Abstract
Background: Calcific aortic valve stenosis (CAVS) is seen in a large proportion of individuals over 60 years. It is an active process, influenced by lipid accumulation, mechanical stress, inflammation, and abnormal extracellular matrix turnover. Various biomarkers (BMs) are studied, as regards mechanisms, diagnosis and prognosis. Methods: In the calcified valves calcium deposition, elastin fragmentation and disorganization of cellular matrix were assessed, together with expression of OPN, OPG, osteocalcin (OCN) and RL2.
We prospectively studied the following serum BMs in 60 patients with CAVS and compared them to 20 healthy controls, free from any cardiac disease: Matrix metalloproteinases (MMP) 2 and 9 and tissue inhibitor of metalloproteinase 1 (TIMP1), which regulate collagen turnover, inflammatory factors, i.e. tumor necrosis factor a (TNFa), interleukin 2 (IL2), transforming growth factor β1 (TGF-β1) which regulates fibrosis, fetuin-A (fet-A), osteopontin (OPN), osteoprotegerin (OPG), sclerostin (SOST), and relaxin-2 (RL2) which positively or negatively regulate calcification. Monocyte chemoattractant protein 1 (MCP-1) which regulates migration and infiltration of monocytes/macrophages was also studied as well as malondialdehyde (MDA) an oxidative marker. Results: Extent of tissue valve calcification (Alizarin Red stain) was negatively correlated with tissue elastin, and RL2, and positively correlated with tissue OCN and serum TIMP1 and MCP-1 and negatively with MMP9.
Tissue OCN was positively correlated with OPN and negatively with the elastin. Tissue OPN was negatively correlated with elastin and OPG. Tissue OPN OPG and RL2 were not correlated with serum levels In the serum we found in patients statistically lower TIMP1, fet-A and RL2 levels, while all other BMs were higher compared to the healthy group. Positive correlations between SOST and IL2, OPG and MDA but negative with TNFa and OPN were found; also MMP9 was negatively correlated with TNFa and MCP-1 was negatively correlated with TIMP1. Conclusion: We found that many BMs expressing calcification, collagen breakdown, or formation, and inflammation are increased in the valve tissue and in the serum of patients with CAVS as compared with healthy group. Our findings may give new insights towards diagnosis but also therapy. Thus antisclerostin, and antiflammatory agents could be tried for preventing aortic calcification progression.
Keywords: aortic valve stenosis, biomarkers, calcification, relaxin-2, Sclerostin
Introduction
Non-rheumatic, calcific aortic valve stenosis (CAVS) is the main cause of aortic valve replacement (AVR) in the elderly, increasingly afflicting our aging population. One third of elderly patients (pts) have echocardiographic or radiological evidence of valve sclerosis, an early and subclinical form of calcific aortic valve disease (CAVD)1, characterized by progressive thickening of the aortic cusps without significant obstruction of the left ventricular outflow2,3 Calcification of the valve is an active process, combining local inflammatory reaction, deposition of lipoprotein components of plasma together with inorganic salts, and diverse chronic biological processes, which lead to the thickening, hardening and finally intense calcification of the aortic cusps4,5 Recent evidence suggests that this process is closely related to coronary artery atherosclerosis and calcification. However, calcification is more pronounced in CAVS than in vessels6. Inflammatory cells, T lymphocytes7,8 and macrophages9 predominate in early lesions of the aortic valve. Helske et al.10 describe inflammation, lipid accumulation, extracellular-matrix remodelling and neovascularization, β1- adrenergic and renin-angiotensin system activation as processes leading to calcification and ossification; they are influenced by flow characteristics, as they are much more prominent on the aortic side of the cusps. Many tissue factors are related to the progression of aortic valve calcification. Activated T lymphocytes in the subendothelial fibrous layer release cytokines such as transforming growth factor β-1 (TGF-β1)11 and interleukin-1 beta (IL-1β), which stimulate the production of metalloproteinases (MMPs), the role of which will be further described12. Olsson et al.7 indicate that IL-2 receptor positive cells were found only in stenotic valves. TGF-β1 regulates cellular proliferation, but also correlates with genes encoding collagen type I and III13; its diverse roles in suppression or activation of osteogenic signaling in vivo and in the initiation and progression of aortic valve calcification are not clear. Fetuin-A (fet-A) is a circulating glycoprotein produced by the liver; it is normally found in high concentrations in human serum14. Its levels are correlated with increased risk for myocardial infarction and cerebral episodes15. It inhibits ectopic calcification in the mitral valve in rheumatic disease16. Lower fet-A concentrations are associated with more extensive vascular atherosclerosis lesions17, and faster stenosis progression and increased valvular calcification in elderly patients with aortic stenosis18. Osteopontin (OPN) is a multifunctional glycol-phospho-protein involved in the biomineralization of dystrophic and ectopic sites, including the aortic valve19; increased plasma levels are found in patients with CAVS20. Patients with early and severe coronary atherosclerosis also have high levels of circulating endothelial progenitor cells with an osteoblastic (osteocalcin [OCN]) phenotype (EPC-OCN)21, a non-collagenous protein responsible for calcification. Osteoprotegerin (OPG) is a glycoprotein involved in bone metabolism and with a regulatory role in immune, skeletal and vascular systems22. There is a complex interaction between OPG and the receptor activator of nuclear factor kappa B (NFκB) (RANK)/RANK ligand (RANKL) system responsible for the inhibition of osteoclastogenesis, which has important consequences for calcification of bone, arteries, and the aortic valve23. The bone morphogenetic proteins (BMPs) belong to the TGF-β superfamily; they regulate cell growth, apoptosis and differentiation of mesenchymal cells, osteoblasts and chondrocytes. From >15 family members, BMPs 2–4 and 6 are expressed in calcified atherosclerotic lesions24,25 Tenascin-C (TN-C) is an extracellular matrix glycoprotein and is associated with increase of bone formation and calcification and also is co-expressed with MMPs and simultaneously overexpressed in calcified aortic cusps26,27 Previous studies indicate that suppression of MMPs downregulates TN-C expression28,29 Tenascin C is found in human calcified cusps together with MMP-2 and alkaline phosphatase, to a much greater degree than in non-calcified cusps. It is also found in macrophage-rich human atherosclerotic plaques26. MMP expression can be stimulated by the pro-inflammatory cytokine tumor necrosis factor alpha (TNFa)30. MMPs mediate the breakdown of collagen; apart from their local concentration and action they are also expressed in the serum. Interestingly, although MMPs break down collagen they are often elevated in osteogenetic conditions10 and are also overexpressed in stenotic valves31. Their action is antagonized by the tissue inhibitors of metalloproteinases (TIMPs). Various MMPs and TIMPs have been suggested to be involved in tissue remodeling in CAVS. The balance between TIMPs and MMPs, expressed by their ratio is crucial for the progression of calcification12,31. MMPs 1, − 2, − 3 and − 9 are expressed in macropophages, lymphocytes and fibroblasts32. Sclerostin is a key negative regulator of bone formation. It is a wingless signaling (Wnt) pathway antagonist regulating osteoblast activity and bone turnover, accordingly to Brandenburg et al. who indicate that sclerostin is locally produced in aortic valve tissue adjacent to areas of calcification33. Patients with CAVS showed increased sclerostin serum levels compared to a healthy reference population, and it was suggested that the severity of calcification may be linked to increased sclerostin serum levels34. Kastellanos et al. found that elevated serum MCP-1 is progressively reduced after aortic valve replacement35. This CCL2 chemokine characteristically attracts T lymphocytes and natural killer cells, and mediates macrophage recruitment into atheromatous lesions36. As already mentioned7–9, these cells are found in stenotic aortic valves. Wallby et al.37 showed that lymphocyte infiltration was similar in both tricuspid and bicuspid stenotic aortic valves, although Moreno et al.38 found a greater density of macrophages/T cells in congenital bicuspid aortic valves than in tricuspid valves.
