Abstract
Rationale
Inflammation in post-myocardial infarct (MI) is necessary for myocyte repair and wound healing. Unfortunately it is also a key component of subsequent heart failure pathology. FoxO4 regulates a variety of biological processes including inflammation. However, its role in MI remains unknown.
Objective
To test the hypothesis that FoxO4 promotes early post-MI inflammation via endothelial Arg1.
Methods and Results
We induced MI in WT and FoxO4−/− mice. FoxO4−/− mice had a significantly higher post-MI survival, better cardiac function, and reduced infarct size. FoxO4−/− hearts had significantly fewer neutrophils, reduced expression of cytokines and competitive nitric oxide synthase (NOS) inhibitor Arginase 1 (Arg1). We generated conditional FoxO4 knockout mice with FoxO4-deleted in cardiac mycoytes (cKO) or endothelial cells (ecKO). FoxO4 ecKO mice showed significant post-MI improvement of cardiac function and reduction of neutrophil accumulation and cytokine expression whereas FoxO4 cKO had no significant difference in cardiac function and post-MI inflammation from those of control littermates. FoxO4 binds the Foxo-binding site in the Arg1 promoter and activates Arg1 transcription. FoxO4-knockdown in human aortic endothelial cells upregulated nitric oxide upon ischemia and suppressed monocyte adhesion that can be reversed by ectopic-expression of Arg1. Furthermore, chemical inhibition of Arg1 in WT mice had similar cardioprotection and reduced inflammation following MI as FoxO4-inactivation and administration of NOS inhibitor to FoxO4 KO mice reversed the beneficial effects of FoxO4-deletion on post-MI cardiac function.
Conclusion
FoxO4 activates Arg1 transcription in endothelial cells in response to MI, leading to downregulation of nitric oxide and upregulation of neutrophil infiltration to the infarct area.
Keywords: FoxO4, Arg1, myocardial infarction, inflammation, transcription, endothelial function, endothelial cell
INTRODUCTION
Myocardial infarction (MI), commonly known as heart attack, is a major public health problem. In response to MI, the left ventricle (LV) undergoes a series of changes in size, shape, and function (referred to as LV remodeling) that can lead to myocardial hypertrophy and eventual heart failure1. Post-MI inflammatory response is part of cardiac repair pathways and plays a critical role in determining the size of the infarct and quality of the repair2. Global non-selective inhibition of inflammation can result in defective wound healing, leading to smaller but weaker scar with tendency to rupture3, 4. Uncontrolled excessive inflammation may activate proapoptotic pathways inducing further loss of cardiac mycoytes, augment matrix degradation causing cardiac rupture, and impair collagen deposition leading to formation of a scar with reduced tensile strength, thus increasing chamber dilation4. There have been great efforts and many clinical trials in the past three decades to find an effective therapy that reduces the length and damage of the inflammatory reaction and in the meantime does not interfere with the reparatory pathways4, 5. However, no adequate therapy for the inflammatory response has yet emerged.
Neutrophil infiltration to the infarct area is part of early inflammatory response to MI. The physiological roles of neutrophil are to phagocytose cell and matrix debris and to activate macrophage for clearance and subsequent resolution of inflammation. Pathologically, activated neutrophils can produce reactive oxygen species (ROS) and reactive nitrogen intermediates. This respiratory burst is part of the innate immunity that is normally aimed at attacking micro-organisms. However, in hypoxic heart it can progressively increase cardiac mycoyte death and thus further exacerbate the immune response. As the range of inflammation depends on the magnitude and density of neutrophils in the infarct area, there has been an effort to restrain/inhibit neutrophils in postinfarction therapy5. Whole-body depletion of neutrophils in dog has been shown to prevent inflammation-induced cardiac damage accompanied by a significantly smaller infarct size6, 7 and to reduce ischemic/reperfusion-damage after coronary revascularization with cardiopulmonary bypass8. However, neutrophil depletion is invasive and impractical for large scale clinical use5. Alternative ways to suppress post-MI neutrophil infiltration are of clinical importance.
FoxO4 is a member of the ubiquitously expressed fork head (Fox) transcription factor O family that also includes FoxO1, O3, and O6. FoxO proteins regulate a variety of biological processes including oxidative stress response, metabolism, immunity, and apoptosis9. FoxO1 and O3 have also been shown to play a protective role against cardiac ischemic injury10 whereas the role of FoxO4 in ischemia remains unknown. We have shown previously that FoxO4 modulates the phenotypes of smooth muscle cells (SMC) by repressing SRF/Myocardin-activated differentiation genes11 and activating the transcription of the matrix metalloproteinase 9 (MMP9)12. Consequently, FoxO4 promotes neointimal formation in vivo in response to carotid artery ligation injury12. FoxO4 can also inhibit NF-κB-activated gene transcription, and inactivation of FoxO4 is associated with elevated susceptibility to chemical-induced colitis13 and high-fat-diet-induced atherosclerosis14. Because FoxO4 can play either a protective or pathological role depending on the cell type and context of the disease model, we wanted to investigate whether and how FoxO4 plays a role in MI.
Here we investigated the function of FoxO4 in post-MI LV remodeling. We show that FoxO4-deficiency has a protective role against MI. Inactivation of FoxO4 in mice resulted in a significantly higher post-MI survival, reduced infarct size, and improved cardiac function. FoxO4−/− heart had significantly fewer neutrophils and attenuated expression of cytokine/chemokines, MMP9, and Arginase 1 (Arg1) in the infarct area compared to infracted heart of WT mice. As in global FoxO4 knockout (KO) mice, endothelial cell (ec)-specific KO (FoxO4 ecKO) mice had similar reduced post-MI neutrophil-infiltration, reduction of cytokines, MMP9, and Arg1 expression, and improved cardiac function compared to control littermates. Mechanistically, we show that Arg1 is a direct transcriptional target of FoxO4. Knockdown of FoxO4 in human aortic endothelial cells (HAECs) by siRNA upregulated nitric oxide (NO) upon ischemia and suppressed TNFα-activated monocyte adhesion that can be rescued by exogenously expressed Arg1. Moreover, chemical inhibition of Arg1 in mice phenocopies the cardioprotective outcome of FoxO4-deletion upon MI and reduction in early inflammation. Since Arg1 is a competitive inhibitor of endothelial nitric oxide (NO) synthase (eNOS) that uses L-arginine as the sole substrate for production of NO15,16, and NO is known to inhibit lymphocyte adhesion and transmigration cross the endothelial barrier17, we propose that activation of Arg1 by FoxO4 may be the underlying mechanism of FoxO4-regulated neutrophil infiltration in post-MI inflammatory response. Our studies indicate that FoxO4 promotes adverse post-MI LV remodeling and thus provides a mechanistic insight for a potential therapeutic modality in ischemic injury.
METHODS
An expanded Materials and Methods section is available in the Online Data Supplement.
MIs were generated using male mice at 8–10 weeks of age by surgical ligation of the left anterior descending coronary artery (LAD). Cardiac functions were measured by echocardiograph using Vevo 2100. For determination of infarct size, histological sections were stained with Masson’s Trichrome and photographed. Infarct size was calculated as the ratio of the infarction length to the perimeter of the left ventricle in each section. Heart cells were isolated from Sham and MI-hearts by the Langendorff method. Non-CMs were stained with PE-conjugated anti-Ly6G antibodies (BD 561104) and subjected to flow cytometry assay. Arg1-luc reporter assays were performed in 293A cells. Monocyte adhesion assays were performed using Human aortic endothelial cells (HAECs) and monocyte U937 cells. All animal usage in this study was approved by the Institutional Animal Care and Use Committee of UT Southwestern Medical Center. Data are presented as means ± SEM unless otherwise stated. All statistical analysis was performed using GraphPad Prism software (San Diego, CA). The two-tailed t-test was used for comparisons between experimental groups. Differences were considered statistically significant as p < 0.05.
