Abstract
Human genetic discoveries offer a powerful method to implicate pathways of major importance to disease pathobiology and hence provide targets for pharmacological intervention. The genetics of pulmonary arterial hypertension (PAH) strongly implicates loss-of-function of the bone morphogenetic protein type II receptor (BMPR-II) signalling pathway and moreover implicates the endothelial cell as a central cell type involved in disease initiation. We and others have described several approaches to restore BMPR-II function in genetic and non-genetic forms of PAH. Of these, supplementation of endothelial BMP9/10 signalling with exogenous recombinant ligand has been shown to hold considerable promise as a novel large molecule biopharmaceutical therapy. Here, we describe the mechanism of action and discuss potential additional effects of BMP ligand therapy.
Introduction
Pulmonary arterial hypertension (PAH) is a disease of pathological vascular remodeling, characterized by medial thickening and the formation of occlusive vascular lesions that obstruct the pulmonary circulation, predominantly at the level of the pre-capillary arterioles. This loss of the pulmonary distal circulation, and the resultant increase in pulmonary vascular resistance (PVR), leads to an elevation of pulmonary arterial pressure and right ventricular hypertrophy. PAH is a rare disease, with a prevalence of 15 to 26 patients per million individuals and an incidence of 2.4 to 7.6 cases per million annually.1,2 It can arise as a primary disease, either in an idiopathic (IPAH) or heritable (HPAH) form, or as a condition associated with immune disorders such as HIV, connective tissues diseases, or exposure to particular drugs or toxins. In its idiopathic and heritable forms, PAH presents in a relatively young patient population, aged 30–50 years, and preferentially in women at a rate of roughly 2.3:1. If left untreated, PAH can lead to death from right-sided heart failure within 3–5 years of diagnosis.
Existing treatments for PAH were developed for this indication because of their effects on vascular tone. The regulation of vascular tone by endothelial cells and vascular smooth muscle cells (SMCs) is mediated by a balance of vasodilators, such as prostacyclin and nitric oxide (NO), and vasoconstrictive agents, including endothelin-1. Established PAH is associated with a shift in this balance towards excessive pulmonary vasoconstriction. Recognition of this imbalance was the driving force behind the development and approval of a range of vasodilatory therapies for PAH.3 These therapies can be divided into three main classes: (i) prostanoids, including epoprostenol and more stable prostacyclin analogues such as iloprost, beraprost and treprostinil; (ii) endothelin receptor antagonists, including bosentan, ambrisentan and macitentan; and (iii) phosphodiesterase 5 inhibitors, such as sildenafil and tadalafil. While these treatments have been successful in improving the haemodynamic parameters and functional status of patients, the three-year survival rate for PAH patients remains poor.4 Although these therapies may also have modest effects on vascular SMC proliferation, available evidence suggests a minimal impact on the process of vascular remodeling in the lungs of patients with PAH.5 Here we propose that more effective therapies for PAH will derive from a greater understanding of the molecular basis of pathological pulmonary vascular remodeling, particularly by targeting pathways identified by human genetics in PAH patients.
The genetic basis of PAH
Heritable PAH (HPAH) is an autosomal dominant disease, marked by a low penetrance (average 20–30%) in at-risk individuals.6 While the existence of this familial form of PAH has been recognized since the first description of the disease, it was only 15 years ago that mutations in BMPR2, the gene encoding the bone morphogenetic protein (BMP) type II receptor (BMPR-II), was identified as the cause of approximately 75% of HPAH cases.7,8 BMPR2 mutations also account for 15–26% of seemingly idiopathic or sporadic cases of PAH, including cases of de novo mutations and parental transmission with no record of a previous family history of disease. As a result of these findings, the definition of HPAH was recently updated to include not only patients in a family with two or more documented cases of PAH, but also includes any PAH patient possessing a mutation in BMPR2.9
A range of mutations in BMPR2 have been reported in PAH patients. The majority of these mutations lead to a state of haploinsufficiency,10 where the mutant allele leads to no production of a protein product. The protein expression from the wild type allele is normal but overall protein expression is reduced by at least 50%. Patients bearing BMPR2 mutations develop PAH earlier, have more severe disease and die sooner than those without mutations.6 Interestingly, PAH patients with BMPR2 mutations exhibit reductions in BMPR-II protein levels of greater than 75% when compared to control subjects, suggesting that the development of PAH can suppress receptor levels to a greater extent than what can be accounted for by haploinsufficiency alone.11 Reduced BMPR-II protein levels and impaired downstream signaling have also been identified in idiopathic PAH patients lacking mutations in BMPR2, as well as in common, non-genetic rodent models of disease,12 further supporting a central role for reduced BMPR-II signaling in most forms of the disease, independent of etiology.
When taken together, these factors make the BMPR-II signaling pathway an extremely attractive target for next-generation therapeutic intervention. However, the translation of this approach into in vivo pre-clinical studies has been limited by uncertainty regarding which cell type or types are critically affected by the loss of BMPR-II signaling, and the complexity of the BMP signalling family. Fortunately, genetic studies describing disease phenotypes associated with mutations in other components of the BMP signaling pathway, coupled with knowledge of the tissue-specific distribution of these proteins, can be used to inform decisions on which cell types, and which BMPs, play important roles in the initiation of PAH.
BMPs are members of the transforming growth factor-β (TGFβ) superfamily, a highly complex family of proteins including over 30 ligands that signal through heteromeric complexes of type I and type II receptors. As a type II receptor of this superfamily, BMPR-II can form complexes with several type I receptors, including the activin like receptor kinases ALK1, ALK2, ALK3 or ALK6, with each receptor complex recognizing a specific subset of BMP ligands.13 Canonical signaling generally involves phosphorylation of the Smad-1, 5 or -8 transcription factors, which complex with the co-smad, Smad-4, and translocate to the nucleus to induce gene expression. The tissue-specific nature of BMP responses is highly dependent upon the components making up the ligand-receptor complex. Numerous accessory receptors, such as endoglin (ENG), also modify signaling, further contributing to the tissue-specificity of BMP responses.
Following the initial identification of BMPR2 mutations in PAH, much of the work investigating the role of deficiency of this receptor in disease pathogenesis focused on the impact of these mutations on pulmonary arterial SMC (PASMC) proliferation and migration.14 In smooth muscle cells, BMP ligands, including BMP2 and BMP4, block serum-induced proliferation via complexes of BMPR-II with ALK3 or ALK6.14 However, mutations in ALK3 and ALK6 do not cause pulmonary vascular disease, but are instead associated with juvenile gastrointestinal polyposis15 and hereditary brachydactyly,16 respectively. This fact calls into question the importance of these receptors, and the signaling complexes that they form, in the pathogenesis of PAH.
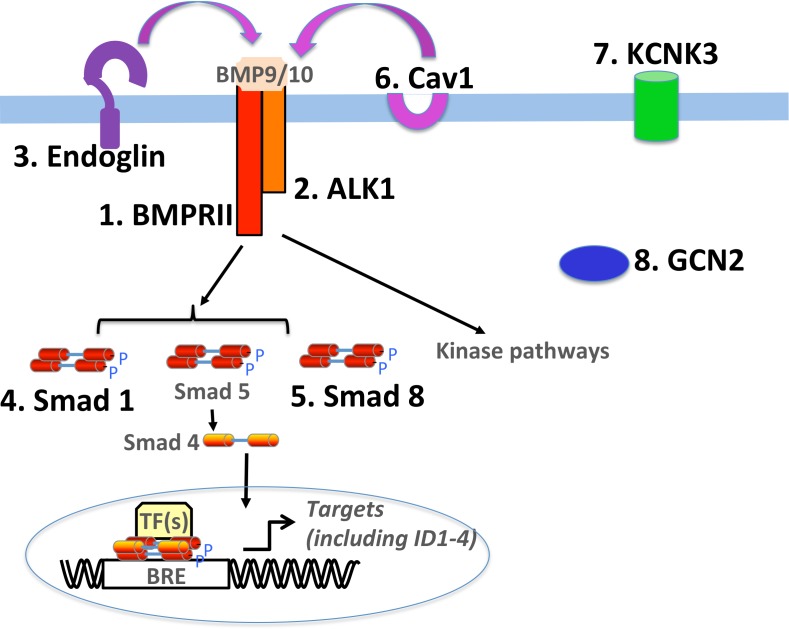
More recently, several studies have demonstrated a role for impaired endothelial BMP signaling in the pathogenesis of PAH. In addition to increased endothelial cell proliferation and enhanced susceptibility to apoptosis,17,18 loss of BMPR-II has also been shown to influence endothelial cell barrier integrity and pulmonary vascular permeability.19 Genetic evidence also supports a role for the pulmonary endothelium as the critical initiating cell type in PAH. Mutations in Endoglin and ALK1 (gene symbol ACVRL1), two proteins found almost exclusively on the endothelium, are primarily associated with hereditary haemorrhagic telangectasia (HHT), a disease that causes vascular abnormalities, including telangiectases in the skin and mucosal regions, and arteriovenous malformations in the lung, liver, gastrointestinal tract and brain. In addition to HHT, ACVRL1 mutations are occasionally associated with PAH, demonstrating a role for these molecules, and the receptor complexes they form, in the maintenance of pulmonary vascular homeostasis.20 To date, mutations in eight genes have been found to play a causal role in idiopathic and heritable PAH (Fig. 1). Notably, of all the known mutations identified to date, six out of eight of these directly or indirectly implicate the BMP signalling pathway, and particularly endothelial BMP signaling, as central to pathobiology. Taken together, these findings strongly support the development of therapeutic strategies that restore expression or function of BMPR-II, particularly in pulmonary vascular endothelial cells.