The purpose of our study is to identify tissue and circulating biomarkers involved in tricuspid valves from patients with CAVS and to find any pertinent correlations among them. Criteria for biomarkers have been proposed by Morrow and de Lemos39. Additionally, Braunwald40 proposes that they should provide important information regarding the pathogenesis of a disease or the identification of subjects at risk and also to be useful in risk stratification and monitoring of therapy. Apart from the ones already mentioned, we also assayed relaxin-2 levels in the valves and serum; its pertinence will be discussed further. Also we compared serum BMs of pts with those of healthy/controls. The identification of novel risk factors may facilitate the discovery of innovative therapies and also have important implication for the selection of patients with CAVS to receive either tissue or mechanical valves. We also measured a simple serum marker of oxidation, malondialdehyde (MDA), which has been found to adversely affect prognosis in pts with CAVS according to Parenica et al.42.
Clinical Study and Methods
We prospectively included 60 patients (pts) who were scheduled to undergo surgery for severe CAVS in the Cardiac Surgery Department of the Onassis Cardiac Surgery Center (OCSC) according to clinical valvular heart disease guidelines. The area of the aortic valve orifice was measured from 0.9 to 0.5 cm2, at the echocardiographic laboratory of the OCSC by standard echocardiographic techniques. Most pts were treated with antihypertensive and all with antilipidemic drugs during the last two years of their course. Exclusion criteria were the presence of rheumatic cardiac disease, a bicuspid aortic valve or connective tissue disorders. Thus from a total of 200 pts, 140 were excluded. The remaning, 60 pts (mean age 66.1 ± 12.5, 50% men) were included. Serum findings from these pts were compared to those of 20 healthy individuals (mean age 34.4 ± 7.5, 50% men). The healthy group was clinically clear of systemic and valvular disease and had no history of cardiac metabolic problems, specifically diabetes mellitus, hyperlipidemia or connective tissue disease. Stenosis or sclerosis of the aortic valve had been excluded by echocardiography. All pts and healthy controls signed informed consent for the use of their clinical and laboratory results for scientific purposes under the condition of anonymity. This study was approved by the Ethics Committee of the OCSC.
Aortic valve trileaflets: tissue preparation and quantification by morphometric analysis
The aortic valve trileaflets (AVTLs) were obtained from the 60 patients undergoing valve replacement. They were rinsed in cold saline to remove blood, placed in 10% formalin buffer overnight at 4°C, washed, dehydrated and embedded in paraffin. Thereafter, 4 μm of serial paraffin sections along the length of the whole AVTLs were cut and mounted on polylysine slides for histochemistry, immunohistochemistry and finally for quantitative morphometric analysis. Results were given as the average of a percentage positively stained AVTL area per patient specimen. Morphometric analysis was performed by using the program Image Pro Plus 4.1 (Media Cybernetics, Inc. Rockville, MD, USA). The intensity measurements of several regions of and the collection data were not known to the clinical researchers, and those performing serum measurements.
Histochemistry, immunohistochemistry and morphometric analysis
Ten sections of the AVTLs (4 μm/section) for each patient, at equal spaces (40 μm) over a distance of about 400 μm, were obtained and stained with Alizarin Red, Orcein and hematoxylin/eosin (H&E) (Sigma-Aldrich Chemie Gmbh, Munich, Germany) in order to evaluate the extent of calcification, elastin degradation (rupture of elastic fibers), and disorganization of cellular valve matrix, respectively.
As previously described, the specimens were also used for immunohistochemistry analysis. Serial paraffin sections (4 μm/section) of the AVTLs were incubated in to antigen retrieval (Sodium Citrate buffer solution, pH = 6), then they were incubated overnight at 4°C either with osteopontin (R&D systems, Minneapolis, MN, USA; aff1433; working concentration 10 μg/ml); antihuman osteocalcin (R&D systems, Minneapolis, MN, USA; MAB1419; working concentration 25 μg/ml); osteoprotegerin (R&D systems, Minneapolis, MN, USA; aff805; working concentration 15 μg/ml); relaxin 2 (K24211R, Meridian Life Science, Biodesign, Memphis, USA, working concentration 10 μg/ml). The procedure was performed by using an ABC kit (Vector Laboratories, CA, USA) and the development was performed with brown chromogen (DAB, CA, DAKO). Negative control sections were processed without primary antibody. Images in figures were photographed with light microscopy (Olympus CX31, Olympus LTD, Hertfordshire, UK), color camera (ALTRA 20 - SIS 2 Megapixel CMOS, Olympus LTD, Hertfordshire. UK), imaging system (Altras Soft Imaging System Olympus LTD, Hertfordshire, UK). In order to photograph the whole area of the tissue for morphometric quantification we used a Leica DM IRB light microscope (Leica Microsystems Wetzlar GmbH, Germany) and Digital images were acquired using a BMF Bioscience Camera (MBF Bioscience, MicroBrightField Europe E.K, Germany) and the assorted stereo investigator version10.0 software (MBF Bioscience, MicroBrightField Europe E.K, Germany). A morphometric analysis was utilized for the quantification of positive stainings by an image-analyzer system (ImageJ is Just, Fiji). Digital images were obtained using the same settings, and the segmentation parameters were held constant for a given marker and experiment.
Serum biomarkers analysis
Blood was obtained by venipuncture after pts and healthy controls fasted overnight. Serum samples were isolated and immediately frozen and stored at − 80°C until analysis was performed in duplicate of the serum biomarkers described in the protocol of the datasheet; dilution was assessed in protocols required. Elisa human kits were used for the following groups of BMs: a) collagen turnover: MMP2 (ELH-MMP2-001, RayBiotech Inc, Norcross, GA, USA); MMP9 (ELH-MMP9, RayBiotech Inc, Norcross, GA, USA); TIMP1 (ab100651, Abcam, Cambridge, USA); b) inflammation: TNFa (ELH-TNFa, RayBiotech Inc, Norcross, GA, USA); TGFβ1 (DB100B, R&D systems, MN, USA); Tenascin C (27751, CA, USA); Interleukin 2 (K4798-100, Biovision, CA, USA); Fetuin A (RD191037100, Biovendor LLC, NC, USA); c) calcification: Sclerostin (TE1023HS, TecoMedical, Quidel Corporation, San Diego, USA); Osteopontin (BMS2066, eBioscience, San Diego, USA); osteoprotegerin (ELH-OPG-001, RayBiotech Inc, Norcross, GA, USA). The novel marker relaxin-2 (CSB-EL019750HU, Cusabio, Hubei P.R China) has vasculoprotective and anticalcifying action, as will be further discussed; MCP-1 (E-EL-H0020, Elabscience, Wuhan, P.R.C); also MDA (MBS728071, Mybiosource, Canada). Of course a large additional number of biomarkers are continuously being studied41,42. Biochemical lipid analysis was also performed but it was not possible to establish a meaningful statistical analysis of total cholesterol and triglycerides in the serum of pts because all of them were taking antilipidemic medications, specifically statins. Thus we could not obtain a valid biochemical comparison of the data between pts and healthy and these data are not described further.