RESULTS
Inactivation of FoxO4 protects mice against ischemia-induced injury
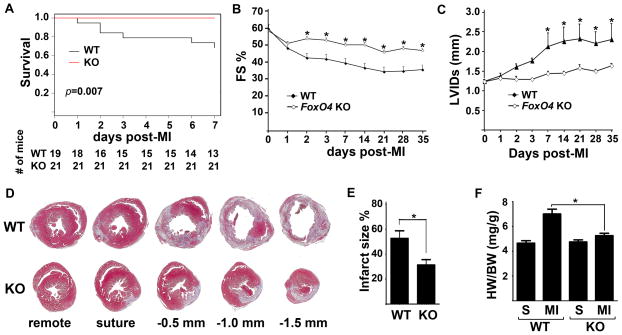
We induced MI in FoxO4 KO and WT littermates by permanent ligation of left anterior descending coronary artery for up to 5 weeks. While we typically observed 30% mortality for WT mice by post-MI day 7, there were no post-MI deaths of FoxO4 KO mice (Fig. 1A). Upon examination of early post-mortem infarct WT mice, we found that many of them have ruptured ventricles (data not shown), suggesting this as the cause of sudden death. Consistent with the improved survival rate, FoxO4-null mice have better-preserved cardiac functions as assayed by echocardiograph (Fig.1, B and C). WT and FoxO4 KO mice have similar fractional shortening (FS) at post-MI day 1. While the cardiac function of infarct WT mice continued to decline, the cardiac function of infarct Foxo4 KO mice remained similar to that of day 1 post-MI. Furthermore, the increase in end systolic left ventricular internal diameter (LVIDs) in infarcted WT mice was also alleviated by deletion of FoxO4 (Fig. 1C). These results indicate that FoxO4 deficiency helps to preserve the cardiac function following MI and prevents left ventricular dilatation.
Figure 1. FoxO4 deletion protects mice against MI-injury.
WT and FoxO4 KO mice underwent permanent ligation of LAD. (A) Survival curves after MI. (B) Fractional shortening (FS) and (C) left ventricular internal diameter at systole (LVIDs) were measured by echocardiograph (n=6–12). (D) Representative images of Masson’s Trichrome-stained transverse cross sections of hearts 7 days post-MI. Sections were cut at the levels indicated. (E) Infarct size quantified from sections shown in D (n=5). (F) HW/BW ratios of mice 7 days after MI (n=5–7 for each group). *, WT vs KO, p<0.05..
As a significant change of cardiac function between infarct WT and FoxO4 KO mice was observed at day 7 post-MI, we focused our histopathological examination of infarct mice at this time point. Masson’s Trichrome, which effectively labels interstitial fibrillar collagen, revealed significant differences in infarct areas between FoxO4 KO and WT mice. The infarct size of FoxO4-null mice (~32%) is significantly smaller than that of WT mice (~52%) at post-MI day 7 (Fig.1, D & E). At 7 days post-MI there was a significant hypertrophic response in WT mice, with 50% increase in heart weight/body weight (HW/BW) ratio, whereas the MI-induced cardiac hypertrophy was significantly attenuated in FoxO4 KO mice (Fig.1F). To test whether reduced infarct size in FoxO4 KO mice may result from a decreased initial propensity to generate an infarct, we determined area at risk (AAR) one day after MI. The AAR is similar between WT and FoxO4 KO mice (Online Figure IA). We noticed that the infarct size of WT mouse heart at post-MI day 7 is significantly larger than AAR at post-MI day 1 (52% vs 32%), indicating that the WT mouse heart underwent extensive post-MI remodeling. We also investigated whether inactivation of FoxO4 affected initial cell death in the infarct zone 24 h after MI. Although staining of histological sections for TUNEL revealed significant cell death throughout the infarct area, the percentage of TUNEL positive nuclei was nearly identical between WT and FoxO4 KO mice (Online Figure IB). This is consistent with similar cardiac functions measured by echocardiograph at one day post-MI (Fig.1, B & C). These results indicate that the reduced infarct size in FoxO4 KO mice is likely due to alterations in post-MI reparative pathways rather than merely a difference in the AAR and cell viability between WT and FoxO4 KO genotypes.
Inactivation of FoxO4 significantly attenuated the early post-MI inflammation and suppressed neutrophil infiltration
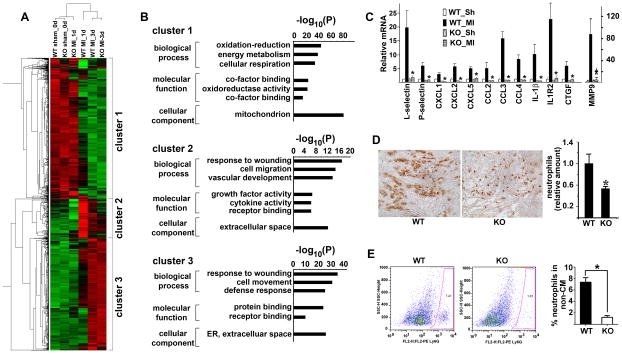
To identify the cellular and molecular events that give rise to the ischemic phenotypes of FoxO4-null mice, we performed microarray gene profiling experiments with cDNAs from the infarct areas of both WT and FoxO4 KO mice one and three days after MI. Cluster analysis showed significant difference of gene expression between WT and FoxO4 KO mouse heart at post-MI day 1; changes of gene expression in WT mouse heart at post-MI day 1 are significantly attenuated in FoxO4-null mice (Fig. 2A). Functional annotation of differentially expressed genes by Toppgene/Toppcluster18 indicated that they are involved in inflammatory response to wound healing, consistent with the fact that acute inflammation is the earliest pathophysiological post-MI response (Fig. 2B). Using qRT-PCR, we confirmed that CXCL1, CXCL2, CXCL5, CCL2, CCL3, CCL4, IL1R2, IL1β, CTGF, as well as neutrophil markers P- and L-selectin, and matrix metalloproteinase MMP9 are upregulated in post-MI day 1 WT mouse hearts, and upregulation of these mRNAs was significantly attenuated in the infarct areas of FoxO4 KO mouse hearts (Fig. 2C). Because FoxO4 was previously shown to inhibit NF-κB-activated cytokine expression, we speculated that down-regulation of the cytokine expression in post-MI FoxO4-null mouse heart is not due to the autonomous function of FoxO4 in immune cells but rather due to a reduction of the amount of immune cells. Downregulation of neutrophil markers in FoxO4-null mice prompted us to measure the number of neutrophils. We stained histological sections of WT and FoxO4 KO mouse hearts 1 day post-MI with the neutrophil marker Ly6G (Fig. 2D). We also isolated neutrophils from the injured heart at post-MI day 2 and subjected them to flow cytometry (FACS) analysis (Fig. 2E). Indeed, both methods showed that the number of neutrophils in the infarct FoxO4-null mouse hearts was significantly decreased compared to that of WT (Fig. 2, D & E).
Figure 2. Deletion of FoxO4 resulted in attenuated early post-MI inflammation.
