Abstract
Objective: To investigate male reproductive parameters via changes of potential testicular protein markers in restraint-stress rats. Methods: Male Sprague-Dawley rats were divided into two groups (non-immobilized control and restraint-immobilized/stress groups, n=8 each group). The stress animals were immobilized (12 h/d) by a restraint cage for 7 consecutive days. All reproductive parameters, morphology and histology were observed and compared between groups. In addition, the expression of steroidogenic acute regulatory (StAR) and phosphotyrosine proteins (previously localized in Sertoli and late spermatid cells) in testicular lysate was assayed by immuno-Western blotting. Results: Testosterone level, sperm concentration and sperm head normality of stress rats were significantly decreased while the corticosterone level was increased as compared with the control (P<0.05). Histologically, stress rats showed low sperm mass in epididymal lumen and some atrophy of seminiferous tubules. Although the expression of testicular StAR protein was not significantly different between groups, changed patterns of the 131, 95, and 75 kDa testicular phosphorylated proteins were observed in the stress group compared with the control group. The intensity of a testicular 95-kDa phosphorylated protein was significantly decreased in stress rats. Conclusions: This study has demonstrated the alteration of testicular phosphorylated protein patterns, associated with adverse male reproductive parameters in stress rats. It could be an explanation of some infertility in stress males.
Keywords: Restraint-stress rats, Steroidogenic acute regulatory (StAR) protein, Testicular phosphorylated protein
1. Introduction
Stress has come to the fore as a major factor adversely affecting the quality of human life. It affects various physiological processes including reproductive functions. Numerous studies in human and experimental animals have shown that stress causes adverse effects in the male reproductive system: (1) erectile dysfunction (Nathan 1986; Ernst et al. 1993; Kennedy et al. 1999) (2) decrease of sperm quality (Almeida et al. 1998; Clarke et al. 1999; Hari Priya and Sreenivasula Reddy 2012; Hari Priya et al. 2014; Rao et al. 2015; Zhang et al. 2015) (3) decrease of testosterone levels (Orr and Mann 1990; Retana-Márquez et al. 2003; Weissman et al. 2009; Lin et al. 2014; Prabsattroo et al. 2015) and (4) damage to testicular tissue (Rai et al. 2003; 2004; Aziz et al. 2013; Prabsattroo et al. 2015). Indeed the corticosterone levels are markedly elevated under a restraint immobilization condition (Bhatia et al. 2011; Prabsattroo et al. 2015). Corroborated with the decrease of testosterone levels Lin et al. (2014) demonstrated that stress could decrease the expression of steroidogenic acute regulatory (StAR) protein and the cytochrome P450 side chain cleavage enzyme (CYP11A1) in rat Leydig cells after acute immobilization stress induction.
Protein tyrosine phosphorylation is a post-transcriptional process that is important for the regulation and coordination of various cell proliferation division growth and differentiation function in both normal and cancer cells (Hunter and Cooper 1985; Hunter 1987; Hanks et al. 1988; Ullrich and Schlessinger 1990). In testicular tissue the phosphorylated proteins have been localized in the Sertoli cells and late (elongated) spermatids (except in the Leydig cells) and these proteins are assumed to have the roles in spermatogenesis (Arad-Dann et al. 1993). In addition sperm capacitation and acrosome reaction in the fertilization steps require protein tyrosine phosphorylation (Kopf and Gerton 1991; Yanagimachi 1994; Visconti and Kopf 1998). Although it has been shown that some drugs or substances can change the expression of testicular phosphorylated proteins (Ballester et al. 2004; Iamsaard et al. 2013; 2014) these changes in stress events have never been reported. This study therefore attempted to demonstrate the alterations of testicular phosphorylation in stress rats.
2. Materials and methods
2.1. Animals and stress procedure
Male Sprague-Dawley rats (200–250 g) were purchased from the National Laboratory Animal Center Salaya Nakhon Pathom Thailand. The rats were administered commercially available pellet and water ad libitum in plastic cages under controlled environmental conditions (temperature (22±2) °C; 12 h light/dark cycles). This study was approved by the Animal Ethics Committee of Khon Kaen University based on the Ethics of Animal Experimentation of National Research Council of Thailand (Ref. No. AEKKU-NLAC 11/2558). After a week of acclimatization animals were randomly divided into two groups (n=8). Group 1 (control group) was not immobilized by a restraint cage and Group 2 (restraint-stress group) was immobilized by a restraint cage (12 h/d to induce acute stress; as described by Ahmad et al. (2012) Retana-Márquez et al. (2003) and Prabsattroo et al. (2015)) followed by weighing the body for 7 consecutive days.
2.2. Plasma corticosterone and testosterone assays
After animals were euthanized the blood was collected by cardiac puncture of the left ventricle and centrifuged at 13 000 r/min at 4 °C for 7 min by Microfuge 22R (Biocompare Inc. USA) to separate the plasma serum from the blood cells. Subsequently all blood sera were sent to the Radiology Unit Srinagarind Hospital >Faculty of Medicine Khon Kaen University Thailand for measurement of serum corticosterone and testosterone levels.
2.3. Morphological and histological studies
At the end of the experiment, all rats were euthanized by cervical dislocation and sacrificed to collect male reproductive organs (testis, penis, epididymis plus vas deferens, and seminal vesicle). Subsequently, these organs were weighed after the fats were removed. The reproductive organ weights were calculated and expressed as the relative reproductive organ weights (g/100 g). Then, all organs were observed for their gross structures and their images captured by digital camera (Nikon Coolpix S2600, Japan). To examine their histology, the testis, caudal epididymis, and penis were fixed in 10% phosphate buffered formalin (pH 7.4) for 24 h and routinely processed for light microscope technique. All the sections of testis, caudal epididymis, and penis were stained with hematoxylin and eosin (H&E), whereas the penis sections were also stained with Masson’s trichrome (Sigma-Aldrich Inc., USA) to investigate collagen fibers. Finally, histological photographs were taken by a Nikon light ECLIPSE E200 microscope (Nikon Inc., Japan) equipped with a DXM1200 digital camera (Nikon Inc., Japan) and an ImageJ program (Version 1.49p) was used to quantify the collagen fiber.