Figure 1.
Simplified schematic summarizing the BMP signalling pathway and genes that have to date been shown to be mutated in PAH and demonstrating that many of these mutations encode protein involved in BMP signalling. Known mutations are numbered and in bold.
Targeting BMPR-II deficiency for the treatment of PAH
Considering the genetic evidence indicating an important role for BMPR-II in the pathobiology of PAH, several groups have begun to use this information to test the potential of next generation therapies that directly target BMPR-II deficiency. Although not limited to the endothelium, a number of these studies directly target pulmonary endothelial BMPR-II for their mode of action. These therapies are directed towards various stages of BMPR-II signaling, including gene expression, translation, surface expression and receptor degradation, as well as enhancing receptor activity through the delivery of BMPR-II agonists. The various strategies can be grouped into three main approaches: (i) rescue of mutant receptor function, (ii) enhancing the expression or longevity of the wild-type receptor or (iii) enhancing BMP signaling through small molecules or recombinant BMP ligands. The published studies examining these three approaches are summarized below and in Table 1.
Table 1.
Summary of experimental PAH therapies targeting BMPR-II
| Therapeutic Strategy | Agent | Model | Citation |
| Rescue of Mutant BMPR-II Receptor | |||
| Chemical Chaperones | 4-PBA, glycerol and thapsigargin | In vitro: HeLa cells | [21] |
| Suppression of Nonsense Mutations | Gentamycin | In vitro: cell-based reporter assay | [23] |
| Ataluren (PTC124) | In vitro: PAH endothelial and smooth muscle cells | [24] | |
| Enhancement of BMPR-II Expression and Longevity | |||
| Inhibition of lysosomal degradation | Chloroquine, hydroxychloroquine | In vitro: PAH endothelial cells In vivo: Monocrotaline rat model | [26, 28] |
| BMPR2 Gene Therapy | Endothelial-targeted adenoviral vector | In vivo: chronic hypoxia and monocrotaline rat models | [29, 30] |
| Enhancement of BMPR-II-mediated Signaling | |||
| Small molecule BMPR-II agonists | Iloprost | In vitro: PAH smooth muscle cells In vivo: Monocrotaline rat model | [32] |
| Sildenafil | In vitro: PAH smooth muscle cells in vivo: Monocrotaline rat model | [33] | |
| FK506 | In vitro: PAH endothelial cells In vivo: Endothelial-specific BMPR2 knockout mouse, Sugen-Hypoxia and monocrotaline rat models | [34] | |
| Recombinant BMP ligand | Recombinant human BMP9 | In vitro: PAH endothelial cells In vivo: bmpr2-R899X mouse, Monocrotaline rat model, Sugen-Hypoxia rat and mouse models | [41] |
Rescuing mutant BMPR-II receptor function: Over 400 different PAH associated BMPR2 mutations have been identified in all functional domains of the BMPR-II protein.6 These include missense mutations within the extracellular domain, transmembrane region, and kinase region, as well as nonsense mutations that encode premature termination codons (PTCs). Strategies designed to enhance BMP signaling by rescuing the mutated copy of the BMPR2 gene can take one of two forms, depending on the type of mutation present.
Chemical or pharmacological chaperones: Missense mutations can lead to the production of misfolded BMPR-II through the replacement of critical cysteine residues that are essential to the folding of the extracellular domain within the endoplasmic reticulum (ER). These mutants can be targeted with chemical chaperones that enhance the transport of the mutant protein from the ER to the cell surface. Some chemical chaperones, such as glycerol, non-selectively stabilize the tertiary structure of the mutant proteins and facilitate folding. Other compounds, including thapsigargin and sodium 4-phenylbutyrate (4-PBA), inhibit the interactions of the mutant protein with the chaperone proteins responsible for their recognition, retention and degradation. In PAH, a panel of chemical chaperones, including 4-PBA, glycerol and thapsigargin, were shown to promote folding and trafficking of mutant BMPR-II protein to the cell surface. Since, in this case, the mutant receptor was still capable of normal signalling, downstream Smad signalling was restored.21 While these results are promising, there are several factors limiting the clinical application of these compounds for PAH. The PAH patient population contains a large variety of missense mutations, only a subset of which encode mutations that lead to the misfolding a receptor that is otherwise functional. Nevertheless, 4-PBA has been used for other indications in man and may be a personalized approach in patients with these specific mutations.
Suppression of nonsense mutations: In contrast to missense mutations, where the mutant protein is produced and can be targeted for rescue, the mRNA transcripts produced by alleles bearing nonsense mutations are typically subject to nonsense-mediated decay (NMD), resulting in reduced BMPR2 mRNA and decreased protein levels. The rescue of these mutations requires the use of compounds that suppress or silence PTCs, allowing for the translation of mutant mRNA into full-length, functional protein.22 A number of compounds belonging to the aminoglycoside family of antibiotics, including gentamicin, have been shown to suppress disease-causing PTCs and partially restore protein function in nonsense mutation-bearing cell culture models of multiple diseases, including cystic fibrosis, Duchenne muscular dystrophy and PAH.23 However, the high doses required to achieve these beneficial effects in vivo are also associated with renal toxicity and hearing loss, thus preventing the widespread use of aminoglycosides in clinical applications.
More recently, high throughput screens have identified other compounds distinct from aminoglycosides that possess the ability to effectively suppress PTCs without affecting the recognition of normal stop signals. One such compound, the small molecule Ataluren (PTC124), is currently in phase III clinical trials for Duchenne muscular dystrophy. In PAH, Ataluren was shown to increase BMPR-II protein levels and downstream signaling in primary cells from patients with nonsense mutations in BMPR2 and SMAD9.24 These effects were accompanied by a restoration of normal proliferation rates in hyperproliferative, patient-derived PAECs and PASMCs.
Enhancing BMPR-II surface expression
Inhibiting BMPR-II degradation: An alternative approach that has been explored for enhancing BMP signalling involves extending the longevity of BMPR-II at the cell surface. BMPR-II is subject to constitutive endocytosis by both clathrin and caveolae mediated pathways25 and is rapidly turned over in endothelial cells. Notable protein loss is observed in these cells as early as 1 hour following the blockade of new protein synthesis.26 BMPR-II can also be targeted for ubiquitination by Kaposi's sarcoma herpes virus K5 E3 ligase, and directed for lysosomal degradation.27 Each of these findings highlights the lysosome as the primary route for BMPR-II turnover and points to lysosomal inhibition as a promising target for preserving BMPR-II longevity and enhancing receptor-mediated signaling. The 4-aminoquinolone, chloroquine, achieves lysosomal inactivation through the prevention of vesicular acidification. Used for decades as a prophylactic antimalarial drug, chloroquine, and its less toxic derivative hydroxychloroquine, have recently been repurposed for use in the treatment of rheumatoid arthritis and cancer. In vitro, chloroquine was shown to enhance BMPR-II protein levels, surface expression and downstream signaling in BMPR2 mutation-bearing endothelial cells.26 In vivo, both chloroquine and hydroxychloroquine inhibited the development of pulmonary hypertension and blocked the progression of established disease in the monocrotaline rat model.28 Although this therapeutic effect was associated with restoration of BMPR-II protein levels, which are decreased in monocrotaline-treated rats, it is likely that the beneficial effect of chloroquine administration may also be linked to the inhibition of excessive autophagy, which has been linked to the aberrant proliferation of vascular cells in PAH. Translation of hydroxychloroquine into a clinical therapy is facilitated by the fact that the compound is inexpensive and presents acceptable toxicity or side effects at therapeutically effective doses. Long-term treatment with hydroxychloroquine has been associated with retinopathy in 0.5–1% of patients. However, this can be avoided with proper monitoring of patients undergoing treatment.