Statistical analysis
a. The Pearson Correlation test was performed for multiple comparisons among tissue biomarkers to evaluate their role in the calcification process. b. The Spearman test was performed for the correlation of tissue to serum biomarkers; and for multiple correlations among serum biomarkers in the patient group. c. Unpaired Student t-test was performed between the healthy and patient groups for serum levels of biomarkers. Apart from measuring MMP2 and 9 and TIMP1 individually, we divided the value of TIMP1 with MMP2 and MMP9; the result of these ratios (e.g. TIMP1/MMP2; TIMP1/MMP9) was also used for the comparisons. The results are presented as means ± standard deviations. A p value of < 0.05 was considered statistically significant.
Results
Tissue biomarkers analysis
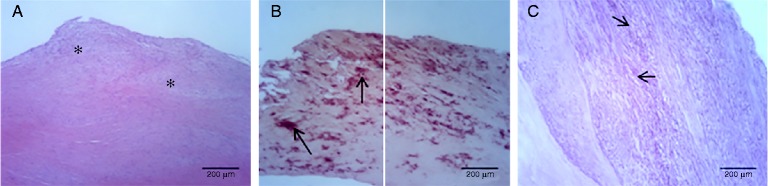
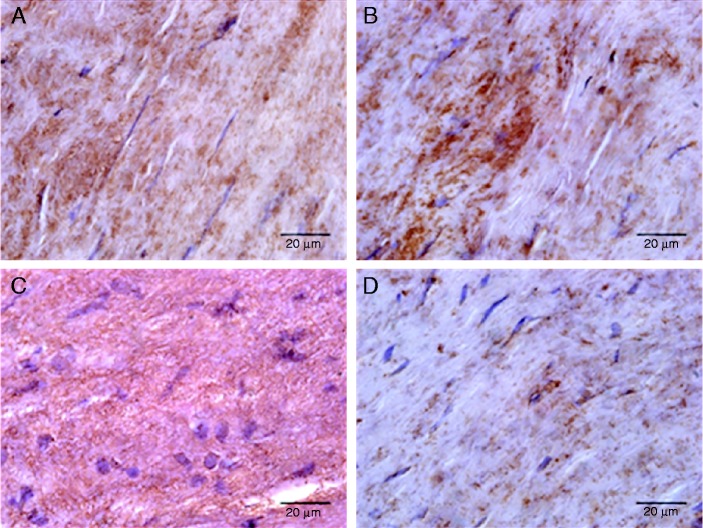
Histologically, the hematoxylin and eosin staining showed a broadened subendothelial space, disruption of fibers and increased cellularity in the aortic valves of the patient group (Fig. 1A); Alizarin red stain showed a typical histological appearance with hardening and deposition of calcium (Fig. 1B); orcein staining demonstrated the disorganization and rupture of elastic fibers (Fig. 1C). The percentage area of positive cells of calcification markers (OCN, OPN, and OPG) and relaxin-2 was estimated from the total surface of each sample. The localization of the above proteins in the subendothelial area of aortic valves is shown in Figure 2. Morphology and immunohistochemistry data are presented in Table 1. A negative correlation found between calcium and elastin deposit. As regards OCN, we found a positive significant correlation with tissue calcification and OPN, but a negative correlation with elastin. There was a negative correlation of OPN with elastin and OPG. Finally, many strong correlations of RL2 were found: positive with elastin, and negative with valve calcification, OPG, OCN and OPN. No statistical correlation was found between tissue and serum levels in OPG, OPN and RL2.
Figure 1.
Histological pathology of aortic valve leaflets from patients with CAS. A. Hematoxylin and eosin staining showing thickening and cellularity in subendothelial space (asterisk). B. Alizarin red staining showing calcium deposits (arrows, red stain). C. Disorganization of elastic fibers showing in dark purple stained with orcein (arrows, purple stain).
Figure 2.
Immunohistochemistry of aortic valves leaflets from patients with CAS. Representative immunopositive sections showed with brown color for A. Osteoprotegerin (OPG); B. Osteocalcin (OCN); C. Osteopontin (OPN); D. Relaxin 2 (RL2). Relaxin 2 localization and calcium deposit increased staining of extracellular matrix and elongated cells in aortic valves.
Table 1.
Valve tissue correlations. AR: Calcium deposit; ORC: Elastin deposit; OPG: osteoprotegerin; OCN: osteocalcin; OPN: osteopontin; RL2: Relaxin 2; Pearson correlation was performed for the percentage area between histology and immunohistochemistry results. r and p value presented. NS = no significant. Calcification positively correlated with OCN and negatively with elastin and RL2. Elastin negatively correlated with OPN and OCN. OPN significantly correlated with osteocalcin. A negative correlation of RL2 was seen with calcium deposits, osteopontin, osteoprotegerin and osteocalcin.
| Tissue biomarkers | Patients (mean ± SD) | r | p-value |
| Area (%) | |||
| AR | 52.95 ± 5.41 | − 0.562 ORC | 0.01 |
| − 0.456 RL2 | 0.044 | ||
| +0.502 OCN | 0.024 | ||
| ORC | 15.74 ± 0.93 | +0.888 RL2 | < 0.0001 |
| − 0.584 OPN | 0.007 | ||
| − 0.685 OCN | 0.001 | ||
| OPG | 17.96 ± 2.95 | +0.284 AR | NS |
| +0.15 OCN | NS | ||
| OCN | 40.09 ± 6.93 | +0.573 OPN | 0.008 |
| OPN | 32.7 ± 6.53 | +0.301 AR | NS |
| − 0.064 OPG | NS | ||
| RL2 | 5.62 ± 0.84 | − 0.523 OPG | 0.018 |
| − 0.740 OCN | < 0.0001 | ||
| − 0.540 OPN | 0.014 |
Serum biomarkers analysis
Statistical analysis data showed significant differences in all serum biomarkers between healthy and patients group (Table 2). Thus, fetuin-A, RL2, TIMP1 and the ratios of TIMP1/MMP2 and TIMP1/MMP9 were lower in patients with CAVS; while MMP2, MMP9, TNFa, TGF-β1, Ten C, IL2, SOST, OPN, OPG, MCP-1 and MDA were higher in patients compared to healthy/control group.
Table 2.