(A) Heat map of differentially expressed genes (>2-fold changes) in WT and FoxO4-nll mouse hearts at sham and post-MI 1 and 3 days. (B) Gene ontology analysis showing the biological processes and molecular functions of differentially expressed genes in (A). (C) qRT-PCR of inflammatory genes that are most attenuated in one-day post-MI FoxO4 KO mice. Expression was normalized against GAPDH and expressed relative to that of WT_Sham (Sh) heart (n=5). *, p<0.05 compared to WT_MI. (D) Representative images of Ly6G staining of sections of WT and FoxO4 KO mouse hearts at one day post-MI. Ly6G staining was quantified by Image-J and expressed relative to the mean value for WT mouse hearts (n=5–7). *, p< 0.05. (E) FACS profiles of non- cardiac mycoytes labeled with Ly6G (n=5–7), *, p<0.05.
We also evaluated the expression of ROS scavenger enzymes such as catalase and Sod2 in WT and FoxO4 KO mouse hearts before and after MI as previous studies indicated that they were downregulated in FoxO1/FoxO3 compound cardiac mycoyte-null mice9. Interestingly, no significant differences in catalase and Sod2 expression were observed in FoxO4 KO mice before and after MI whereas the expression of these two enzymes in WT mice was downregulated significantly 24 h after MI (Online Figure II).
Inactivation of FoxO4 in endothelial cells but not in cardiac mycoytes recapitulated the post-MI inflammatory phenotype of global FoxO4 KO mice
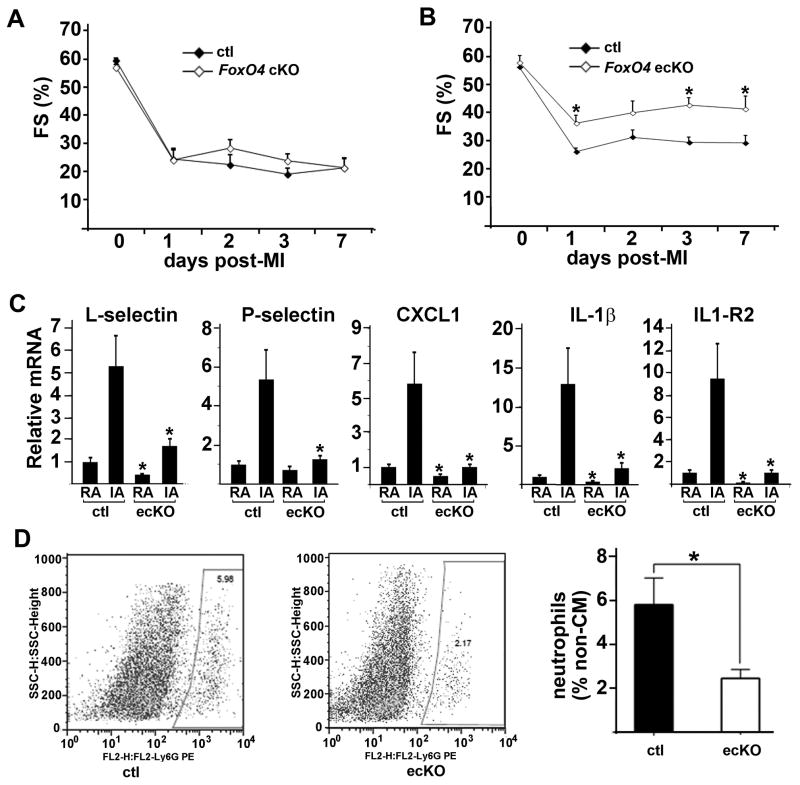
Neutrophils in the infarct area are from the circulation, via infiltration through the endothelial barrier upon MI. Downregulation of the amount of neutrophils in global FoxO4 KO mice could be due to decreased homing chemokines produced by dying cardiac mycoytes and/or to an increased endothelial barrier. To test these two possibilities, we generated conditional knockout mice with FoxO4 deleted specifically in cardiac mycoytes (cKO) (FoxO4f/f; αMHC-cre) or in endothelial cells (ecKO) (FoxO4f/f; Tie2-cre) and subjected these mice to MI. FoxO4 KO, cKO, and ecKO mice had similar cardiac function at baseline (ca. 55% FS) as measured by echocardiography. Unlike global-deletion of FoxO4, inactivation of FoxO4 in cardiac mycoyte did not improve post-MI cardiac function (Fig. 3A). Expression of inflammatory cytokine/chemokines in FoxO4 cKO mouse heart at post-MI day 1 was not significantly different from those of control (FoxO4f/f) littermates (Online Figure IIIA). FACS analysis of isolated non- cardiac mycoytes from injured hearts at post-MI day 2 showed similar numbers of neutrophils for both WT and FoxO4 cKO mice (Online Figure IIIB). In contrast, deletion of FoxO4 in endothelial cells resulted in better post-MI cardiac function (Fig. 3B), attenuated inflammatory cytokine expression (Fig. 3C), and suppression of neutrophil infiltration (Fig. 3D) compared to those of control littermates. We also analyzed the infarct size of FoxO4 cKO and ecKO mice 7 days post-MI. Both cKO and ecKO mice had similar infarct size compared to their respective littermate controls (Online Figure IIIC). While FoxO4 cKO and ecKO mice had better survival trend compared to control littermates, the p value did not reach significance, possibly due to the small sample size (Online Figure IIID).
Figure 3. Inactivation of FoxO4 in endothelial cells but not in cardiac mycoytes downregulates post-MI inflammation and preserves post-infarct cardiac function.
(A, B) Fractional shortenings of (A) FoxO4 cKO (FoxO4f/f;_MHC-cre) and (B) FoxO4 ecKO mice (FoxO4f/f;Tie2-cre) and their respective control littermates (FoxO4f/f) before and after MI (n=6–8). *, p<0.05. (C) qRT-PCR of selective cytokines from remote area (RA) and infarct area (IA) of FoxO4 ecKO and control littermates at post-MI day 1. (D) FACS profile of non- cardiac mycoytes labeled with Ly6G from hearts of FoxO4 ecKO and control littermates post-MI 2 days. Neutrophil fractions gated on the two left panel were quantified (right panel) (n=3). *, p<0.05.
We also noticed that the infarction had variable consequences on cardiac function in control groups of FoxO4 cKO, ecKO, and KO. This may be attributed to strain differences as a wide variation in stress-induced heart failure phenotype has been previously observed between the strains of the hybrid mouse diversity panel19. The outcome of infarct healing including infarct rupture and cardiac function in mice has also been shown to be dependent on genetic background 20.
Since Tie2-cre is also active in hematopoietic cells21, we investigated the potential contribution of these cells to FoxO4 ecKO phenotype. We transplanted bone marrow of FoxO4f/f;CAG-cre (chimera) or FoxO4f/f (control) mice into irradiated WT mice. After 8 weeks of recovery, we performed MI on these mice and observed no significant difference of post-MI cardiac function between the chimera and control mice (Online Figure IIIE), excluding the possibility of FoxO4-expressed immune cell contribution to the post-MI phenotypes of FoxO4 ecKO.
Tie2-cre has also shown to be active in endothelial-derived cardiac fibroblasts (cFbs)22. We speculate that these cells may not contribute significantly to the early inflammatory phenotype of FoxO4 ecKO for the following reasons. The majority of Tie2-cre-labled cFbs are in the septum and few in the left ventricle where the infarct area is. Although it remains to be tested whether these cells can migrate into the left ventricle upon MI, they proliferate locally and do not migrate to other regions of the heart in response to transverse aortic constriction-induced pressure overload. Moreover, cFbs are normally activated after the early post-MI inflammation. Taken together, these data suggest that the early inflammatory phenotype of post-MI FoxO4 ecKO mice may be indeed due to the loss of FoxO4 in endothelial cells.