2.4. Sperm concentration and head morphology assessment
The left caudal epididymis and vas deferens were operated on gently and squeezed for sperm fluid. Epididymal sperm fluid was dipped and suspended in 1 ml of phosphate buffer saline (PBS; 37 °C, pH 7.4). Subsequently, the diluted sperm were centrifuged at 5000 r/min at 25 °C for 2 min to wash and separate mature sperm pellets from the fluid. The sperm pellets were re-suspended with 1 ml PBS (37 °C, pH 7.4). Then the sperm suspension was diluted with PBS (1:20 (v/v) dilution) before a count was made of the mature sperm using a Neubauer counting chamber under a light microscope (Nikon ECLIPSE E200, Japan) in triplicate examinations (Iamsaard et al., 2013). To examine sperm head abnormalities, described by Wyrobek and Bruce (1975) and Sakr et al. (2014), the diluted sperm (10 μl) were smeared onto a cleaned glass slide. The smears were air-dried and incubated in a hot air oven at 50 °C overnight. The dried sperm were fixed in methyl alcohol and stained by H&E. Abnormal sperm heads were classified and explained by Sakr et al. (2014). All sperm heads were captured by a Nikon light ECLIPSE E200 microscope equipped with a DXM1200 digital camera. To quantify sperm head abnormalities, a total of 600 sperms were counted in each animal. Then the abnormalities were calculated and represented as a percentage of sperm head abnormality.
2.5. Western blot analysis of StAR and phosphotyrosine protein expression
Testicular tissue was homogenized with radioimmunoprecipitation assay (RIPA) buffer (Cell Signaling Technology Inc., USA) containing a cocktail of protease inhibitors (Sigma Inc., USA) to extract and maintain the proteins. Then the testicular homogenate was centrifuged at 12 000 r/min at 4 °C for 10 min to separate testicular lysate from pellet. The total protein concentrations of the testicular lysate were measured using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies Inc., USA) at an absorbance of 280 nm. To determine testicular StAR protein expression, the total testicular proteins (80 μg) of both groups were loaded and separated on 10% polyacrylamide gel by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Separated proteins were transferred onto nitrocellulose membrane. Subsequently the membrane was incubated with 5% skim milk in 0.1% PBST (0.1% Tween-20, 0.01 mol/L PBS, pH 7.4) for 1 h to block non-specific binding proteins and then incubated with StAR antibody (1:1000 (v/v) dilution; Santa Cruz Biotechnology Inc., USA) or β-actin antibody (1:2000 (v/v) dilution; Santa Cruz Biotechnology Inc., USA) at 4 °C overnight. The membrane was washed in 0.05% PBST (0.05% Tween-20, 0.01 mol/L PBS, pH 7.4) for 5 min (three times) and incubated with goat anti-rabbit IgG or goat anti-mouse conjugated horseradish peroxidase (HRP) secondary antibody for 1 h at room temperature. For the analysis of testicular phosphotyrosine protein expression as described by Iamsaard et al. (2003; 2004), the transferred-protein membrane was incubated with anti-phosphotyrosine primary antibody (1:2000 (v/v); Millipore Co., USA) at 4 °C overnight followed by washing and then incubated with anti-mouse conjugated with HRP secondary antibody for 2 h at room temperature. Then, all membranes were washed in 0.05% PBST before detection of StAR or β-actin using enhanced chemiluminescence (ECL) substrate under gel doct 4 (ImageQuant 400, GH Healthcare, USA). For protein expression, the ImageJ program (Version 1.49p) was used to analyze the relative intensity of target proteins.
2.6. Statistical analysis
All quantitative data were represented as mean±standard deviation (SD). The independent sample t-test was performed to examine the significant difference between two groups using SPSS statistics 19.0 software. A P-value of <0.05 was considered as a significant difference.
3. Results
3.1. Effect of stress on rat body weights and male reproductive organs
Daily changes of the body weight in the control and restraint-stress groups are shown in Fig. 1a. This result demonstrated that the body weights of stress group were significantly decreased (P<0.05) in consecutive 7 d as compared with the control group. Effect of stress on morphology of male reproductive organs was also investigated. The results showed that the testis of restraint-stress group was smaller than that of the control group (Fig. 1b). The testicular size (width×length) was (1.31±0.02) cm×(2.26±0.02) cm for the control and (1.25±0.01) cm×(2.08±0.01) cm for the stress group, respectively. However, morphologies of the penis, epididymis plus vas deferens, and seminal vesicles in both groups were not significantly different (Fig. 1b).
Fig. 1.