Serum Biomarkers; Comparison between healthy and patients. MMP2: metalloproteinase 2; MMP9: metalloproteinase 9; TIMP1: tissue inhibitor 1 of metalloproteinase; TNFa: tumor necrosis factor a; TGFb1: transforming growth factor, beta 1; Ten C: Tenascin C; IL2: interleukin 2; Fet A: Fetuin A; SOST: Sclerostin; OPN: osteopontin; OPG: osteoprotegerin; RL2: Relaxin 2; MCP-1: monocyte chemoattractant protein 1; MDA: malondialdehyde. Results from serum biomarkers and TIMP1/MMP2 and TIMP1/MMP9 ratios between patients and healthy/controls tested with unpaired student t-test statistical analysis. Asterisk * represents p < 0.0001.
| Healthy/Controls | Patients | p-value | |
| Serum biomarkers | (mean ± SD) | (mean ± SD) | Patients/Healthy |
| MMP2 (ng/ml) | 169.3 ± 15.39 | 193.6 ± 48.49 | 0.03 |
| MMP9 (ng/ml) | 351 ± 30.29 | 464.3 ± 75.69 | * |
| TIMP1 (ng/ml) | 153.8 ± 32.63 | 106.7 ± 4.3 | * |
| TIMP1/MMP2 | 0.91 ± 0.21 | 0.57 ± 0.1 | * |
| TIMP1/MMP9 | 0.43 ± 0.089 | 0.23 ± 0.049 | * |
| TNFa (ng/ml) | 0.51 ± 0.29 | 17.06 ± 5.97 | * |
| TGFβ1 (ng/ml) | 0.136 ± 0.028 | 0.44 ± 0.12 | * |
| Ten C (ng/ml) | 48.24 ± 21.8 | 71.57 ± 25.7 | 0.0005 |
| IL2 (ng/ml) | 0.76 ± 0.2 | 1.57 ± 0.28 | * |
| Fet A (ng/ml) | 79.5 ± 7.68 | 48.05 ± 9 | * |
| SOST (ng/ml) | 0.6 ± 0.0.26 | 2.59 ± 0.79 | * |
| OPN (ng/ml) | 24.92 ± 3.5 | 58.53 ± 6.97 | * |
| OPG (ng/ml) | 6.71 ± 2.06 | 31.04 ± 13.67 | * |
| RL2 (ng/ml) | 0.5 ± 0.08 | 0.02 ± 0.005 | * |
| MCP-1 (ng/ml) | 0.015 ± 0.003 | 0.45 ± 0.21 | * |
| MDA (ng/ml) | 0.078 ± 0.008 | 2.076 ± 0.39 | * |
Statistical correlation analysis of the serum data showed that MMP2, TGF-β1, Tenascin C, fetuin-A, and relaxin-2 are not correlated among them and with the other serum biomarkers. Sclerostin is negatively significantly correlated with TNFa (r2 = − 0.427, p = 0.0007) and osteopontin (r = − 0.286, p = 0.027). Furthermore, sclerostin is significantly positively correlated with interleukin 2 (r = 0.296, p = 0.022) and osteoprotegerin (r = 0.324, p = 0.012) and MDA (r = 0.269, p = 0.037). Also, MMP9 was negatively significantly correlated with TNFa (r = − 0.360, p = 0.005). MCP-1 is negatively correlated with TIMP1 (r = − 0.319, p = 0.042) (Data shown in Table 3). Also, the extent of valve calcification as evaluated by alizarin red was positively correlated with TIMP1 (r = 0.304, p = 0.01), MCP-1 (r = 0.296, p = 0.02) and negatively with MMP-9 (r = − 0.325 and p = 0.01).
Table 3.
Correlation between serum biomarkers in patients. Pearson correlation was performed between serum biomarkers of patient group. Bottom left values (bold italics) presenting significant r values, while up right values (bold) presenting p values.
| MMP2 | MMP9 | TIMP1 | TNFa | TGFβ1 | Ten C | IL2 | Fet A | SOST | OPN | OPG | RL2 | MCP1 | MDA | |
| MMP2 | 0.420 | 0.915 | 0.492 | 0.676 | 0.146 | 0.101 | 0.286 | 0.622 | 0.995 | 0.503 | 0.078 | 0.254 | 0.557 | |
| MMP9 | − 0.106 | 0.558 | 0.005 | 0.863 | 0.341 | 0.525 | 0.373 | 0.188 | 0.056 | 0.602 | 0.548 | 0.757 | 0.250 | |
| TIMP1 | 0.014 | − 0.077 | 0.377 | 0.225 | 0.882 | 0.300 | 0.647 | 0.399 | 0.790 | 0.351 | 0.184 | 0.042 | 0.768 | |
| TNFa | 0.091 | − 0.360 | 0.116 | 0.288 | 0.554 | 0.588 | 0.477 | 0.0007 | 0.059 | 0.313 | 0.292 | 0.338 | 0.647 | |
| TGFβ1 | − 0.055 | − 0.023 | 0.159 | 0.139 | 0.221 | 0.750 | 0.725 | 0.618 | 0.301 | 0.139 | 0.174 | 0.052 | 0.767 | |
| Ten C | 0.190 | − 0.125 | − 0.020 | − 0.078 | − 0.160 | 0.718 | 0.476 | 0.298 | 0.442 | 0.460 | 0.783 | 0.516 | 0.632 | |
| IL2 | − 0.214 | − 0.084 | 0.136 | − 0.071 | − 0.042 | − 0.048 | 0.267 | 0.022 | 0.386 | 0.343 | 0.381 | 0.980 | 0.961 | |
| Fet A | 0.140 | 0.117 | 0.060 | − 0.094 | 0.046 | 0.094 | 0.146 | 0.138 | 0.200 | 0.459 | 0.675 | 0.477 | 0.994 | |
| SOST | − 0.065 | 0.172 | 0.111 | − 0.427 | − 0.066 | − 0.136 | 0.296 | 0.194 | 0.027 | 0.012 | 0.662 | 0.948 | 0.037 | |
| OPN | − 0.001 | − 0.249 | − 0.035 | 0.245 | 0.136 | 0.101 | − 0.114 | 0.168 | − 0.286 | 0.096 | 0.147 | 0.178 | 0.484 | |
| OPG | 0.088 | − 0.069 | − 0.123 | − 0.132 | − 0.193 | − 0.097 | 0.124 | − 0.097 | 0.324 | − 0.217 | 0.286 | 0.645 | 0.694 | |
| RL2 | 0.229 | 0.177 | 0.441 | 0.018 | − 0.004 | − 0.226 | − 0.247 | 0.195 | − 0.127 | 0.034 | 0.050 | 0.702 | 0.314 | |
| MCP1 | − 0.182 | 0.05 | − 0.319 | − 0.154 | − 0.306 | 0.104 | 0.004 | − 0.114 | 0.01 | 0.215 | − 0.074 | − 0.062 | 0.914 | |
| MDA | − 0.077 | − 0.151 | 0.039 | 0.060 | 0.039 | − 0.063 | 0.006 | − 0.001 | 0.269 | 0.092 | 0.052 | 0.132 | − 0.014 |
Discussion
Histological evidence for calcification
We believe that our findings add some novel data on the pathogenesis of CAVS. As regards valvular tissue measurements we found OPN, OCN and OPG in the calcified valves as expected and correlated to calcium deposits. Interestingly, Kadden et al. found that OPG is decreased but still present in the calcific aortic valves43. However, some correlations are interesting. We further found that the degree of aortic calcification was negatively correlated with elastin, and positively correlated with calcifying proteins. We additionally found that elastin preservation was significantly positively correlated with RL2, while both were negatively correlated with calcification factors, osteopontin, osteocalcin and OPG. Thus, RL2-relaxin a novel biomarker described here for the first time gave very interesting correlations which all testify towards its role as promoting elastin preservation and inhibiting calcification. Relaxin represents a potent inhibitory factor in the stimulated multi-step cascade of early vascular inflammation, mitigating expression of endothelial adhesion molecules, decreasing chemokine expression and suppressing firm monocyte adhesion to the endothelium40. Brecht et al. indicate that it may be of relevance for the prevention and treatment of atherosclerosis and of other pro-inflammatory states and it could be a new promising candidate drug for vascular protection in the future44. Relaxin also represses inflammation during pregnancy and reduces the recruitment of inflammatory leukocytes in the reperfused myocardium45. Thus, its negative correlation with aortic calcification is not surprising. Its therapeutic potential will be discussed further.