Upregulation of Arg1 in response to MI is attenuated in both FoxO4 KO and ecKO mouse hearts compared to their respective control littermates
To identify the molecular mechanism(s) by which FoxO4 regulates the endothelial barrier function, we focused on Arg1 since it is the top differentially expressed gene between WT and FoxO4 KO hearts at post-MI day 1 (top gene in cluster 3 of Fig. 2A), thus it is a potential novel transcriptional target of FoxO4. Arg1 is a critical regulator for NO production by competing with eNOS for L-arginine in the endothelium23. Arg1 is implicated in various cardiovascular pathologies and ischemic injuries24–31. Increased Arginase activity has been shown to diminish the bioavailability of NO28, 29 that is a known major regulator of cardiovascular functions including neutrophil adhesion and transmigration across endothelial barrier17. We speculated that downregulation of neutrophil infiltration in post-MI FoxO4 KO and ecKO mouse hearts could be due to a loss of Arg1, thus increasing NO and endothelial barrier function.
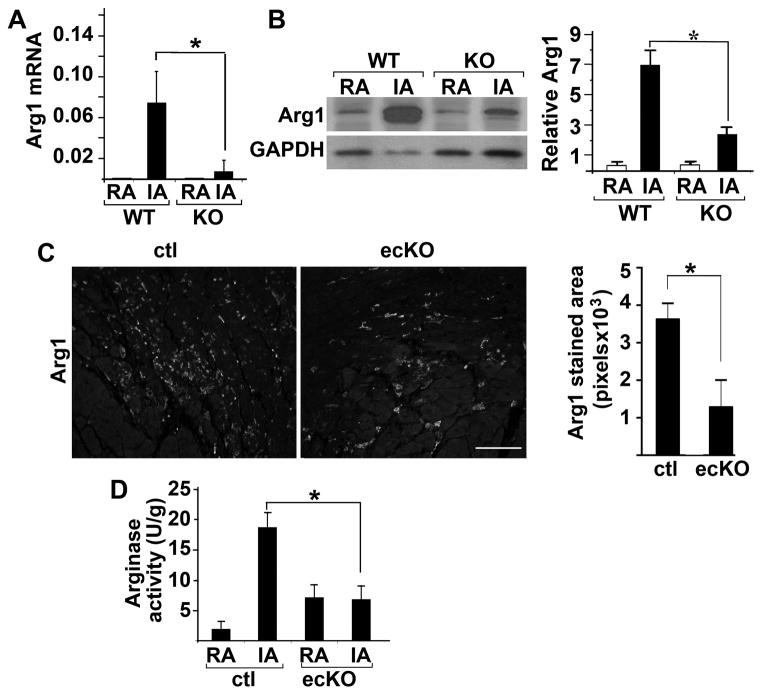
To test this hypothesis, we first confirmed downregulation of Arg1 in post-MI FoxO4 KO and ecKO mouse heart. Arg1 is expressed at low level in the heart at baseline and dramatically upregulated in the infarct area upon MI in WT mouse (Fig. 4A). This upregulation was significantly attenuated in post-MI FoxO4 KO mouse hearts at both mRNA and protein levels (Fig. 4, A & B). Upregulation of Arg1 upon MI is significantly attenuated in FoxO4 ecKO mouse hearts as well (Fig. 4C). Arginase activity in FoxO4 ecKO mouse hearts was also significantly attenuated at post-MI day 2 (Fig. 4D). Since there are two isoforms of arginase (Arg1 and Arg2) in the cell, they both likely contribute to the changes of arginase activity after MI injury. We tested whether deletion of FoxO4 had any effect on Arg2 expression. Arg2 transcription is upregulated after MI significantly, but the fold increase (ca. 3-fold) is much smaller than that of Arg1. Deletion of FoxO4 resulted in a significantly attenuated Arg2 expression (Online Figure IV). It will be worthwhile to test whether Arg2 is a direct transcriptional-target of FoxO4 in the future and whether it influences post-MI remodeling as well.
Figure 4. Upregulation of post-MI Arg1 expression was significantly attenuated in FoxO4 KO and FoxO4 ecKO mouse hearts.
(A) Relative mRNA of Arg1 in the remote (RA) and infarct area (IA) of FoxO4 KO and WT littermate mouse hearts post-MI 1 day as measured by qRT-PCR. mRNAs were normalized against internal GAPDH (n=6). *, p<0.05. (B) Western blot of Arg1 from tissues described in (A) (n=3). *, p<0.05. Protein levels were quantified by densitometry and normalized against GAPDH. (C) Immunofluorescent micrographs of IA from histological heart sections of FoxO4 ecKO and control littermates stained with Arg1 antibody post-MI day 2. Arg1 immunofluorescence from 5 different sections of each genotype mouse was quantified by Image-J, and averaged values are shown (n=3) *, p<0.05. Scale bar=100μM. (D) Arginase activity of lysates from remote area (RA) and infarct area (IA) of FoxO4 ecKO or control littermates (n=3). *, p<0.05.
FoxO4 knockdown in endothelial cells upregulates NO and downregulates monocyte adhesion that can be rescued by ectopic expression of Arg1
We investigated how FoxO4 activity may be regulated in post-MI remodeling. We measured the expression level of FoxO4 before and after MI and observed little change in the expression (Online Figure VA). We next tested whether FoxO4 can alter its cellular location in response to ischemic stimuli. GFP-FoxO4 was transduced into primary human aortic endothelial cells (HAECs) via lentiviruses. GFP-FoxO4 was located in the cytoplasm under baseline condition and translocated to the nucleus of HAECs upon stimulation by ischemia and TNFα (Fig. 5A). We knocked down FoxO4 in HAECs using multiple independent siRNAs (Online Figure VB). FoxO4 knockdown significantly upregulated NO production (Fig. 5B) and downregulated adhesion of monocyte to endothelial cells (Fig. 5, C & D). Similarly decreased monocyte adhesion was also observed in Arg1-knockdown cells (Fig. 5, C & D). To test whether Arg1 could mediate the FoxO4-regulated endothelial barrier function, we ectopically re-expressed Arg1 in FoxO4-knockdown cells using lentiviruses that express Arg1. Indeed, we observed a rescue of monocyte adhesion in FoxO4 knockdown cells (Fig. 5, E & F), suggesting that Arg1 is a downstream effector of FoxO4.
Figure 5. FoxO4 knockdown upregulates NO and suppresses monocyte adhesion that can be rescued by ectopic expression of Arg1.
(A) GFP-FoxO4 was transduced into the HAECS and stimulated with or without ischemia +TNFα. (B) HAECs cells transfected with control or FoxO4 siRNA were incubated with DAF-FM DA to visualize NO production and stimulated with ischemia for 1 hr. (C) Representative micrographs of monocyte adhesion to HAECs that were transfected with control, FoxO4, or Arg1 siRNA and stimulated with or without TNFα (D) Monocyte adhesions in (C) were quantified and averaged from 4 randomly chosen fields, and expressed as percentage relative to cells transfected with control siRNA-transfected and stimulated with TNFα (n=3). *, p<0.05. (D) Control or FoxO4 siRNA-transfected HAECs were transduced with lentiviruses expressing GFP, Arg1, or FoxO4 before adhesion assays were performed in the presence or absence of TNFα. Representative micrographs from multiple experiments (N>3) and two independent siRNA duplexes were shown. (E) Monocyte adhesion from (D) was quantified and expressed as percentage relative to that of ctl-siRNA transfected and TNFα/GFP-treated cells. (n=3). *, p<0.05.