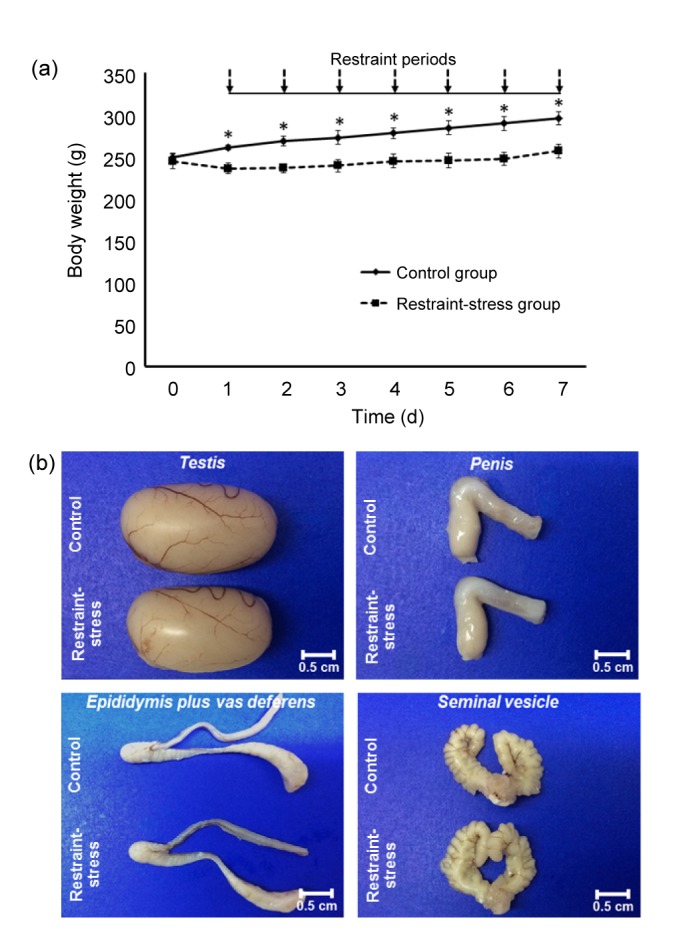
Body weights (a) and comparative morphologies of testis, penis, epididymis plus vas deferens, and seminal vesicle (b) between the control and restraint-stress groups
Each data point in (a) is represented as mean±SD (n=8 rats each group). * P<0.05, vs. restraint-stress group
3.2. Effect of stress on sperm head morphology
The sperm head morphologies of the control and restraint-stress groups are shown in Fig. 2. Normal sperm heads were mostly observed in the control group (Fig. 2a). In contrast, abnormal sperm heads (without the hook (Fig. 2b), pinhead (Fig. 2c), and crooked neck (Fig. 2d)) were increased in the restraint-stress group.
Fig. 2.
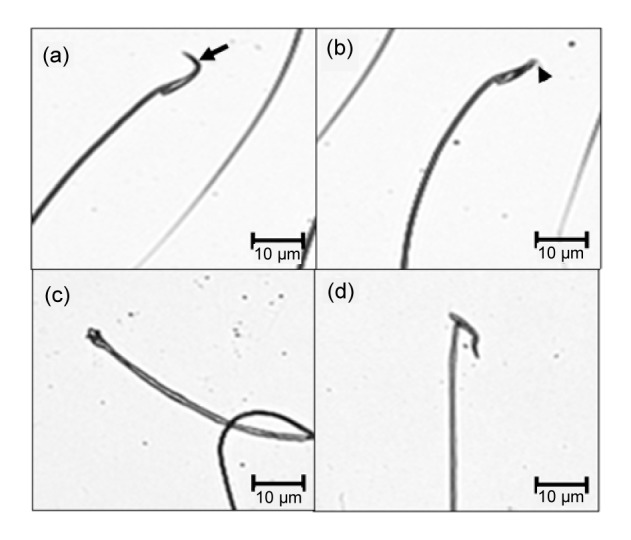
Photographs showing sperm head morphologies observed in the control and restraint-stress groups
Normal sperm head with normal hook (arrow) (a); abnormal sperm heads without hook (arrow head) (b), pinhead (c), and crooked neck (d)
3.3. Effect of stress on the weights of reproductive organs, sperm concentration, sperm head abnormality, and corticosterone hormone
The absolute and relative weights of the testis, epididymis plus vas deferens, and seminal vesicle in the restraint-stress and control groups were shown in Table 1. Significantly, the absolute and relative weights of testes were reduced (P<0.05) in the restraint-stress group. However, the rest parameters were not significantly different between both groups. In contrast, the restraint stress significantly decreased (P<0.05) sperm concentration and testosterone level while it significantly increased (P<0.05) the percentage of sperm head abnormality and corticosterone level compared with the control group (Table 1).
Table 1.
Effect of stress on the weights of male reproductive organs, sperm concentration, sperm head abnormality, and corticosterone levels in rats
| Group | Absolute weight (g) |
Relative weight (g/100 g) |
c s (106 cells/ml) | Sperm head abnormality (%) | c c (ng/ml) | c t (ng/ml) | ||||
| Testis | Epididymis plus vas deferens | Seminal vesicle | Testis | Epididymis plus vas deferens | Seminal vesicle | |||||
| Control | 1.60±0.09 | 0.40±0.01 | 0.64±0.04 | 0.54±0.04 | 0.14±0.01 | 0.22±0.01 | 167.00±2.53 | 1.38±0.86 | 389.00±23.00 | 1.08±0.46 |
| RS | 1.26±0.15* | 0.38±0.01 | 0.63±0.07 | 0.49±0.06* | 0.15±0.00 | 0.25±0.03 | 69.50±8.98* | 2.75±0.66* | 506.20±57.47* | 0.47±0.26* |
RS: restraint-stress; c s: sperm concentration; c c: corticosterone level; c t: testosterone level.
Significant differences (P<0.05), compared with the control group. Data are represented as mean±SD (n=8 rats each group)
3.4. Effect of stress on histology of testis, caudal epididymis, and penis
The histology of male reproductive organs is shown in Fig. 3. The result showed that the stress moderately damaged seminiferous tubules by increasing tubular atrophy and interstitial space compared with the control group (Figs. 3a and 3b). In line with sperm concentration (Table 1), the density of caudal sperm in the restraint-stress group was markedly lower than that in the control group (Figs. 3c and 3d). However, the histology of penis and penile collagen fibers stained by H&E and Masson’s trichrome was not different between the control and restraint groups (Figs. 3e–3h).