Role of serum markers in aortic valve calcification process
As regards serum levels, we verified previously reported findings such as decreased fetuin-A17,18. We also found decreased TIMP1 and increased MMP2 and MMP9 levels. Interestingly, the TIMP1/MMP2 and TIMP1/MMP9 ratios also gave very strong differences between pts and healthy controls. TIMPs regulate ECM deposition through inhibition of MMPs46–48. We also found increased TNFa, TGF-β1, Tenascin C, IL2, OPN, OPG levels as already reported in other studies. We found an increase of serum MMP2 and MMP9 and a decrease of TIMP1 in our patients as compared to controls. The role of MMPs in aortic stenosis is stressed by Helske et al.10 together with the importance of the imbalance of TIMP1 and MMP. The same authors also stress that TNFa and tenascin-C may exert their action by modulating MMP expression and activation. Le Maire et al. studied pts with ascending aortic aneurysms that had either trileaflet aortic valves (TAVs) or biscuspid aortic valves (BAVs)49. They found an increased MMP9 expression in the former and increased MMP2 in the latter. We found that both MMP2 and 9 were increased. Together with TGF-β1, it must be reminded that many of these markers reflect not only valvular but also myocardial fibrosis13.
There were few, but interesting, correlations among serum markers. Our finding of a negative correlation between TNFa and MMP9 is intriguing given the report of Galis et al.30 that MMP expression is stimulated by TNFa: a biofeedback mechanism can be postulated. In fact, it has been pointed out that many feedback and counter-regulatory mechanisms are operable in the calcification process10,29. We found a 4-fold increase of sclerostin. This is higher than that reported by Koos et al.34, who also studied patients with calcified tricuspid aortic valves and found just below a 2-fold increase. However, from their data it emerges that their patients were selected on the basis of valve calcification only. Thus, the degree of stenosis was probably lower in their population, since our patients were selected on the basis of severity. The role of sclerostin is intriguing. As stated, it is increased in CAVS, but has been found to associate both with increased risk of bone fracture50 and thus is described as a calcification inhibitor by Claes et al.51. These authors postulate that it may participate in a counter regulatory mechanism to suppress the progression of vascular and probably valvular calcification. Its negative relation to osteopontin and positive to osteoprotegerin is supportive of this postulation. It must be realized that many authors point out that aortic calcification is paralleled by bone demineralization. Inflammation and oxidative stress are postulated as unifying factors52–54. The positive correlations of sclerostin and interleukin 2 may support this concept.
We also found that MDA levels were increased. Parenica et al.42, in pts undergoing transcatheter aortic valve placement or surgical replacement, found that it was negatively associated with one-year prognosis. This is not unexplained, since oxidative stress is a main component of the calcifying process. Its positive correlation with sclerostin further supports this hypothesis.
We cannot readily explain the negative association of MCP-1 and TIMP1. It is known that both MCP-1 and TIMPs are elevated in inflammation and can be upregulated by some inducers, such as nicotinamide phosphoribosyltransferase55. Thus, a negative feedback may also be involved.
We also found increased OPG levels, as Ueland et al. have56. Osteoprotegerin serum levels are associated with the extent of vascular calcification. This may well explain the positive correlation that we found with sclerostin. However, treatment with OPG attenuates calcification56; again highlighting feedback and counter-regulatory mechanisms involved in the calcification process. Finally, the positive correlation of calcification extent with TIMP1 and MCP-1 is not surprising in view of the data discussed. Also, the negative correlation with MMP-9 sets into focus the arguments of Fondard et al.31, that this metalloproteinase primarily involves collagen breakdown and probably secondarily calcification.
Therapeutic considerations
In this vein, some therapeutic postulations could emerge from our study. Up to now, no effective preventive therapy for CAVS exists, since statins and anti-angiotensin interventions have failed. To find a widely applicable anti-inflammatory agent would be difficult. The very strong differences (34-fold) of TNFa in our patients could give a thought as regards anti-TNFa treatment. Additionally, Isoda et al. found that IL-1Ra deficiency in inflammatory cells induced aortic valve inflammation and TNFa participates importantly in the development of AS in IL-1Ra (-/-) mice57. However, anti-inflammatory treatment has largely disappointed in heart failure studies.
Antisclerostin antibodies are being tried in osteoporotic disease58. According to our and Koos et al.34 findings they could be of value in preventing progression to severe calcification. Denosumab, an antibody against RANKL, can also be considered a potential player59, in view of the increased OPG levels; Serelaxin has been found to be beneficial in acute heart failure60, while relaxin-2 is employed in systemic sclerosis61. It could be considered in early aortic calcification. Also our findings on MDA, which are in agreement with these of Parenica et al.42, raise the question of antioxidative treatment, although such efforts have largely failed in atherosclerotic disease treatment. Finally our, findings may also have an application in the choice of the prosthesis to replace the stenotic valve. Shetty et al. have shown that bioprosthetic valves show increased calcification in pts with an elevated proportion of small, dense low-density lipoprotein particles62. Taking this step further we may postulate that in “vulnerable’’ pts, i.e. those with increased pro- and decreased anti-calcific factors in their serum, a bioprosthesis for AVR should not be preferred. Yeghiazaryan et al. gave a very detailed study towards this goal, identifying many factors associated with early tissue valve degeneration63.
Study limitations
In our study, the controls were younger, but we had to ensure that they were free of heart disease. We did not study healthy valves. Thus, we could not verify or refute the findings of Kaden et al. that OPG is decreased but still present in calcified valves43. However, two of the markers found in the stenotic valves (OPN, OPG) were also increased in the serum, although their levels were not correlated. Additionally, the study of the expression of relaxin 2 in healthy valve tissue and its comparison to sclerotic leaflets would be interesting. We did not study the diverse collagen forms or BMPs in valve tissue, or any other circulating markers found in aortic valvular disease, such as osteogenic precursor cellshomocysteine of ADMA as Ferrari et al. did64, or C-reactive protein (CRP). Additionally, we did not address the very novel subject of microRNAs. A distinctive miRNA profile has been underlined as promoting calcification, but in BAVs. Thus, Nigam et al. found decreased levels in calcific BAV valves of the following miRNAs: 26a, 195,30b65 Moreover, Yanagawa et al. found decreased miRNA-141 levels, but only in BAV, by 14.5-fold. This decrease was associated with an increase of the BMP-2 protein in BAV as compared to TAV66 miRNA therapeutic manipulation is already a reality in many conditions.
Another question which has not been addressed in our study is the change of serum biomarkers after aortic valve replacement. Gerber et al. have found that serum CRP is decreased after AVR67. Also Kastellanos et al. found that CRP, TNFa and MCP-1 decrease after aortic valve replacement35. However, in an opposite manner, Askevold et al. did not find a significant long-term change of secreted procalcific Wnt modulators after aortic valve replacement68.
We are currently studying a new series of pts to see if the markers that we measured will show changes after valve replacement. Their behavior may give some further insight, to the relationship between vulnerable patient and vulnerable valve, specifically if valve processes affect serum levels, or if a systemic pro-osteogenetic profile of the patient induces valvular calcification, as seen in the metabolic syndrome69. Actually, both pathways may be operative and counter reacting.