BMPR2 gene therapy: Gene therapy techniques have also been explored in animal models of PAH as a means to restore BMPR-II receptor levels. The targeted delivery of an adenoviral vector containing the BMPR2 gene to the pulmonary vascular endothelium of rats substantially reduced the severity of pulmonary hypertension in the chronic hypoxia and monocrotaline models of PAH.29,30 For these studies, targeting of the adenoviral vectors to the pulmonary endothelium appeared to be a key feature of therapeutic efficacy, as similar studies using aerosol vector delivery and a non-specific promoter failed to demonstrate any measurable benefit in the monocrotaline rat model.31
Enhancement of BMPR-II downstream signalling: One alternative to enhancing BMPR-II receptor expression or longevity involves enhancing signaling through the action of small molecules. Existing PAH therapies, including prostanoids and sildenafil, have been shown to partially restore BMP signaling in PASMCs bearing BMPR2 mutations and prevent the development of PAH in animal models of disease.32,33 More recently, a high throughput screening approach demonstrated that the calcineurin inhibitor, tacrolimus (FK506), potentiates BMPR-II-mediated signaling in endothelial cells, rescues dysfunctional BMP signaling in endothelial cells from PAH patients with BMPR2 mutations and reverses established disease in multiple rodent models of PAH.34 Early clinical trials are currently underway to establish the viability of this strategy as a treatment for PAH. However, this approach is likely to lead to widespread and non-specific activation of BMP receptors, as well as having off target effects independent of BMP signalling.
BMP9 and BMP10 as potential therapies for PAH
One of the most direct strategies for targeting BMPR-II deficiency in PAH involves the delivery of exogenous BMP ligand to enhance signaling via the remaining functional receptor. Proof-of-concept studies using patient-derived pulmonary arterial smooth muscle cells (PASMCs) have shown that the addition of increasing concentrations of BMP ligand can overcome the functional defects associated with BMPR2 mutation in vitro.14 However, identifying the appropriate BMP ligand or ligands to selectively target the endothelium in vivo presents a significant challenge. The selective expression of ALK1 on the endothelium makes the ALK1 /BMPR-II complex an ideal target for exogenous ligand therapy. Although ALK1 was originally thought to be an orphan receptor, two studies in 2007 identified BMP9 and BMP10 as ligands that signal via complexes of ALK1 with either BMPR-II or type II activin receptors (ActRII).35,36
Further examination of the receptor selectivity of these ligands has primarily focused on BMP9. Endogenously, BMP9 activates ALK1 with high affinity (EC50 = 50 pg/ml). BMP9 also activates ALK2 but with much a lower affinity in the ng/ml range.35–39 Biochemical analysis has also revealed discrete differences in the type II receptor selectivity of BMP9 and BMP10. Although BMP9 and BMP10 both bind to BMPRII, ActRIIA and ActRIIB, BMP9 exhibits a preference towards ActRIIB compared to BMPRII and ActRIIA while BMP-10 binds to all type II receptors with similar affinities.40 In endothelial cells, type II receptor redundancy means that loss of either BMPR-II or ActRIIA has a minor impact on the stimulation of Smad1/5 phosphorylation due to compensation by the remaining type II receptor.37 Loss of both receptors, however, abolished the Smad phopshorylation responses. Of note, some transcriptional responses to BMP9, such as E-selectin and IL8 induction are more reliant on BMPR-II signaling as ActRIIA does not compensate.37 This implies that there are BMPR-II-specific signals that may contribute more than others to the pathology of PAH. Another feature of the BMPR-II selectivity of BMP9 relates to the transcriptional regulation of the receptors themselves. BMP9 induces the expression of BMPR-II, but not ALK1 or ActRIIA, suggesting that this ligand switches the balance of signaling in the endothelial cell towards BMPR-II.35,37 Furthermore, this induction is ALK1-dependent and Smad1-dependent, suggesting a feed forward signaling mechanism.37,41 It was recently shown that the therapeutic effects of BMP9 include restoration of BMPR-II expression and signaling of BMPR-II in animal models of PAH.41 Therefore, BMP9 represents a therapy that promotes its key downstream signalling pathways partly via rescue of the deficient BMPR-II expression that underlies the pathogenesis of PAH. A proposed mechanism for this upregulation is shown in Fig. 2.
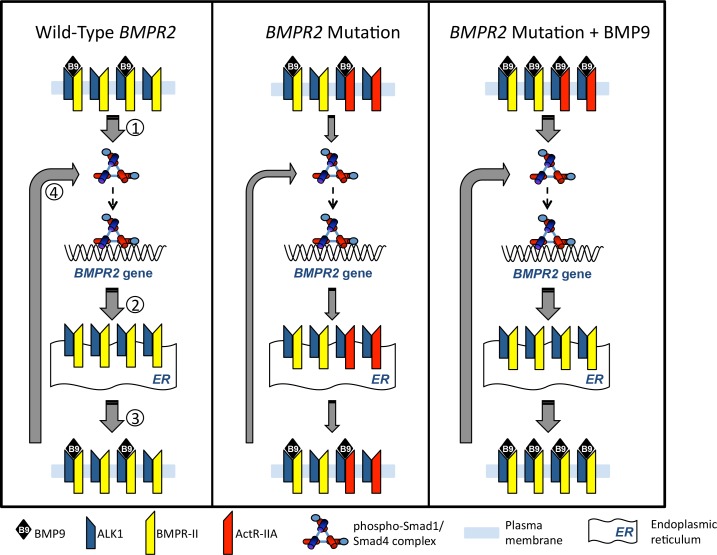
Figure 2.
Proposed mechanism for the restoration of cell surface BMPR-II expression and signalling using exogenous BMP9 therapy. Left panel: Under normal conditions an individual possesses two wild-type alleles for BMPR2. Under these circumstances BMP9 signalling involves signalling via the ALK1:BMPR-II ligand receptor complex and activation of the Smad1/4 transcriptional complex.1 This promotes BMPR-II mRNA transcription and synthesis2 and trafficking of newly-synthesised BMPR-II to the cell surface where it complexes with ALK1, which has been recycled via the endosomal pathway.3 In the presence of BMP9, this feed forward pathway continues in an autoregulatory loop.4 Middle panel: in patients with a heterozygous mutation in BMPR2 leading to haploinsufficiency, cell surface BMPR-II is reduced and in its place, ActRII-A can form a complex with available ALK1 but this does not promote autoregulatory BMPR-II production in response to endogenous concentrations of BMP9, which remain unchanged. The reduced signalling through BMPR-II leads to reduced BMPR-II levels of the receptor at the cell surface. Right Panel: Administration of exogenous BMP9 to PAH patients with a heterozygous mutation in BMPR2, increases the circulating concentration of BMP9 which increases signalling via the Smad1/4 complex to induce BMPR-II protein expression. This shifts the equilibrium of the BMPR-II:ActR-IIA ratio in favour of BMPR-II associating with the available ALK1 and thus restores the autoregulatory production of BMPR-II in response to BMP9, thus restoring normal endothelial BMP9 signalling.
Based on our studies of BMPR-II dysfunction in PAH and the emerging role of BMP9 as a key circulating regulator of endothelial function and positive regulator of BMPR-II expression, we recently examined the therapeutic delivery of BMP9 to target endothelial dysfunction in PAH.41 In vitro, BMP9 prevents the enhanced apoptosis observed in BMPR2 mutation-bearing endothelial cells and promotes monolayer integrity through the formation of tight junctions. In vivo, BMP9 prevents and reverses established disease in a range of rodent models, including spontaneous disease in a mouse model bearing a knock-in of the PAH-associated R899X-Bmpr2 mutation. From these studies, we propose that BMP9 constitutes a potential therapy that overcomes the underlying endothelial dysfunction that causes PAH and can overcome the deficient BMPR-II signaling that is a consequence of the major genetic defect in most HPAH patients (Fig. 3). Our anticipation is that, by restoring the balance in BMP signaling, this approach will redress the imbalance of other dysregulated pathways that are targeted by current therapies.