Fig. 3.
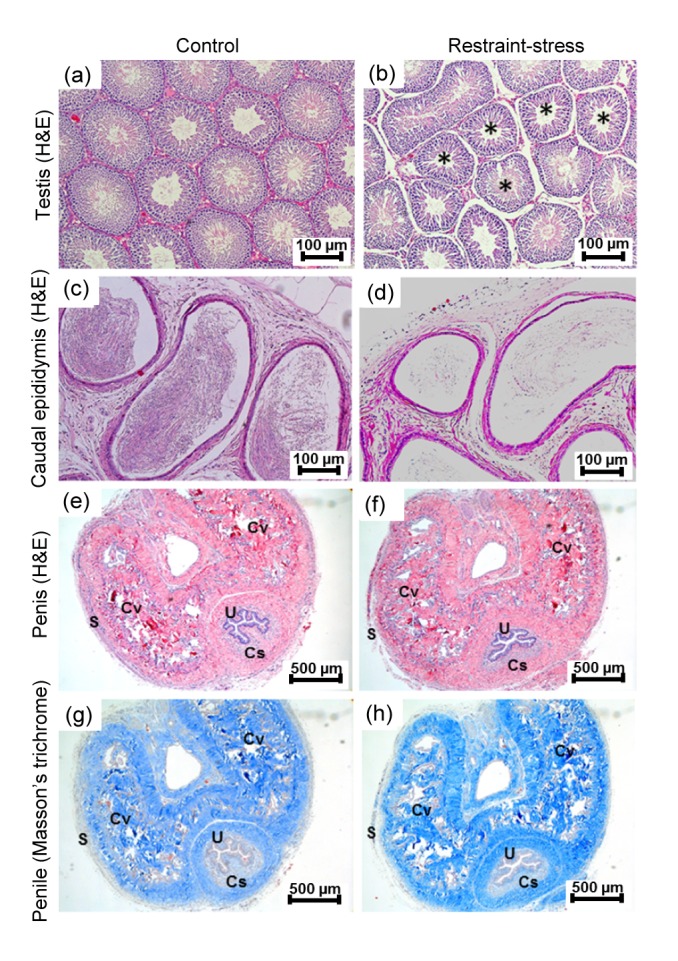
Photomicrographs showing histologies of testis (a, b), caudal epididymis (c, d), and penis (e, f) stained by H&E and penile section stained by Masson’s trichrome (g, h) of the control and restraint-stress groups, respectively
Asterisks: atrophic seminiferous tubules; S: sheath of tunica albugenia; Cv: corpora cavernosa; Cs: corpus spinosum; U: urethra
3.5. Effect of stress on testicular StAR protein
StAR protein expression was shown by immuno-Western blotting (Fig. 4a). The results showed that restraint stress did not affect testicularStAR protein expression compared with the control (Fig. 4a). As confirmed by the relative intensity of StAR protein and the ratio of StAR/β-actin (Figs. 4b and 4c), the results showed that StAR protein levels were not significantly different in both groups.
Fig. 4.
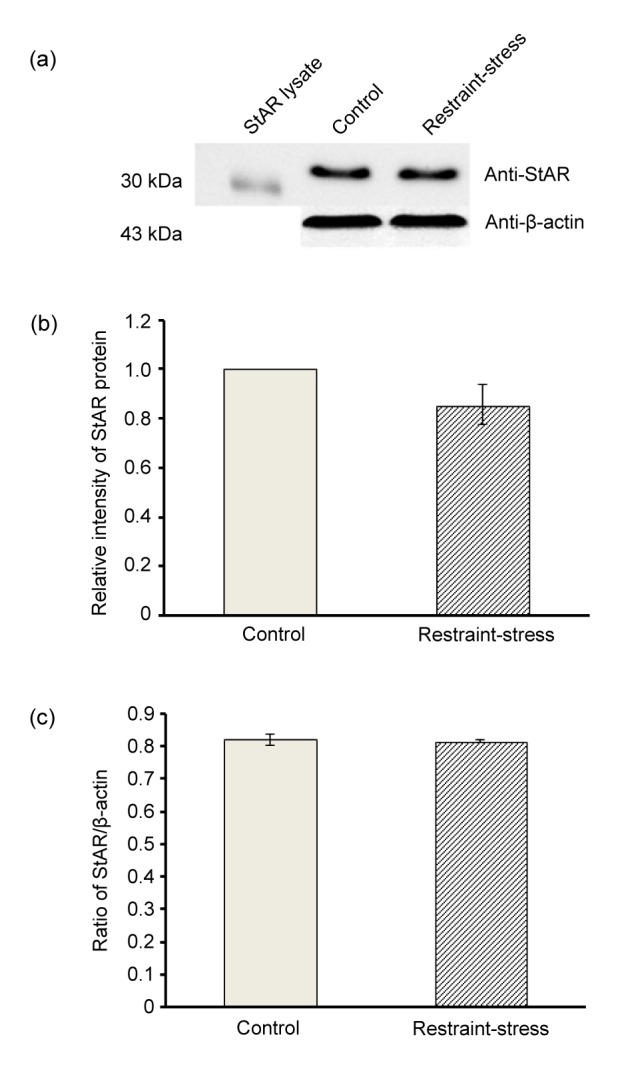
Representative Western-blot analysis of StAR protein expression in testicular lysates (a), relative intensity of testicular StAR protein (b), and ratio of StAR/β-actin (c) in the control and restraint-stress groups
StAR lysate was used as a positive control. Data are represented as mean±SD (n=4 rats each group)
3.6. Effect of stress on testicular tyrosine protein phosphorylation
The effect of stress on testicular phosphotyrosine expression is shown in Fig. 5a. The result showed that six testicular phosphorylated protein bands (48, 65, 75, 95, 131, and 200 kDa) were clearly detected in both control and restraint-stress groups (Fig. 5a). Intriguingly, we found the decreased expression of a phosphorylated 95-kDa protein in the restraint-stress group with normal protein profiles by SDS-PAGE compared with control (Fig. 5b). This result was confirmed by its relative intensity (Fig. 5c).