Conclusion
In our study we again validated that many inflammatory, collagen turnover and osteogenic factors are present in CAVS. We provided a verification of the importance of sclerostin and described for the first time the protective effect of relaxin-2. Finally, we offer some thoughts on therapeutic possibilities based on our findings.
Abbreviations
(AVTLs) aortic valve trileaflets
(BAVs) bicuspid aortic valves
(BMPs) bone morphogenetic proteins
(CAVD) calcific aortic valve disease
(CAVS) calcific aortic valve stenosis
(CRP) C-reactive protein
(DAB) 3, 3′-diaminobenzidine
(EPC-OCN) endothelial progenitor cells with osteoblastic phenotype
(fet-A) fetuin-A
(IL-1Ra) interleukin-1 receptor antagonist
(IL-1b) interleukin-1 beta
(MCP-1) monocyte chemoattractant protein 1
(MDA) malondialdehyde
(miRNAs) microRNAs
(MMPs) matrix metalloproteinases
(MMP2) matrix metalloproteinase 2
(MMP9) matrix metalloproteinase 9
(NFκB) nuclear factor kappa B
(OPC) osteocalcin
(OPG) osteoprotegerin
(OPN) osteopontin
(RANK) receptor activator of nuclear factor kappa-B
(RANKL) receptor activator of nuclear factor kappa-B ligand
(RL2) relaxin-2
(TAVs) trileaflet aortic valves
(TIMPs) tissue inhibitor or metalloproteinases
(TGF-β1) transforming growth factor β1
(TNFa) tumor necrosis factor alpha
(Pts) patients
(Wnt) wingless proteins
Conflicts of Interest
None.
Funding Sources
The study was co-funded by a grant of the Alexander Onassis Public Benefit Foundation.
Acknowledgements
The technical support of Anna Agapaki in tissue preparation; Chrysostomos Aravanis in image analysis is gratefully acknowledged.
References
- 1.Towler DA. Molecular and cellular aspects of calcific aortic valve disease. Circ Res. 2013 Jul 5;113(2):198–208. doi: 10.1161/CIRCRESAHA.113.300155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chester AH, El-Hamamsy I, Butcher JT, Latif N, Bertazzo S, Yacoub MH. The living aortic valve: From molecules to function. Glob Cardiol Sci Pract. 2014 Jan 29;2014(1):52–77. doi: 10.5339/gcsp.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sainger R, Grau JB, Branchetti E, Poggio P, Lai E, Koka E, Vernick WJ, Gorman RC, Bavaria JE, Ferrari G. Comparison of transesophageal echocardiographic analysis and circulating biomarker expression profile in calcific aortic valve disease. J Heart Valve Dis. 2013 Mar;22(2):156–165. [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with aortic valve calcification. J Am Col Card. 1997 Mar 1;29(3):630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 5.Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly. J Am Coll Card. 1993 Apr;21(5):1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 6.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001 Mar 20;103(11):1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 7.Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Rydén L, Nilsson J. Accumulation of T-lymphocytes and expression of IL-2 receptors in nonrheumatic aortic valve stenosis. J Am Coll Card. 1994 Apr;23(5):1162–1170. doi: 10.1016/0735-1097(94)90606-8. [DOI] [PubMed] [Google Scholar]
- 8.Wallby L, Janerot-Sjöberg B, Steffensen T, Broqvist M. T lymphocyte infiltration in nonrheumatic aortic stenosis: a comparative descriptive study between tricuspid and bicuspidaortic valves. Heart. 2002 Oct;88(4):348–351. doi: 10.1136/heart.88.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of degenerative aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994 Aug;90(2):844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 10.Helske S, Kupari M, Lindstedt KA, Kovanen PT. Aortic valve stenosis: an active atheroinflammatory process. Curr Opin Lipidol. 2007 Oct;18(5):483–491. doi: 10.1097/MOL.0b013e3282a66099. [DOI] [PubMed] [Google Scholar]
- 11.Jian B, Narula N, Li QY, Mohler ER, 3rd, Levy RJ. Progression of aortic valve stenosis:TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003 Feb;75(2):465–466. doi: 10.1016/s0003-4975(02)04312-6. discussion 465-6. [DOI] [PubMed] [Google Scholar]
- 12.Kaden JJ, Dempfle CE, Grobholz R, Tran HT, Kiliç R, Sarikoç A, Brueckmann M, Vahl C, Hagl S, Haase KK, Borggrefe M. IL-1 beta promotes matrixmetalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. 2003 Oct;170(2):205–211. doi: 10.1016/s0021-9150(03)00284-3. [DOI] [PubMed] [Google Scholar]
- 13.Villar AV, Llano M, Cobo M, Expósito V, Merino R, Martín-Durán R, Hurlé MA, Nistal JF. Gender differences of echocardiographic and gene expression patterns in human pressure overload left ventricular hypertrophy. J Mol Cell Cardiol. 2009 Apr;46(4):526–535. doi: 10.1016/j.yjmcc.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley MA. Fetuin-A and kidney function in persons with coronary artery disease: data from the Heart and Soul Study. Nephrol DialTransplant. 2006 Aug;21(8):2144–2151. doi: 10.1093/ndt/gfl204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, Häring HU, Boeing H, Fritsche A. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008 Dec 9;118(24):2555–2562. doi: 10.1161/CIRCULATIONAHA.108.814418. [DOI] [PubMed] [Google Scholar]
- 16.Mukhopadhyay S, Pandit BN, Saran RK, Mazumdar K, Yusuf J, Minhas HS, Trehan V, Tyagi S. Systemic and local levels of fetuin-a in calcified mitral valves of rheumatic heart disease. J Heart Valve Dis. 2014 Jan;23(1):55–65. [PubMed] [Google Scholar]
- 17.Wang AY, Woo J, Lam CW, Wang M, Chan IH, Gao P, Lui SF, Li PK, Sanderson JE. Associations of serum fetuin-A with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol Dial Transplant. 2005 Aug;20(8):1676–1685. doi: 10.1093/ndt/gfh891. [DOI] [PubMed] [Google Scholar]
- 18.Mohty D, Côté N, Pibarot P, Fournier D, Pépin A, Audet A, Després JP, Mathieu P. Reduced Fetuin A serum levels is associated with faster stenosis progression and increased valvular calcification in elderly patients with aortic stenosis. J Clinic Experiment Cardiol. 2011;11:8. [Google Scholar]
- 19.Cho HJ, Cho HJ, Kim HS. Osteopontin: a multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep. 2009 May;11(3):206–213. doi: 10.1007/s11883-009-0032-8. [DOI] [PubMed] [Google Scholar]
