Abstract
Background
To investigate concerns about a potential association between quadrivalent human papillomavirus vaccination (HPV4) and venous thromboembolism (VTE), we conducted a self-controlled case series study in adolescents and young adults 9–26 years of age in the Vaccine Safety Datalink.
Methods
We identified potential VTE cases diagnosed in 2008 through 2011 who had also received at least one HPV4 dose during that period. We confirmed each presumptive diagnosis by medical record review. We calculated incidence rate ratios (IRRs) and 95% confidence intervals (CI) to estimate the risk in the 1–60 day period following HPV4 exposure and in subsets of that period. IRRs were stratified by age, gender, hormonal contraceptive use, and recent surgery or trauma.
Results
We identified 313 potential cases of VTE among HPV4 vaccinees, and 291 (93%) had sufficient medical records for review. Of these, we confirmed 156 (54%) cases. VTE was uncommon among males (n = 3) and 9–12 year olds (n = 4). Nearly all confirmed cases (97%) had at least one known risk factor for VTE, including hormonal contraceptive use, obesity, and hypercoagulability. Sixteen (10%) confirmed cases occurred in the 1–60 days following HPV4 exposure. The risk of VTE varied from 1.47 (95% CI: 0.47–4.64) in the 1–7 days following HPV4 exposure to 0.92 (95% CI: 0.54–1.57) in the 1–60 days following vaccination. It was not possible to calculate a stratified IRR for males due to small sample size; the other risk factors evaluated did not significantly affect the risk of VTE after HPV4 exposure.
Conclusion
The risk of developing VTE among 9- to 26-year-olds was not elevated following HPV4 exposure. Sample size limited our ability to rigorously evaluate potential effect modifiers, such as gender, through stratified analysis.
Keywords: Human papillomavirus vaccine, Safety, Venous thromboembolism, Adolescents
1. Introduction
Two post-licensure monitoring studies from the United States have reported a potential association between quadrivalent human papillomavirus vaccination (HPV4) and venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE). An increased rate of VTE reporting with HPV4 vaccination (0.2 per 100,000 HPV4 doses distributed) compared to other adolescent vaccines was observed in the U.S. Vaccine Adverse Event Reporting System [1], and an elevated risk of VTE (relative risk (RR) = 1.98) was noted in the 1–7 days following HPV4 vaccination among females 9–17 years old in the Vaccine Safety Datalink (VSD) [2]. The number of cases included in these U.S. studies was small, and cases had multiple known risk factors for VTE, primarily oral contraceptive use.
In contrast to these early observations, later studies from North America and Scandinavia have reported no increased risk of VTE following HPV4 vaccination in young women [3–6]. No VTE was reported among 12–15 year old Canadian girls, and no increased risk of VTE was observed in a cohort of 9–26 year old females in California [3,5]. Using national registry data from Denmark and Sweden, Arnheim-Dahlstrom et al. reported a RR of 0.86 in the 90-day exposure period following vaccination in a cohort of females 10–17 years old, and Scheller et al. reported a RR of 0.77 in the 42 days after exposure among Danish females 10–44 years old using a self-controlled case series design [4,6]. An important limitation of these studies was they did not review medical records to confirm VTE diagnoses, which may have resulted in misclassification of case status and onset date. Moreover, none of the studies to date have included males, although HPV4 has been licensed for males in the United States since October 2009 [7].
The primary objective of our study was to assess the risk of confirmed VTE following HPV4 exposure in a large population-based cohort of female and male adolescents and young adults in the VSD. Our secondary objective was to identify potential effect modification between known VTE risk factors and HPV4 exposure in this population.
2. Methods
The VSD is a collaboration between nine integrated healthcare delivery systems (“sites”) and the Centers for Disease Control and Prevention (CDC) [8]. Each site creates annual study data files using available electronic medical records and administrative databases. The files contain information about member demographics and health plan enrollment, diagnoses, procedures, and immunizations.