Fig. 5.
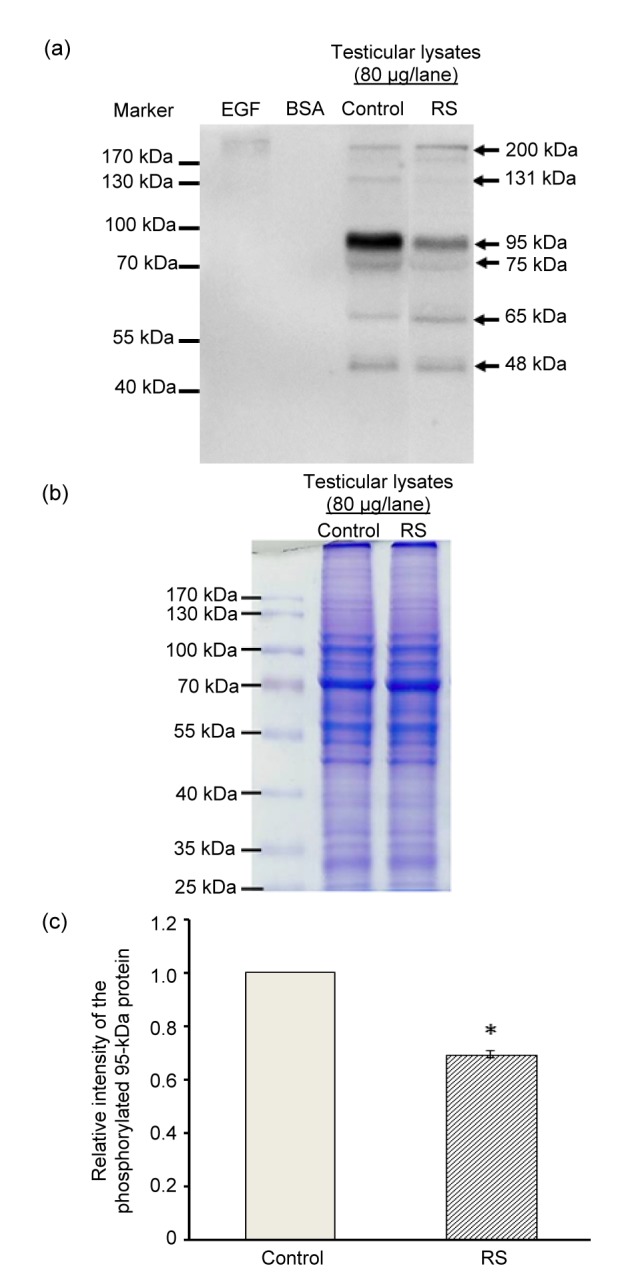
Representative Western-blot analysis of tyrosine protein phosphorylation in testicular lysates (a), protein profiles (SDS-PAGE) (b), and relative intensity of the testicular phosphorylated 95-kDa protein (c) in the control and restraint-stress (RS) groups
Bovine serum albumin (BSA) and epidermal growth factor (EGF)-like growth factors were used as negative and positive controls, respectively. Data were represented as mean±SD (n=4 rats each group). * P<0.05, compared with the control group
4. Discussion
Stress is a major factor in male infertility. It has been reported as inducing erectile dysfunction, premature ejaculation, orgasmic difficulty, and low sperm quality (Nathan, 1986; Ernst et al., 1993; Clarke et al., 1999; Kennedy et al., 1999; Rao et al., 2015; Zhang et al., 2015). In the recent study, all parameters of adverse male reproductive system including decreased body weight in rats exposed to restraint stress (Fig. 1a and Table 1) were similar to those parameters previously documented (Orr and Mann, 1990; Carrasco and van de Kar, 2003; Zardooz et al., 2006; Weissman et al., 2009; Hari Priya and Sreenivasula Reddy, 2012; Lin et al., 2014; Prabsattroo et al., 2015). In explanation of weight loss (Table 1), it is known that testosterone activates the protein synthetic apparatus muscles and other organs while corticosterone increases protein catabolism and decreases protein synthesis. In addition, many previous studies showed that increased corticosterone mediated restraint-induced weight loss via increasing glycolysis and lipolysis (Arner, 1992; Lafontan et al., 1997; Chotiwat and Harris, 2008; Scherer et al., 2011). Consistent with previous studies (Hari Priya and Sreenivasula Reddy, 2012; Bitgul et al., 2013), this study also demonstrated atrophy of seminiferous tubules in stress testes (Fig. 3b). In addition, the low density of epididymal sperm mass was also shown in stress rats (Fig. 3d), and it was associated with a significant decrease of sperm concentration (Table 1). Interestingly, we found that sperm head abnormality of stress rats was significantly greater than that of control (Table 1 and Fig. 2). Although restraint stress can suppress the activities of monoamine oxidase type B and phosphodiesterase type 5 in the rat penis (Prabsattroo et al., 2015), it affects neither penile morphology nor the amount of collagen fibers (Figs. 1b and 3e–3h). This result indicated that stress interrupted penile function but not its morphology.
