Abstract
The pulmonary circulation is a high-flow/low-pressure system, coupled with a flow generator chamber–the right ventricle–, which is relatively unable to tolerate increases in afterload. A right heart catheterization, using a fluid-filled, balloon-tipped Swan-Ganz catheter allows the measurement of all hemodynamic parameters characterizing the pulmonary circulation: the inflow pressure, an acceptable estimate the outflow pressure, and the pulmonary blood flow. However, the study of the pulmonary circulation as a continuous flow system is an oversimplification and a thorough evaluation of the pulmonary circulation requires a correct understanding of the load that the pulmonary vascular bed imposes on the right ventricle, which includes static and dynamic components. This is critical to assess the prognosis of patients with pulmonary hypertension or with heart failure.
Pulmonary compliance is a measure of arterial distensibility and, either alone or in combination with pulmonary vascular resistance, gives clinicians the possibility of a good prognostic stratification of patients with heart failure or with pulmonary hypertension. The measurement of pulmonary arterial compliance should be included in the routine clinical evaluation of such patients.
Keywords: pulmonary circulation, pulmonary arterial compliance
Physiology of the pulmonary circulation
The pulmonary circulation can be defined as a high-flow/low-pressure system in which the right ventricle represents a thin wall flow generator; meaning the right ventricle can accommodate large changes in volume loading (as it may happen in congenital, systemic-to-pulmonary shunts), but has a limited contractile reserve to match rapid increases in afterload (as may happen in acute pulmonary embolism).1,2
A right heart catheterization using a fluid-filled, balloon-tipped Swan-Ganz catheter allows the measurement of all hemodynamic parameters characterizing the pulmonary circulation. The inflow pressure is the pulmonary arterial pressure (PAP); the outflow pressure is the left atrial pressure (Pla), an acceptable estimate of which is provided by the pulmonary capillary wedge pressure (PCWP), obtained by inflating the balloon at the tip of the catheter (there is an average gradient of 3 mmHg between wedge pressure and end-diastolic left ventricular pressure); and the pulmonary blood flow (as measured by cardiac output, Q) is assessed by a number of clinical methods, such as thermodilution or the Fick principle.3
A simple and clinically useful description of the functional state of the pulmonary circulation is provided by the calculation of pulmonary vascular resistance (PVR):
Where (PAPm − PCWP) is the transpulmonary pressure gradient and Q is the cardiac output.
According to the Hagen-Poiseuille law, (which states that for laminar flows of a Newtonian fluid through rigid tubes, the resistance to flow in a single tube is equal to 8 times the product of the length of the tube times the viscosity, η, of the flow, divided by the product of pi (π) and the fourth power of the radius of the pipe itself) the calculation of pulmonary vascular resistance becomes:
Obviously, the arteries are not circular rigid tubes, the flow through human arteries is not laminar, and the blood is not an ideal fluid whose viscosity is independent of flow velocity. The fact that the radius in the equation is raised to the fourth power explains why PVR is extremely sensitive to even the smallest changes in diameter of the resistive pulmonary arterioles, which is also the site of most pulmonary vascular diseases. For this reason PVR is a good hemodynamic marker of the state of constriction or dilation of the small pulmonary vessels, useful to detect alterations of the calibre of the arteries due to changes in their tone and/or structure.
However, the study of the pulmonary circulation as a continuous flow system is an oversimplification since right ventricular contraction generates pulsatile pulmonary pressure and flow waves.4 The force generated from the right ventricle to propel the blood in the pulmonary circulation is consists of two components; a stationary component, required to produce a net forward flow, and an oscillatory component (about 25% of the total power generated) necessary to produce the pulsatile flow and pressure. Unlike the PVR, which represents the static component of the right ventricular afterload, the pulmonary impedance (PVZ) and compliance (PCa) include both static and dynamic pulsatile components.
The right ventricular afterload and its static and dynamic components
The concept of afterload is frequently used in routine clinical practice, but its measurement and quantification in vivo is somewhat difficult. Simplified models of the arterial circulation have been proposed, the most recognized being the 3-element Windkessel model introduced by Frank in 1899.5 The name derives from the old fashioned fire extinguishing pumps (“Windkessel” in German means air tank), where the rhythmic water output of the plunger strokes was transformed in a continuous water-jet downstream (Figure 1). This model describes the hemodynamics of the pulmonary and systemic arterial circulation: the resistance of small distal arteries and arterioles (describing the viscous and inertial properties of the vascular bed), the elastic properties (total compliance or capacitance) of the entire arterial system and the impedance of blood and large proximal arteries.6
Figure 1.