We used these VSD datasets to conduct a self-controlled case series analysis of VTE risk following HPV vaccination among adolescents and young adults 9–26 years of age, the ages for which the vaccine is licensed. The self-controlled case series method involves identifying cases, dividing each case's observation time into pre-defined exposed and unexposed periods based on dates of vaccination, and calculating the incidence rate ratio (IRR) using these periods [9].
2.1. Case ascertainment and validation
We identified potential cases of VTE (DVT and PE) defined as persons with an ICD-9 diagnosis code of 415.1x (pulmonary embolism), 451.1x (phlebitis and thrombophlebitis of deep vessels of lower extremities), or 453.x (deep vein thrombosis and thrombophlebitis) assigned to outpatient, inpatient, and emergency room encounters from January 1, 2008 through December 31, 2011 at six VSD sites (Kaiser Permanente Northwest, Group Health, Kaiser Permanente Colorado, Kaiser Permanente Southern California, Kaiser Permanente Northern California, and Marshfield Clinic). We limited potential cases to those who did not have a VTE diagnosis assigned between 2001 and 2007 in order to focus on incident cases and exclude follow-up care for VTE or recurrent diagnoses. We further limited potential cases to those who had received at least one HPV4 dose during the 2008–2011 study period, and were enrolled in the health plan at the time of both VTE symptom onset and vaccination.
Trained medical records abstractors manually reviewed the electronic medical record for all selected potential cases to confirm the incident VTE diagnosis, estimate the date of onset of VTE symptoms, and collect additional information on known VTE risk factors. Reviewers were blinded to the vaccination status and date of the potential case. We used the abstracted information to further classify cases into four groups based on criteria used in other epidemiologic studies of VTE: definite, probable, possible, and not VTE (Table 1) [10]. Radiologic confirmation was required to meet the definition of a definite DVT or PE case. Complicated cases were reviewed and adjudicated by consensus by a committee of physician study investigators (T.H., C.V., N.K., L.J.).
Table 1.
Case criteria for defining venous thromboembolism events (from Spencer et al., 2006 [8]).
| Deep vein thrombosis (DVT) |
| Definite - if confirmed by venography, compression/duplex ultrasound, CT scan, MRI scan, or at autopsy. |
| Probable - if the above tests were not performed, or were indeterminate, but impedance plethysomography, radionuclide venography, or radiolabelled fibrinogen scan test results were reported as positive. |
| Possible - if all of these confirmatory tests were not performed, or were indeterminate, and 2 of the following criteria were satisfied - medical record indicates that the physician made a diagnosis of DVT, signs and/or symptoms of DVT were documented, and the patient underwent therapy with anticoagulants, or an IVC filter was placed. |
| Pulmonary embolism (PE) |
| Definite - if confirmed by pulmonary angiography, spiral CT scan, MRI scan, or pathology. |
| Probable - if the above tests were not performed, or were indeterminate, but ventilation-perfusion scan findings were of high probability. |
| Possible - if all of the above confirmatory tests were not performed, or were indeterminate, and 2 of the following criteria were satisfied - medical record indicates that the physician made a diagnosis of PE, signs and/or symptoms of PE were documented, and the patient underwent therapy with anticoagulants, or an IVC filter was placed. |
2.2. Exposure and risk factor ascertainment
We extracted dose number and administration date for all HPV4 doses received during the study period from the study data files. We manually abstracted information about known risk factors for VTE from the electronic medical records, including pneumonia, cancer, or hypercoagulability (e.g., Factor V Leiden, Protein S deficiency); pregnancy, surgery, trauma, international or long-distance travel, and long-term care facility residence within 60 days prior to VTE diagnosis; hormonal contraceptive use; smoking status; family history of VTE; and presence of a peripherally inserted central catheter (PICC) or central venous catheter (CVC) [11–15]. Abstracted height and weight were used to calculate body mass index (BMI), which was categorized as underweight or normal (<25.0 m/kg2), overweight (25.0–29.9 m/kg2), or obese (≥30.0 m/kg2). We also extracted additional pregnancy information from the VSD data files, including pregnancy dates and outcomes, using a previously validated algorithm [16].