This study attempted to explain the decrease of testosterone levels in stress rats by observing StAR protein expression using Western bolt (Fig. 4). Unexpectedly, the expression of StAR levels in both groups was not significantly different (Figs. 4a–4c). It is possible that StAR expression is very sensitive to acute immobilization for a day (Lin et al., 2014). In the subacute stress for consecutive 7 d (present study), this expression might be already corrected to be at normal levels (Fig. 4). To additionally clarify the decreased testosterone levels, the disturbances of other steroidogenic machineries such as cytochrome P-450 cholesterol side-chain cleavage enzymes, scavenger receptor class B, and hydroxysteroid dehydrogenases need to be further investigated. In various studies, the increase of malondialdehyde levels and the decrease of enzymatic activities of catalase, glutathione, and superoxide dismutase have been demonstrated in stress testicular tissue (Zayachkivska et al., 2006; Brzozowski et al., 2008; Bhatia et al., 2011; Warzecha et al., 2011; Kwiecien et al., 2012; Hari Priya and Sreenivasula Reddy, 2012; Xu et al., 2014). The alteration of oxidative stress markers might affect normal spermatogenesis, resulting in decreased sperm concentration (Table 1 and Fig. 3d). When probed with an anti-phosphotyrosine monoclonal antibody, the phosphorylation of testicular proteins has been localized in only Sertoli and elongated spermatid cells (Arad-Dann et al., 1993). It indicates that testicular tyrosine-phosphorylated proteins play some roles in spermatogenesis since this post-translational process is involved in proliferation and differentiation (Hunter and Cooper, 1985; Hunter, 1987; Hanks et al., 1988; Ullrich and Schlessinger, 1990). Moreover, it is well documented that sperm phosphorylated proteins are essential for capacitation and acrosome reaction (Kopf and Gerton, 1991; Yanagimachi, 1994; Visconti and Kopf, 1998). The patterns of testicular phosphorylated proteins have also been previously shown in hyperglycemic rats (Ballester et al., 2004) and they are changed when treated with some drugs or substances, resulting in alterations of sperm concentration (Iamsaard et al., 2013; 2014). In the same vein, we have demonstrated for the first time that restraint stress could change the pattern of testicular phosphorylated protein of stress rats compared with the control healthy rats (Fig. 5). The relative intensity of a phosphorylated 95-kDa protein band in stress testicular lysate was significantly decreased (Figs. 5a–5c), which was associated with the decrease of sperm concentration and sperm head morphology (Table 1 and Fig. 2). Taken together, we speculate that this phosphorylated protein pattern might play an important role in spermatogenesis, especially in the differentiation process of spermatogenesis.
Footnotes
Project supported by the Postgraduate Study Support Grant and Invitation Research Grant (IN59134), Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
Compliance with ethics guidelines: Supatcharee ARUN, Jaturon BURAWAT, Wannisa SUKHORUM, Apichakan SAMPANNANG, Nongnut UABUNDIT, and Sitthichai IAMSAARD declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Ahmad A, Rasheed N, Gupta P, et al. Novel Ocimumoside A and B as anti-stress agents: modulation of brain monoamines and antioxidant systems in chronic unpredictable stress model in rats. Phytomedicine. 2012;19:639–647. doi: 10.1016/j.phymed.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Almeida SA, Petenusci SO, Anselmo-Franci JA, et al. Decreased spermatogenic and androgenic testicular functions in adult rats submitted to immobilization-induced stress from prepuberty. Braz J Med Biol Res. 1998;31(11):1443–1448. doi: 10.1590/s0100-879x1998001100013. (Available from: http://dx.doi.org/10.1590/S0100-879X1998001100013) [DOI] [PubMed] [Google Scholar]
- 3.Arad-Dann H, Beller U, Haimovitch R, et al. Immunohistochemistry of phosphotyrosine residues: identification of distinct intracellular patterns in epithelial and steroidogenic tissues. J Histochem Cytochem. 1993;41(4):513–519. doi: 10.1177/41.4.7680679. (Available from: http://dx.doi.org/10.1177/41.4.7680679) [DOI] [PubMed] [Google Scholar]
- 4.Arner P. Adrenergic receptor function in fat cells. Am J Clin Nutr. 1992;55:228S–236S. doi: 10.1093/ajcn/55.1.228s. [DOI] [PubMed] [Google Scholar]
- 5.Aziz NM, Ragy MM, Gayyed MF. Effect of acute immobilization stress with or without a heme oxygenase inducer on testicular structure and function in male albino rats. J Basic Clin Physiol Pharmacol. 2013;24(4):255–262. doi: 10.1515/jbcpp-2012-0066. (Available from: http://dx.doi.org/10.1515/jbcpp-2012-0066) [DOI] [PubMed] [Google Scholar]
- 6.Ballester J, Munoz MC, Dominguez J, et al. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl. 2004;25(5):706–719. doi: 10.1002/j.1939-4640.2004.tb02845.x. (Available from: http://dx.doi.org/10.1002/j.1939-4640.2004.tb02845.x) [DOI] [PubMed] [Google Scholar]