- 20.Yu PJ, Skolnick A, Ferrari G, Heretis K, Mignatti P, Pintucci G, Rosenzweig B, Diaz-Cartelle J, Kronzon I, Perk G, Pass HI, Galloway AC, Grossi EA, Grau JB. Correlation between plasma osteopontin levels and aortic valve calcification: potential insights into the pathogenesis of aortic valve calcification and stenosis. J Thorac Cardiovasc Surg. 2009 Jul;138(1):196–199. doi: 10.1016/j.jtcvs.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 21.Gössl M, Mödder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol. 2008 Oct;52(16):1314–1325. doi: 10.1016/j.jacc.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjerre M. Osteoprotegerin (OPG) as a biomarker for diabetic cardiovascular complications. Springerplus. 2013 Dec 6;2:658. doi: 10.1186/2193-1801-2-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Amelio P, Isaia G, Isaia GC. The osteoprotegerin/rank/Rankl system: A bone key to vascular disease. J Endocrinol Invest. 2009;32(4 Suppl):6–9. [PubMed] [Google Scholar]
- 24.Lind M, Schumacker B, Søballe K, Keller J, Melsen F, Bünger C. Transforming growth factor-beta enhances fracture healing in rabbit tibiae. Acta Orthop Scand. 1993 Oct;64(5):553–556. doi: 10.3109/17453679308993691. [DOI] [PubMed] [Google Scholar]
- 25.Sefat F, Denyer MC, Youseffi M. Imaging via widefield surface plasmon resonance microscope for studying bone cell interactions with micropatterned ECM proteins. J Microsc. 2011 Mar;241(3):282–290. doi: 10.1111/j.1365-2818.2010.03430.x. [DOI] [PubMed] [Google Scholar]
- 26.Jian B, Jones PL, Li Q, Mohler ER, 3rd, Schoen FJ, Levy RJ. MMPs-2 is associated with tenacin-C in calcific aortic valve. Am J Path. 2001 Jul;159(1):321–327. doi: 10.1016/S0002-9440(10)61698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satta J, Melkko J, Pöllänen R, Tuukkanen J, Pääkkö P, Ohtonen P, Mennander A, Soini Y. Progression of human aortic valve stenosis is associated with tenascin-C expression. J Am Coll Cardiol. 2002 Jan;39(1):96–101. doi: 10.1016/s0735-1097(01)01705-3. [DOI] [PubMed] [Google Scholar]
- 28.Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol. 1997 Oct 6;139(1):279–293. doi: 10.1083/jcb.139.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowan KN, Jones PL, Rabinovitch M. Regression of hypertrophied rat pulmonary arteries in organ culture is associated with suppression of proteolytic activity, inhibition of tenascin-C, and smooth muscle cell apoptosis. Circ Res. 1999 May 28;84(10):1223–1233. doi: 10.1161/01.res.84.10.1223. [DOI] [PubMed] [Google Scholar]
- 30.Galis ZS, Muszynski M, Sukhova GK, Simon-Morrissey E, Unemori EN, Lark MW, Amento E, Libby P. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ Res. 1994 Jul;75(1):181–189. doi: 10.1161/01.res.75.1.181. [DOI] [PubMed] [Google Scholar]
- 31.Fondard O, Detaint D, Iung B, Choqueux C, Adle-Biassette H, Jarraya M, Hvass U, Couetil JP, Henin D, Michel JB, Vahanian A, Jacob MP. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J. 2005 Jul;26(13):1333–1341. doi: 10.1093/eurheartj/ehi248. [DOI] [PubMed] [Google Scholar]
- 32.Edep ME, Shirani J, Wolf P, Brown DL. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc Pathol. 2000 Sep–Oct;9(5):281–286. doi: 10.1016/s1054-8807(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 33.Brandenburg VM, Kramann R, Koos R, Krüger T, Schurgers L, Mühlenbruch G, Hübner S, Gladziwa U, Drechsler C, Ketteler M. Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: a cross-sectional study. BMC Nephrol. 2013 Oct 10;14:219. doi: 10.1186/1471-2369-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koos R, Brandenburg V, Mahnken AH, Schneider R, Dohmen G, Autschbach R, Marx N, Kramann R. Sclerostin as a potential novel biomarker for aortic valve calcification: an in-vivo and ex-vivo study. J Heart Valve Dis. 2013 May;22(3):317–325. [PubMed] [Google Scholar]
- 35.Kastellanos SS, Toumpoulis IK, Aggeli C, Zezas S, Chlapoutakis E, Kastellanos S, Stefanadis CI. Time course of C-reactive protein, tumour necrosis factor-alpha and monocyte chemoattractant protein-1 following the surgical treatment of patients with aortic valve stenosis. Hellenic J Cardiol. 2007 Jan–Feb;48(1):5–14. [PubMed] [Google Scholar]
- 36.Gonzalez-Quesada C, Frangogiannis NG. Monocyte chemoattractant protein-1/CCL2 as a biomarker in acute coronary syndromes. Curr Atheroscler Rep. 2009 Mar;11(2):131–138. doi: 10.1007/s11883-009-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallby L, Janerot-Sjöberg B, Steffensen T, Broqvist M. T lymphocyte infiltration in non-rheumatic aortic stenosis: a comparative descriptive study between tricuspid and bicuspid aortic valves. Heart. 2002 Oct;88(4):348–351. doi: 10.1136/heart.88.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno PR, Astudillo L, Elmariah S, Purushothaman KR, Purushothaman M, Lento PA, Sharma SK, Fuster V, Adams DH. Increased macrophage infiltration and neovascularization in congenital bicuspid aortic valve stenosis. Thorac Cardiovasc Surg. 2011 Oct;142(4):895–901. doi: 10.1016/j.jtcvs.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. 2007 Feb 27;115(8):949–952. doi: 10.1161/CIRCULATIONAHA.106.683110. [DOI] [PubMed] [Google Scholar]
- 40.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008 May 15;358(20):2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 41.Beckmann E, Grau JB, Sainger R, Poggio P, Ferrari G. Insights into the use of biomarkers in calcific aortic valve disease. J Heart Valve Dis. 2010 Jul;19(4):441–452. [PMC free article] [PubMed] [Google Scholar]
- 42.Parenica J, Nemec P, Tomandl J, Ondrasek J, Pavkova-Goldbergova M, Tretina M, Jarkovsky J, Littnerova S, Poloczek M, Pokorny P, Spinar J, Cermakova Z, Miklik R, Malik P, Pes O, Lipkova J, Tomandlova M, Kala P. Prognostic utility of biomarkers in predicting of one-year outcomes in patients with aortic stenosis treated with transcatheter or surgical aortic valve implantation. Plos one. 2012 Dec;7(12):e48851. doi: 10.1371/journal.pone.0048851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarikoç A, Kiliç R, Brueckmann M, Lang S, Zahn I, Vahl C, Hagl S, Dempfle CE, Borggrefe M. Receptor activator of nuclear factor kappa B ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004 Jan;36(1):57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Brecht A, Bartsch C, Baumann G, Stangl K, Dschietzig T. Relaxin inhibits early steps in vascular inflammation. Regul Pept. 2011 Jan 17;166(1–3):76–82. doi: 10.1016/j.regpep.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Mu X, Urso ML, Murray K, Fu F, Li Y. Relaxin regulates MMP expression and promotes satellite cell mobilization during muscle healing in both young and aged mice. Am J Pathol. 2010 Nov;177(5):2399–2410. doi: 10.2353/ajpath.2010.091121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee KW, Everett TH, 4th, Rahmutula D, Guerra JM, Wilson E, Ding C, Olgin JE. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006 Oct 17;114(16):1703–1712. doi: 10.1161/CIRCULATIONAHA.106.624320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardin S, Pelletier P, Libby E, Le Bouter S, Xiao L, Kääb S, Demolombe S, Glass L, Nattel S. Marked differences between atrial and ventricular gene-expression remodeling in dogs with experimental heart failure. J Mol Cell Cardiol. 2008 Dec;45(6):821–831. doi: 10.1016/j.yjmcc.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Hoit BD, Takeishi Y, Cox MJ, Gabel M, Kirkpatrick D, Walsh RA, Tyagi SC. Remodeling of the left atrium in pacing-induced atrial cardiomyopathy. Mol Cell Biochem. 2002 Sep;238(1–2):145–150. doi: 10.1023/a:1019988024077. [DOI] [PubMed] [Google Scholar]