2.3. Analysis
Our analysis included only chart-confirmed cases (definite, probable, and possible VTE) who were vaccinated during the study period (2008–2011). Observation time began January 1, 2008 or the case's first health plan enrollment date thereafter. Observation time was terminated at the end of the study period (December 31, 2011) or health plan disenrollment, whichever occurred first. We then divided this observation time into exposure periods (1–7, 1–14, 1–28, 1–42, and 1–60 days following HPV4 vaccination); all other observation time was considered unexposed. We selected the 1–60 day exposure period to encompass the theoretical period during which inflammatory or coagulation pathways may be activated following an exposure and result in medically recognized VTE.
Using the date of VTE symptom onset, we calculated IRRs and 95% confidence intervals (CIs) with conditional Poisson regression to compare the incidence of VTE in HPV4 exposed periods to the incidence in unexposed periods. We decided a priori to calculate age- and gender-stratified IRRs, and to calculate IRRs stratified by exposure to hormonal contraceptives at the time of VTE diagnosis, assuming that these might be important effect modifiers. We also calculated IRRs stratified by exposure to surgery or trauma within the 60 days preceding VTE diagnosis.
Institutional Review Boards at each participating site and the Centers for Disease Control and Prevention reviewed and approved the study protocol and abstraction form.
3. Results
3.1. Case ascertainment and validation
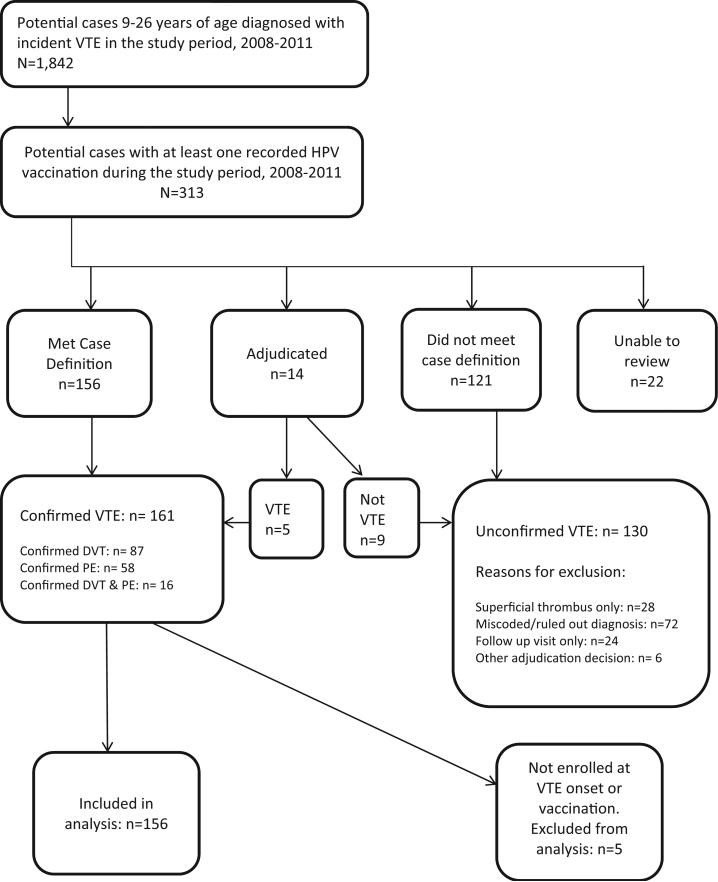
During the study period, there were about 1.24 million doses of HPV4 administered to 650,737 persons 9–26 years of age enrolled in the VSD. We identified 313 persons with a diagnosis of VTE who had received at least one HPV4 dose during the study period (Fig. 1). Of these, 291 (93%) had sufficient medical records for review. After medical record review, 156 (54%) of the cases reviewed met the VTE definition, 121 (42%) did not meet the definition, and 14 (5%) required further review and adjudication. We confirmed 161 cases of VTE, including 87 DVT cases, 58 PE cases, and 16 cases with both DVT and PE. Among the 130 potential cases that were not confirmed, reasons for exclusion were miscoded or ruled out diagnoses (n = 72), encounters for VTE follow-up care rather than new onset cases (n = 24), thromboses in superficial rather than deep veins (n = 28), and adjudication decision (n = 6).