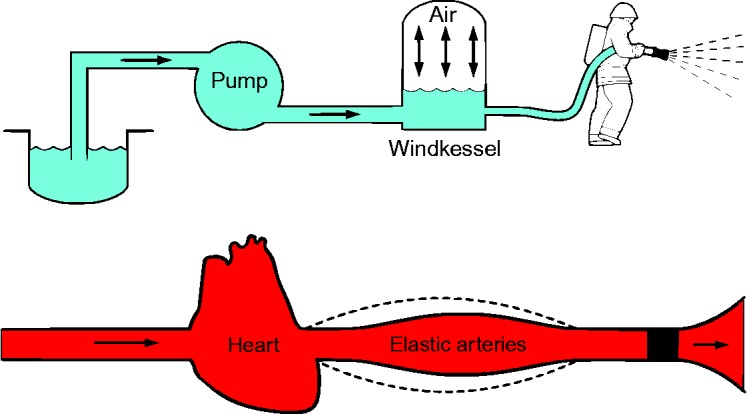
The Windkessel Effect explained. By Kurzon (Own work) [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons.
Pulmonary vascular resistance
The resistance of the model corresponds to the pulmonary vascular resistance (PVR); it is an extrapolation of the pulmonary circulation of Poiseuille's law, which completely ignores the pulsatility imposed by the cardiac cycle. PVR is the parameter most frequently used as a measure of right ventricular afterload because it is easy to define and interpret, and has a clear physiological significance - being highly dependent on the diameter of resistance arterioles and small distal arteries.
However, there is undoubtedly a limitation in the calculation of PVR using the formula PVR = (PAPm − PCWP)/Q. While in healthy subjects the measurement of PCWP may be a reasonable estimate of Pla, in patients with pulmonary disease flow through the capillaries it is reduced to zero at a positive pressure, which is substantially different from PCWP (so called “critical pressure”). As a consequence, a single point determination of pressure and flow is insufficient for an accurate calculation of PVR, because the assumption of linearity and zero-crossing of the (PAPm − Pla)/Q is incorrect. In cases such as this, multipoint pressure and flow coordinates should be used to avoid this error.7,8
Pulmonary vascular impedance
Pulmonary artery input impedance (PVZ), is describes the ratio of the arterial pressure waveform to the blood flow waveform over an entire cardiac cycle and thus includes both mean and pulsatile data. The calculation of PVZ requires synchronized recordings of high-fidelity pulmonary artery pressure and flow curves, a spectral analysis of pressure and flow waves and a mathematical reworking (via Fourier analysis) of the relationship between these two elements to derive a PVZ spectrum, which is expressed as a ratio of pressure and flow moduli and a phase angle, both as a function of frequency9:
The impedance spectra have a similar pattern in systemic and pulmonary circulation, with a high 0-Hertz value (Z0), followed by a decrease and oscillations at higher frequencies. Z0 is the input impedance in the absence of flow oscillations and thus corresponds to PVR, being essentially a measure of distal pulmonary vascular resistance. The average of the impedance modulus at higher harmonics is used as an estimation of the characteristics impedance (Zc), i.e. the input impedance in the absence of wave reflections. Zc is determined by the ratio of the stiffness of the proximal arteries and fluid inertia. Another parameter that can be derived by the impedance spectrum is pulse wave reflection.
From a clinical point of view, the relevance of the determination of PVZ in patients with pulmonary hypertension or heart failure is yet unknown.
Pulmonary arterial compliance
Compliance is a measure of arterial distensibility. From a mechanical point of view, it refers to the structural changes occurring in the pulmonary vasculature as a consequence of pulmonary diseases, which make the vessels stiffer than normal. There are a huge number of direct and indirect methods to quantify vessel stiffness and comprehensive and focused reviews have already been published in the literature, which are summarized in Table 1.10 Direct measurements of pulmonary vascular stiffness can be obtained in vivo quantifying arterial diameter as a function of pressure and generating pressure-diameter curves (the stiffer the vessel, the steeper the slope of the curves). In vitro, this tests allows a fine check of direct analysis of the material properties of the vessel as the effect of a single parameter on stiffness can be determined (e.g. collagen or elastin content, tone of smooth muscle cells, etc.).
Table 1.
A list of parameters used to measure arterial stiffness.
| Definition (unit) | Formula | Notes |
| Pressure-strain modulus, Eρ (Pa) |
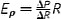
|
R can also be replaced with diameter (D). Extrinsic mechanical property |
| Elastic modulus, E (Pa) |
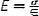
|
The slope of stress-strain (σ − ε) curve, assuming linear homogenous, incompressible wall material. Thin-wall or thick-wall assumptions lead to different calculations of σ and ε. Intrinsic material property |
| Incremental elastic modulus (1), E inc (Pa) |
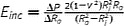
|
R o and R i are external and internal radii and can be replaced with corresponding diameters (D). Assuming locally linear, homogenous, incompressible (if ν = 0.5) wall material. Thick wall assumption. Intrinsic material property |
| Incremental elastic modulus (2), E inc (Pa) |
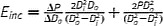
|
Modified E inc assuming orthotropic cylindrical tube. Intrinsic material property |
| Stiffness constant, β (dimensionless) |
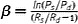
|
P s and P d are systolic and diastolic pressures. Radius R can be replaced with diameter (D). Assume homogenous, incompressible, isotropic material. Extrinsic mechanical property |
| Distensibility, D, (1/Pa) |
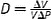
|
V (volume) can be replaced with A (area). Extrinsic mechanical property |
| Compliance, C, (m 3/Pa) |
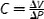
|
V (volume) can be replaced with A (area). Extrinsic mechanical property |
P = pressure, R = radius, D = diameter, V = volume, A = area. Adapted from Wang Z, Chesler NC. Pulmonary vascular wall stiffness: An important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ. 2011;1(2):212–223.