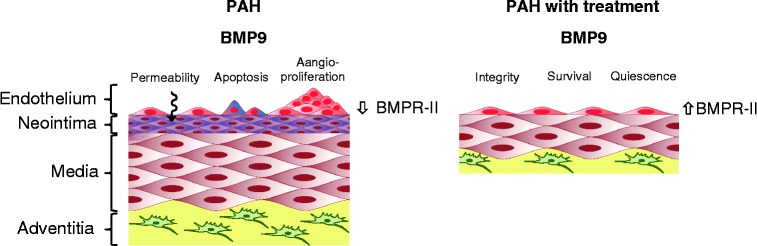
Figure 3.
Impact of BMP9 therapy on pulmonary endothelial cell function. In the lungs of PAH patients, loss of BMPR-II leads to endothelial dysfunction, including increased vascular permeability, apoptosis and aberrant angioproliferation. Therapeutic delivery of recombinant BMP9 promotes endothelial quiescence, survival and vascular integrity, while simultaneously enhancing BMPR-II expression.
Another theoretical approach to activate the endothelial ALK1/BMPR-II pathway would be to develop a peptide mimetic that possesses BMP9/10-like function. An example of such an approach was recently demonstrated for a peptide derived from BMP7.42 Although it is considered unlikely by some that a small peptide mimetic would be able to substitute for a BMP dimer in the assembly of two type I and two type II receptors, all of which are required for signalling,43–45 the BMP7 peptide mimetic was shown to have similar effects to full-length BMP7 ligand in reversing established fibrosis in five mouse models of acute and chronic renal injury.42 Although the findings of this study have been challenged by researchers from several groups, synthetic peptides that specifically activate the endothelial ALK1/BMPR-II pathway still represent promising potential drug candidates for treating PAH.
Since BMP9 exerts its beneficial effect on the endothelium in an ALK1-dependent manner, recombinant BMP10 protein, which is the only other BMP ligand that signals through ALK1, could also have a similar therapeutic potential for treating PAH. There are several advantages of BMP10-based therapy. Firstly, unlike BMP9, it does not show osteogenic activity either in vitro signalling assays, or in an in vivo bone-forming screen,46 and may therefore be potentially safer than BMP9 for treating cardiovascular disease. Secondly, BMP10 binds to ALK1 and BMPR-II with higher affinities than BMP9,40 making it likely to be more potent and more specific for ALK1 and BMPR-II.
In spite of these advantages, there are still several hurdles to overcome before BMP10 can be developed into a therapy for PAH. For example, the activity of circulating BMP10 in humans is still under investigation. BMP10 is bound tightly by its prodomain, which inhibits BMP10 signalling activity when applied in molar excess.47 It has therefore been proposed that BMP10, similar to TGFβ, exists in a latent form in the circulation and requires an additional activation step to achieve bioactivity, such as cleavage of the prodomain by BMP1.47 This hypothesis is further supported by the fact that, although circulating BMP10 could be consistently detected and measured by ELISA48,49 and by pull-down coupled with proteomics,50 early studies could not detect BMP10 activity in the circulation.48,51 However, one recent report was able to demonstrate BMP10 activity in mouse serum.49
A further question to be addressed is whether the presence of the prodomain on therapeutically administered BMP10 is important for the stability and half-life of the ligand. A better understanding of the physiology of BMP10 is required in vivo, since BMP10 is a much less well-studied BMP ligand and most of the data are from embryos of mouse and zebrafish. The data on the role of BMP10 in adult physiology and in PAH are limited, and such information will inform the development and testing of BMP10-based drugs.
Physiological roles of BMP9 and BMP10
During embryonic development and into adult life, BMP9 and BMP10 are both expressed in a highly tissue-specific pattern. BMP9 is expressed in the mouse fcomment liver from E9.75–1049 and expression remains high in the adult liver.52,53 The highest levels of expression are observed in mRNA from intrahepatic biliary epithelial cells and hepatocytes.53 In contrast, BMP10 is highly expressed in the developing embryonic mouse heart, with much lower levels of expression detected in lung and liver.54 The expression of BMP10 is high in the trabecular myocardium between days E9.5 – E13.5, a period that coincides with cardiac growth and chamber maturation.54 The expression of BMP10 becomes restricted to the atria by day E18.5 and in adults, only the right atrium expresses BMP10.49,55
BMP9 is secreted and circulates at levels of 2–12 ng/ml in human plasma56 and 1.18–1.84 ng/ml in human serum,51 based on cell-based luciferase bioassays. Plasma BMP9 levels in mice increase just before birth and peak postnatally at levels of 6 ng/ml on Day 15 before declining to a steady state of approximately 1.5–2 ng/ml.53 Similar to other BMPs, BMP9 is synthesised as a 429 amino acid precursor (pre-pro-BMP9) comprising a 22 amino acid signal peptide, a 297 amino acid prodomain and a 110 amino acid mature protein.53,57 The precursor is then cleaved by serine endoproteases to produce a mature protein dimer (25 kDa) which can remain non-covalently associated with two pro-domains (33 kDa each) forming a 100 kDa complex.53 Unlike the pro-regions of TGFβs and GDF8, which whilst bound, inhibit their ligands in vitro and in vivo,58–61 the mature BMP9 dimer retains its biological activity when in complex with the pro-domain.62,63 The bound pro-domain is proposed to enhance the stability of BMP9 in vivo.62 Approximately 60% of the 100 kDa circulating pro-BMP9 complex is cleaved and active, whereas the remaining 40% is unprocessed and may be activated through a local furin cleavage.53
The role of BMP10 as a secreted ligand is more controversial, since some studies suggest that BMP9 is solely responsible for the ALK1-activating BMP activity in plasma.48,56 However, a recent study has reported that BMP10 is present in mouse (0.5–2 ng/ml) and human (1–3 ng/ml) serum, suggesting that BMP10 may be present in the circulation at physiologically important levels.49 Whether BMP10 in the circulation is processed and circulates in a similar manner to BMP9 is not yet clear. However, evidence supporting a functional role for circulating BMP10 has been revealed in zebrafish embryos, showing that BMP10 is necessary for maintaining vascular stability and endothelial Smad signalling in the vessels proximal to the heart.64 Loss of BMP10 phenocopies the cranial vascular abnormalities of the Alk1 zebrafish mutants,65 although as discussed later, this differs from the phenotype of the Bmp10 knockout mouse.
The overlapping expression of BMP10 and Alk1 at about E8.549, 66 compared to the expression of BMP9 in the liver at E9.75-10 has been argued as evidence for the developmental regulation of BMP10 signalling through Alk1.49 As BMP9 and BMP10 both activate ALK1, this may represent temporally important roles of the ligands. For example, Alk1-/- mice die at E10.5 due to defects in angiogenesis,67 which may represent a failure of both BMP9 and BMP10 signalling, as discussed below. In comparison, Alk1+/ − mice develop normally, but exhibit nosebleeds and vascular anomalies. This reflects the pathology of ALK1 mutations causing HHT in man, although the disease penetrance is lower in heterozygous mouse models.
Intriguingly, BMP9 knockout mice develop to adulthood without any overt phenotype,48 although further analysis has revealed lymphatic vessel defects68 and delayed closure of the ductus arteriosus.69 The lack of a severe vascular phenotype in BMP9-/- mice appears to be due to functional redundancy with BMP10, confirmed by defective retinal vascularisation in BMP9-/- mice when BMP10 is also neutralised.48,49 Of note, ALK1-Fc administration, which inhibits both BMP9 and BMP10, phenocopies this sprouting defect.49 As circulating BMP10 levels are elevated slightly in BMP9-/- mice, one might speculate that BMP10 may compensate for the absence of BMP9 in the circulation.48
Consistent with the restricted cardiac expression, BMP10-/- embryos die around E9.5–E10.5 due to hypoplastic cardiac development, probably as a result of reduced myocyte proliferation.55 Although original reports reported an absence of vascular defects,55 recent data indicate that the dorsal aorta and cardinal veins are fused in BMP10-/- mice.49 Furthermore, cloning of the BMP9 coding region into the BMP10 knockout mouse does not rescue the cardiac defect whereas early vascular development appears normal, suggesting that BMP10 mediates cardiac development via a mechanism that is independent of the signalling capacity of BMP9.49 This could be due to differences in the selectivity for type II receptors.49 Intriguingly, ectopic expression of BMP10 driven by the alpha-myosin heavy chain promoter in the mouse myocardium leads to cardiac hypotrophy by 6 weeks of age.70 The level of BMP10 expression achieved in the hearts was high, so it is not clear whether this represents a physiologically relevant effect of BMP10.70 Table 2 summarizes the similarity and the difference between BMP9 and BMP10 in physiology.
Table 2.