Fig. 1.
Identification and confirmation of vaccinated venous thromboembolism cases.
We excluded an additional 5 of the 161 confirmed cases because they had incomplete health plan enrollment records at the time of VTE onset or vaccination to allow for the calculation of observation time, leaving 156 confirmed cases for analysis. Of these 156 confirmed cases, nine (6%) occurred in the 1–60 days following exposure to HPV4 dose 1, five (3%) following dose 2, two (1%) following dose 3, and 140 (90%) during pre- or post-vaccination unexposed observation time.
3.2. Case characteristics
Ninety-seven percent of the 101 confirmed DVTs included in our analysis were categorized as definite, 0% as probable, and 3% as possible. Seventy percent of confirmed DVTs were located in the lower extremities. Eighty-nine percent of the 71 confirmed PEs included in our analysis were categorized as definite, 0% as probable, and 11% as possible. On average, there was a delay of 4.4 days between symptom onset and diagnosis. Confirmed cases of VTE among males (n = 3) and 9–12 year olds (n = 4) were uncommon (Table 2). Most cases (97%) had at least one known VTE risk factor, and 47% had three or more risk factors. The most common risk factors in this population were hormonal contraceptive use, obesity, surgery or trauma in the 60 days prior to VTE diagnosis, and hypercoagulability. Among the 92 women using hormonal contraceptives, 26 (28%) initiated contraceptive use within three months of VTE onset, 56 (61%) initiated more than three months before onset, and 10 (11%) had unknown initiation dates.
Table 2.
Characteristics of confirmed cases of venous thromboembolism among HPV4 vaccinees, Vaccine Safety Datalink, 2008-2011.
| VTE Total (156) | DVT (101) | PE (71) | |
|---|---|---|---|
| Age | |||
| 9-12 years | 4 (3%) | 3 (3%) | 1 (1%) |
| 13-17 years | 33 (21%) | 21 (21%) | 15 (21%) |
| 18-22 years | 70 (45%) | 43 (43%) | 34 (48%) |
| 23-26 years | 49 (31%) | 34 (34%) | 21 (30%) |
| Gender | |||
| Female | 153 (98%) | 100 (99%) | 69 (97%) |
| Male | 3 (2%) | 1 (1%) | 2 (3%) |
| Risk factors | |||
| PICC or CVC | 13 (8%) | 8 (8%) | 6 (8%) |
| Pneumoniaa | 11 (7%) | 5 (5%) | 8 (11%) |
| Malignancyb | 6 (4%) | 5 (5%) | 2 (3%) |
| Surgery or Traumaa | 49 (31%) | 39 (39%) | 16 (23%) |
| Pregnancya | 12 (8%) | 8 (8%) | 8 (11%) |
| LTCFa | 2 (1%) | 1 (1%) | 1 (1%) |
| International/Long-Distance Travela | 17 (11%) | 9 (9%) | 9 (13%) |
| Hypercoagulability | 70 (45%) | 40 (40%) | 42 (59%) |
| Smoking (current) | 20 (13%) | 15 (15%) | 7 (10%) |
| Hormonal contraceptives | 92 (59%) | 56 (55%) | 45 (63%) |
| Obesity (BMI ≥ 30.0 m/kg2) | 48 (31%) | 27 (27%) | 27 (38%) |
| Risk factor (sum) | |||
| 0 | 5 (3%) | 5 (5%) | 0 |
| 1 | 26 (17%) | 15 (15%) | 11 (15%) |
| 2 | 51 (33%) | 33 (33%) | 21 (30%) |
| 3-5 | 74 (47%) | 48 (48%) | 39 (55%) |
DVT = deep vein thrombosis; PE = pulmonary embolism; PICC = peripherally inserted central catheter; CVC = central venous catheter; LTCF = long-term care facility.