The simple hemodynamic measurements obtained during routine right heart catheterization allow calculation of the compliance of the entire pulmonary circulation – that is the capacity of all arteries and arterioles to accumulate blood in systole and release it in diastole. If we assume that the pulmonary circulation is closed at the peripheral level, then the increase in pulmonary pressure (PP) determined by a single cardiac systole (stroke volume, SV) depends on the compliance of the vascular bed according to the formula:
PP is directly proportional to SV and inversely proportional to PCa; in other words, the lower the pulmonary compliance, the greater the increase in pressure for a given SV.
In vivo, other parameters that require less invasive information have been introduced to investigate pulmonary arterial stiffness during the routine clinical evaluations of patients11,12 (Table 2). An example is the relative change of vessel area, measured at cardiac magnetic resonance, which does not require simultaneous pressure measurements. Obviously this parameter does not express a structural property, but is a simple geometrical property of the vessel. However, imaging data may be combined with pressure data obtained at right heart catheterization (with the limit of the two exams not being simultaneous) to obtain the compliance of the proximal pulmonary artery (substituting volume changes with area changes).
Table 2.
A list of parameters which can be calculated to measure pulmonary compliance in vivo, using hemodynamic and/or imaging data.
| Parameter | Units | Formula | Definition |
| Pulsatility | % | maxA − minA/minA × 100 | Relative change in lumen area during the cardiac cycle |
| Compliance | mm2/mm Hg | [(maxA − minA)/PP] | Absolute change in lumen area for a given change in pressure |
| Capacitance | mm3/mm Hg | SV/PP | Change in volume associated with a given change in pressure |
| Distensibility | %/mm Hg | [(maxA − minA)/PP × minA] × 100 | Relative change in lumen area for a given change in pressure |
| Elastic modulus | mm Hg | PP × minA/(maxA − minA) | Pressure change driving a relative increase in lumen area |
| Stiffness index β | N/A | Ln(sPAP/dPAP)/[(maxA − minA)/minA] | Slope of the function between distending arterial pressure and arterial distension |
Adapted from Sanz J, Kariisa M, Dellegrottaglie S, Prat-González S, Garcia MJ, Fuster V, Rajagopalan S. Evaluation of Pulmonary Artery Stiffness in Pulmonary Hypertension With Cardiac Magnetic Resonance. JACC: Cardiovascular Imaging. 2009;2(3):286–295.
The inverse relationship between pulmonary vascular resistance and pulmonary arterial compliance
A compliant vascular bed allows the arteries to expand passively during systole and to return to original conformation during diastole. This has two important effects: 1) compliant arteries accumulate blood volume pumped in systole and release this volume during diastole, causing a continuous peripheral blood flow during the entire cardiac cycle; 2) compliant arteries attenuate pressure oscillations, so that the diastolic pressure in the pulmonary artery decreases significantly less than in the right ventricle.6
As a matter of fact, the decrease in pulmonary artery pressure during diastole depends both on PVR and on PCa. Elevated PVR slows down flow through the peripheral circulation, while at the same time a preserved PCa determines a good volume accumulation in systole and release in diastole, also contributing to a slow reduction in diastolic pulmonary artery pressure. This combined effect can be summarized by the product of PVR and PCa which results in a time (units = seconds), the time constant tau “τ”, called RC-time (R was borrowed from physics as a resistor-capacitor):
which represents the time constant of the mono-exponential pressure decay of pulmonary artery pressure in diastole, as shown in Figure 2.13
Figure 2.
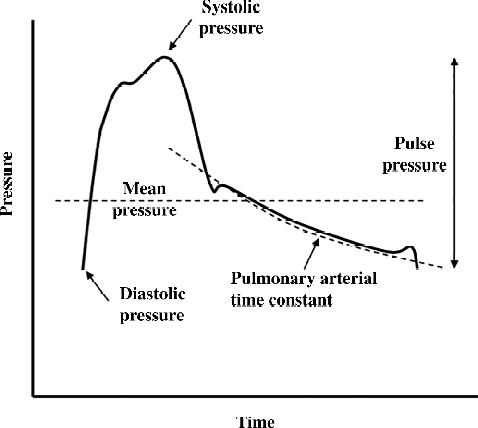
Illustration of the pulmonary artery pressure curve showing the significance of the pulmonary arterial time constant (RC-time). Adapted from Ross RVM, Toshner MR, Soon E, Naeije R, Pepke-Zaba J. Decreased time constant of the pulmonary circulation in chronic thromboembolic pulmonary hypertension. American Journal of Physiology - Heart and Circulatory Physiology. 2013;305(2):H259–H264.