Summary of similarities and differences between BMP9 and BMP10 in physiology
| BMP9 | BMP10 | |
| -/- mice phenotype | Normal, lymphatic vessel defect | Lethal, impaired cardiac development |
| Adult expression | Liver, into circulation | Right atrium |
| Circulating form and levels | 2–10 ng/ml (by activity) | Presence shown by ELISA and proteomics; |
| ∼300 pg/ml (ELISA)108 | Only 1 in 3 reports can detect activity | |
| Function | Vascular quiescence factor | Flow-dependent arterial quiescence |
| Bone-forming activity | Highest among 14 BMPs | Undetected |
| Endothelial cell signalling | Controlling a similar set of target genes with similar potency48 | |
| Affinity for ALK1/BMPRII | Higher |
Potential side effects of BMP9/10 therapy
One potential concern associated with the delivery of exogenous BMP ligands is the activation of other BMP receptors in non-endothelial cells, much of which is a dose-related phenomenon. It is therefore important to consider the roles of BMP9 in tissue ossification/calcification, hepatic function and tumour regulation.
Osteogenesis, chrondrogenesis and adipogenesis: The potent orthotopic bone-forming activity of BMP9 may be a potential complication of BMP9 therapy in PAH. BMP9 induces the differentiation of mesenchymal stem cells (MSCs - adult stem cells found in bone marrow) into osteocytes, chondrocytes and adipocytes46 and is one of the most potent osteogenesis-inducing BMP ligands in MSCs.46,71–74 The osteogenic induction of bone marrow MSCs is mediated via both ALK1 and ALK275 and is reported to involve BMP9 dependent induction of angiogenic HIF1a signaling.76
The osteogenic response to BMP9 in skelcomment muscle has been achieved through several modes of local ligand expression. Delivery of BMP9 into skelcomment muscle via direct sonoporation,77 injection of transfected MSCs78 or C2C12 cells46,79 or injection of an adenovirus engineered to express BMP946 all elicited ectopic bone formation in muscle tissue. These processes are attributed to the differentiation of local multipotent MSCs or osteoblastic progenitor cells.80–82 Ad-BMP9 stimulates lamellar bone in mice by 3 months,83 although studies have reported evidence of calcification after 9 days of exposure.84 It is notable that the majority of these studies involve a local inflammatory stimulus and it appears that the heterotopic ossification response to BMP9 requires injury to skelcomment muscle.85 Another important consideration would be the concentration of BMP9 achieved during therapeutic administration. All of the above studies involved high concentrations of BMP9 or uncontrolled local overexpression. Our recent study employed intraperitoneal dosing of BMP9 injections, and we observed no evidence of heterotopic calcification after 4 weeks of daily intraperitoneal BMP9 injection, or indeed 3 weeks of intramuscular injection.41 The concentration of BMP9 is likely to be important to achieve therapeutic activation of BMPR-II/ALK1, but avoidance of ALK2 activation at higher concentrations.
A recent study demonstrated that BMP9 promotes calcification of vascular smooth muscle cells in the context of high phosphate levels.86 In this study, the authors propose ALK1 as the receptor mediating this calcification, although this conclusion was derived from the use of an ALK1-Fc ligand trap to inhibit BMP9 rather than molecular dissection of the receptor signalling in VSMCs. BMP9 stimulated calcification at concentrations of 5 ng/ml or greater, representing concentrations at which BMP9 can stimulate both ALK1 and ALK2. In contrast, no calcification was observed at 0.5 ng/ml BMP9, a concentration that would exclusively activate ALK1. Indeed, BMP9 and BMP10 might be expected to exert endothelial-mediated vascular protective effects in atherosclerosis, since endothelial-specific knockout of BMPR-II led to enhanced inflammation and atherosclerosis in ApoE-knockout mice.87 As BMP10 appears to lack osteogenic activity in muscle tissue, at least when transduced with an adenovirus,46 BMP10 may represent an attractive therapeutic alternative to BMP9, providing that BMP10 proves an effective therapy in animal models of PAH.
Liver function: The ability of BMP9 to stimulate hepatocyte proliferation is long established, being one of the earliest observations of BMP9 function.88 Early studies demonstrated that BMP9 binds to specific receptors in HepG2 cells,89 consistent with the expression of the low affinity type I receptor, ALK2 and the type II receptors, BMPRII, ActRIIA and ActRIIB.90 Furthermore, both the HLE (human, non-differentiated hepatoma) cell line and hepatic stellate cells express ALK1, indicating these cells may exhibit high sensitivity to BMP9, though this has yet to be established.91,92 Of note, BMP9 stimulates canonical Smad signalling and proliferation in HepG2, Hep3B and HuH7 cells.93 The lowest BMP9 concentration examined in this report was 1 ng/ml and the responses were completely inhibited by low concentrations of the ALK2/3/6 inhibitor, LDN193189, implying these responses are not through ALK1.93 The data from normal hepatocytes suggest that BMP9 may participate in liver repair but in conditions of liver carcinogenesis, BMP9 may promote tumour growth as discussed below.
In vivo studies have highlighted a positive role for hepatic BMP9 in energy metabolism. BMP9 inhibits hepatic glucose production and activates the expression of key enzymes of lipid metabolism.63 Furthermore, recombinant BMP9 improved glucose homeostasis in vivo in diabetic and non-diabetic rodents.63 However, these effects were observed at doses of BMP9 that were at least 1000 times higher than those employed in our PAH models.41 Conversely, BMP9 is reduced in the livers of insulin-resistant rats and administration of an anti-BMP9 antibody induced glucose intolerance and insulin resistance in fasted rats.94 Consistent with this observation, administration of insulin in combination with high glucose induced BMP9 expression in the livers of 12h-fasted rats.94 This promotion of hepatic insulin sensing is associated with reduced expression of a rate-limiting enzyme of gluconeogenesis, phosphoenolpyruvate carboxykinase (PEPCK) in hepatocytes.63
In addition to effects on the liver, BMP9 may regulate glucose utilization by skelcomment muscle as it activates of Akt2 kinase in differentiated myotubes.63 Akt2 is essential for the activation of insulin-induced glucose uptake by muscle and Akt2 signalling is impaired in insulin resistance.95–97 In differentiated L6 myotubes, Akt2 is activated by Smad5 and this has been proposed as the mechanism for BMP9 action,98 though a direct effect of BMP9 signalling through Smad5 was not confirmed. Overall, the positive effect of BMP9 on hepatic glucose sensing may be of benefit in patients given the perspective that metabolic dysfunction is a component of the pathophysiology of PAH.99,100
Tumour regulation: Variable effects of BMP9 on tumours and tumour cell growth have been reported. For example BMP9 promotes proliferation of tumour-derived cell lines from ovary101 and liver93 but induces apoptosis in prostate cancer cells102 and restricts osteosarcoma cell proliferation and migration.103,104 Furthermore, neutralisation of BMP9 with Endoglin-Fc restricts colonic tumours in mice.105 The key receptor promoting the proliferative response is ALK2 in ovarian and liver cancer cell lines, shown through siRNA transfection and inhibition by low concentrations of the ALK2/3/6 inhibitor, LDN193189.93,101 The tumour promoting and inhibitory responses are unlikely to be due to ALK1 activation, as prostate cancer cells do not express ALK1 yet BMP9 promotes apoptosis.102 In some instances, the proliferation of tumour cell lines is promoted by autocrine BMP9 production.101 Thus the impact of BMP9 on tumour cells appears to predominantly via ALK2 and at high local concentrations of BMP9.
In addition to effects on tumour cells, it is proposed that BMP9, and possibly BMP10, promote tumour angiogenesis.106,107 The proangiogenic response to BMP9 appears to be context dependent, as BMP9 is reported to promote proliferation and angiogenic processes in embryonic mouse endothelial cells and transformed endothelial cell lines,106,107 but inhibits angiogenesis in primary adult endothelial cell lines.35,36,41 The possible role of BMP9 in tumour angiogenesis has led to the development of Dalantercept, a soluble ALK1 ligand trap, as an anti-tumour angiogenesis therapy. When applied clinically, the potential capacity of BMP9/10 to sustain tumour angiogenesis would need to be balanced against the possible transformational effect of BMP9 in treating patients with life-limiting PAH.
In summary, despite the potential challenges of enhancing signalling downstream of loss-of-function mutations in BMPR-II there are now several approaches that could be taken forwards into the clinic as novel therapeutic approaches in PAH. Of these, the direct enhancement of endothelial BMPR-II/ALK1 signalling with BMP9/10 offers an immediate solution that could be rapidly tested with the appropriate cautions in patients with this life-limiting disease.