Occurred at the time of, or within 60 days prior to, VTE diagnosis.
Occurred at the time of, or within 6 months prior to, VTE diagnosis.
3.3. VTE risk associated with HPV4
We observed no increased risk of VTE in any exposure period; risk varied from 0.72 (95% CI: 0.31–1.63) in the 1–28 days following HPV4 exposure to 1.47 (95% CI: 0.47–4.64) in the 1–7 days following vaccination (Table 3). There were no males who had received HPV4 within 60 days prior to VTE symptom onset, so their risk estimates could not be calculated. Risk ranged across the five exposure periods from 0.59 (95% CI: 0.08–4.27) to 1.20 (95% CI: 0.16–8.71) among 9–18 year-olds, and from 0.60 (95% CI: 0.19–1.92) to 1.66 (95% CI: 0.40–6.81) among 19–26 year olds. Among females using hormonal contraceptives at the time of diagnosis, VTE risk ranged from 0.41 (95% CI: 0.10–1.69) to 1.16 (95% CI: 0.60–2.23); risk among non-users ranged from 2.44 (95% CI: 0.59–10.12) in the 1–7 days after vaccination to 0.67 (95% CI: 0.26–1.72) in the 1–60 day period. There were no cases of VTE in the 1–14 days after vaccination with a recent surgery or trauma; the IRR ranged from 0.68 (95% CI: 0.25–1.88) to 2.15 (95% CI: 0.67–6.84) following vaccination among cases without a recent surgery or trauma.
Table 3.
Number of exposed cases and risk of VTE following vaccination overall and stratified by gender, age, hormonal contraceptive use, and recent surgery or trauma.
| Exposure periods following HPV vaccination |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1-7 days |
1-14 days |
1-28 days |
1-42 days |
1-60 days |
||||||
| Cases | 1RR (95% CI) | Cases | 1RR (95% CI) | Cases | 1RR (95% CI) | Cases | 1RR (95% CI) | Cases | 1RR (95% CI) | |
| Overall | 3 | 1.47 (0.47-4.64) | 4 | 0.97 (0.36-2.65) | 6 | 0.72 (0.31-1.63) | 10 | 0.80 (0.42-1.54) | 16 | 0.92 (0.54-1.57) |
| Males | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - |
| Females | 3 | 1.51 (0.48-4.76) | 4 | 1.00 (0.37-2.71) | 6 | 0.73 (0.32-1.68) | 10 | 0.82 (0.43-1.58) | 16 | 0.95 (0.55-1.62) |
| Ages 9-18 | 1 | 1.20 (0.16-8.71) | 1 | 0.59 (0.08-4.27) | 3 | 0.89 (0.28-2.87) | 5 | 1.01 (0.40-2.55) | 8 | 1.18 (0.55-2.52) |
| Ages 19-26 | 2 | 1.66 (0.40-6.81) | 3 | 1.25 (0.39-3.99) | 3 | 0.60 (0.19-1.92) | 5 | 0.66 (0.26-1.66) | 8 | 0.74 (0.35-1.58) |
| Contraceptive Use | 1 | 0.86 (0.12-6.18) | 1 | 0.42 (0.06-3.04) | 2 | 0.41 (0.10-1.69) | 6 | 0.85 (0.37-1.99) | 11 | 1.16 (0.60-2.23) |
| No Contraceptive Use | 2 | 2.44 (0.59-10.12) | 3 | 1.84 (0.57-5.97) | 4 | 1.21 (0.43-3.40) | 4 | 0.78 (0.28-2.19) | 5 | 0.67 (0.26-1.72) |
| Surgery/Trauma | 0 | - | 0 | - | 2 | 0.79 (0.19-3.29) | 3 | 0.78 (0.24-2.56) | 4 | 0.73 (0.26-2.05) |
| No Surgery/Trauma | 3 | 2.15 (0.67-6.84) | 4 | 1.43 (0.52-3.92) | 4 | 0.68 (0.25-1.88) | 7 | 0.81 (0.37-1.77) | 12 | 1.01 (0.54-1.89) |
4. Discussion
In this population-based cohort of adolescents and young adults, we observed no increased risk of VTE in the 1–60 days following HPV4 exposure. Despite the large population of adolescents and young adults included in the VSD, we identified only a small number of exposed VTE cases and were limited in our ability to rigorously evaluate potential effect modifiers through stratified analysis. Additional study of VTE risk in males, in particular, may be warranted since our study only identified three HPV4-exposed male confirmed cases.