Arg1 is a direct transcriptional target of FoxO4
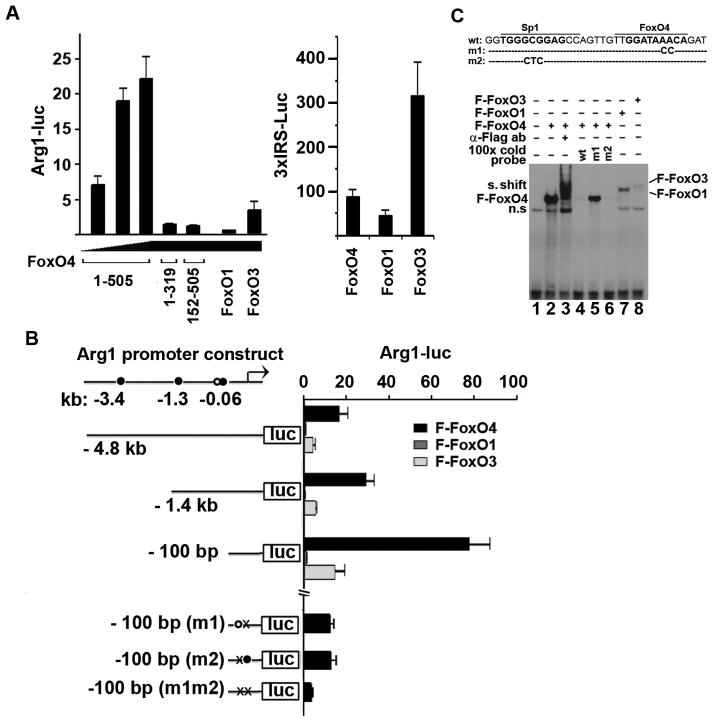
Finally, we tested whether Arg1 is a direct transcriptional target of FoxO4 by investigating the ability of FoxO4 to activate a luciferase reporter driven by the Arg1-promoter. FoxO4 activated the Arg1-luc reporter in a dose-dependent manner (Fig. 6A, left panel), and the activation requires both the DNA binding domain (amino acids 89 to 270) and transactivation domain (amino acids 450–505) of FoxO4 as deletion of either domain failed to activate the Arg1-luc reporter (Fig. 6A, left panel). Interestingly, although FoxO1 and FoxO3 can activate robustly the 3xIRS-luciferase reporter that contains 3 copies of insulin responsive sequence (Fig. 6A, right panel), they are much weaker activators of Arg1 transcription compared to FoxO4. We identified several potential FoxO4 binding sites in the 4.8-kb promoter region of Arg1 using the online program PROMO32 (Fig. 6B). Further luciferase reporter assays with a series of promoter constructs containing progressive deletions from the 5’ end of the promoter sequence and point mutations identified a sequence around −60 bp as the functional FoxO4 binding site. Gel shift assay with a probe containing this FoxO4 binding site showed that FoxO4 has a strong affinity for this site whereas FoxO1 or FoxO3 bind weakly (Fig. 6C). The 100-bp Arg1-promoter fragment has a much greater response to FoxO4 than the 4.8-kb or 1.4-kb promoter constructs, suggesting that deletion of upstream regions may have removed some negative regulatory elements. We also identified a Sp1 binding site near the FoxO4 binding site in the Arg1 promoter. Mutation of the Sp1 binding site attenuated the FoxO4-activated transcription to the same extent as mutation of the nearby FoxO4 site, and mutation of both sites almost completely abolished the transcriptional response to FoxO4 (Fig. 6B). The gel shift assay also suggested that the Sp1 site may contribute to the FoxO4 binding (Fig. 6C). These results are consistent with our previous observation that FoxO4 can interact with Sp1 and activate the transcription of MMP912.
Figure 6. FoxO4 activates Arg1 transcription.
(A) Arg1 promoter (4.8kb)-driven luciferase (Luc) reporter construct (left panel) or 3xIRS-luc (right panel) were co- transfected into 293A cells with the indicated FoxO expression plasmids and internal control CMV-LacZ. The luciferase activities were normalized against co-transfected β-galactosidase and expressed relative to that from vector Flag-pcDNA transfected cells (n=3). (B) One potential Sp1- (open circle) and three FoxO4-binding sites (solid circles) were identified in the Arg1 promoter. Arg1-luc reporters with different 5’ends and point mutations in the Sp1- (m2) and FoxO4-binding site (m1) were used to identify the functional Sp1 and FoxO4 binding sites in the Arg1 promoter (n=3). (C) A gel shift assay was performed with 32P-labeled oligonucleotide probe containing the Sp1 and FoxO4-binding sequence in the Arg1 promoter and lysates of cells transfected with Flag-FoxO proteins as indicated.
Chemical inhibition of arginase phenocopies the improvement of cardiac function of FoxO4-deletion
Inhibition of arginase has been shown to reduce infarct size after ischemia-reperfusion (I/R) in rat31, improve basal endothelial function33 and endothelium-dependent vasodilatation following I/R in patients with coronary artery disease34. The benefit of arginase inhibitor in mouse MI model has not been demonstrated. Treatment with the arginase-specific inhibitor BEC in WT mice upon MI resulted in cardiac protection similar to that of FoxO4-deletion (Fig. 7A). BEC treatment also reduced expression of several inflammatory cytokines (Fig. 7B) and suppressed neutrophil accumulation in the infarct area at post-MI day 2 (Fig. 7C). The effect of BEC on post-MI heart is likely mediated by both Arg1 and Arg2 since BEC is a non-selective inhibitor of Arginase. Unlike its effect in I/R injury model, BEC-treatment did not reduce infarct size in our MI model (data not shown). This is not unexpected since additional damage to myocardium and endothelial vasculature caused by reperfusion in I/R model is dominated by ROS and loss of bio-availability of NO; thus, it is more sensitive to arginase inhibition35.
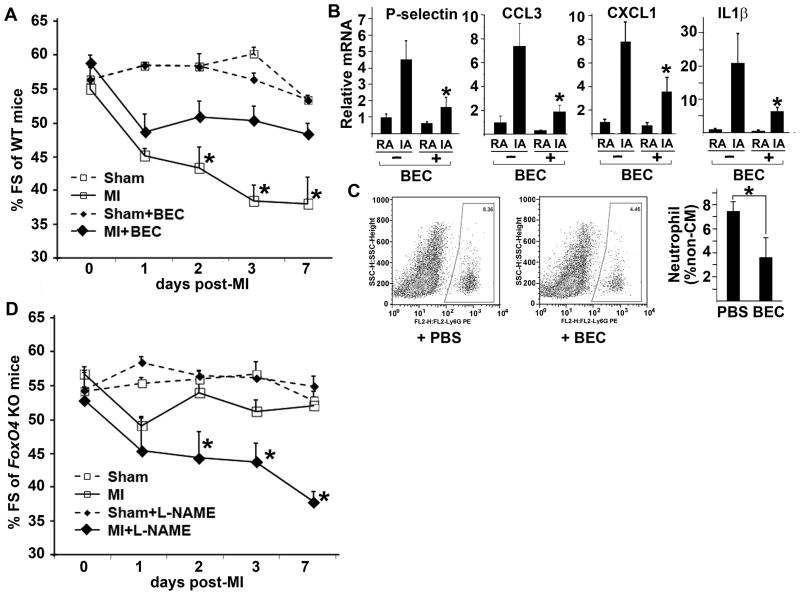
Figure 7. Arg1 may mediate the pathological function of FoxO4.