A few decades ago, Reuben first described this interesting inverse relationship between PCa and PVR in physiological and pathological pulmonary circulation.14 In recent years, to understand the characteristics of the pulmonary hemodynamics in pulmonary arterial hypertension, these concepts were re-addressed and new studies confirmed the inverse hyperbolic correlation between the two parameters and the constancy of the product of PVR and PCa.
There are two reasons that might explain the constancy of RC-time: 1) the first is based on the anatomical and physiological properties of arterial vessels: an increase of vascular resistance leads to an increase in intravascular pressure and, according to the non-linear elasticity of the arteries, the high internal pressure results in an increase in the rigidity (stiffness) and a reduction in arterial compliance12,15; 2) the second is based on the peculiarity of the pulmonary circulation; it is well known that the pulmonary artery with the two main branches account for only 15–20% of the total pulmonary arterial compliance, the rest being distributed throughout the entire pulmonary arterial vessels.6,16 This would make the correlation between resistance and compliance anatomically inseparable throughout the entire pulmonary vascular bed, resulting in a constant RC-time. However, the interpretation of these data requires caution since the picture might be more complicated than depicted, and this issue is still a matter of debate. In post-capillary pulmonary hypertension, the RC time has been demonstrated to be shorter than in pre-capillary pulmonary hypertension.18 A shorter RC time has also been calculated in normal subjects as compared to patients with pulmonary arterial hypertension.15 Finally, in most studies a significant scatter of the data is observed around the hyperbolic curve fit, questioning the “constancy” of RC-time.18
Further studies are needed to understand the usefulness of the calculation of RC-time for a physiological characterization of the pulmonary circulation.
Why we should measure PCa in pulmonary arterial hypertension
Pulmonary arterial hypertension is mainly due to a severe and progressive remodeling of the vascular bed: proliferation of endothelial cells and of smooth muscle cells leading to a thickening of the tunica intima and of the tunica media, change in composition of the extracellular matrix protein with accumulation of proteins such as collagen, formation of plexiform lesions and occlusion of small pulmonary arterioles.19 The mechanical consequences of these processes are; an increase in pulmonary vascular resistance, an increase in stiffness, and a reduction in pulmonary arterial compliance. In other words, there is a marked increase in right ventricular afterload,20 causing a progressive impairment in RV function. The leading cause of death for this disease is, in fact, right heart failure.21
In clinical practice and in randomized clinical trials, the right ventricular afterload is most frequently described in terms of pulmonary vascular resistance, and an improvement in right ventricular afterload following specific therapy is described in terms of a reduction in pulmonary vascular resistance. As stated above, PVR constitutes only one of the three components of the ventricular afterload. PVZ is a better descriptor of right ventricular load; however, usage of this variable is limited by the high complexity of measurement, analysing both the frequency and the time domain, and the difficulty of transferring the results into the clinical practice. There are indeed data in the literature suggesting that PVZ can be calculated in routine clinical practice using Doppler echocardiography to record the pulmonary flow, and that this measure could be more useful than PVR as a prognostic parameter.22,23
A reduced PCa is a powerful marker of poor prognosis in idiopathic pulmonary arterial hypertension. This has been demonstrated both for the standard measure of hemodynamic compliance, SV/PP, and for a non-invasive, Doppler echocardiographic estimate of pulmonary capacitance.24,25 However, the use of echocardiographic parameters to calculate compliance is not frequent; on the contrary, the use of cardiac magnetic resonance imaging has been proposed to measure pulmonary arterial stiffness in patients with pulmonary arterial hypertension. Several studies have shown that PCa calculated using CMR is significantly associated with survival.11,12,15
Importantly, it has been suggested that the combination of PVR with PCa may outline the right ventricular afterload better than each of the two variables separately.10,26
Figure 3 shows the inverse hyperbolic relationship between PVR and PCa. This relationship predicts that in the early stages of pulmonary arterial hypertension a small increase in pulmonary vascular resistance is accompanied by a fairly significant reduction in compliance; on the contrary, in more advanced stages of the disease, vascular stiffness has already reached the maximum limits and any further increase in PVR is not associated with further reduction in PCa. Therefore it seems that the earliest stages of the disease could be better diagnosed through an alteration in PCa, rather than through the increase in PVR.
Figure 3.