Our findings of no elevated VTE risk following HPV4 vaccination are consistent with the recently published Scandinavian studies, which reported risk estimates in the range of 0.77–0.86 [4,6]. These prior studies did not validate cases with medical record review so they may have been biased toward the null hypothesis due to misclassification of both exposure periods and outcomes. Previous studies have demonstrated that the predictive values of ICD-9 codes for VTE range from 29% to 95% depending on the individual code, diagnosis setting (outpatient vs. inpatient), and the position of the code (primary vs. secondary) [17]. Medical record review is especially important since the case confirmation rate we observed in this study was low (54%) and symptom onset was on average more than four days before VTE diagnosis. Our study overcomes these previous limitations by using chart-reviewed cases that met a standard case definition and symptom onset date rather than the diagnosis date.
Recent findings from the U.S. Sentinel System are also consistent with the VSD results reported here [18]. The Sentinel System reported an overall positive predictive value of 24% for three VTE ICD-9 codes (415.1x, 451.x, and 453.x) that ranges from 6% in the outpatient setting to 64% in the inpatient setting. Using a self-controlled risk interval design and chart-confirmed cases, the risk of VTE in the 1–28 day period following HPV4 exposure in females 9–26 years of age was 0.70 (95%CI: 0.33–1.44).
VTE is uncommon in the age group for whom HPV4 is licensed and recommended, with an estimated incidence of 1–7 cases per 10,000 in persons less than 30 years old [11–15]. The cases we identified had many other known risk factors for VTE, though small sample size limited our ability to investigate potential effect modification by these risk factors. The self-controlled case series design inherently controls for risk factors, such as underlying genetic susceptibility to VTE, that do not change with time [9]. The method is less robust for time-varying exposures, such as age, duration of contraceptive use, or acute events such as surgery or trauma. None of these exposures appeared to have significantly affected the risk of VTE following vaccination in our stratified models, although the baseline risk of VTE may have varied within the strata.
HPV4 vaccination could hypothetically induce thrombus formation and VTE by triggering inflammatory or coagulation pathways [19]. We selected the 1–60 day exposure period to encompass the period during which these pathways may be activated following an exposure and result in medically recognized VTE; however, previous studies have used risk periods as short as 7 days and as long as 90 days following vaccination. We saw small elevations in risk in the 1–7 day period following HPV4 exposure, but the number of cases exposed in these periods was small, and the corresponding confidence intervals were wide and not statistically significant. This elevation in the 1–7 days following vaccination is consistent with the RR of 1.98 observed in the Gee et al. VSD study [2]. Risk generally decreased as the exposure period lengthened and the number of exposed cases increased.
In conclusion, the risk of developing VTE among 9- to 26-year-olds was not elevated following HPV4 exposure based on this large U.S. study and on data from several other population-based studies. These findings should assure healthcare providers, parents, and patients about the safety of this vaccine.
Acknowledgments
Funding statement
This study was funded by the Centers for Disease Control and Prevention (contract number 200-2012-53584).
Dr. Naleway reports receiving research support from Glaxo-SmithKline and Pfizer. Dr. Klein reports receiving research support from Merck, GlaxoSmithKline, Pfizer, Sanofi Pasteur, Novartis, Nuron Biotech, MedImmune, and Protein Science.