References
- 1.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–1030. doi: 10.1164/rccm.200510-1668OC. et al. [DOI] [PubMed] [Google Scholar]
- 2.Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30(1):104–109. doi: 10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 3.Seferian A, Simonneau G. Therapies for pulmonary arterial hypertension: where are we today, where do we go tomorrow? European respiratory review: an official journal of the European Respiratory Society. 2013;22(129):217–226. doi: 10.1183/09059180.00001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Audit of Pulmonary Hypertension. United Kingdom: Health and Social Care Information Centre; 2013. In: Centre HaSCI, editor. [Google Scholar]
- 5.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186(3):261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin ED, Loyd JE. The genetics of pulmonary arterial hypertension. Circ Res. 2014;115(1):189–202. doi: 10.1161/CIRCRESAHA.115.303404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet. 2000;26(1):81–84. doi: 10.1038/79226. et al. [DOI] [PubMed] [Google Scholar]
- 8.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67(3):737–744. doi: 10.1086/303059. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soubrier F, Chung WK, Machado R, Grunig E, Aldred M, Geraci M. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25):D13–D21. doi: 10.1016/j.jacc.2013.10.035. et al. [DOI] [PubMed] [Google Scholar]
- 10.Machado RD, Aldred MA, James V, Harrison RE, Patel B, Schwalbe EC. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat. 2006;27(2):121–132. doi: 10.1002/humu.20285. et al. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105(14):1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. et al. [DOI] [PubMed] [Google Scholar]
- 12.Long L, Crosby A, Yang X, Southwood M, Upton PD, Kim DK. Altered bone morphogenetic protein and transforming growth factor-beta signaling in rat models of pulmonary hypertension: potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation. 2009;119(4):566–576. doi: 10.1161/CIRCULATIONAHA.108.821504. et al. [DOI] [PubMed] [Google Scholar]
- 13.Upton PD, Morrell NW. TGF-beta and BMPR-II pharmacology–implications for pulmonary vascular diseases. Curr Opin Pharmacol. 2009;9(3):274–280. doi: 10.1016/j.coph.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Davies RJ, Southwood M, Long L, Yang X, Sobolewski A. Mutations in bone morphogenetic protein type II receptor cause dysregulation of Id gene expression in pulmonary artery smooth muscle cells: implications for familial pulmonary arterial hypertension. Circ Res. 2008;102(10):1212–1221. doi: 10.1161/CIRCRESAHA.108.173567. et al. [DOI] [PubMed] [Google Scholar]
- 15.Howe JR, Bair JL, Sayed MG, Anderson ME, Mitros FA, Petersen GM. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet. 2001;28(2):184–187. doi: 10.1038/88919. et al. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann K, Seemann P, Stricker S, Sammar M, Meyer B, Suring K. Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2. Proc Natl Acad Sci U S A. 2003;100(21):12277–12282. doi: 10.1073/pnas.2133476100. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009;180(8):780–787. doi: 10.1164/rccm.200810-1662OC. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavoie JR, Ormiston ML, Perez-Iratxeta C, Courtman DW, Jiang B, Ferrer E. Proteomic analysis implicates translationally controlled tumor protein as a novel mediator of occlusive vascular remodeling in pulmonary arterial hypertension. Circulation. 2014;129(21):2125–2135. doi: 10.1161/CIRCULATIONAHA.114.008777. et al. [DOI] [PubMed] [Google Scholar]
- 19.Burton VJ, Ciuclan LI, Holmes AM, Rodman DM, Walker C, Budd DC. Bone morphogenetic protein receptor II regulates pulmonary artery endothelial cell barrier function. Blood. 2011;117(1):333–341. doi: 10.1182/blood-2010-05-285973. [DOI] [PubMed] [Google Scholar]
- 20.Trembath RC, Thomson JR, Machado RD, Morgan NV, Atkinson C, Winship I. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2001;345(5):325–334. doi: 10.1056/NEJM200108023450503. et al. [DOI] [PubMed] [Google Scholar]
- 21.Sobolewski A, Rudarakanchana N, Upton PD, Yang J, Crilley TK, Trembath RC. Failure of bone morphogenetic protein receptor trafficking in pulmonary arterial hypertension: potential for rescue. Hum Mol Genet. 2008;17(20):3180–3190. doi: 10.1093/hmg/ddn214. et al. [DOI] [PubMed] [Google Scholar]
- 22.Keeling KM, Bedwell DM. Suppression of nonsense mutations as a therapeutic approach to treat genetic diseases. Wiley interdisciplinary reviews RNA. 2011;2(6):837–852. doi: 10.1002/wrna.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasim MT, Ghouri A, Patel B, James V, Rudarakanchana N, Morrell NW. Stoichiometric imbalance in the receptor complex contributes to dysfunctional BMPR-II mediated signalling in pulmonary arterial hypertension. Hum Mol Genet. 2008;17(11):1683–1694. doi: 10.1093/hmg/ddn059. et al. [DOI] [PubMed] [Google Scholar]
- 24.Drake KM, Dunmore BJ, McNelly LN, Morrell NW, Aldred MA. Correction of nonsense BMPR2 and SMAD9 mutations by ataluren in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2013;49(3):403–409. doi: 10.1165/rcmb.2013-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartung A, Bitton-Worms K, Rechtman MM, Wenzel V, Boergermann JH, Hassel S. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol Cell Biol. 2006;26(20):7791–7805. doi: 10.1128/MCB.00022-06. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunmore BJ, Drake KM, Upton PD, Toshner MR, Aldred MA, Morrell NW. The lysosomal inhibitor, chloroquine, increases cell surface BMPR-II levels and restores BMP9 signalling in endothelial cells harbouring BMPR-II mutations. Hum Mol Genet. 2013;22(18):3667–3679. doi: 10.1093/hmg/ddt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durrington HJ, Upton PD, Hoer S, Boname J, Dunmore BJ, Yang J. Identification of a lysosomal pathway regulating degradation of the bone morphogenetic protein receptor type II. J Biol Chem. 2010;285(48):37641–37649. doi: 10.1074/jbc.M110.132415. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res. 2013;112(8):1159–1170. doi: 10.1161/CIRCRESAHA.111.300483. et al. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds AM, Xia W, Holmes MD, Hodge SJ, Danilov S, Curiel DT. Bone morphogenetic protein type 2 receptor gene therapy attenuates hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1182–L1192. doi: 10.1152/ajplung.00020.2006. et al. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds AM, Holmes MD, Danilov SM, Reynolds PN. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur Respir J. 2012;39(2):329–343. doi: 10.1183/09031936.00187310. [DOI] [PubMed] [Google Scholar]
- 31.McMurtry MS, Moudgil R, Hashimoto K, Bonnet S, Michelakis ED, Archer SL. Overexpression of human bone morphogenetic protein receptor 2 does not ameliorate monocrotaline pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L872–L878. doi: 10.1152/ajplung.00309.2006. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Li X, Al-Lamki RS, Southwood M, Zhao J, Lever AM. Smad-dependent and smad-independent induction of id1 by prostacyclin analogues inhibits proliferation of pulmonary artery smooth muscle cells in vitro and in vivo. Circ Res. 2010;107(2):252–262. doi: 10.1161/CIRCRESAHA.109.209940. et al. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Li X, Al-Lamki RS, Wu C, Weiss A, Berk J. Sildenafil potentiates bone morphogenetic protein signaling in pulmonary arterial smooth muscle cells and in experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2013;33(1):34–42. doi: 10.1161/ATVBAHA.112.300121. et al. [DOI] [PubMed] [Google Scholar]
- 34.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123(8):3600–3613. doi: 10.1172/JCI65592. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109(5):1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 36.Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120(Pt 6):964–972. doi: 10.1242/jcs.002949. et al. [DOI] [PubMed] [Google Scholar]