(A) WT mice were given IV vehicle PBS or the arginase inhibitor BEC at one day before, the day of MI, and one day after MI and sham surgeries. The arginase inhibitor had a similar cardioprotective effect post-MI (filled-diamond with solid line vs open-square with solid line) as inactivation of FoxO4 (open-square with solid line in D vs open-square with sold line in A) (n=5–8). *, BEC vs PBS-treated groups following MI. *, p<0.05. (B) mRNA of selective cytokines expressed in RA and IA of post-MI day 1 WT mouse hearts with or without BEC treatment (n=6–7), *, p<0.05, BEC vs PBS-treated groups. (C) FACS profiles of neutrophils from post-MI day 2 WT mouse hearts treated with or without BEC (n=5–7), *, p<0.05. (D) FoxO4 KO mice were given IV PBS or the NOS inhibitor L-NAME as the same time points as (A). The improved cardiac function due to inactivation of FoxO4 over that of WT mice (open-square with solid line in D vs open-square with solid line in A) is significantly reversed by L-NAME (filled diamond with solid line in D) (n=4–5). *, p<0.05. PBS vs L-NAME treated groups after MI.
The effect of arginase on endothelial function and myocardial ischemia injury has been attributed to its regulation of NO bioavailability25, 28–31. To test whether this may also be the underlying mechanism mediating the function of FoxO4/Arg1, we subjected the FoxO4 KO mice to the NOS inhibitor L-NAME at time of MI. Administration of L-NAME to FoxO4-null mice upon MI abolished the improvement of cardiac function in post-MI FoxO4-null mice over WT mice (Fig. 7D). However, no significant difference of cytokine expression was observed before or after L-NAME treatment (data not shown), suggesting that other NO-dependent mechanisms may be involved in the protective function of NO in post-MI remodeling.
DISCUSSION
The heart is a multi-cellular organ that contains not only myocytes but also non-myocytes, including cells comprising the vasculature (endothelial cells, smooth muscle cells), fat, connective tissue (fibroblasts), nerves and immune system. Emerging evidence indicates that these cardiac non-myocytes play active roles in healthy and diseased hearts. In this study, we have identified novel functions of FoxO4 in promoting adverse post-MI cardiac remodeling. Genetic deletion of FoxO4 in mice reduces inflammation and infarct size, and improves survival and cardiac function. Our studies suggest that these post-MI phenotypes are caused by FoxO4 mainly in the non-myocytes as cardiac mycoyte-deletion of FoxO4 had a minimal effect on post-MI remodeling. In particular, we demonstrated the role of endothelial FoxO4 in regulating post-MI inflammatory response. We show that downregulation of early post-MI inflammation and improvement of cardiac function can be recapitulated in mice with FoxO4 depletion in endothelial cells; knockdown of FoxO4 in endothelial cells attenuated the adhesion of monocytes in response to TNFα, which can be rescued by ectopic expression of Arg1. Although it remains to be tested, we speculate that the post-MI infarct size may be determined by FoxO4 in fibroblasts/fibroblast-like cells since, unlike global-deletion of FoxO4, FoxO4-deletion in cardiac mycoytes, endothelial cells, or immune cells did not change the infarct size. Alternatively, the post-MI infarct size may be determined by concerted action of FoxO4 in all cell types in the heart.
The unique pathophysiological function of FoxO4 in ischemic diseases
The pathophysiological function of FoxO4 in post-MI cardiac remodeling is different from that of other FoxO family members, FoxO1 and FoxO3. Inactivation of FoxO1 and FoxO3 in cardiac mycoytes resulted in decreased cardiac mycoyte survival and adverse post-MI remodeling 9 whereas deletion of FoxO4 in cardiac mycoytes did not. Sod2 and catalase are well known transcriptional targets of FoxO proteins and are implicated in FoxO1/O3-mediated cardiac mycoyte survival upon MI. Their expression in FoxO1/FoxO3 KO mice was downregulated following MI injury but remained unchanged in FoxO4 KO mice (Online Figure II). We also observed no significant difference in myocyte apoptosis between infarcted WT and Foxo4-null mouse hearts at the early time point of MI. These results suggest that FoxO4 has a unique function that is different from that of FoxO1/O3 in ischemic diseases. This is consistent with the emerging view that the function of FoxO proteins is context- and cell-type dependent 25–32. It is interesting to note that the expression of catalase and Sod2 in MI-injured WT mouse hearts is downregulated (Online Figure II). Although it remains to be determined, the relatively higher amount of antioxidant enzymes in FoxO4 KO mice compared to that in WT may also contribute to the overall improved outcome of post-MI LV remodeling in FoxO4 KO mice.
Mechanism(s) by which FoxO4 promotes pathological post-MI remodeling
In this study, we identified Arg1 as a novel FoxO4 transcriptional target that may mediate the pathological function of FoxO4 in post-MI remodeling. Arg1 is an emerging player in vascular pathology and ischemic heart diseases. We propose that endothelial Arg1 may mediate the pro-inflammatory function of FoxO4 in post-MI remodeling based on the following evidence: (1) upregulation of post-MI Arg1 is significantly attenuated in FoxO4 KO mouse heart with reduced neutrophil recruitment (Fig. 2) that can be recapitulated in FoxO4 ecKO mice (Fig. 3); (2) Arg1 is a direct transcriptional target of FoxO4 (Fig. 6); (3) knockdown of FoxO4 in ECs upregulated NO and suppressed monocyte adherence to a similar degree as that caused by knockdown of Arg1, and downregulation of monocyte adhesion in FoxO4 knockdown cells can be rescued by add-back of Arg1 (Fig. 5); (4) chemical inhibition of arginase activity in the early phase of the MI injury protects mice from adverse post-MI remodeling (Fig. 7A), and the NOS inhibitor L-NAME could reverse the beneficial effects of FoxO4-inactivation following MI-injury (Fig. 7D). These results suggest that downregulation of early post-MI inflammation in FoxO4-null mice may be due to the reduced neutrophil infiltration that was suppressed by increased endothelial NO as a result of decreased Arg1. That being said, it is possible that other aspects beyond Arg1 and NO signaling might also be involved in FoxO4-regulated post-MI inflammatory response since NOS inhibitor L-NAME treatment did not change the inflammatory cytokine expression in FoxO4 ecKO mice.
Arg1 is expressed ubiquitously, including endothelial cells, immune cells, and myofibroblasts (Online Figure VI). Arginase activity is also expressed in red blood cells that are involved in post-I/R cardiac functional recovery36. Although we focused on endothelial Arg1 in this study, Arg1 in cell types other than endothelial may be involved in FoxO4-regulated post-MI reparative mechanisms. Another caveat of our study is that FoxO4 KO, cKO, and ecKO mice are in different genetic backgrounds. Thus, one can’t compare different groups of KO mice to assess the relative contribution of FoxO4-regulated specific cell type to post-MI remodeling. It may be worthwhile in future studies to backcross all the cre-transgenic lines to the FVB background in which FoxO4f/f mice are maintained.
In our studies, we found that unlike global FoxO4 deletion, deletion of FoxO4 in endothelial cells did not reduce the infarct size, even though it resulted in decreased inflammation. This is not unexpected since it was previously shown that post-MI cardiac function and infarct size may be regulated by two different mechanisms. For example, genetic deletion of CCL2 or IL1R in mice, although improving cardiac function, did not reduce infarct size 37, 38. Conversely, Moelker et al. showed that bone marrow transplantation in a porcine model of I/R did not improve cardiac function although it reduced infarct size39. Inflammation may not extend ischemic myocyte injury but rather cause prolonged activation of myofibroblasts and/or enhanced activation of matrix metalloproteinase and subsequent matrix loss, resulting in reduced tensile strength of the area of infarct and loss of cardiac function2, 5.