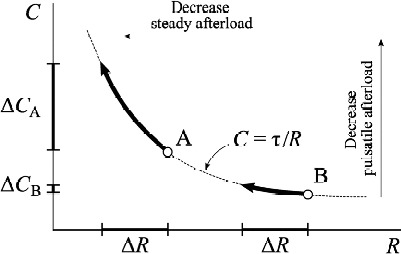
Inverse hyperbolic relationship between PVR and PCa. The figure shows the changes in PCa for a similar reduction in PVR in patients with different PVR values at baseline. Adapted from Jan-Willem Lankhaar et al. Eur Heart J 2008;29:1688–1695.
On the other hand, this relationship predicts that patients with higher baseline PVR will require a greater reduction of the PVR to achieve the same increase of PCa. We may also predict a greater reduction in right ventricular afterload and a consequent greater hemodynamic (and possibly clinical) improvement if the reduction of PVR is accompanied by an increase of Ca, rather than in case of reduction of PVR only. In fact, the combination of PVR and PCA in a single parameter was better associated with an increase in cardiac index after the beginning of therapy13 (Figure 4).
Figure 4.
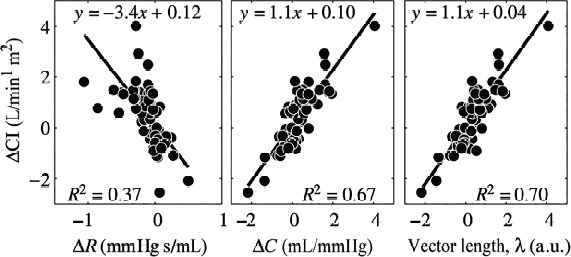
The vector lambda better explain changes in cardiac index after therapy in pulmonary hypertension patients. Adapted from Jan-Willem Lankhaar et al. Eur Heart J 2008;29:1688–1695.
Why we should measure PCa in heart failure
In heart failure patients, the attention of researchers initially focused on the relationship between outcome and systemic arterial compliance. In patients with heart failure and left ventricular dysfunction, a reduced of thoracic aorta distensibility was found to be associated with reduced exercise tolerance and poor outcome.27,28 Recently, the attention shifted to the pulmonary circulation. In a huge clinical database including right heart hemodynamic data of patients with pre-capillary and post-capillary pulmonary hypertension, the presence of elevated pulmonary capillary wedge pressure was found to have a large impact on the relationship between PCa and PVR, since PCa was lower for any PVR values. This would mean that in pulmonary hypertension due to heart failure the pulsatile, relative to the resistive load is increased, and the net right ventricular afterload is therefore higher at any level of PVR17 (Figure 5). The authors hypothesized that this mechanism might be an important determinant of right ventricular dysfunction in patients with pulmonary hypertension secondary to heart failure. The prognostic role of PCa in heart failure has been underlined in several studies. In a population of patients with heart failure due to left ventricular systolic dysfunction, the presence of pulmonary hypertension was associated with lower PCa, and PCa was useful to refine risk assessment.29
Figure 5.
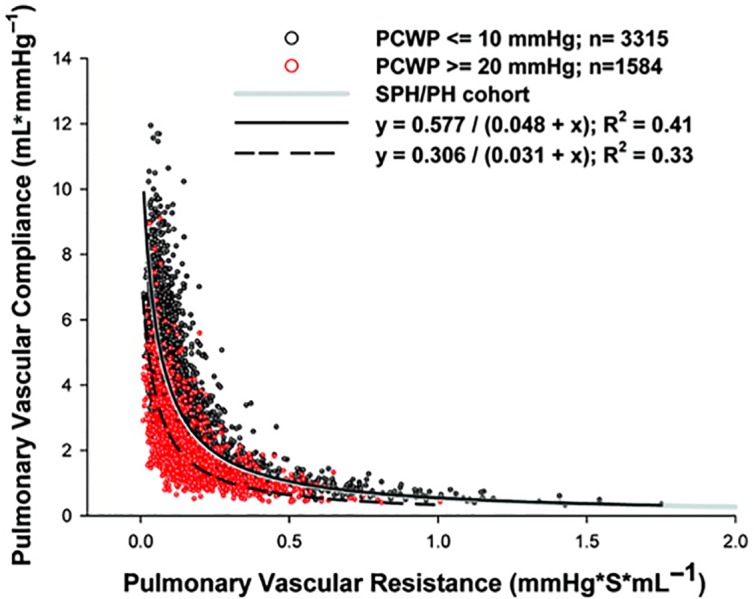
In patients with elevated pulmonary capllary wedge pressure (red points) the relationship betwee PVR and PCa is shifted downward. Adapted from Ryan J. Tedford et al. Circulation. 2012;125289-297.