- 7.Bhatia N, Jaggi AS, Singh N, et al. Adaptogenic potential of curcumin in experimental chronic stress and chronic unpredictable stress-induced memory deficits and alterations in functional homeostasis. J Nat Med. 2011;65(3-4):532–543. doi: 10.1007/s11418-011-0535-9. (Available from: http://dx.doi.org/10.1007/s11418-011-0535-9) [DOI] [PubMed] [Google Scholar]
- 8.Bitgul G, Tekmen I, Keles D, et al. Protective effects of resveratrol against chronic immobilization stress on testis. Urology. 2013;6:1–10. doi: 10.1155/2013/278720. (Available from: http://dx.doi.org/10.1155/2013/278720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brzozowski T, Konturek PC, Chlopicki S, et al. Therapeutic potential of 1-methylnicotinamide against acute gastric lesions induced by stress: role of endogenous prostacyclin and sensory nerves. J Pharmacol Exp Ther. 2008;326(1):105–116. doi: 10.1124/jpet.108.136457. (Available from: http://dx.doi.org/10.1124/jpet.108.136457) [DOI] [PubMed] [Google Scholar]
- 10.Carrasco GA, van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463(1-3):235–272. doi: 10.1016/s0014-2999(03)01285-8. (Available from: http://dx.doi.org/10.1016/S0014-2999(03)01285-8) [DOI] [PubMed] [Google Scholar]
- 11.Chotiwat C, Harris RB. Antagonism of specific corticotropin-releasing factor receptor subtypes selectively modifies weight loss in restrained rats. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R1762–R1773. doi: 10.1152/ajpregu.00196.2008. (Available from: http://dx.doi.org/10.1152/ajpregu.00196.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke RN, Klock SC, Geoghegan A, et al. Relationship between psychological stress and semen quality among in-vitro fertilization patients. Hum Reprod. 1999;14(3):753–758. doi: 10.1093/humrep/14.3.753. (Available from: http://dx.doi.org/10.1093/humrep/14.3.753) [DOI] [PubMed] [Google Scholar]
- 13.Ernst C, Foldenyi M, Angst J. The Zurich Study: XXI. Sexual dysfunctions and disturbances in young adults. Data of a longitudinal epidemiological study. Eur Arch Psychiatry Clin Neurosci. 1993;243(3-4):179–188. doi: 10.1007/BF02190725. (Available from: http://dx.doi.org/10.1007/BF02190725) [DOI] [PubMed] [Google Scholar]
- 14.Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241(4861):42–52. doi: 10.1126/science.3291115. (Available from: http://dx.doi.org/10.1126/science.3291115) [DOI] [PubMed] [Google Scholar]
- 15.Hari Priya P, Sreenivasula Reddy P. Effect of restraint stress on lead-induced male reproductive toxicity in rats. J Exp Zool A Ecol Genet Physiol. 2012;317(7):455–465. doi: 10.1002/jez.1738. (Available from: http://dx.doi.org/10.1002/jez.1738) [DOI] [PubMed] [Google Scholar]
- 16.Hari Priya P, Girish BP, Sreenivasula Reddy P. Restraint stress exacerbates alcohol-induced reproductive toxicity in male rats. Alcohol. 2014;48(8):781–786. doi: 10.1016/j.alcohol.2014.07.014. (Available from: http://dx.doi.org/10.1016/j.alcohol.2014.07.014) [DOI] [PubMed] [Google Scholar]
- 17.Hunter T. A thousand and one protein kinases. Cell. 1987;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. (Available from: http://dx.doi.org/10.1016/0092-8674(87)90509-5) [DOI] [PubMed] [Google Scholar]
- 18.Hunter T, Cooper JA. Protein tyrosine kinases. Annu Rev Biochem. 1985;54(1):897–930. doi: 10.1146/annurev.bi.54.070185.004341. (Available from: http://dx.doi.org/10.1146/annurev.bi.54.070185.004341) [DOI] [PubMed] [Google Scholar]
- 19.Iamsaard S, Prabsattroo T, Sukhorum W, et al. Anethum graveolens Linn. (dill) extract enhances the mounting frequency and level of testicular tyrosine protein phosphorylation in rats. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2013;14(3):247–252. doi: 10.1631/jzus.B1200287. (Available from: http://dx.doi.org/10.1631/jzus.B1200287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iamsaard S, Arun S, Burawat J, et al. Phenolic contents and antioxidant capacities of Thai-Makham Pom (Phyllanthus emblica L.) aqueous extracts. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2014;15(4):405–408. doi: 10.1631/jzus.B1300284. (Available from: http://dx.doi.org/10.1631/jzus.B1300284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy SH, Dickens SE, Eisfeld BS, et al. Sexual dysfunction before antidepressant therapy in major depression. J Affect Disord. 1999;56(2-3):201–208. doi: 10.1016/s0165-0327(99)00050-6. (Available from: http://dx.doi.org/10.1016/S0165-0327(99)00050-6) [DOI] [PubMed] [Google Scholar]
- 22.Kopf G, Gerton G. The mammalian sperm acrosome and the acrosome reaction. In: Wassarman P, editor. Elements of Mammalian Fertilization. Boca Raton, USA: CRC Press; 1991. pp. 154–203. [Google Scholar]
- 23.Kwiecien S, Ptak-Belowska A, Krzysiek-Maczka G, et al. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, interacts with gastric oxidative metabolism and enhances stress-induced gastric lesions. J Physiol Pharmacol. 2012;63(5):515–524. [PubMed] [Google Scholar]
- 24.Lafontan M, Barbe P, Galitzky J, et al. Adrenergic regulation of adipocyte metabolism. Hum Reprod. 1997;12(Suppl. 1):6–20. doi: 10.1093/humrep/12.suppl_1.6. (Available from: http://dx.doi.org/10.1093/humrep/12.suppl_1.6) [DOI] [PubMed] [Google Scholar]