Because global FoxO4-deletion resulted in a reduced infarct size, the inability of FoxO4-deletion in endothelial cells to change the scar size suggests that other cell type(s) in the heart may be involved. We speculate FoxO4 in fibroblast/myofibroblasts may play a role. Arg1 is expressed in myofibroblasts (Online Figure VI). The metabolic product of Arg1, ornithine, is a precursor for proline, an essential amino acid for collagen synthesis, and also precursor for polyamines23. Upregulation of Arg1 could result in more proline production and thereby lead to increased collagen deposition and fibrosis. It remains to be tested whether the smaller fibrotic scar in FoxO4-null mice is due to the downregulation of Arg1 in myofibroblasts or caused by decreased numbers of myofibroblasts. Upregulation of Arg1 also may promote proliferation of cardiac fibroblasts via increased polyamine synthesis27. FoxO4 may modulate the phenotypes of myofibroblasts via its role in TGFβ signaling pathway40. A concerted action of various cell types and yet-to-be identified new downstream effectors may also contribute to the mechanism(s) of FoxO4-regulated scar formation. In the future, it will be interesting to determine whether FoxO4 plays a role in the activation of cardiac fibroblasts, transdifferentiation and/or proliferation of myofibroblasts following MI.
FoxO4 as a therapeutic target in post-MI LV remodeling
Post-MI remodeling consists of both early pro-inflammatory and later anti-inflammatory phases. The biphasic and seemingly paradoxical functions of many molecules involved in post-MI remodeling often complicates the targeting strategy for minimizing the adverse remodeling process. For example, arginase inhibitors have been used in animal and human studies of cardiovascular diseases to improve NO production41. However, arginase may also play a protective role against post-ischemic injuries even though this remains to be firmly established. For example, arginase-mediated L-arginine depletion in M2 macrophages in the later anti-inflammatory phase of post-MI remodeling might suppress T cell immune response and promote scar formation. Because the cytokine IL4 is a potent stimulus for arginase expression in macrophages42, 43, we tested whether FoxO4-deletion has any effect on IL4-stimulated Arg1 expression in macrophages. No difference in IL4-induced Arg1 expression was observed between WT and Foxo4−/ − macrophages (M.Z. and Z.P.L., unpublished data), suggesting that FoxO4 is not involved in IL4-induced Arg1 transcription in macrophages. Thus, FoxO4 may offer an alternative target for inhibition of post-MI LV remodeling. Inactivation of FoxO4 could function as a brake to attenuate Arg1 expression in order to protect the heart against MI-induced injury via enhancement of NO production, reduced inflammation and fibrosis. However, since FoxO3/O1 has a beneficial function in post-MI remodeling, selective inhibitors of FoxO4 may be necessary.
Supplementary Material
Novelty and Significance.
What Is Known?
Inflammation is part of the reparative response to myocardial infarction (MI), but also participates in the pathogenesis of adverse post-MI left ventricular (LV) remodeling.
The extent of post-MI remodeling is an important predictor of mortality due to heart failure after infarction.
It has been suggested that recruitment of circulating neutrophils in the infarct may extend inflammatory injury.
What New Information Does This Article Contribute?
Global deletion of FoxO4 protects mice against adverse post-MI LV remodeling, and is associated with improved survival, preserved cardiac function, reduced scar size, and attenuated post-MI hypertrophic remodeling.
Deletion of FoxO4 in endothelial cells recapitulates some of the post-MI cardiac phenotypes of global FoxO4 knockout mice, and is associated with attenuated inflammation and reduced neutrophil number in the infarct area.
Endothelial FoxO4 can activate Arg1 expression in response to ischemia, resulting in decreased nitric oxide (NO) and impaired endothelial barrier function.
The post-MI inflammatory response participates in cardiac repair, but is also implicated in the pathogenesis of adverse remodeling and heart failure. Efforts to improve outcome in patients with myocardial infarction, by targeting the inflammatory reaction without disrupting repair have been unsuccessful. In this study, we report that endothelium-specific deletion of FoxO4 promotes the early inflammatory response following myocardial infarction by suppression of endothelial barrier function, a previously unrecognized function. We also document a novel link between FoxO4 and Arginase 1 (Arg1) and show that inhibition of arginase activity in MI can reduce post-MI inflammation and preserve post-MI cardiac function. Our results suggest that inactivation of FoxO4 may reduce inflammation and attenuate adverse remodeling, without disrupting repair. Thus, FoxO4 may be a potential therapeutic target for post-MI heart repair.
Acknowledgments
SOURCES OF FUNDING
Work in the laboratory of Z.P.L was supported by grants from the NIH (RO1 HL085749 and HL109471), American Heart Association, and Cancer prevention and research institute of Texas (CPRIT-RP120717); work in the laboratory of S.M.M. was supported by NIH RO1 GM057384. M.Z. was supported by an AHA post-doctoral fellowship 10POST3260014.
Nonstandard Abbreviations and Acronyms
- AAR
area at risk
- Arg1
Arginase 1
- BEC
S-(2-boronoethyl)-L-cysteine
- cFbs
cardiac fibroblasts
- cKO
cardiac mycoyte specific knockout
- CM
cardiac mycoyte
- DAF-FM
- DA
4-amino-5-methylamino-29,79-difluororescein diacetate
- ecKO
endothelial cell specific knockout
- eNOS
endothelial nitric oxide (NO) synthase
- FACS
flow cytometry
- Fox
fork head
- FoxO
fork head transcription factor O family
- FS
fractional shortening
- HAECs
human aortic endothelial cells
- HW/BW
heart weight/body weight
- I/R
ischemia-reperfusion
- KO
knockout
- LAD
left anterior descending coronary artery
- L-NAME
N5-[imino(nitroamino)methyl]-L-ornithine, methyl ester, monohydrochloride
- LV
left ventricle
- LVIDs
end systolic left ventricular internal diameter
- MI
myocardial infarction
- MMP9
matrix metalloproteinase 9
- NO
nitric oxide
- NOS
nitric oxide synthase
- ROS
reactive oxygen species
- SMC
smooth muscle cell
Footnotes
DISCLOSURES
None.