Footnotes
Conflicts of interest
None of the remaining authors have conflicts of interest to disclose.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, et al. Postlicen-sure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750–7. doi: 10.1001/jama.2009.1201. [DOI] [PubMed] [Google Scholar]
- 2.Gee J, Naleway A, Shui I, Baggs J, Yin R, Li R, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011;29:8279–84. doi: 10.1016/j.vaccine.2011.08.106. [DOI] [PubMed] [Google Scholar]
- 3.Klein NP, Hansen J, Chao C, Velicer C, Emery M, Slezak J, et al. Safety of quadrivalent human papillomavirus vaccine administered routinely to females. Arch Pediatr Adolesc Med. 2012;166:1140–8. doi: 10.1001/archpediatrics.2012.1451. [DOI] [PubMed] [Google Scholar]
- 4.Arnheim-Dahlstrom L, Pasternak B, Svanstrom H, Sparen P, Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunization of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. Br Med J. 2013;347:f5906. doi: 10.1136/bmj.f5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris ST, Williams DM, Fediurek J, Scott T, Deeks SL. Adverse events following immunization in Ontario's female school-based HPV program. Vaccine. 2014;32:1061–6. doi: 10.1016/j.vaccine.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Scheller NM, Pasternak B, Svanstrom H, Hviid A. Quadrivalent human papillomavirus vaccine and the risk of venous thromboembolism. JAMA. 2014;312:187–8. doi: 10.1001/jama.2014.2198. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration [18.02.15];Approval Letter – Gardasil. 2009 Oct 16; Available at: www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186991.htm.
- 8.Baggs J, Gee J, Lewis E, Fowler F, Benson P, Lieu T, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics. 2011;127(Suppl. 1):S45–53. doi: 10.1542/peds.2010-1722H. [DOI] [PubMed] [Google Scholar]
- 9.Whitaker HJ, Hocine MN, Farrington CP. The methodology of self-controlled case series studies. Stat Methods Med Res. 2009;18:7–26. doi: 10.1177/0962280208092342. [DOI] [PubMed] [Google Scholar]
- 10.Spencer FA, Emery C, Lessard D, Anderson F, Emani S, Aragam J, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21:722–7. doi: 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton JL. Trends in the incidence of deep vein thrombosis and pulmonary embolism. A 25-year population-based study. Arch Intern Med. 1998;158:585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 12.Fowkes FJ, Price JF, Fowkes FG. Incidence of diagnosed deep vein thrombosis in the general population: systematic review. Eur J Vasc Endovasc Surg. 2003;25:1–5. doi: 10.1053/ejvs.2002.1778. [DOI] [PubMed] [Google Scholar]
- 13.Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328–36. doi: 10.1016/j.contraception.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Ageno W, Squizzato A, Garcia D, Imberti D. Epidemiology and risk factors of venous thromboembolism. Semin Thromb Hemost. 2006;32:651–8. doi: 10.1055/s-2006-951293. [DOI] [PubMed] [Google Scholar]
- 15.Boulet SL, Grosse SD, Thornburg CD, Yusuf H, Tsai J, Hooper WC. Trends in venous thromboembolism-related hospitalizations, 1994–2009. Pediatrics. 2012;130:e812–20. doi: 10.1542/peds.2012-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naleway AL, Gold R, Kurosky S, Riedlinger K, Henninger ML, Nordin JD, et al. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine. 2013;31:2898–903. doi: 10.1016/j.vaccine.2013.03.069. [DOI] [PubMed] [Google Scholar]
- 17.White RH, Garcia M, Sadeghi B, Tancredi DJ, Zrelak P, Cuny J, et al. Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thromb Res. 2010;126:61–7. doi: 10.1016/j.thromres.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Yih WH, Greene SK, Zichittella L, deJong JL, Gil-Prieto R, Griffin MR, et al. [April 29.04.15];Mini-sentinel medical product assessment: evaluation of the risk of venous thromboembolism after gardasil vaccination. Available at: http://www.mini-sentinel.org/assessments/medical events/default.aspx.
- 19.IOM (Institute of Medicine) Adverse effects of vaccines: evidence and causality. National Academies Press; Washington, DC: 2012. [PubMed] [Google Scholar]