- 25.Lin H, Yuan KM, Zhou HY, et al. Time-course changes of steroidogenic gene expression and steroidogenesis of rat Leydig cells after acute immobilization stress. Int J Mol Sci. 2014;15(11):21028–21044. doi: 10.3390/ijms151121028. (Available from: http://dx.doi.org/10.3390/ijms151121028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan SG. The epidemiology of the DSM-III psychosexual dysfunctions. J Sex Marital Ther. 1986;12(4):267–281. doi: 10.1080/00926238608415413. (Available from: http://dx.doi.org/10.1080/00926238608415413) [DOI] [PubMed] [Google Scholar]
- 27.Orr TE, Mann DR. Effects of restraint stress on plasma LH and testosterone concentrations, Leydig cell LH/HCG receptors, and in vitro testicular steroidogenesis in adult rats. Horm Behav. 1990;24(3):324–341. doi: 10.1016/0018-506x(90)90013-n. (Available from: http://dx.doi.org/10.1016/0018-506X(90)90013-N) [DOI] [PubMed] [Google Scholar]
- 28.Prabsattroo T, Wattanathorn J, Iamsaard S, et al. Moringa oleifera extract enhances sexual performance in stressed rats. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(3):179–190. doi: 10.1631/jzus.B1400197. (Available from: http://dx.doi.org/10.1631/jzus.B1400197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rai J, Pandey SN, Srivastava RK. Effect of immobilization stress on spermatogenesis of albino rats. J Anat Soc India. 2003;52(1):55–57. [Google Scholar]
- 30.Rai J, Pandey S.N. Srivastava, R.K. Testosterone hormone level in albino rats following restraint stress of long duration. J Anat Soc India. 2004;53(1):17–19. [Google Scholar]
- 31.Rao M, Zhao XL, Yang J, et al. Effect of transient scrotal hyperthermia on sperm parameters, seminal plasma biochemical markers, and oxidative stress in men. Asian J Androl. 2015;17(4):668–675. doi: 10.4103/1008-682X.146967. (Available from: http://dx.doi.org/10.4103/1008-682X.146967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Retana-Márquez S, Bonilla-Jaime H, Vazquez-Palacios G, et al. Changes in masculine sexual behavior, corticosterone and testosterone in response to acute and chronic stress in male rats. Horm Behav. 2003;44(4):327–337. doi: 10.1016/j.yhbeh.2003.04.001. (Available from: http://dx.doi.org/10.1016/j.yhbeh.2003.04.001) [DOI] [PubMed] [Google Scholar]
- 33.Sakr SA, Zowail ME, Marzouk AM. Effect of saffron (Crocus sativus L.) on sodium valporate induced cytogenetic and testicular alterations in albino rats. Anat Cell Biol. 2014;47(3):171–179. doi: 10.5115/acb.2014.47.3.171. (Available from: http://dx.doi.org/10.5115/acb.2014.47.3.171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherer IJ, Holmes PV, Harris RB. The importance of corticosterone in mediating restraint-induced weight loss in rats. Physiol Behav. 2011;102(2):225–233. doi: 10.1016/j.physbeh.2010.11.014. (Available from: http://dx.doi.org/10.1016/j.physbeh.2010.11.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. (Available from: http://dx.doi.org/10.1016/0092-8674(90)90801-K) [DOI] [PubMed] [Google Scholar]
- 36.Visconti PE, Kopf GS. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod. 1998;59(1):1–6. doi: 10.1095/biolreprod59.1.1. (Available from: http://dx.doi.org/10.1095/biolreprod59.1.1) [DOI] [PubMed] [Google Scholar]
- 37.Warzecha Z, Dembinski A, Ceranowicz P, et al. Role of sensory nerves in gastroprotective effect of anandamide in rats. J Physiol Pharmacol. 2011;62(2):207–217. [PubMed] [Google Scholar]
- 38.Weissman BA, Sottas CM, Holmes M, et al. Normal responses to restraint stress in mice lacking the gene for neuronal nitric oxide synthase. J Androl. 2009;30(5):614–620. doi: 10.2164/jandrol.108.007443. (Available from: http://dx.doi.org/10.2164/jandrol.108.007443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyrobek AJ, Bruce WR. Chemical induction of sperm abnormalities in mice. PNAS. 1975;72(11):4425–4429. doi: 10.1073/pnas.72.11.4425. (Available from: http://dx.doi.org/10.1073/pnas.72.11.4425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu M, Wei Q, Zheng K, et al. Protective effects of Big-leaf mulberry and physiological roles of nitric oxide synthases in the testis of mice following water immersion and restraint stress. Acta Histochem. 2014;116(8):1323–1330. doi: 10.1016/j.acthis.2014.08.003. (Available from: http://dx.doi.org/10.1016/j.acthis.2014.08.003) [DOI] [PubMed] [Google Scholar]
- 41.Yanagimachi R. Mammalian fertilization. In: Knobil E, editor. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 189–317. [Google Scholar]
- 42.Zardooz H, Zahedi Asl S, Gharib Naseri MK, et al. Effect of chronic restraint stress on carbohydrate metabolism in rat. Physiol Behav. 2006;89(3):373–378. doi: 10.1016/j.physbeh.2006.06.023. (Available from: http://dx.doi.org/10.1016/j.physbeh.2006.06.023) [DOI] [PubMed] [Google Scholar]
- 43.Zayachkivska OS, Gzhegotsky MR, Terletska OI, et al. Influence of Viburnum opulus proanthocyanidins on stress-induced gastrointestinal mucosal damage. J Physiol Pharmacol. 2006;5:155–167. [PubMed] [Google Scholar]
- 44.Zhang MH, Shi ZD, Yu JC, et al. Scrotal heat stress causes sperm chromatin damage and cysteinyl aspartate-spicific proteinases 3 changes in fertile men. J Assist Reprod Genet. 2015;32(5):747–755. doi: 10.1007/s10815-015-0451-0. (Available from: http://dx.doi.org/10.1007/s10815-015-0451-0) [DOI] [PMC free article] [PubMed] [Google Scholar]