References
- 1.Gajarsa JJ, Kloner RA. Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev. 2011;16:13–21. doi: 10.1007/s10741-010-9181-7. [DOI] [PubMed] [Google Scholar]
- 2.Frangogiannis NG. Inflammation in cardiac injury, repair and regeneration. Curr Opin Cardiol. 2015;30:240–5. doi: 10.1097/HCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts R, DeMello V, Sobel BE. Deleterious effects of methylprednisolone in patients with myocardial infarction. Circulation. 1976;53:I204–6. [PubMed] [Google Scholar]
- 4.Christia P, Frangogiannis NG. Targeting inflammatory pathways in myocardial infarction. Eur J Clin Invest. 2013;43:986–95. doi: 10.1111/eci.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Akker F, Deddens JC, Doevendans PA, Sluijter JP. Cardiac stem cell therapy to modulate inflammation upon myocardial infarction. Biochim Biophys Acta. 2013;1830:2449–58. doi: 10.1016/j.bbagen.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–23. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- 7.Litt MR, Jeremy RW, Weisman HF, Winkelstein JA, Becker LC. Neutrophil depletion limited to reperfusion reduces myocardial infarct size after 90 minutes of ischemia. Evidence for neutrophil-mediated reperfusion injury. Circulation. 1989;80:1816–27. doi: 10.1161/01.cir.80.6.1816. [DOI] [PubMed] [Google Scholar]
- 8.Palatianos GM, Balentine G, Papadakis EG, Triantafillou CD, Vassili MI, Lidoriki A, Dinopoulos A, Astras GM. Neutrophil depletion reduces myocardial reperfusion morbidity. Ann Thorac Surg. 2004;77:956–61. doi: 10.1016/j.athoracsur.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 10.Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiac mycoyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–78. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu ZP, Wang Z, Yanagisawa H, Olson EN. Phenotypic modulation of smooth muscle cells through interaction of Foxo4 and myocardin. Dev Cell. 2005;9:261–270. doi: 10.1016/j.devcel.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Liang J, Castrillon DH, DePinho RA, Olson EN, Liu ZP. FoxO4 regulates tumor necrosis factor alpha-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene transcription. Mol Cell Biol. 2007;27:2676–2686. doi: 10.1128/MCB.01748-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou W, Cao Q, Peng Y, Zhang QJ, Castrillon DH, DePinho RA, Liu ZP. FoxO4 Inhibits NF-κ B and Protects Mice Against Colonic Injury and Inflammation. 2009;137:1403–14. doi: 10.1053/j.gastro.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu M, Zhang QJ, Wang L, Li H, Liu ZP. FoxO4 inhibits atherosclerosis through its function in bone marrow derived cells. Atherosclerosis. 2011;219:492–8. doi: 10.1016/j.atherosclerosis.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris SM., Jr Arginases and arginine deficiency syndromes. Curr Opin Clin Nutr Metab Care. 2012;15:64–70. doi: 10.1097/MCO.0b013e32834d1a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris CR, Gladwin MT, Kato GJ. Nitric oxide and arginine dysregulation: a novel pathway to pulmonary hypertension in hemolytic disorders. Curr Mol Med. 2008;8:620–32. doi: 10.2174/156652408786241447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao JK. Linking endothelial dysfunction with endothelial cell activation. J Clin Invest. 2013;123:540–1. doi: 10.1172/JCI66843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Research. 2009;37:W305–W311. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rau CD, Wang J, Avetisyan R, Romay MC, Martin L, Ren S, Wang Y, Lusis AJ. Mapping genetic contributions to cardiac pathology induced by Beta-adrenergic stimulation in mice. Circ Cardiovasc Genet. 2015;8:40–9. doi: 10.1161/CIRCGENETICS.113.000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Borne SW, van de Schans VA, Strzelecka AE, Vervoort-Peters HT, Lijnen PM, Cleutjens JP, Smits JF, Daemen MJ, Janssen BJ, Blankesteijn WM. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res. 2009;84:273–82. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- 21.Gitler Aaron D, Kong Yi, Choi John K, Zhu Yuan, Pear Warren S, Epstein Jonathan A. Tie2-Cre–Induced Inactivation of a Conditional Mutant Nf1 Allele in Mouse Results in a Myeloproliferative Disorder that Models Juvenile Myelomonocytic Leukemia. Pediatric Research. 2004;55:581–584. doi: 10.1203/01.PDR.0000113462.98851.2E. [DOI] [PubMed] [Google Scholar]
- 22.Moore-Morris T, Guimarães-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J, Evans SM. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124:2921–34. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris SM., Jr Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol. 2009;157:922–30. doi: 10.1111/j.1476-5381.2009.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bekpinar S, Gurdol F, Unlucerci Y, Develi S, Yilmaz A. Serum levels of arginase I are associated with left ventricular function after myocardial infarction. Clin Biochem. 2011;44:1090–3. doi: 10.1016/j.clinbiochem.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Jung C, Gonon AT, Sjöquist PO, Lundberg JO, Pernow J. Arginase inhibition mediates cardioprotection during ischaemia-reperfusion. Cardiovasc Res. 2010;85:147–54. doi: 10.1093/cvr/cvp303. [DOI] [PubMed] [Google Scholar]
- 26.Sediri Y, Kallel A, Ben Ali S, Omar S, Mourali MS, Elasmi M, Taieb SH, Sanhaji H, Feki M, Mechmeche R, Jemaa R, Kaabachi N. Association of rs2781666 G/T polymorphism of arginase I gene with myocardial infarction in Tunisian male population. Clin Biochem. 2010;43:106–9. doi: 10.1016/j.clinbiochem.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 27.Schlüter KD, Schulz R, Schreckenberg R. Arginase induction and activation during ischemia and reperfusion and functional consequences for the heart. Front Physiol. 2015;6:65. doi: 10.3389/fphys.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh M, Padhy G, Vats P, Bhargava K, Sethy NK. Hypobaric hypoxia induced arginase expression limits nitric oxide availability and signaling in rodent heart. Biochim Biophys Acta. 2014;1840:1817–24. doi: 10.1016/j.bbagen.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Bhatta A, Yao L, Toque HA, Shatanawi A, Xu Z, Caldwell RB, Caldwell RW. Angiotensin II-Induced Arterial Thickening, Fibrosis and Stiffening Involves Elevated Arginase Function. PLoS One. 2015;10:e0121727. doi: 10.1371/journal.pone.0121727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tratsiakovich Y, Yang J, Gonon AT, Sjöquist PO, Pernow J. Arginase as a target for treatment of myocardial ischemia-reperfusion injury. Eur J Pharmacol. 2013;720:121–3. doi: 10.1016/j.ejphar.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 31.Grönros J, Kiss A, Palmér M, Jung C, Berkowitz D, Pernow J. Arginase inhibition improves coronary microvascular function and reduces infarct size following ischaemia-reperfusion in a rat model. Acta Physiol (Oxf) 2013;208:172–9. doi: 10.1111/apha.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farré D, Roset R, Huerta M, Adsuara JE, Roselló L, Albà MM, Messeguer X. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003;31:3651–3. doi: 10.1093/nar/gkg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shemyakin A, Kövamees O, Rafnsson A, Böhm F, Svenarud P, Settergren M, Jung C, Pernow J. Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus. Circulation. 2012;126:2943–50. doi: 10.1161/CIRCULATIONAHA.112.140335. [DOI] [PubMed] [Google Scholar]
- 34.Kövamees O, Shemyakin A, Pernow J. Effect of arginase inhibition on ischemia-reperfusion injury in patients with coronary artery disease with and without diabetes mellitus. PLoS One. 2014;9:e103260. doi: 10.1371/journal.pone.0103260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darra E, Rungatscher A, Carcereri de Prati A, Podesser BK, Faggian G, Scarabelli T, Mazzucco A, Hallström S, Suzuki H. Dual modulation of nitric oxide production in the heart during ischaemia/reperfusion injury and inflammation. Thromb Haemost. 2010;104:200–6. doi: 10.1160/TH09-08-0554. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Gonon AT, Sjöquist PO, Lundberg JO, Pernow J. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc Natl Acad Sci U S A. 2013;110:15049–54. doi: 10.1073/pnas.1307058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–9. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 38.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moelker AD, Baks T, van den Bos EJ, van Geuns RJ, de Feyter PJ, Duncker DJ, van der Giessen WJ. Reduction in infarct size, but no functional improvement after bone marrow cell administration in a porcine model of reperfused myocardial infarction. Eur Heart J. 2006;27:3057–64. doi: 10.1093/eurheartj/ehl401. [DOI] [PubMed] [Google Scholar]
- 40.Gomis RR, Alarcón C, He W, Wang Q, Seoane J, Lash A, Massagué J. A FoxO-Smad synexpression group in human keratinocytes. Proc Natl Acad Sci U S A. 2006;103:12747–52. doi: 10.1073/pnas.0605333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pernow J, Jung C. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovasc Res. 2013;98:334–43. doi: 10.1093/cvr/cvt036. [DOI] [PubMed] [Google Scholar]
- 42.Gray MJ, Poljakovic M, Kepka-Lenhart D, Morris SM., Jr Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPbeta. Gene. 2005;353:98–106. doi: 10.1016/j.gene.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Sheldon KE, Shandilya H, Kepka-Lenhart D, Poljakovic M, Ghosh A, Morris SM., Jr Shaping the murine macrophage phenotype: IL-4 and cyclic AMP synergistically activate the arginase I promoter. J Immunol. 2013;191:2290–8. doi: 10.4049/jimmunol.1202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.