- 37.Upton PD, Davies RJ, Trembath RC, Morrell NW. Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells. J Biol Chem. 2009;284(23):15794–15804. doi: 10.1074/jbc.M109.002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei Z, Salmon RM, Upton PD, Morrell NW, Li W. Regulation of Bone Morphogenetic Protein 9 (BMP9) by Redox-dependent Proteolysis. J Biol Chem. 2014;289(45):31150–31159. doi: 10.1074/jbc.M114.579771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wooderchak-Donahue WL, McDonald J, O'Fallon B, Upton PD, Li W, Roman BL. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet. 2013;93(3):530–537. doi: 10.1016/j.ajhg.2013.07.004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Townson SA, Martinez-Hackert E, Greppi C, Lowden P, Sako D, Liu J. Specificity and structure of a high affinity activin receptor-like kinase 1 (ALK1) signaling complex. J Biol Chem. 2012;287(33):27313–27325. doi: 10.1074/jbc.M112.377960. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long L, Ormiston ML, Yang X, Southwood M, Graf S, Machado RD. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21(7):777–785. doi: 10.1038/nm.3877. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugimoto H, LeBleu VS, Bosukonda D, Keck P, Taduri G, Bechtel W. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat Med. 2012;18(3):396–404. doi: 10.1038/nm.2629. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitman M, Rosen V, Brivanlou AH, Groppe JC, Sebald W, Mueller T. Regarding the mechanism of action of a proposed peptide agonist of the bone morphogenetic protein receptor activin-like kinase 3. Nat Med. 2013;19(7):809–810. doi: 10.1038/nm.3080. [DOI] [PubMed] [Google Scholar]
- 44.Knaus P, Sebald W. Cooperativity of binding epitopes and receptor chains in the BMP/TGFbeta superfamily. Biol Chem. 2001;382(8):1189–1195. doi: 10.1515/BC.2001.149. [DOI] [PubMed] [Google Scholar]
- 45.Isaacs MJ, Kawakami Y, Allendorph GP, Yoon BH, Izpisua Belmonte JC, Choe S. Bone morphogenetic protein-2 and -6 heterodimer illustrates the nature of ligand-receptor assembly. Mol Endocrinol. 2010;24(7):1469–1477. doi: 10.1210/me.2009-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene therapy. 2004;11(17):1312–1320. doi: 10.1038/sj.gt.3302298. et al. [DOI] [PubMed] [Google Scholar]
- 47.Sengle G, Ono RN, Sasaki T, Sakai LY. Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: extracellular matrix interactions and growth factor bioavailability. J Biol Chem. 2011;286(7):5087–5099. doi: 10.1074/jbc.M110.188615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119(25):6162–6171. doi: 10.1182/blood-2012-01-407593. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen H, Brady Ridgway J, Sai T, Lai J, Warming S, Chen H. Context-dependent signaling defines roles of BMP9 and BMP10 in embryonic and postnatal development. Proc Natl Acad Sci U S A. 2013;110(29):11887–11892. doi: 10.1073/pnas.1306074110. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Souza TA, Chen X, Guo Y, Sava P, Zhang J, Hill JJ. Proteomic identification and functional validation of activins and bone morphogenetic protein 11 as candidate novel muscle mass regulators. Mol Endocrinol. 2008;22(12):2689–2702. doi: 10.1210/me.2008-0290. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herrera B, Inman GJ. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC cell biology. 2009;10:20. doi: 10.1186/1471-2121-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller T, Williams K, Johnstone RW, Shilatifard A. Identification, cloning, expression, and biochemical characterization of the testis-specific RNA polymerase II elongation factor ELL3. J Biol Chem. 2000;275(41):32052–32056. doi: 10.1074/jbc.M005175200. [DOI] [PubMed] [Google Scholar]
- 53.Bidart M, Ricard N, Levet S, Samson M, Mallet C, David L. BMP9 is produced by hepatocytes and circulates mainly in an active mature form complexed to its prodomain. Cellular and molecular life sciences: CMLS. 2012;69(2):313–324. doi: 10.1007/s00018-011-0751-1. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neuhaus H, Rosen V, Thies RS. Heart specific expression of mouse BMP-10 a novel member of the TGF-beta superfamily. Mech Dev. 1999;80(2):181–184. doi: 10.1016/s0925-4773(98)00221-4. [DOI] [PubMed] [Google Scholar]
- 55.Chen H, Shi S, Acosta L, Li W, Lu J, Bao S. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131(9):2219–2231. doi: 10.1242/dev.01094. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102(8):914–922. doi: 10.1161/CIRCRESAHA.107.165530. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Constam DB, Robertson EJ. Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. J Cell Biol. 1999;144(1):139–149. doi: 10.1083/jcb.144.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98(16):9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thies RS, Chen T, Davies MV, Tomkinson KN, Pearson AA, Shakey QA. GDF-8 propeptide binds to GDF-8 and antagonizes biological activity by inhibiting GDF-8 receptor binding. Growth factors. 2001;18(4):251–259. doi: 10.3109/08977190109029114. et al. [DOI] [PubMed] [Google Scholar]
- 60.Yang J, Ratovitski T, Brady JP, Solomon MB, Wells KD, Wall RJ. Expression of myostatin pro domain results in muscular transgenic mice. Molecular reproduction and development. 2001;60(3):351–361. doi: 10.1002/mrd.1097. [DOI] [PubMed] [Google Scholar]
- 61.Jiang MS, Liang LF, Wang S, Ratovitski T, Holmstrom J, Barker C. Characterization and identification of the inhibitory domain of GDF-8 propeptide. Biochem Biophys Res Commun. 2004;315(3):525–531. doi: 10.1016/j.bbrc.2004.01.085. et al. [DOI] [PubMed] [Google Scholar]
- 62.Brown MA, Zhao Q, Baker KA, Naik C, Chen C, Pukac L. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J Biol Chem. 2005;280(26):25111–25118. doi: 10.1074/jbc.M503328200. et al. [DOI] [PubMed] [Google Scholar]
- 63.Chen C, Grzegorzewski KJ, Barash S, Zhao Q, Schneider H, Wang Q. An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nat Biotechnol. 2003;21(3):294–301. doi: 10.1038/nbt795. et al. [DOI] [PubMed] [Google Scholar]
- 64.Laux DW, Young S, Donovan JP, Mansfield CJ, Upton PD, Roman BL. Circulating Bmp10 acts through endothelial Alk1 to mediate flow-dependent arterial quiescence. Development. 2013;140(16):3403–3412. doi: 10.1242/dev.095307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roman BL, Pham VN, Lawson ND, Kulik M, Childs S, Lekven AC. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development. 2002;129(12):3009–3019. doi: 10.1242/dev.129.12.3009. et al. [DOI] [PubMed] [Google Scholar]
- 66.Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003;93(7):682–689. doi: 10.1161/01.RES.0000095246.40391.3B. [DOI] [PubMed] [Google Scholar]
- 67.Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97(6):2626–2631. doi: 10.1073/pnas.97.6.2626. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levet S, Ciais D, Merdzhanova G, Mallet C, Zimmers TA, Lee SJ. Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation. Blood. 2013;122(4):598–607. doi: 10.1182/blood-2012-12-472142. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levet S, Ouarne M, Ciais D, Coutton C, Subileau M, Mallet C. BMP9 and BMP10 are necessary for proper closure of the ductus arteriosus. Proc Natl Acad Sci U S A. 2015;112(25):E3207–E3215. doi: 10.1073/pnas.1508386112. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen H, Yong W, Ren S, Shen W, He Y, Cox KA. Overexpression of bone morphogenetic protein 10 in myocardium disrupts cardiac postnatal hypertrophic growth. J Biol Chem. 2006;281(37):27481–27491. doi: 10.1074/jbc.M604818200. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2007;25(5):665–677. doi: 10.1002/jor.20359. et al. [DOI] [PubMed] [Google Scholar]
- 72.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) The Journal of bone and joint surgery American volume. 2003;85-A(8):1544–1552. doi: 10.2106/00004623-200308000-00017. et al. [DOI] [PubMed] [Google Scholar]
- 73.Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279(53):55958–55968. doi: 10.1074/jbc.M407810200. et al. [DOI] [PubMed] [Google Scholar]
- 74.Peng Y, Kang Q, Cheng H, Li X, Sun MH, Jiang W. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. Journal of cellular biochemistry. 2003;90(6):1149–1165. doi: 10.1002/jcb.10744. et al. [DOI] [PubMed] [Google Scholar]