In a similar population of patients with advanced heart failure, PCa but not PVR was an independent predictor of prognosis in multivariate analysis.30 Finally, in a population of heart failure due to left ventricular systolic dysfunction, PCa significantly outperformed all hemodynamic parameters and emerged as the strongest predictor of cardiovascular death or hard events, regardless of the presence or absence of pulmonary hypertension.31
This finding is highly relevant from a clinical perspective, since it suggests that structural changes of large vessels might precede the increase in PVR in patients with heart failure; this is in agreement with the hypothesis formulated in pulmonary arterial hypertension patients that the early phase of pulmonary vascular disease cannot be detected by right heart catheterization with elevation of PVR but only with a reduction in PCa (Figure 6).10 However, we need histologic data from pulmonary arteries to confirm confirm this hypothesis of early alteration in vessel stiffness in heart failure patients with no pulmonary hypertension.32
Figure 6.
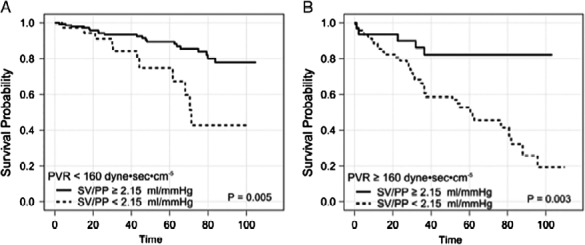
In patients with heart failure, a PCa < 2.15 is associated with poorer survival, regardless of presence or absence of pulmonary hypertension. Adapted from Chest. 2014;145(5):1064–1070. doi:10.1378/chest.13-1510.
Why we should measure PCa in chronic thromboembolic pulmonary hypertension
The study of patients in whom pulmonary hypertension is due to thromboembolism occluding pulmonary arteries (CTEPH) and the comparison with data obtained in patients in whom a distal vasculopathy causes idiopathic pulmonary arterial hypertension (IPAH) has long fascinated researchers, as this is a perfect model to test the relative importance of the pulsatile and of the steady components of opposition to flow in the pulmonary circulation. In fact, the abnormal mechanical properties and geometry of proximal pulmonary arteries in CTEPH patients would theoretically lead to earlier and enhanced wave reflection, lower PCa, and different pulmonary pulse pressure as compared to IPAH patients having comparable mean pulmonary pressure. The hypothesis that this could help clinicians in the differential diagnosis between IPAH and CTEPH patients has not been confirmed.33,34 However, the study of the relationship between PVR and PCa in patients with pulmonary hypertension of different aetiology is extremely helpful for a better understanding of the physiology of the pulmonary circulation.35,36
The evaluation of PCa may help to explain the symptoms (dyspnoea) complained of by patients with chronic thromboembolic pulmonary hypertension who have undergone pulmonary endarterectomy. Endarterectomy is the treatment of choice to relieve pulmonary artery obstruction in such patients.37 In expert centers surgery is associated with low mortality, even in patients with distal segmental chronic thromboembolic disease.38 On the other hand, patients can continue to suffer from a limitation in exercise capacity despite improvement or even normalization of cardiac output and mean pulmonary artery pressure after successful surgery.39
In thirteen successfully-operated patients complaining of persistent dyspnoea, a decreased PCa during effort was shown to be associated with a limited exercise capacity.40 More recently, hemodynamic, functional and echocardiographic parameters were retrospectively analysed in a large population of patients affected by thromboembolic pulmonary hypertension who underwent surgery in a single center and were followed-up for 5 years.41 In a multivariable model PCa was found to be an independent predictor of exercise capacity, in association with other parameters such as age, gender, tricuspid annular plane systolic excursion, arterial oxygen tension and carbon monoxide transfer factor.
Conclusions
A thorough evaluation of the pulmonary circulation requires a correct understanding of the load that the pulmonary vascular bed imposes on the right ventricle, which includes static and dynamic components. Pulmonary compliance is a measure of arterial distensibility and, either alone or in combination with standard pulmonary vascular resistance, it gives to clinicians the possibility of a good prognostic stratification of patients with heart failure or with pulmonary hypertension. Despite the fact that our knowledge on pulmonary circulation has greatly improved over the last few years, there are a lot of issues which need to be further explored. In particular, to identify the cells and the cellular signaling pathways that control pulmonary arterial stiffening and to assess the efficiency of the entire right ventricle–pulmonary arterial system.
Competing interests
The authors have no competing interests.
Funding sources
The work has not been funded.
Authors' contributions
Each of the authors listed on the paper has given a substantive intellectual contribution to the work and all have given final approval to the manuscript version submitted for publication.
