scholar]
- 44.Shen F, Li DG, Zhou HQ. An epidemiological investigation of irritable bowel syndrome in Shanghai Songjiang communities. Chin J Dig. 2011;31(10):663-668(in Chinese):663–668 (in Chinese). (Available from: http://dx.doi.org/10.3760/cma.j.issn.0254-) [Google Scholar]
- 45.Shulman RJ, Jarrett ME, Cain KC, et al. Associations among gut permeability, inflammatory markers, and symptoms in patients with irritable bowel syndrome. J Gastroenterol. 2014;49(11):1467–1476. doi: 10.1007/s00535-013-0919-6. (Available from: http://dx.doi.org/10.1007/s00535-013-0919-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skaper SD, Pollock M, Facci L. Mast cells differentially express and release active high molecular weight neurotrophins. Mol Brain Res. 2001;97(2):177–185. doi: 10.1016/s0169-328x(01)00314-x. (Available from: http://dx.doi.org/10.1016/S0169-328X(01)00314-X) [DOI] [PubMed] [Google Scholar]
- 47.Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20(4):629–632. doi: 10.1016/s0896-6273(00)81003-x. (Available from: http://dx.doi.org/10.1016/S0896-6273(00)81003-X) [DOI] [PubMed] [Google Scholar]
- 48.Stein AT, Ufret-Vincenty CA, Hua L, et al. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128(5):509–522. doi: 10.1085/jgp.200609576. (Available from: http://dx.doi.org/10.1085/jgp.200609576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiseo PJ, Kivitz AJ, Ervin JE, et al. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double-blind, placebo-controlled exploratory study in osteoarthritis of the knee. Pain. 2014;155(7):1245–1252. doi: 10.1016/j.pain.2014.03.018. (Available from: http://dx.doi.org/10.1016/j.pain.2014.03.018) [DOI] [PubMed] [Google Scholar]
- 50.van den Wijngaard RM, Klooker TK, Welting O, et al. Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterol Motil. 2009;21(10):1107–e94. doi: 10.1111/j.1365-2982.2009.01339.x. (Available from: http://dx.doi.org/10.1111/j.1365-2982.2009.01339.x) [DOI] [PubMed] [Google Scholar]
- 51.Wang WL, Wu WC. Nerve growth factor and irritable bowel syndrome. Chin J Gastroenterol Hepatol. 2010;19(3):285-287(in Chinese):285–287 (in Chinese). (Available from: http://dx.doi.org/10.3969/j.issn.1006-5709.2010.03.029) [Google Scholar]
- 52.Willot S, Gauthier C, Patey N, et al. Nerve growth factor content is increased in the rectal mucosa of children with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24(8):734–e347. doi: 10.1111/j.1365-2982.2012.01933.x. (Available from: http://dx.doi.org/10.1111/j.1365-2982.2012.01933.x) [DOI] [PubMed] [Google Scholar]
- 53.Yao X, Yang YS, Zhao KB, et al. Clinical features and subtypes of irritable bowel syndrome based on Rome III diagnostic criteria. World Chin J Digestol. 2008;16(5): 563-566(in Chinese):563–566 (in Chinese). (Available from: http://dx.doi.org/10.3969/j.issn.1009-3079.2008.05.023) [Google Scholar]
- 54.Yu Y, Gao J, Zhang W, et al. Effect of nerve growth factor on colorectal visceral hypersensitivity in rats. Chin J Dig. 2008;28(11):764-766(in Chinese):764–766 (in Chinese). (Available from: http://dx.doi.org/10.3760/j.issn:0254-1432.2008.11.011) [Google Scholar]
- 55.Zhang L, Duan LP, Liu YX, et al. A meta-analysis of the prevalence and risk factors of irritable bowel syndrome in Chinese community. Chin J Intern Med. 2014;53(12):969-975(in Chinese):969–975 (in Chinese). (Available from: http://dx.doi.org/10.3760/cma.j.issn.0578-1426.2014.12.011) [PubMed] [Google Scholar]
- 56.Zhou QQ, Zhang BY, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146(1-2):41–46. doi: 10.1016/j.pain.2009.06.017. (Available from: http://dx.doi.org/10.1016/j.pain.2009.06.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou XP, Li XL. Advances in studies on visceral hypersensitivity of irritable bowel syndrome. Chin J Gastroenterol. 2014;19(2):117-119(in Chinese):117–119 (in Chinese). (Available from: http://dx.doi.org/10.3969/j.issn.1008-7125.2014.02.011) [Google Scholar]
- 58.Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci. 2007;34(4):689–700. doi: 10.1016/j.mcn.2007.01.005. (Available from: http://dx.doi.org/10.1016/j.mcn.2007.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhuang ZY, Xu H, Clapham DE, et al. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24(38):8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. (Available from: http://dx.doi.org/10.1523/JNEUROSCI.2893-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]