- 75.Luo J, Tang M, Huang J, He BC, Gao JL, Chen L. TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J Biol Chem. 2010;285(38):29588–29598. doi: 10.1074/jbc.M110.130518. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu N, Jiang D, Huang E, Liu X, Li R, Liang X. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. J Cell Sci. 2013;126(Pt 2):532–541. doi: 10.1242/jcs.114231. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheyn D, Kimelman-Bleich N, Pelled G, Zilberman Y, Gazit D, Gazit Z. Ultrasound-based nonviral gene delivery induces bone formation in vivo. Gene therapy. 2008;15(4):257–266. doi: 10.1038/sj.gt.3303070. [DOI] [PubMed] [Google Scholar]
- 78.Aslan H, Zilberman Y, Arbeli V, Sheyn D, Matan Y, Liebergall M. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006;12(4):877–889. doi: 10.1089/ten.2006.12.877. et al. [DOI] [PubMed] [Google Scholar]
- 79.Luther G, Wagner ER, Zhu G, Kang Q, Luo Q, Lamplot J. BMP-9 induced osteogenic differentiation of mesenchymal stem cells: molecular mechanism and therapeutic potential. Current gene therapy. 2011;11(3):229–240. doi: 10.2174/156652311795684777. et al. [DOI] [PubMed] [Google Scholar]
- 80.Bosch P, Musgrave D, Ghivizzani S, Latterman C, Day CS, Huard J. The efficiency of muscle-derived cell-mediated bone formation. Cell transplantation. 2000;9(4):463–470. doi: 10.1177/096368970000900403. [DOI] [PubMed] [Google Scholar]
- 81.Lee JY, Musgrave D, Pelinkovic D, Fukushima K, Cummins J, Usas A. Effect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. The Journal of bone and joint surgery American volume. 2001;83-A(7):1032–1039. doi: 10.2106/00004623-200107000-00008. et al. [DOI] [PubMed] [Google Scholar]
- 82.Lee JY, Peng H, Usas A, Musgrave D, Cummins J, Pelinkovic D. Enhancement of bone healing based on ex vivo gene therapy using human muscle-derived cells expressing bone morphogenetic protein 2. Hum Gene Ther. 2002;13(10):1201–1211. doi: 10.1089/104303402320138989. et al. [DOI] [PubMed] [Google Scholar]
- 83.Varady P, Li JZ, Cunningham M, Beres EJ, Das S, Engh J. Morphologic analysis of BMP-9 gene therapy-induced osteogenesis. Hum Gene Ther. 2001;12(6):697–710. doi: 10.1089/104303401300057423. et al. [DOI] [PubMed] [Google Scholar]
- 84.Li JZ, Hankins GR, Kao C, Li H, Kammauff J, Helm GA. Osteogenesis in rats induced by a novel recombinant helper-dependent bone morphogenetic protein-9 (BMP-9) adenovirus. The journal of gene medicine. 2003;5(9):748–756. doi: 10.1002/jgm.412. [DOI] [PubMed] [Google Scholar]
- 85.Leblanc E, Trensz F, Haroun S, Drouin G, Bergeron E, Penton CM. BMP-9-induced muscle heterotopic ossification requires changes to the skelcomment muscle microenvironment. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2011;26(6):1166–1177. doi: 10.1002/jbmr.311. et al. [DOI] [PubMed] [Google Scholar]
- 86.Zhu SF, Hu HB, Xu HY, Fu XF, Peng DX, Su WY. Human umbilical cord mesenchymal stem cell transplantation restores damaged ovaries. J Cell Mol Med. 2015 doi: 10.1111/jcmm.12571. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim CW, Song H, Kumar S, Nam D, Kwon HS, Chang KH. Anti-inflammatory and antiatherogenic role of BMP receptor II in endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(6):1350–1359. doi: 10.1161/ATVBAHA.112.300287. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song JJ, Celeste AJ, Kong FM, Jirtle RL, Rosen V, Thies RS. Bone morphogenetic protein-9 binds to liver cells and stimulates proliferation. Endocrinology. 1995;136(10):4293–4297. doi: 10.1210/endo.136.10.7664647. [DOI] [PubMed] [Google Scholar]
- 89.Miller AF, Harvey SA, Thies RS, Olson MS. Bone morphogenetic protein-9. An autocrine/paracrine cytokine in the liver. J Biol Chem. 2000;275(24):17937–17945. doi: 10.1074/jbc.275.24.17937. [DOI] [PubMed] [Google Scholar]
- 90.Xia Y, Babitt JL, Sidis Y, Chung RT, Lin HY. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood. 2008;111(10):5195–5204. doi: 10.1182/blood-2007-09-111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Q, Gu X, Weng H, Ghafoory S, Liu Y, Feng T. Bone morphogenetic protein-9 induces epithelial to mesenchymal transition in hepatocellular carcinoma cells. Cancer Sci. 2013;104(3):398–408. doi: 10.1111/cas.12093. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wiercinska E, Wickert L, Denecke B, Said HM, Hamzavi J, Gressner AM. Id1 is a critical mediator in TGF-beta-induced transdifferentiation of rat hepatic stellate cells. Hepatology. 2006;43(5):1032–1041. doi: 10.1002/hep.21135. et al. [DOI] [PubMed] [Google Scholar]
- 93.Herrera B, Garcia-Alvaro M, Cruz S, Walsh P, Fernandez M, Roncero C. BMP9 is a proliferative and survival factor for human hepatocellular carcinoma cells. PloS one. 2013;8(7):e69535. doi: 10.1371/journal.pone.0069535. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caperuto LC, Anhe GF, Cambiaghi TD, Akamine EH, do Carmo Buonfiglio D, Cipolla-Neto J. Modulation of bone morphogenetic protein-9 expression and processing by insulin, glucose, and glucocorticoids: possible candidate for hepatic insulin-sensitizing substance. Endocrinology. 2008;149(12):6326–6335. doi: 10.1210/en.2008-0655. et al. [DOI] [PubMed] [Google Scholar]
- 95.George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304(5675):1325–1328. doi: 10.1126/science.1096706. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hussain K, Challis B, Rocha N, Payne F, Minic M, Thompson A. An activating mutation of AKT2 and human hypoglycemia. Science. 2011;334(6055):474. doi: 10.1126/science.1210878. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB., 3rd Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292(5522):1728–1731. doi: 10.1126/science.292.5522.1728. et al. [DOI] [PubMed] [Google Scholar]
- 98.Anhe FF, Lellis-Santos C, Leite AR, Hirabara SM, Boschero AC, Curi R. Smad5 regulates Akt2 expression and insulin-induced glucose uptake in L6 myotubes. Molecular and cellular endocrinology. 2010;319(1–2):30–38. doi: 10.1016/j.mce.2010.01.003. et al. [DOI] [PubMed] [Google Scholar]
- 99.Bogaard HJ, Al Husseini A, Farkas L, Farkas D, Gomez-Arroyo J, Abbate A. Severe pulmonary hypertension: The role of metabolic and endocrine disorders. Pulm Circ. 2012;2(2):148–154. doi: 10.4103/2045-8932.97592. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cottrill KA, Chan SY. Metabolic dysfunction in pulmonary hypertension: the expanding relevance of the Warburg effect. European journal of clinical investigation. 2013;43(8):855–865. doi: 10.1111/eci.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Herrera B, van Dinther M, Ten Dijke P, Inman GJ. Autocrine bone morphogenetic protein-9 signals through activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Res. 2009;69(24):9254–9262. doi: 10.1158/0008-5472.CAN-09-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ye L, Kynaston H, Jiang WG. Bone morphogenetic protein-9 induces apoptosis in prostate cancer cells, the role of prostate apoptosis response-4. Molecular cancer research: MCR. 2008;6(10):1594–1606. doi: 10.1158/1541-7786.MCR-08-0171. [DOI] [PubMed] [Google Scholar]
- 103.Li B, Yang Y, Jiang S, Ni B, Chen K, Jiang L. Adenovirus-mediated overexpression of BMP-9 inhibits human osteosarcoma cell growth and migration through downregulation of the PI3K/AKT pathway. Int J Oncol. 2012;41(5):1809–1819. doi: 10.3892/ijo.2012.1617. [DOI] [PubMed] [Google Scholar]
- 104.Lv Z, Yang D, Li J, Hu M, Luo M, Zhan X. Bone morphogenetic protein 9 overexpression reduces osteosarcoma cell migration and invasion. Molecules and cells. 2013;36(2):119–126. doi: 10.1007/s10059-013-0043-8. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Castonguay R, Werner ED, Matthews RG, Presman E, Mulivor AW, Solban N. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J Biol Chem. 2011;286(34):30034–30046. doi: 10.1074/jbc.M111.260133. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cunha SI, Pardali E, Thorikay M, Anderberg C, Hawinkels L, Goumans MJ. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J Exp Med. 2010;207(1):85–100. doi: 10.1084/jem.20091309. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suzuki Y, Ohga N, Morishita Y, Hida K, Miyazono K, Watabe T. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J Cell Sci. 2010;123(Pt 10):1684–1692. doi: 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- 108.van Baardewijk LJ, van der Ende J, Lissenberg-Thunnissen S, Romijn LM, Hawinkels LJ, Sier CF. Circulating bone morphogenetic protein levels and delayed fracture healing. International orthopaedics. 2013;37(3):523–527. doi: 10.1007/s00264-012-1750-z. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]