References
- 1.Naeije R. Physiology of the pulmonary circulation and the right heart. Curr Hypertens Rep. 2013;15:623–631. doi: 10.1007/s11906-013-0396-6. [DOI] [PubMed] [Google Scholar]
- 2.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB, National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure Right ventricular function and failure: Report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 3.Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow-directed catheter. N Engl J Med. 1970;283:447–451. doi: 10.1056/NEJM197008272830902. [DOI] [PubMed] [Google Scholar]
- 4.Nichols WW, O'Rourke MF, editors. Theoretical, Experimental and Clinical Principles. 5th edition. London: Hodder Arnold; 2005. McDonald's blood flow in arteries. [Google Scholar]
- 5.Frank O. The basic shape of the arterial pulse. First treatise: Mathematical analysis. 1889. J Mol Cell Cardiol. 1990;22:255–277. doi: 10.1016/0022-2828(90)91460-o. [DOI] [PubMed] [Google Scholar]
- 6.Saouti N, Westerhof N, Postmus PE, Vonk-Noordegraaf A. The arterial load in pulmonary hypertension. Eur Respir Rev. 2010;19:197–203. doi: 10.1183/09059180.00002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janicki JS, Weber KT, Likoff MJ, Fishman AP. The pressure-flow response of the pulmonary circulation in patients with heart failure and pulmonary vascular disease. Circulation. 1985;72:1270–1278. doi: 10.1161/01.cir.72.6.1270. [DOI] [PubMed] [Google Scholar]
- 8.Chemla D, Castelain V, Hervé P, Lecarpentier Y, Brimioulle S. Haemodynamic evaluation of pulmonary hypertension. Eur Respir J. 2002;20:1314–1331. doi: 10.1183/09031936.02.00068002. [DOI] [PubMed] [Google Scholar]
- 9.O'Rourke MF. Vascular impedance in studies of arterial and cardiac function. Physiol Review. 1982;62:570–623. doi: 10.1152/physrev.1982.62.2.570. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Chesler NC. Pulmonary vascular wall stiffness: An important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ. 2011;1:212–223. doi: 10.4103/2045-8932.83453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lankhaar JW, Noordegraaf AV, Marcus JT. A computed method for non invasive MRI assessment of pulmonary arterial hypertension. J Appl Physiol. 2004;97:794–795. doi: 10.1152/japplphysiol.00225.2004. [DOI] [PubMed] [Google Scholar]
- 12.Sanz J, Kariisa M, Dellegrottaglie S, Prat-González S, Garcia MJ, Fuster V, Rajagopalan S. Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. JACC Cardiovasc Imaging. 2009;2:286–295. doi: 10.1016/j.jcmg.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Lankhaar JW, Westerhof N, Faes TJ, Gan CT, Marques KM, Boonstra A, van den Berg FG, Postmus PE, Vonk-Noordegraaf A. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J. 2008;29:1688–1695. doi: 10.1093/eurheartj/ehn103. [DOI] [PubMed] [Google Scholar]
- 14.Reuben SR. Compliance of the pulmonary arterial system in disease. Circ Res. 1971;29:40–50. doi: 10.1161/01.res.29.1.40. [DOI] [PubMed] [Google Scholar]
- 15.Gan CT, Lankhaar JW, Westerhof N, Marcus JT, Becker A, Twisk JW, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary hypertension. Chest. 2007;132:1906–1912. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 16.Saouti N, Westerhof N, Helderman F, Marcus JT, Stergiopulos N, Westerhof BE, Boonstra A, Postmus PE, Vonk-Noordegraaf A. RC-time constant of single lung equals that of both lungs together: A study in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;297:H2154–H2160. doi: 10.1152/ajpheart.00694.2009. [DOI] [PubMed] [Google Scholar]
- 17.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125:289–297. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tedford RJ. Determinants of right ventricular afterload (2013 Grover conference series) Pulmonary Circulation. 2014;4(2):211–219. doi: 10.1086/676020. doi:10.1086/676020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43(Suppl. 12):25S–32S. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 20.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(Suppl. 12):13S–124. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 21.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 22.Brimioulle S, Naeije R, Vachiéry JL. Feasibility of routine pulmonary arterial impedance measurements in pulmonary hypertension. Chest. 2004;125:2121–2128. doi: 10.1378/chest.125.6.2121. [DOI] [PubMed] [Google Scholar]