- 49.LeMaire SA, Wang X, Wilks JA, Carter SA, Wen S, Won T, Leonardelli D, Anand G, Conklin LD, Wang XL, Thompson RW, Coselli JS. Matrix metalloproteinase in ascending aortic aneurysms: bicuspid versus trileaflet aortic valves. J Surg Res. 2005 Jan;123(1):40–48. doi: 10.1016/j.jss.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Arasu A, Cawthon PM, Lui LY, Do TP, Arora PS, Cauley JA, Ensrud KE, Cummings SR. Study of Osteoporotic Fractures Research Group. Serum sclerostin and risk of hip fracture in older Caucasian women. J Clin Endocrinol Metab. 2012 Jun;97(6):2027–2032. doi: 10.1210/jc.2011-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Claes KJ, Viaene L, Heye S, Meijers B, d'Haese P, Evenepoel P. Sclerostin: Another vascular calcification inhibitor? J Clin Endocrinol Metab. 2013 Aug;98(8):3221–3228. doi: 10.1210/jc.2013-1521. [DOI] [PubMed] [Google Scholar]
- 52.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(-/-) mice. Circulation. 2008 Jan 22;117(3):411–420. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res. 2011 May 27;108(11):1392–1412. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warburton DE, Nicol CW, Gatto SN, Bredin SS. Cardiovascular disease and osteoporosis: balancing risk management. Vasc Health Risk Manag. 2007;3(5):673–689. [PMC free article] [PubMed] [Google Scholar]
- 55.Nokhbehsaim M1, Eick S, Nogueira AV, Hoffmann P, Herms S, Fröhlich H, Jepsen S, Jäger A, Cirelli JA, Deschner J. Stimulation of MMP-1 and CCL2 by NAMPT in PDL cells. Mediators Inflamm. 2013;2013:437123. doi: 10.1155/2013/437123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ueland T, Aukrust P, Dahl CP, Husebye T, Solberg OG, Tønnessen T, Aakhus S, Gullestad L. Osteoprotegerin levels predict mortality in patients with symptomatic aortic stenosis. J Intern Med. 2011 Nov;270(5):452–460. doi: 10.1111/j.1365-2796.2011.02393.x. [DOI] [PubMed] [Google Scholar]
- 57.Isoda K, Matsuki T, Kondo H, Iwakura Y, Ohsuzu F. Deficiency of interleukin-1 receptor antagonist induces aortic valve disease in BALB/c mice. Arterioscler Thromb Vasc Biol. 2010 Apr;30(4):708–715. doi: 10.1161/ATVBAHA.109.201749. [DOI] [PubMed] [Google Scholar]
- 58.Lewiecki EM. Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther Adv Musculoskelet Dis. 2014 Apr;6(2):48–57. doi: 10.1177/1759720X13510479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quemerais-Durieu MA, Kerlan V, Chabre O. [Therapeutic innovation in osteoporosis (antisclerostin antibody and denosumab)]. Ann Endocrinol (Paris) 2011 Oct;72(Suppl 1):S15–S22. doi: 10.1016/S0003-4266(11)70005-1. [DOI] [PubMed] [Google Scholar]
- 60.Filippatos G, Teerlink JR, Farmakis D, Cotter G, Davison BA, Felker GM, Greenberg BH, Hua T, Ponikowski P, Severin T, Unemori E, Voors AA, Metra M. Serelaxin in acute heart failure patients with preserved left ventricular ejection fraction: results from the RELAX-AHF trial. Eur Heart J. 2014 Apr;35(16):1041–1050. doi: 10.1093/eurheartj/eht497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khanna D, Clements PJ, Furst DE, Korn JH, Ellman M, Rothfield N, Wigley FM, Moreland LW, Silver R, Kim YH, Steen VD, Firestein GS, Kavanaugh AF, Weisman M, Mayes MD, Collier D, Csuka ME, Simms R, Merkel PA, Medsger TA, Sanders ME, Jr, Maranian P, Seibold JR. A Randomized, Double-Blind, Placebo-Controlled Trial of Recombinant Human Relaxin in the Treatment of Systemic Sclerosis with Diffuse Scleroderma. Arthritis Rheum. 2009 April;60(4):1102–1111. doi: 10.1002/art.24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shetty R, Girerd N, Côté N, Arsenault B, Després JP, Pibarot P, Mathieu P. Elevated proportion of small, dense low-density lipoprotein particles and lower adiponectin blood levels predict early structural valve degeneration of bioprostheses. Cardiology. 2012;121(1):20–26. doi: 10.1159/000336170. [DOI] [PubMed] [Google Scholar]
- 63.Yeghiazaryan K, Skowasch D, Bauriedel G, Schild HH, Golubnitschaja O. Degenerative valve disease and bioprostheses: risk assessment, predictive diagnosis, personalised treatments. EPMA J. 2011 Mar;2(1):91–105. doi: 10.1007/s13167-011-0072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrari G, Sainger R, Beckmann E, Keller G, Yu PJ, Monti MC, Galloway AC, Weiss RL, Vernick W, Grau JB. Validation of plasma biomarkers in degenerative calcific aortic stenosis. J Surg Res. 2010 Sep;163(1):12–17. doi: 10.1016/j.jss.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nigam V, Sievers HH, Jensen BC, Sier HA, Simpson PC, Srivastava D, Mohamed SA. Altered microRNAs in bicuspid aortic valve: a comparison between stenotic and insufficient valves. J Heart Valve Dis. 2010 Jul;19(4):459–465. [PMC free article] [PubMed] [Google Scholar]
- 66.Yanagawa B, Lovren F, Pan Y, Garg V, Quan A, Tang G, Singh KK, Shukla PC, Kalra NP, Peterson MD, Verma S. miRNA-141 is a novel regulator of BMP-2-mediated calcification in aortic stenosis. J Thorac Cardiovasc Surg. 2012 Jul;144(1):256–262. doi: 10.1016/j.jtcvs.2011.10.097. [DOI] [PubMed] [Google Scholar]
- 67.Gerber IL, Stewart RA, Hammett CJ, Legget ME, Oxenham H, West TM, French JK, White HD. Effect of aortic valve replacement on c-reactive protein in nonrheumatic aortic stenosis. Am J Cardiol. 2003 Nov 1;92(9):1129–1132. doi: 10.1016/j.amjcard.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 68.Askevold ET, Gullestad L, Aakhus S, Ranheim T, Tønnessen T, Solberg OG, Aukrust P, Ueland T. Secreted Wnt modulators in symptomatic aortic stenosis. J Am Heart Assoc. 2012 Dec;1(6):e002261. doi: 10.1161/JAHA.112.002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mathieu P, Després JP, Pibarot P. The ‘valvulo-metabolic’ risk in calcific aortic valve disease. Can J Cardiol. 2007 Oct;23(Suppl B):32B–39B. doi: 10.1016/s0828-282x(07)71008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]