- 23.Hunter KS, Lee PF, Lanning CJ, Ivy DD, Kirby KS, Claussen LR, Chan KC, Shandas R. Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. Am Heart J. 2008;155:166–174. doi: 10.1016/j.ahj.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 25.Mahapatra S, Nishimura RA, Oh JK, McGoon MD. The prognostic value of pulmonary vascular capacitance determined by Doppler echocardiography in patients with pulmonary arterial hypertension. J Am Soc Echocardiogr. 2006;19:1045–1050. doi: 10.1016/j.echo.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Lankhaar JW, Westerhof N, Faes TJ, Marques KM, Marcus JT, Postmus PE, Vonk-Noordegraaf A. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2006;291:H1731–H1737. doi: 10.1152/ajpheart.00336.2006. [DOI] [PubMed] [Google Scholar]
- 27.Domanski MJ, Mitchell GF, Norman JE, Exner DV, Pitt B, Pfeffer MA. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J Am Coll Cardiol. 1999;33:951–958. doi: 10.1016/s0735-1097(98)00679-2. [DOI] [PubMed] [Google Scholar]
- 28.Bonapace S, Rossi A, Cicoira M, Franceschini L, Golia G, Zanolla L, Marino P, Zardini P. Aortic distensibility independently affects exercise tolerance in patients with dilated cardiomyopathy. Circulation. 2003;107:1603–1608. doi: 10.1161/01.CIR.0000051458.39176.43. [DOI] [PubMed] [Google Scholar]
- 29.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction. J Am Coll Cardiol HF. 2013;1:290–299. doi: 10.1016/j.jchf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Dupont M, Mullens W, Skouri HN, Abrahams Z, Wu Y, Taylor DO, Starling RC, Tang WH. Prognostic role of pulmonary arterial capacitance in advanced heart failure. Circ Heart Fail. 2012;5:778–785. doi: 10.1161/CIRCHEARTFAILURE.112.968511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pellegrini P, Rossi A, Pasotti M, Raineri C, Cicoira M, Bonapace S, Dini FL, Temporelli PL, Vassanelli C, Vanderpool R, Naeije R, Ghio S. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest. 2014;145:1064–1070. doi: 10.1378/chest.13-1510. [DOI] [PubMed] [Google Scholar]
- 32.Delgado JF, Conde E, Sánchez V, López-Ríos F, Gómez-Sánchez MA, Escribano P, Sotelo T, Gómez de la Cámara A, Cortina J, de la Calzada CS. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail. 2005;7:1011–1016. doi: 10.1016/j.ejheart.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama Y, Nakanishi N, Hayashi T, Nagaya N, Sakamaki F, Satoh N, Ohya H, Kyotani S. Pulmonary artery reflection for differentially diagnosing primary pulmonary hypertension and chronic pulmonary thromboembolism. J Am Coll Cardiol. 2001;38:214–218. doi: 10.1016/s0735-1097(01)01365-1. [DOI] [PubMed] [Google Scholar]
- 34.Castelain V, Hervé P, Lecarpentier Y, Duroux P, Simonneau G, Chemla D. Pulmonary artery pulse pressure and wave reflection in chronic pulmonary thromboembolism and primary pulmonary hypertension. J Am Coll Cardiol. 2001;37:1085–1092. doi: 10.1016/s0735-1097(00)01212-2. [DOI] [PubMed] [Google Scholar]
- 35.Pagnamenta A, Vanderpool R, Brimioulle S, Naeije R. Proximal pulmonary arterial obstruction decreases the time constant of the pulmonary circulation and increases right ventricular afterload. J Appl Physiol. 2013;114:1586–1592. doi: 10.1152/japplphysiol.00033.2013. [DOI] [PubMed] [Google Scholar]
- 36.MacKenzie Ross RV, Toshner MR, Soon E, Naeije R, Pepke-Zaba J. Decreased time constant of the pulmonary circulation in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H259–H264. doi: 10.1152/ajpheart.00128.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer E, Jenkins D, Lindner J, D'Armini A, Kloek J, Meyns B, Ilkjaer LB, Klepetko W, Delcroix M, Lang I, Pepke-Zaba J, Simonneau G, Dartevelle P. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141:702–710. doi: 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 38.Madani MM, Auger WR, Pretorius V, Sakakibara N, Kerr KM, Kim NH, Fedullo PF, Jamieson SW. Pulmonary endarterectomy: Recent changes in a single institution's experience of more than 2.700 patients. Ann Thorac Surg. 2012;94:97–103. doi: 10.1016/j.athoracsur.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Corsico AG, D'Armini AM, Cerveri I, Klersy C, Ansaldo E, Niniano R, Gatto E, Monterosso C, Morsolini M, Nicolardi S, Tramontin C, Pozzi E, Viganò M. Long-term outcome after pulmonary endarterectomy. Am J Respir Crit Care Med. 2008;178:419–424. doi: 10.1164/rccm.200801-101OC. [DOI] [PubMed] [Google Scholar]
- 40.Bonderman D, Martischnig AM, Vonbank K, Nikfardjam M, Meyer B, Heinz G, Klepetko W, Naeije R, Lang IM. Right ventricular load at exercise is a cause of persistent exercise limitation in patients with normal resting pulmonary vascular resistance after pulmonary endarterectomy. Chest. 2011;139:122–127. doi: 10.1378/chest.10-0348. [DOI] [PubMed] [Google Scholar]
- 41.Ghio S, Morsolini M, Corsico A, Klersy C, Mattiucci G, Raineri C, Scelsi L, Vistarini N, D'Armini AM. Pulmonary arterial compliance and exercise capacity after pulmonary endarterectomy. Eur Respir J. 2014;43:1403–1409. doi: 10.1183/09031936.00195313. [DOI] [PubMed] [Google Scholar]


